Development and Characterization of a Pilot-Scale Model Cocoa Fermentation System Suitable for Studying the Impact of Fermentation on Putative Bioactive Compounds and Bioactivity of Cocoa
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Pilot-Scale Fermentation Model
2.3. Microbial Enumeration
2.4. Cut Test
2.5. Fermentation Index
2.6. Bean pH
2.7. High-Performance Liquid Chromatography (HPLC) Analysis of Fermentation Metabolites
2.7.1. Pulp Media Sample Preparation
2.7.2. Bean Sample Preparation
2.7.3. Analysis
2.8. Polyphenol Extraction and Quantification
2.9. Individual Polyphenol Analysis by Reversed Phase UPLC-MS
2.10. HILIC UPLC-MS/MS
2.11. Data Analysis and Statistics
3. Results
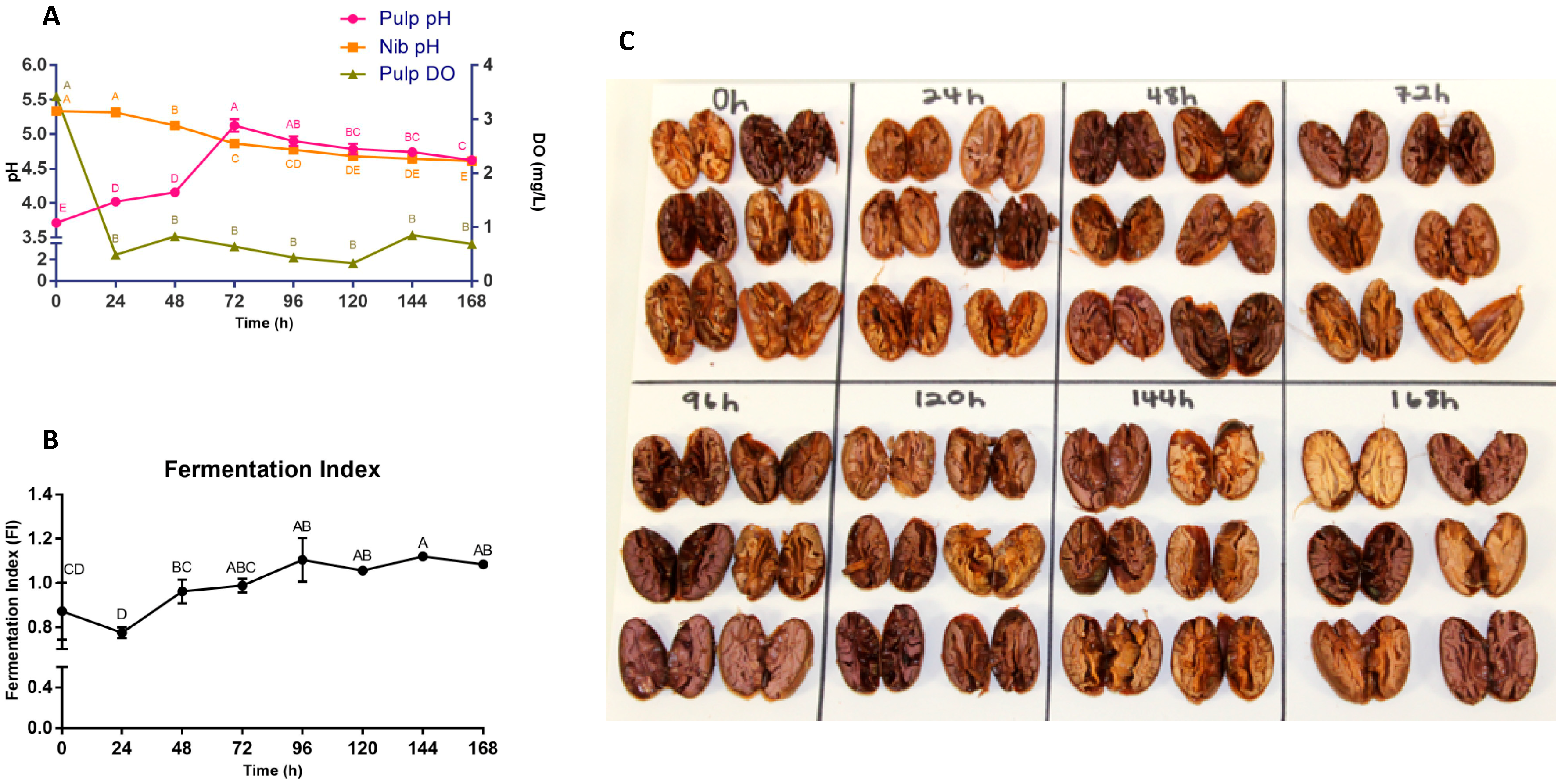
3.1. pH, DO, FI, Cut Test
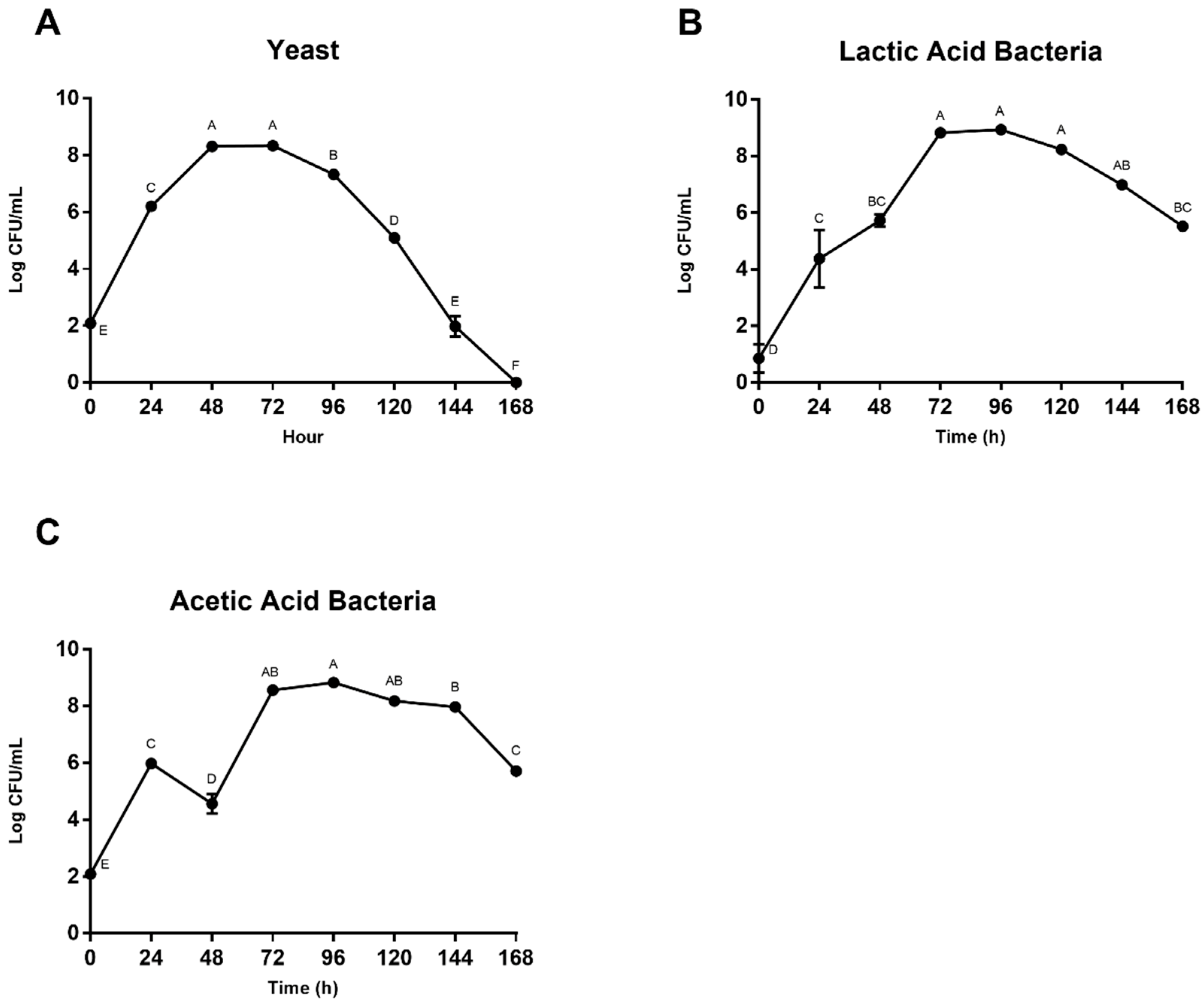
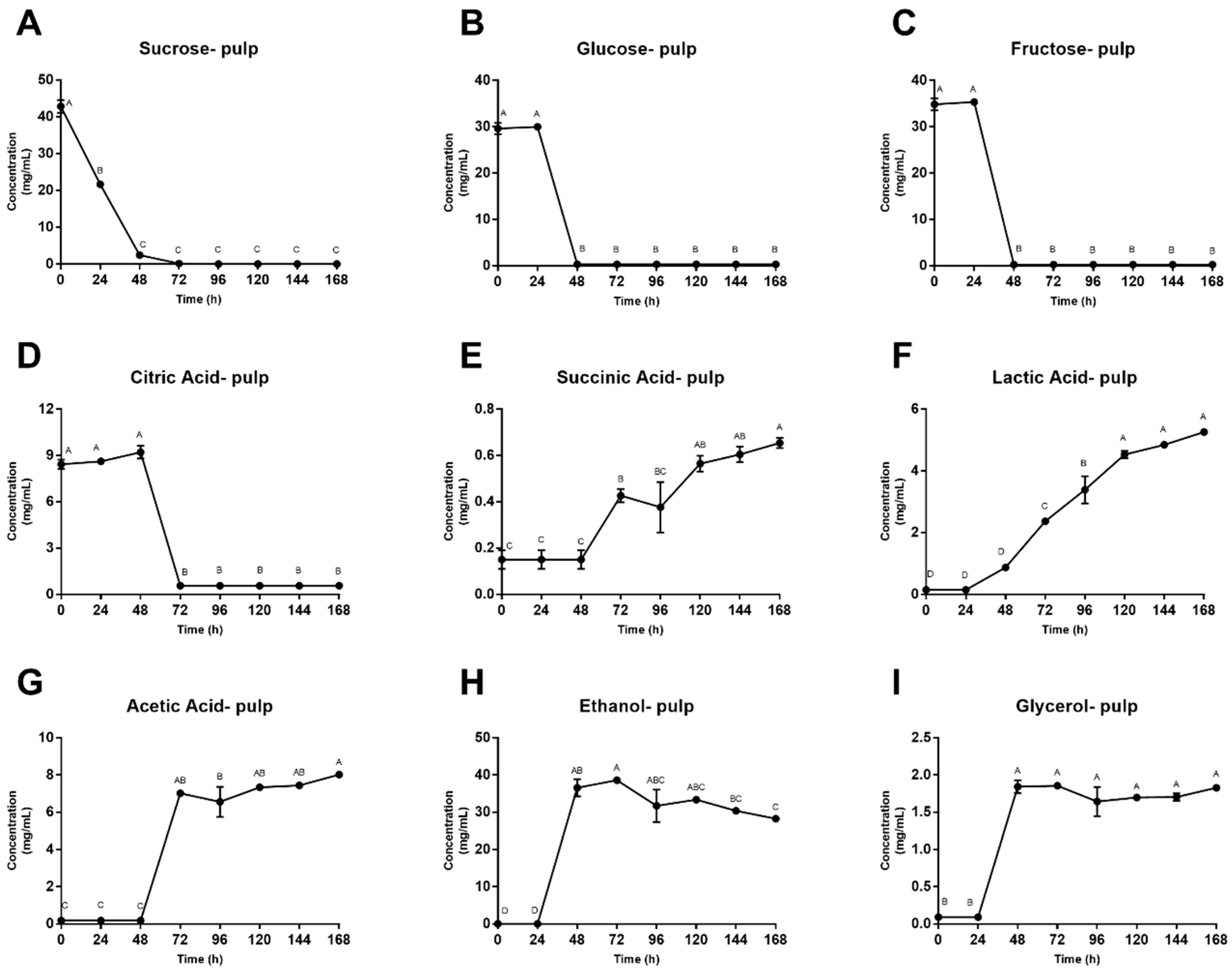
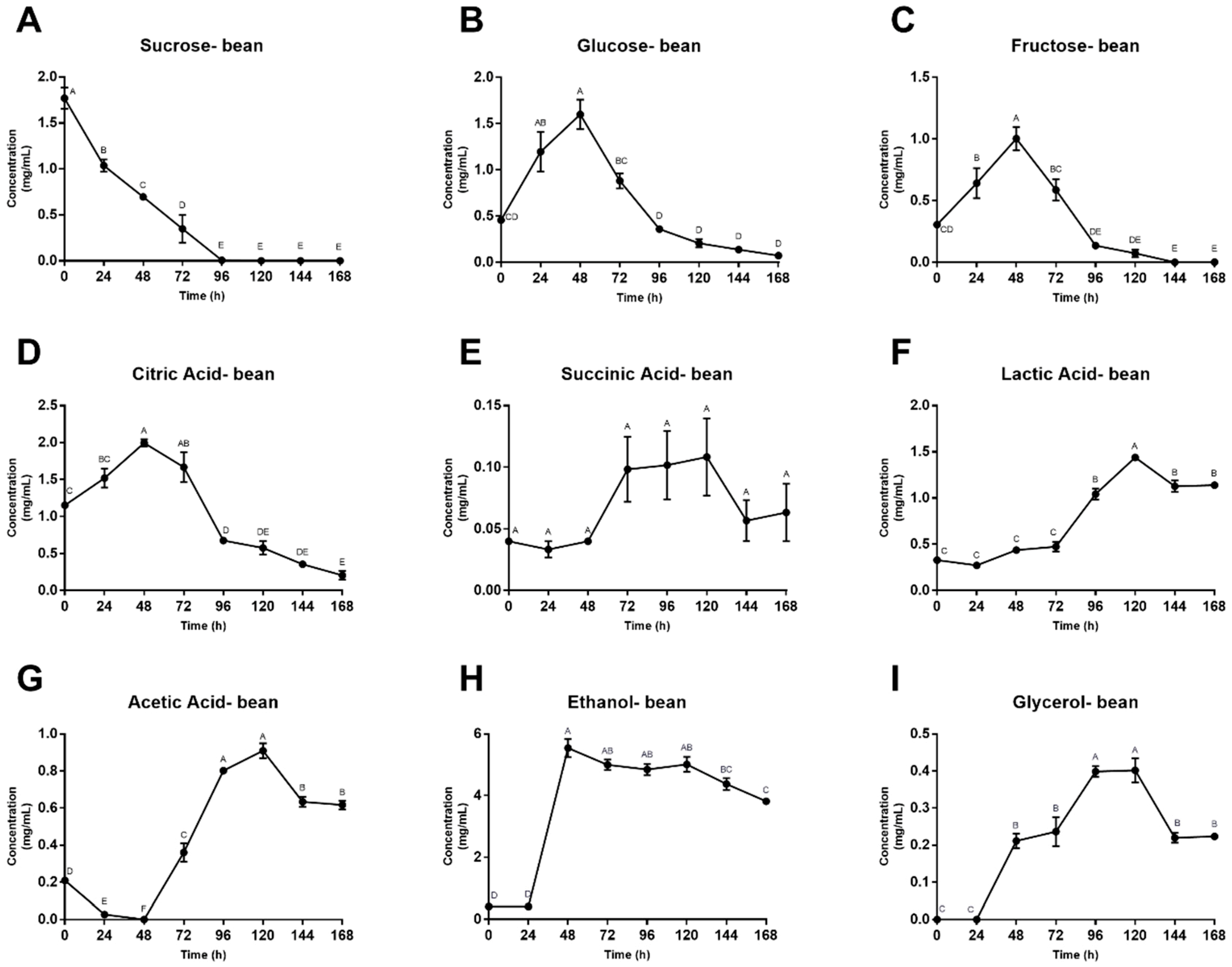
3.2. Microbial Enumeration and Fermentation Products
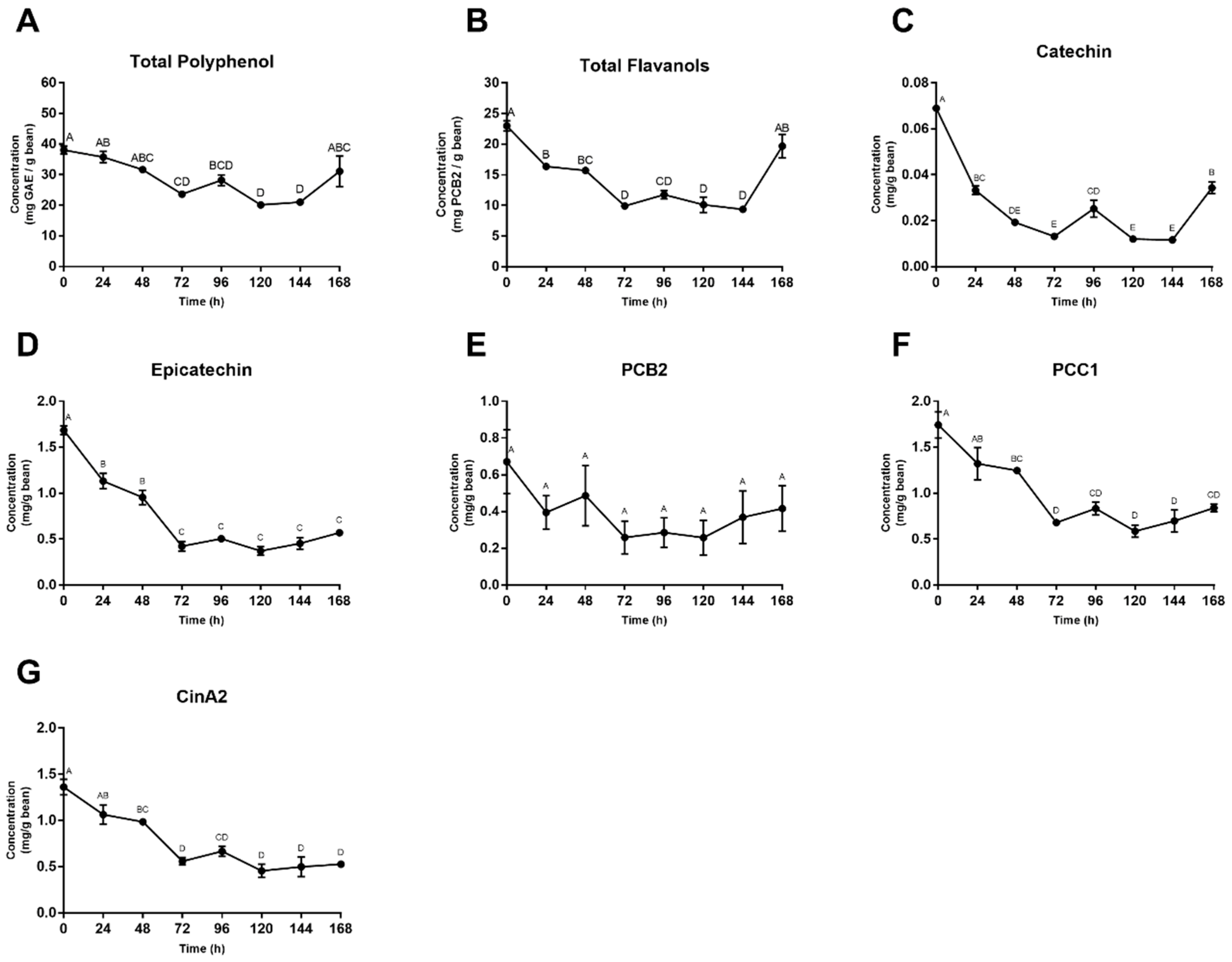
3.3. Total Polyphenol and Flavanol Content
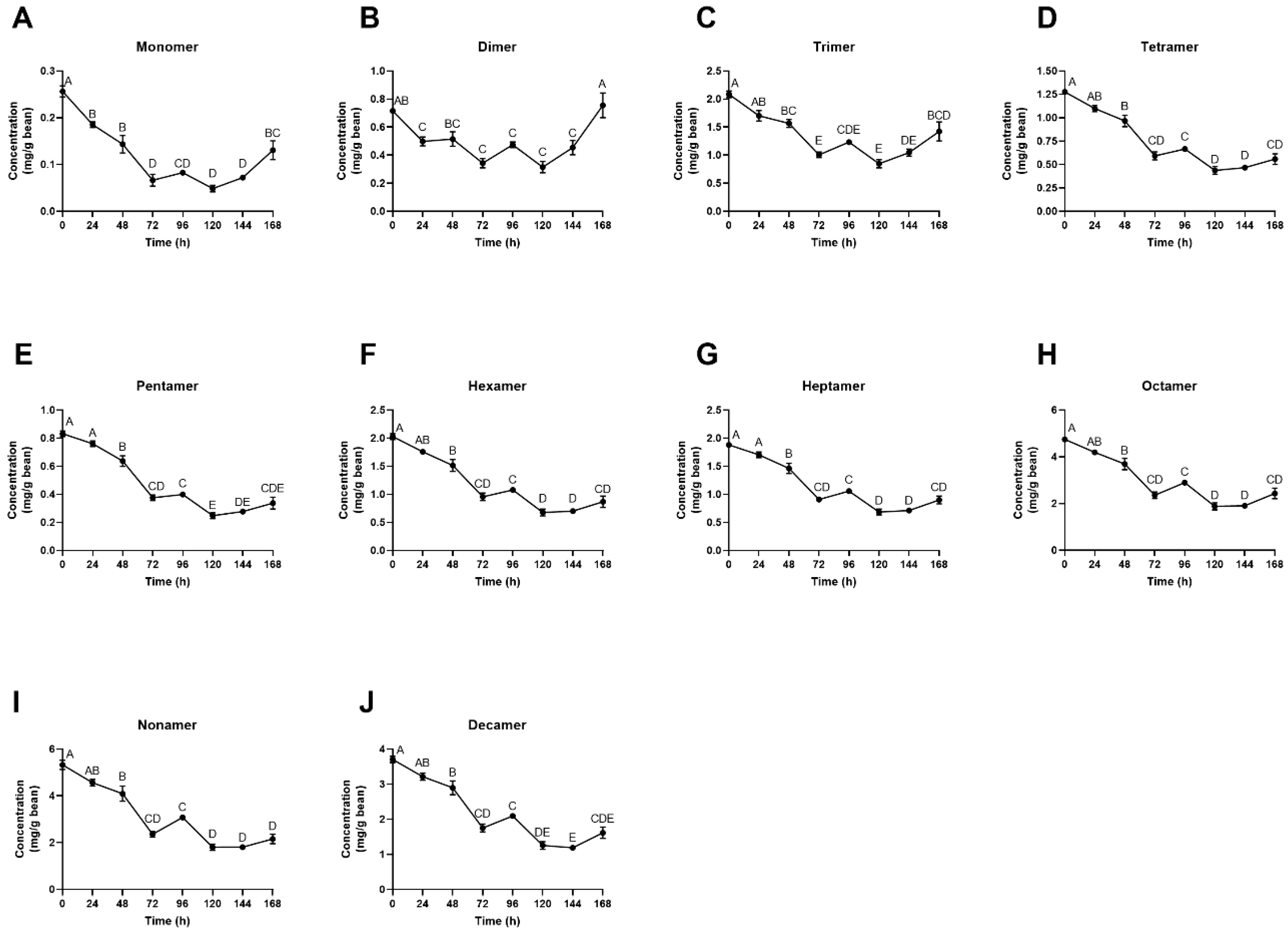
3.4. Individual Polyphenol Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andújar, I.; Recio, M.C.; Giner, R.M.; Ríos, J.L. Cocoa polyphenols and their potential benefits for human health. Oxid. Med. Cell. Longev. 2012, 2012, 906252. [Google Scholar] [CrossRef] [PubMed]
- Wollgast, J.; Anklam, E. Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Camu, N.; De Winter, T.; Addo, S.K.; Takrama, J.S.; Bernaert, H.; De Vuyst, L. Fermentation of cocoa beans: Influence of microbial activities and polyphenol concentrations on the flavour of chocolate. J. Sci. Food Agric. 2008, 88, 2288–2297. [Google Scholar] [CrossRef]
- Romanens, E.; Näf, R.; Lobmaier, T.; Pedan, V.; Leischtfeld, S.F.; Meile, L.; Schwenninger, S.M. A lab-scale model system for cocoa bean fermentation. Appl. Microbiol. Biotechnol. 2018, 102, 3349–3362. [Google Scholar] [CrossRef]
- Hurst, W.J.; Krake, S.H.; Bergmeier, S.C.; Payne, M.J.; Miller, K.B.; Stuart, D.A. Impact of fermentation, drying, roasting and Dutch processing on flavan-3-ol stereochemistry in cacao beans and cocoa ingredients. Chem. Cent. J. 2011, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- De Taeye, C.; Bodart, M.; Caullet, G.; Collin, S. Roasting conditions for preserving cocoa flavan-3-ol monomers and oligomers: Interesting behaviour of Criollo clones. J. Sci. Food Agric. 2017, 97, 4001–4008. [Google Scholar] [CrossRef] [PubMed]
