Evaluation of Antimicrobial Interventions against E. coli O157:H7 on the Surface of Raw Beef to Reduce Bacterial Translocation during Blade Tenderization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
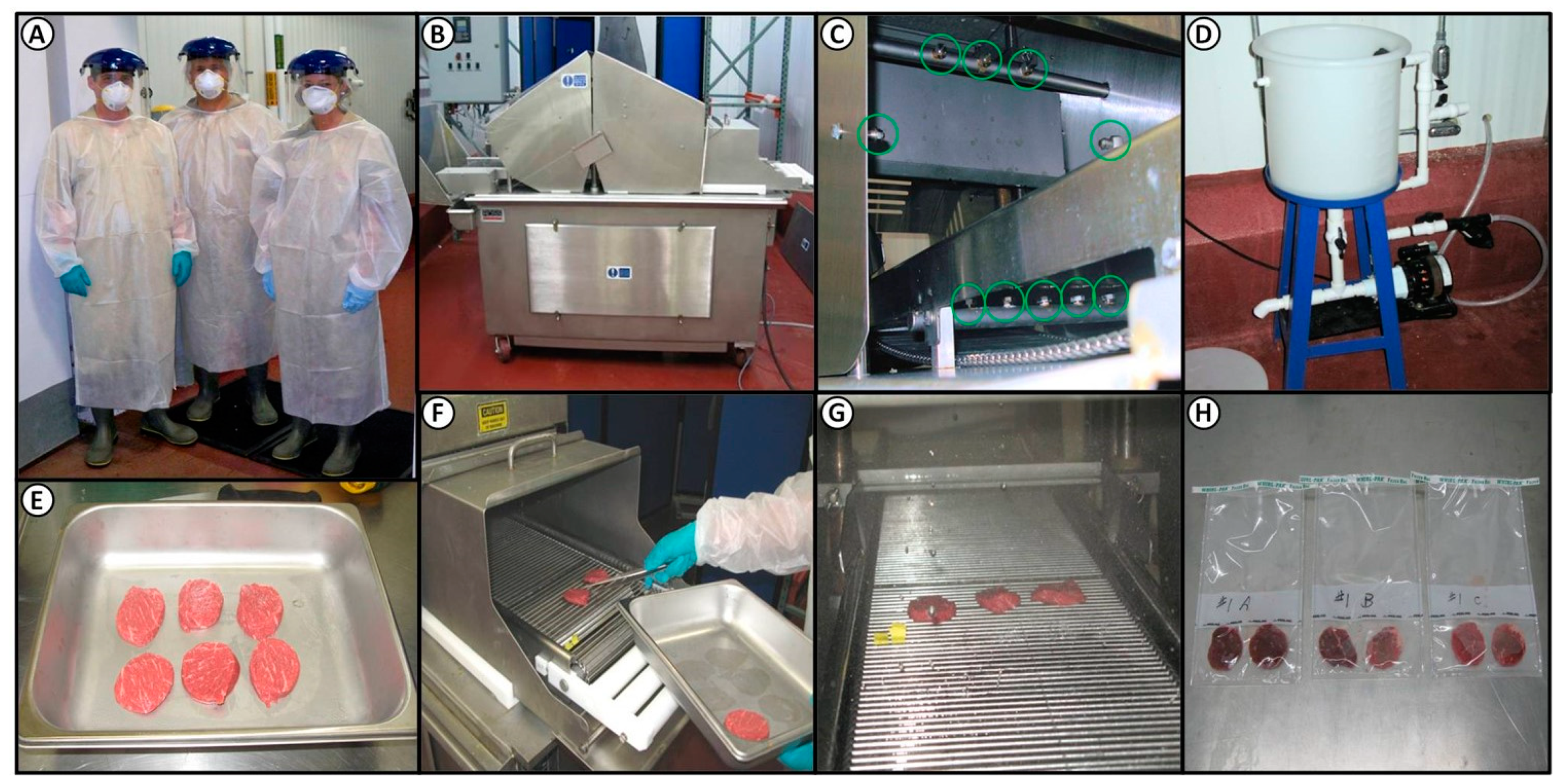
2.2. Biosafety Level 2 (BSL-2) Laboratory Precautions
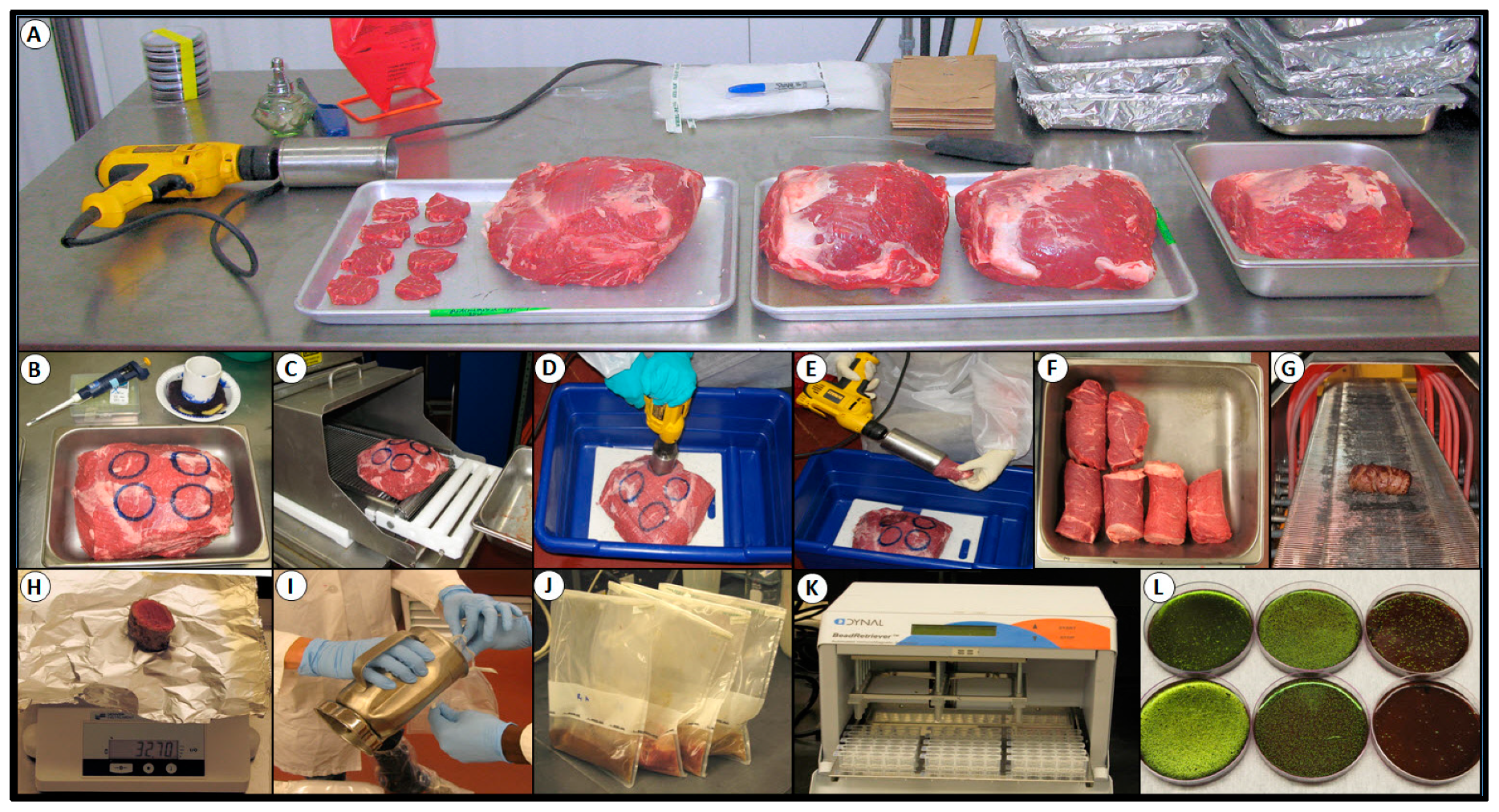
2.3. Processing Lean Beef Wafers and Beef Cores
2.4. Antimicrobial Spray and Blade Tenderization System
2.5. Antimicrobial Solutions—Phase 1 (Lean Beef Wafers)
2.6. Antimicrobial Solutions—Phase 2 (Blade Tenderization)
2.7. Inoculation and Spray Treatment of Lean Beef Wafers
2.8. Inoculation and Mechanical Tenderization of Beef Subprimals
2.9. Microbial Sampling, Isolation, and Confirmation
2.10. Statistical Analysis
3. Results
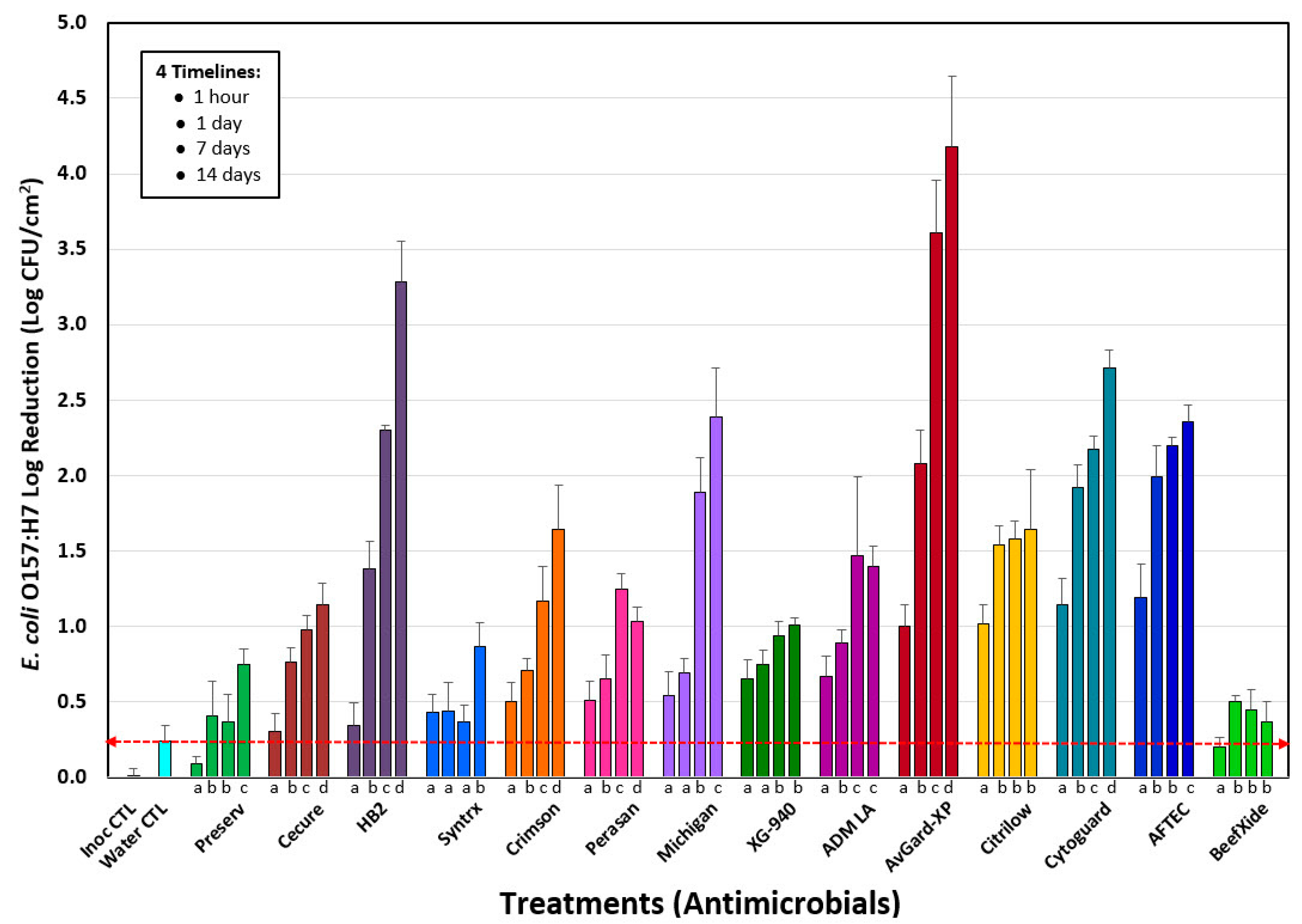