- Ioannone, F.; Di Mattia, C.D.; De Gregorio, M.; Sergi, M.; Serafini, M.; Sacchetti, G. Flavanols, proanthocyanidins and antioxidant activity changes during cocoa (Theobroma cacao L.) roasting as affected by temperature and time of processing. Food Chem. 2015, 174, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Camu, N.; Winter, T.D.; Verbrugghe, K.; Cleenwerck, I.; Vandamme, P.; Takrama, J.S.; Vancanneyt, M.; Vuyst, L.D. Dynamics and Biodiversity of Populations of Lactic Acid Bacteria and Acetic Acid Bacteria Involved in Spontaneous Heap Fermentation of Cocoa Beans in Ghana. Appl. Environ. Microbiol. 2007, 73, 1809–1824. [Google Scholar] [CrossRef]
- Albertini, B.; Schoubben, A.; Guarnaccia, D.; Pinelli, F.; Della Vecchia, M.; Ricci, M.; Di Renzo, G.C.; Blasi, P. Effect of fermentation and drying on cocoa polyphenols. J. Agric. Food Chem. 2015, 63, 9948–9953. [Google Scholar] [CrossRef]
- Dorenkott, M.R.; Griffin, L.E.; Goodrich, K.M.; Thompson-Witrick, K.A.; Fundaro, G.; Ye, L.; Stevens, J.R.; Ali, M.; O’Keefe, S.F.; Hulver, M.W.; et al. Oligomeric Cocoa Procyanidins Possess Enhanced Bioactivity Compared to Monomeric and Polymeric Cocoa Procyanidins for Preventing the Development of Obesity, Insulin Resistance, and Impaired Glucose Tolerance during High-Fat Feeding. J. Agric. Food Chem. 2014, 62, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.M.; Khoo, W.; Ye, L.; Lambert, J.D.; O’Keefe, S.F.; Neilson, A.P. Loss of Native Flavanols during Fermentation and Roasting Does Not Necessarily Reduce Digestive Enzyme-Inhibiting Bioactivities of Cocoa. J. Agric. Food Chem. 2016, 64, 3616–3625. [Google Scholar] [CrossRef]
- Schwan, R.F.; Wheals, A.E. The Microbiology of Cocoa Fermentation and its Role in Chocolate Quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Żyżelewicz, D.; Bojczuk, M.; Budryn, G.; Zduńczyk, Z.; Juśkiewicz, J.; Jurgoński, A.; Oracz, J. Influence of diet based on bread supplemented with raw and roasted cocoa bean extracts on physiological indices of laboratory rats. Food Res. Int. 2018, 112, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Camu, N.; González, A.; De Winter, T.; Van Schoor, A.; De Bruyne, K.; Vandamme, P.; Takrama, J.S.; Addo, S.K.; De Vuyst, L. Influence of turning and environmental contamination on the dynamics of populations of lactic acid and acetic acid bacteria involved in spontaneous cocoa bean heap fermentation in Ghana. Appl. Environ. Microbiol. 2008, 74, 86–98. [Google Scholar] [CrossRef]
- Lefeber, T.; Gobert, W.; Vrancken, G.; Camu, N.; De Vuyst, L. Dynamics and species diversity of communities of lactic acid bacteria and acetic acid bacteria during spontaneous cocoa bean fermentation in vessels. Food Microbiol. 2011, 28, 457–464. [Google Scholar] [CrossRef] [PubMed]
- De Melo Pereira, G.V.; Magalhães, K.T.; de Almeida, E.G.; da Silva Coelho, I.; Schwan, R.F. Spontaneous cocoa bean fermentation carried out in a novel-design stainless steel tank: Influence on the dynamics of microbial populations and physical–chemical properties. Int. J. Food Microbiol. 2013, 161, 121–133. [Google Scholar] [CrossRef]
- Di Mattia, C.; Sacchetti, G.; Seghetti, L.; Piva, A.; Mastrocola, D. “Vino Cotto” Composition and Antioxidant Activity as Affected by Non Enzymatic Browning. Influenza Dellimbrunimento non Enzimatico Sulla Compos. e Attiv. Antiossidante Vino Cotto 2007, 19, 413–424. [Google Scholar]
- De Taeye, C.; Caullet, G.; Eyamo Evina, V.J.; Collin, S. Procyanidin A2 and Its Degradation Products in Raw, Fermented, and Roasted Cocoa. J. Agric. Food Chem. 2017, 65, 1715–1723. [Google Scholar] [CrossRef]
- Eyamo Evina, V.J.; De Taeye, C.; Niemenak, N.; Youmbi, E.; Collin, S. Influence of acetic and lactic acids on cocoa flavan-3-ol degradation through fermentation-like incubations. LWT Food Sci. Technol. 2016, 68, 514–522. [Google Scholar] [CrossRef]
- Kadow, D.; Niemenak, N.; Rohn, S.; Lieberei, R. Fermentation-like incubation of cocoa seeds (Theobroma cacao L.)–Reconstruction and guidance of the fermentation process. LWT Food Sci. Technol. 2015, 62, 357–361. [Google Scholar] [CrossRef]