3.1. Phase 1: Evaluation of 14 Antimicrobials against E. coli O157:H7 on the Surface of Inoculated Lean Beef Discs
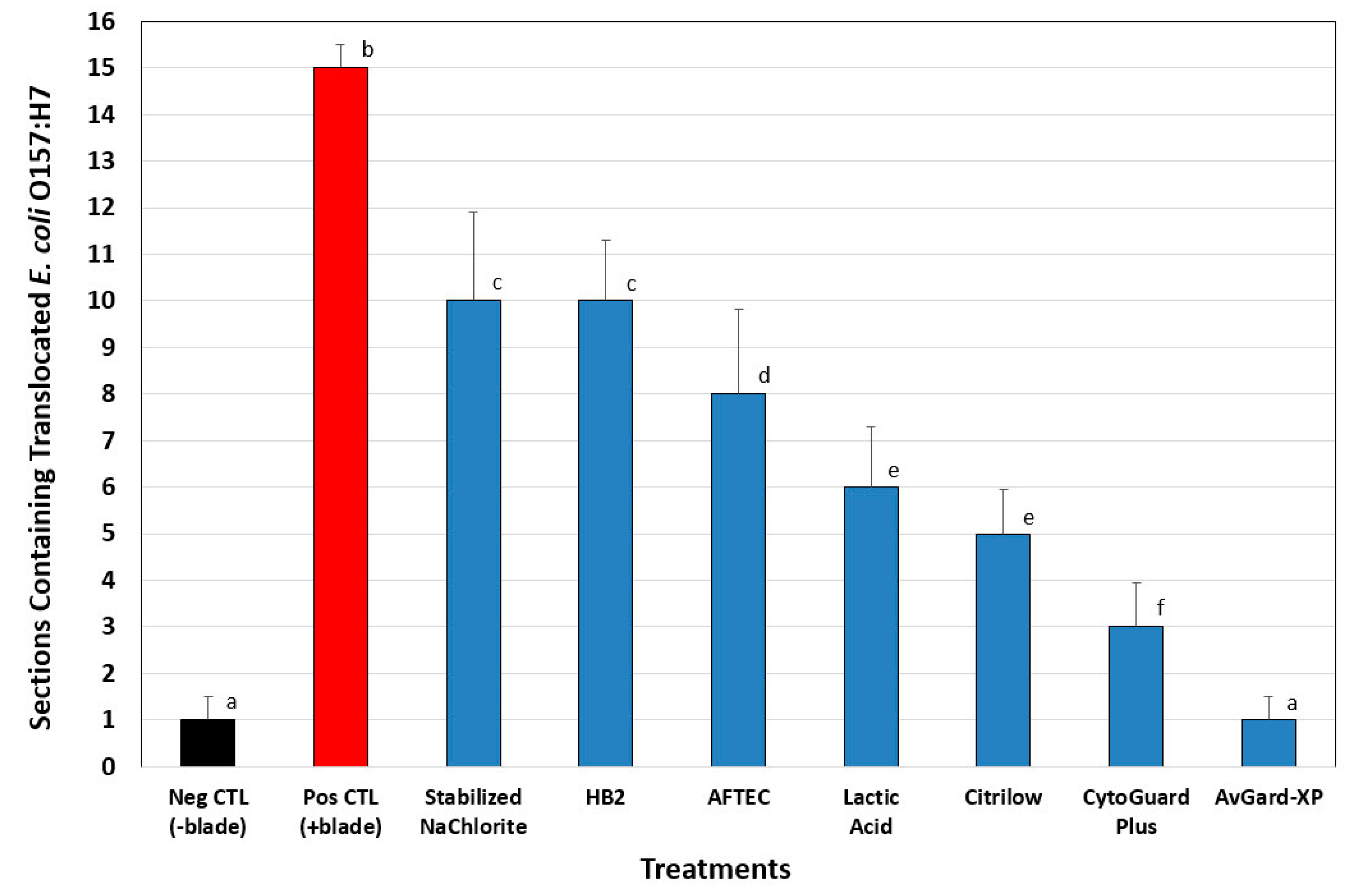
3.2. Phase 2: Translocation of E. coli O157:H7 into Beef Cores
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- NCBA. Beef Industry Long Range Plan; National Cattlemen’s Beef Assoc.: Englewood, CO, USA, 1998. [Google Scholar]
- Harrison, A.; Smith, M.; Allen, D.M.; Hunt, M.C.; Klastner, C.; Kroft, D. Nutritional regime effects on quality and yield characteristics of beef. J. Anim. Sci. 1978, 47, 383–388. [Google Scholar] [CrossRef]
- Davis, K.; Huffman, D.; Cordray, J. Effect of mechanical tenderization, aging and pressing on beef quality. J. Food Sci. 1975, 40, 1222–1224. [Google Scholar] [CrossRef]