- De Melo Pereira, G.V.; Miguel, M.G.; Ramos, C.L.; Schwan, R.F. Microbiological and Physicochemical Characterization of Small-Scale Cocoa Fermentations and Screening of Yeast and Bacteria Strains for the Development of a Defined Starter Culture. Appl. Environ. Microbiol. 2012, 78, 5395–5405. [Google Scholar] [CrossRef]
- Lee, A.H.; Neilson, A.P.; O’Keefe, S.F.; Ogejo, J.A.; Huang, H.; Ponder, M.; Chu, H.S.S.; Jin, Q.; Pilot, G.; Stewart, A.C. A laboratory-scale model cocoa fermentation using dried, unfermented beans and artificial pulp can simulate the microbial and chemical changes of on-farm cocoa fermentation. Eur. Food Res. Technol. 2019, 245, 511–519. [Google Scholar] [CrossRef]
- Kim, H.; Keeney, P.G. (−)-Epicatechin Content in Fermented and Unfermented Cocoa Beans. J. Food Sci. 1984, 49, 1090–1092. [Google Scholar] [CrossRef]
- De Brito, E.S.; García, N.H.P.; Gallão, M.I.; Cortelazzo, A.L.; Fevereiro, P.S.; Braga, M.R. Structural and chemical changes in cocoa (Theobroma cacao L.) during fermentation, drying and roasting. J. Sci. Food Agric. 2001, 81, 281–288. [Google Scholar] [CrossRef]
- Mazor Jolić, S.; Radojčić Redovniković, I.; Marković, K.; Ivanec Šipušić, Đ.; Delonga, K. Changes of phenolic compounds and antioxidant capacity in cocoa beans processing. Int. J. Food Sci. Technol. 2011, 46, 1793–1800. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Cienfuegos-Jovellanos, E.; Marin, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerda, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; et al. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J. Agric. Food Chem. 2007, 55, 3926–3935. [Google Scholar] [CrossRef]
- Cienfuegos-Jovellanos, E.; Quiñones, M.D.; Muguerza, B.; Moulay, L.; Miguel, M.; Aleixandre, A. Antihypertensive effect of a polyphenol-rich cocoa powder industrially processed to preserve the original flavonoids of the cocoa beans. J. Agric. Food Chem. 2009, 57, 6156–6162. [Google Scholar] [CrossRef]
- Stahl, L.; Miller, K.B.; Apgar, J.; Sweigart, D.S.; Stuart, D.A.; McHale, N.; Ou, B.; Kondo, M.; Hurst, W.J. Preservation of cocoa antioxidant activity, total polyphenols, flavan-3-ols, and procyanidin content in foods prepared with cocoa powder. J. Food Sci. 2009, 74, C456–C461. [Google Scholar] [CrossRef]