- Glover, E.; Forrest, J.; Johnson, H.; Bramblett, V.; Judge, M. Palatability and cooking characteristics of mechanically tenderized beef. J. Food Sci. 1977, 42, 871–874. [Google Scholar] [CrossRef]
- Schwartz, W.; Mandigo, R. Effect of conveyor speed on mechanical tenderization of beef inside rounds. J. Anim. Sci. 1977, 44, 581–584. [Google Scholar] [CrossRef]
- Boyd, K.J.; Ockerman, H.W.; Plimpton, R.F. Sensory characteristics and microbialogical evaluation of stored mechanizally tenderized beef semimembranosus muscle. J. Food Sci. 1978, 43, 670–672. [Google Scholar] [CrossRef]
- Mandigo, R.W.; Olson, D.G. Effect of blade size for mechanically tenderizing beef rounds. J. Food Sci. 1982, 47, 2095–2096. [Google Scholar] [CrossRef]
- Miller, S.G. Mechanical Tenderization of Meat in the HRI Trade. Available online: https://meatscience.org/docs/default-source/publications-resources/rmc/1975/mechanical-tenderization-of-meat-in-the-hri-trade.pdf?sfvrsn=eb08bbb3_2 (accessed on 19 February 2019).
- Bell, B.P.; Goldoft, M.; Griffin, P.M.; Davis, M.A.; Gordon, D.C.; Tarr, P.I.; Bartleson, C.A.; Lewis, J.H.; Barrett, T.J.; et al. A multistate outbreak of Escherichia coli O157:H7—associated bloody diarrhea and hemolytic uremic syndrome from hamburgers: the Washington experience. JAMA 1994, 272, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- USDA-FSIS. Beef products contaminated with Escherichia coli O157:H7. Fed. Regist. 1999, 64, 2803–2805. [Google Scholar]
- USDA-FSIS. Shiga toxin-producing Escherichia coli in certain raw beef products (final rule, implementation). Fed. Regist. 2012, 77, 31975–31981. [Google Scholar]
- USDA-FSIS. HACCP plan reassessment for mechanically tenderized beef products. Fed. Regist. 2005, 70, 30331–30334. [Google Scholar]
- Phebus, R.; Thippareddi, H.; Sporing, S.; Marsden, J.; Kastner, C. Escherichia coli O157: H7 risk assessment for blade-tenderized beef steaks. Cattlem. Day 2000, 2000, 117–118. [Google Scholar]
- Laine, E.S.; Scheftel, J.M.; Boxrud, D.J.; Vought, K.J.; Danila, R.N.; Elfering, K.M.; Smith, K.E. Outbreak of Escherichia coli O157:H7 infections associated with nonintact blade-tenderized frozen steaks sold by door-to-door vendors. J. Food Prot. 2005, 68, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Multistate Outbreak of E. coli O157:H7 Infections Associated with Beef from National Steak and Poultry (Final Update). Available online: https://www.cdc.gov/ecoli/2010/national-steak-poultry-1-6-10.html (accessed on 19 February 2019).
- USDA-FSIS. Descriptive designation for needle- or blade-tenderized (mechanically tenderized) beef products. Fed. Regist. 2015, 80, 28153–28172. [Google Scholar]
- Luchansky, J.B.; Phebus, R.K.; Thippareddi, H.; Call, A.E. Translocation of surface-inoculated Escherichia coli O157:H7 into beef subprimals following blade tenderization. J. Food Prot. 2008, 71, 2190–2197. [Google Scholar] [CrossRef] [PubMed]
- Huang, L. Growth kinetics of Escherichia coli O157:H7 in mechanically-tenderized beef. Int. J. Food Microb. 2010, 140, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Luchansky, J.B.; Porto-Fett, A.C.S.; Shoyer, B.; Phebus, R.K.; Thippareddi, H.; Call, J.E. Thermal inactivation of Escherichia coli O157:H7 in blade-tenderized beef steaks cooked on a commercial open-flame gas grill. J. Food Prot. 2009, 72, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Luchansky, J.B.; Porto-Fett, A.C.S.; Shoyer, B.A.; Call, J.E.; Schlosser, W.; Shaw, W.; Bauer, N.; Latimer, H. Fate of shiga toxin-producing O157:H7 and Non-O157:H7 Escherichia coli cells within blade-tenderized beef steaks after cooking on a commercial open-flame gas grill. J. Food Prot. 2012, 75, 62–70. [Google Scholar] [CrossRef]
- Berry, E.D.; Cutter, C.N. Effects of acid adaptation of Escherichia coli O157:H7 on efficacy of acetic acid spray washes to decontaminate beef carcass tissue. Appl. Environ. Microb. 2000, 66, 1493–1498. [Google Scholar] [CrossRef]
- USDA-FSIS. Pathogen Reduction, Hazard Analysis and Critical Control Point (HACCP) Systems. Final Rule. Fed. Regist. 1996, 61, 28805–38855. [Google Scholar]
- Cherrington, C.A.; Hinton, M.; Mead, G.C.; Chopra, I. Organic Acids: Chemistry, Antibacterial Activity and Practical Applications. In Advances in Microbial Physiology; Rose, A.H., Tempest, D.W., Eds.; Academic Press: New York, NY, USA, 1991; Volume 32, pp. 87–108. [Google Scholar]
- Mani-López, E.; García, H.S.; López-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microb. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microb. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Sierra, M.; Sanhueza, A.; Alcántara, R.; Sánchez, G. Antimicrobial evaluation of copper sulfate (II) on strains of Enterococcus faecalis. In vitro study. J. Oral Res. 2013, 2, 3. [Google Scholar] [CrossRef]
- Sharma, C.S.; Williams, S.K.; Schneider, K.R.; Schmidt, R.H.; Rodrick, G.E. Mechanism of antimicrobial action of sodium metasilicate against Salmonella enterica serovar Typhimurium. Foodborne Pathog. Dis. 2013, 10, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Williams, S.K.; Sims, C.A.; Simmone, A. Sodium metasilicate affects antimicrobial, sensory, physical, and chemical characteristics of fresh commercial chicken breast meat stored at 4 °C for 9 days. Poult. Sci. 2011, 90, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Post, R.; Budak, C.; Canavan, J.; Duncan-Harrington, T.; Jones, B.J.; Kegley, M. A Guide to Federal Food Labeling Requirements for Meat and Poultry Products. Available online: https://www.fsis.usda.gov/wps/wcm/connect/f4af7c74-2b9f-4484-bb16-fd8f9820012d/Labeling_Requirements_Guide.pdf?MOD=AJPERES (accessed on 19 February 2019).
- United States Food and Drug Administration. CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=101.17 (accessed on 19 February 2019).