- Kealey, K.S.; Snyder, R.M.; Romanczyk, L.J., Jr.; Geyer, H.M.; Myers, M.E.; Whitacre, E.J.; Hammerstone, J.F., Jr.; Schmitz, H.H. Cocoa Components, Edible Products Having Enriched Polyphenol Content, Methods of Making Same and Medical Uses. U.S. Patent No. 6,312,753, 6 November 2001. [Google Scholar]
- Kealey, K.S.; Snyder, R.M.; Romanczyk, L.J., Jr.; Hammerstone, J.F., Jr.; Buck, M.M.; Cipolla, G.G. Foods Containing Cocoa Solids Having High Cocoa Polyphenol Contents. U.S. Patent No. 6,372,267, 16 April 2002. [Google Scholar]
- Zapp, L.; Slaga, T.J.; Zhao, J.; Lang, M. Method for Enhancing Post-Processing Content of Beneficial Compounds in Foodstuffs Made with Cocoa Beans. U.S. Patent No. 6,660,322, 9 December 2003. [Google Scholar]
- García-Alamilla, P.; Salgado-Cervantes, M.A.; Barel, M.; Berthomieu, G.; Rodríguez-Jimenes, G.C.; García-Alvarado, M.A. Moisture, acidity and temperature evolution during cacao drying. J. Food Eng. 2007, 79, 1159–1165. [Google Scholar] [CrossRef]
- Jinap, S.; Thien, J.; Yap, T.N. Effect of drying on acidity and volatile fatty acids content of cocoa beans. J. Sci. Food Agric. 1994, 65, 67–75. [Google Scholar] [CrossRef]
- Nielsen, D.S.; Teniola, O.D.; Ban-Koffi, L.; Owusu, M.; Andersson, T.S.; Holzapfel, W.H. The microbiology of Ghanaian cocoa fermentations analysed using culture-dependent and culture-independent methods. Int. J. Food Microbiol. 2007, 114, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, S.L.; Loiseau, G.; Paredes, J.L.; Barel, M.; Guiraud, J.-P. Study on the microflora and biochemistry of cocoa fermentation in the Dominican Republic. Int. J. Food Microbiol. 2007, 114, 124–130. [Google Scholar] [CrossRef]
- Guehi, S.T.; Dabonne, S.; Ban-Koffi, L.; Kedjebo, D.K.; Zahouli, G.I.B. Effect of turning beans and fermentation method on the acidity and physical quality of raw cocoa beans. Adv. J. Food Sci. Technol. 2010, 2, 163–171. [Google Scholar]
- Hansen, C.E.; del Olmo, M.; Burri, C. Enzyme activities in cocoa beans during fermentation. J. Sci. Food Agric. 1998, 77, 273–281. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Zhao, J.; Fleet, G. Yeasts are essential for cocoa bean fermentation. Int. J. Food Microbiol. 2014, 174, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Schwan, R.F. Cocoa fermentations conducted with a defined microbial cocktail inoculum. Appl. Environ. Microbiol. 1998, 64, 1477–1483. [Google Scholar]
- Elwers, S.; Zambrano, A.; Rohsius, C.; Lieberei, R. Differences between the content of phenolic compounds in Criollo, Forastero and Trinitario cocoa seed (Theobroma cacao L.). Eur. Food Res. Technol. 2009, 229, 937–948. [Google Scholar] [CrossRef]
- Romero-Cortes, T.; Salgado-Cervantes, M.A.; García-Alamilla, P.; García-Alvarado, M.A.; del C Rodríguez-Jimenes, G.; Hidalgo-Morales, M.; Robles-Olvera, V. Relationship between fermentation index and other biochemical changes evaluated during the fermentation of Mexican cocoa (Theobroma cacao) beans. J. Sci. Food Agric. 2013, 93, 2596–2604. [Google Scholar] [CrossRef]
- Harborne, J. Spectral methods of characterizing anthocyanins. Biochem. J. 1958, 70, 22. [Google Scholar] [CrossRef]
- Racine, K.C.; Lee, A.H.; Stewart, A.C.; Blakeslee, K.W.; Neilson, A.P. Development of a rapid ultra performance hydrophilic interaction liquid chromatography tandem mass spectrometry method for procyanidins with enhanced ionization efficiency. J. Chromatogr. A 2019. [Google Scholar] [CrossRef]
- Wollgast, J.; Anklam, E. Polyphenols in chocolate: Is there a contribution to human health? Food Res. Int. 2000, 33, 449–459. [Google Scholar] [CrossRef]
- Ardhana, M.M.; Fleet, G.H. The microbial ecology of cocoa bean fermentations in Indonesia. Int. J. Food Microbiol. 2003, 86, 87–99. [Google Scholar] [CrossRef]
- Nazaruddin, R.; Seng, L.K.; Hassan, O.; Said, M. Effect of pulp preconditioning on the content of polyphenols in cocoa beans (Theobroma cacao) during fermentation. Ind. Crops Prod. 2006, 24, 87–94. [Google Scholar] [CrossRef]
- Emmanuel, O.A.; Jennifer, Q.; Agnes, S.B.; Jemmy, S.T.; Firibu, K.S. Influence of pulp-preconditioning and fermentation on fermentative quality and appearance of Ghanaian cocoa (Theobroma cacao) beans. Int. Food Res. J. 2012, 19, 127–133. [Google Scholar]
- Selamat, J.; Mordingah Harun, S. Formation of methyl pyrazine during cocoa bean fermentation. Pertanika 1994, 17, 27–32. [Google Scholar]