- Heller, C.E.; Scanga, J.A.; Sofos, J.N.; Belk, K.E.; Warren-Serna, W.; Bellinger, G.R.; Bacon, R.T.; Rossman, M.L.; Smith, G.C. Decontamination of beef subprimal cuts intended for blade tenderization or moisture enhancement. J. Food Prot. 2007, 70, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Adler, J.M.; Geornaras, I.; Belk, K.E.; Smith, G.C.; Sofos, J.N. Thermal Inactivation of Escherichia coli O157:H7 Inoculated at Different Depths of Non-Intact Blade-Tenderized Beef Steaks. J. Food Sci. 2012, 77, M108–M114. [Google Scholar] [CrossRef] [PubMed]
- Saha, J.; Jaroni, D.; Nelson, J.; Willoughby, C.; McDaniel, C.; Jadeja, R. Influences of weight and thickness on cooking time required for various mechanically tenderized beef steaks to reach minimum safe internal temperature without resting. LWT 2018, in press. [Google Scholar] [CrossRef]
- Ma, A.; Chui, L. Identification of heat resistant Escherichia coli by qPCR for the locus of heat resistance. J. Microb. Method 2017, 133, 87–89. [Google Scholar] [CrossRef] [PubMed]
- USDA-FSIS. Compliance Guidelines to Control Listeria monocytogenes in Post-lethality Exposed Ready-to-eat Meat and Poultry Products; USDA-FSIS: Washington, DC, USA, 2012.
| Product/Trade Name | Active Ingredient(s) | pH | Application Strength | |
|---|---|---|---|---|
| 1. | AvGard-XP | Disodium metasilicate | 13.1 | 60,000 ppm SMS |
| 2. | HB2 | Hydrobromic acid | 7.5 | 300 ppm |
| 3. | Cecure | Cetylpyridinium chloride | 7.0 | 4000 ppm |
| 4. | Preserv | Copper sulfate pentahydrate | 6.8 | 30% of concentrate * |
| 5. | Stabilized Sodium Chlorite | Sodium chlorite, citric acid, sodium hydroxide | 6.5 | <1%, <1%, <1% each * |
| 6. | XG-940 | Acidified sodium chlorite | 6.5 | 200 ppm |
| 7. | Perasan MP2 | Peroxyacetic acid | 3.2 | 220 ppm |
| 8. | CytoGuard Plus | Lauric arginate (LAE), peroxyacetic acid (PAA) | 3.0 | 5,000 ppm LAE; 220 ppm PAA |
| 9. | Acidified Sodium Chlorite | Sodium chlorite acidified with citric acid | 2.7 | 1100 ppm |
| 10. | BeefXide | Lactic acid, citric acid | 2.1 | 2.4% of concentrate * |
| 11. | Lactic Acid | Hydroxypropanoic acid | 1.9 | 50,000 ppm |
| 12. | Syntrx 3300 | HCl, citric acid | 1.2 | 3% of concentrate * |
| 13. | AFTEC 3000 | Buffered sulfuric acid | 1.0 | 17,500 ppm |
| 14. | Citrilow | HCl, citric acid | 0.8 | 18% of concentrate * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muriana, P.M.; Eager, J.; Wellings, B.; Morgan, B.; Nelson, J.; Kushwaha, K. Evaluation of Antimicrobial Interventions against E. coli O157:H7 on the Surface of Raw Beef to Reduce Bacterial Translocation during Blade Tenderization. Foods 2019, 8, 80. https://doi.org/10.3390/foods8020080
Muriana PM, Eager J, Wellings B, Morgan B, Nelson J, Kushwaha K. Evaluation of Antimicrobial Interventions against E. coli O157:H7 on the Surface of Raw Beef to Reduce Bacterial Translocation during Blade Tenderization. Foods. 2019; 8(2):80. https://doi.org/10.3390/foods8020080
Chicago/Turabian StyleMuriana, Peter M., Jackie Eager, Brent Wellings, Brad Morgan, Jacob Nelson, and Kalpana Kushwaha. 2019. "Evaluation of Antimicrobial Interventions against E. coli O157:H7 on the Surface of Raw Beef to Reduce Bacterial Translocation during Blade Tenderization" Foods 8, no. 2: 80. https://doi.org/10.3390/foods8020080
APA StyleMuriana, P. M., Eager, J., Wellings, B., Morgan, B., Nelson, J., & Kushwaha, K. (2019). Evaluation of Antimicrobial Interventions against E. coli O157:H7 on the Surface of Raw Beef to Reduce Bacterial Translocation during Blade Tenderization. Foods, 8(2), 80. https://doi.org/10.3390/foods8020080