- Misnawi, S.; Jamilah, B.; Nazamid, S. Effects of incubation and polyphenol oxidase enrichment on colour, fermentation index, procyanidins and astringency of unfermented and partly fermented cocoa beans. Int. J. Food Sci. Technol. 2003, 38, 285–295. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Changes in the flavan-3-ols, anthocyanins, and flavanols composition of cocoa beans of different Theobroma cacao L. groups affected by roasting conditions. Eur. Food Res. Technol. 2015, 241, 663–681. [Google Scholar] [CrossRef]
- Da Veiga Moreira, I.M.; Miguel, M.G.; Duarte, W.F.; Dias, D.R.; Schwan, R.F. Microbial succession and the dynamics of metabolites and sugars during the fermentation of three different cocoa (Theobroma cacao L.) hybrids. Food Res. Int. 2013, 54, 9–17. [Google Scholar] [CrossRef]
- Vuyst, L.D.; Weckx, S. The cocoa bean fermentation process: From ecosystem analysis to starter culture development. J. Appl. Microbiol. 2016, 121, 5–17. [Google Scholar] [CrossRef]
- Oracz, J.; Zyzelewicz, D.; Nebesny, E. The Content of Polyphenolic Compounds in Cocoa Beans (Theobroma cacao L.), Depending on Variety, Growing Region, and Processing Operations: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1176–1192. [Google Scholar] [CrossRef]
- Saltini, R.; Akkerman, R.; Frosch, S. Optimizing chocolate production through traceability: A review of the influence of farming practices on cocoa bean quality. Food Control 2013, 29, 167–187. [Google Scholar] [CrossRef]
- Payne, M.J.; Hurst, W.J.; Miller, K.B.; Rank, C.; Stuart, D.A. Impact of Fermentation, Drying, Roasting, and Dutch Processing on Epicatechin and Catechin Content of Cacao Beans and Cocoa Ingredients. J. Agric. Food Chem. 2010, 58, 10518–10527. [Google Scholar] [CrossRef]
- Aikpokpodion, P.E.; Dongo, L.N. Effects of fermentation intensity on polyphenols and antioxidant capacity of cocoa beans. Int. J. Sustain. Crop Prod. 2010, 5, 66–70. [Google Scholar]
- De Taeye, C.; Eyamo Evina, V.J.; Caullet, G.; Niemenak, N.; Collin, S. Fate of anthocyanins through cocoa fermentation. Emergence of new polyphenolic dimers. J. Agric. Food Chem. 2016, 64, 8876–8885. [Google Scholar] [CrossRef]

| Compound | tRa | [M–H]– b |
|---|---|---|
| (min) | (m/z) | |
| (±)-catechin | 2.946 | 288.95 |
| (–)-epicatechin | 3.625 | 289.01 |
| PCB2 | 3.366 | 576.84 |
| PCC1 | 3.904 | 864.85 |
| CinA2 | 4.063 | 1153.19 |
| Compound | tRa | MW | [M–H]– b | Daughter Ion |
|---|---|---|---|---|
| (min) | (g mol−1) | (m/z) | (m/z) | |
| Monomer | 0.61 | 290.27 | 289.03 | 245.06 |
| Epigallocatechin | 0.74 | 458.37 | 305.04 | 124.98 |
| Dimer | 2.03 | 578.52 | 577.14 | 425.10 |
| Trimer | 3.05 | 866.77 | 865.22 | 287.07 |
| Tetramer | 3.73 | 1155.02 | 576.40 | 125.02 |
| Pentamer | 4.26 | 1443.28 | 720.41 | 125.02 |
| Hexamer | 4.66 | 1731.53 | 864.52 | 125.02 |
| Heptamer | 5.00 | 2017.81 | 1008.40 | 125.17 |
| Octamer | 5.28 | 2308.03 | 1152.58 | 125.17 |
| Nonamer | 5.53 | 2596.54 | 864.12 | 125.17 |
| Decamer | 5.75 | 2884.54 | 960.18 | 125.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racine, K.C.; Lee, A.H.; Wiersema, B.D.; Huang, H.; Lambert, J.D.; Stewart, A.C.; Neilson, A.P. Development and Characterization of a Pilot-Scale Model Cocoa Fermentation System Suitable for Studying the Impact of Fermentation on Putative Bioactive Compounds and Bioactivity of Cocoa. Foods 2019, 8, 102. https://doi.org/10.3390/foods8030102
Racine KC, Lee AH, Wiersema BD, Huang H, Lambert JD, Stewart AC, Neilson AP. Development and Characterization of a Pilot-Scale Model Cocoa Fermentation System Suitable for Studying the Impact of Fermentation on Putative Bioactive Compounds and Bioactivity of Cocoa. Foods. 2019; 8(3):102. https://doi.org/10.3390/foods8030102
Chicago/Turabian StyleRacine, Kathryn C., Andrew H. Lee, Brian D. Wiersema, Haibo Huang, Joshua D. Lambert, Amanda C. Stewart, and Andrew P. Neilson. 2019. "Development and Characterization of a Pilot-Scale Model Cocoa Fermentation System Suitable for Studying the Impact of Fermentation on Putative Bioactive Compounds and Bioactivity of Cocoa" Foods 8, no. 3: 102. https://doi.org/10.3390/foods8030102
APA StyleRacine, K. C., Lee, A. H., Wiersema, B. D., Huang, H., Lambert, J. D., Stewart, A. C., & Neilson, A. P. (2019). Development and Characterization of a Pilot-Scale Model Cocoa Fermentation System Suitable for Studying the Impact of Fermentation on Putative Bioactive Compounds and Bioactivity of Cocoa. Foods, 8(3), 102. https://doi.org/10.3390/foods8030102






