Biofilm Challenge: Lactic Acid Bacteria Isolated from Bovine Udders versus Staphylococci
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of the Strains
2.2. Biofilm Assay
2.2.1. Preformation of Biofilms by Staphylococci
2.2.2. Biofilm Challenge
2.2.3. Assessment of Biofilm Formation
2.3. Statistical Analysis
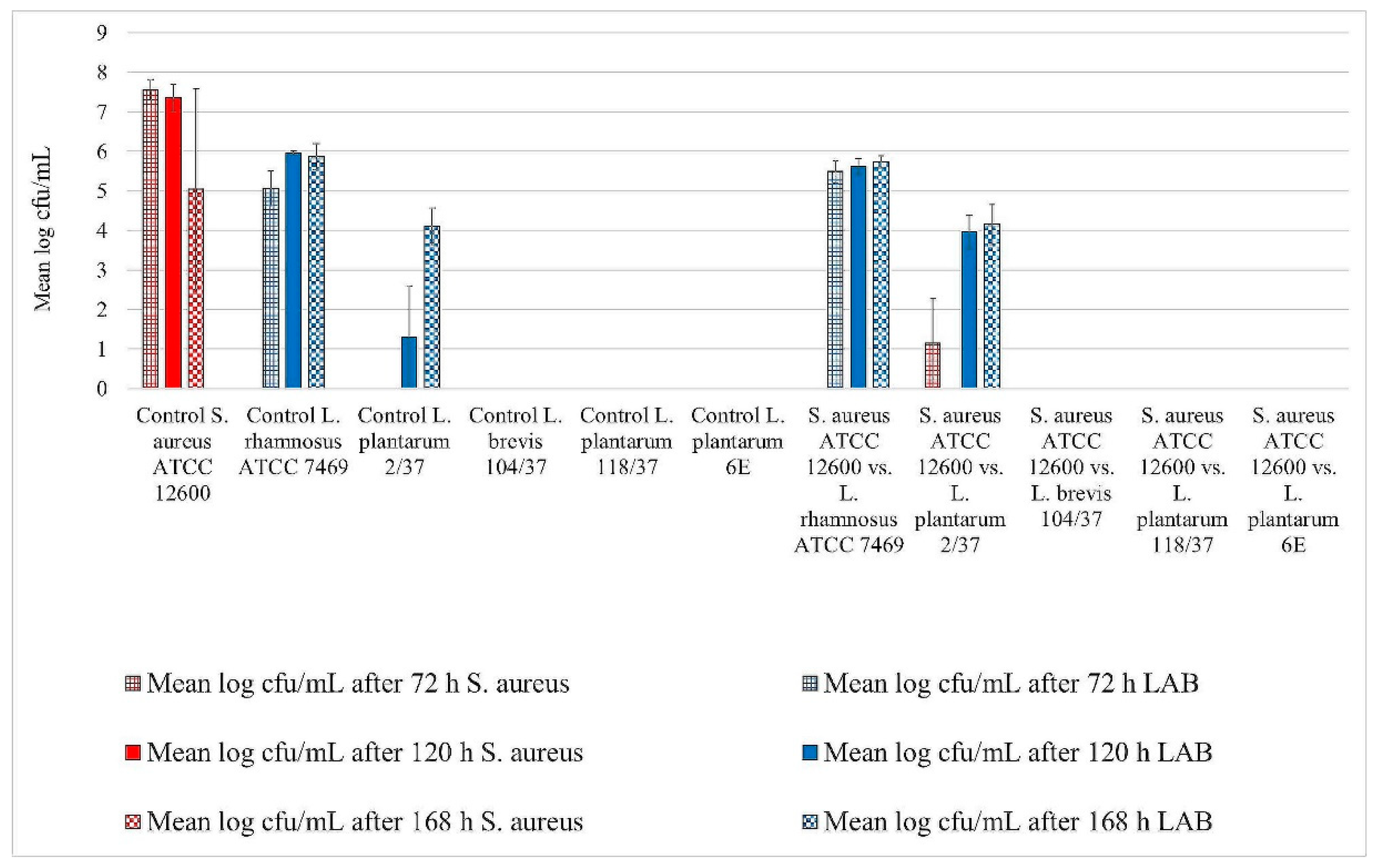
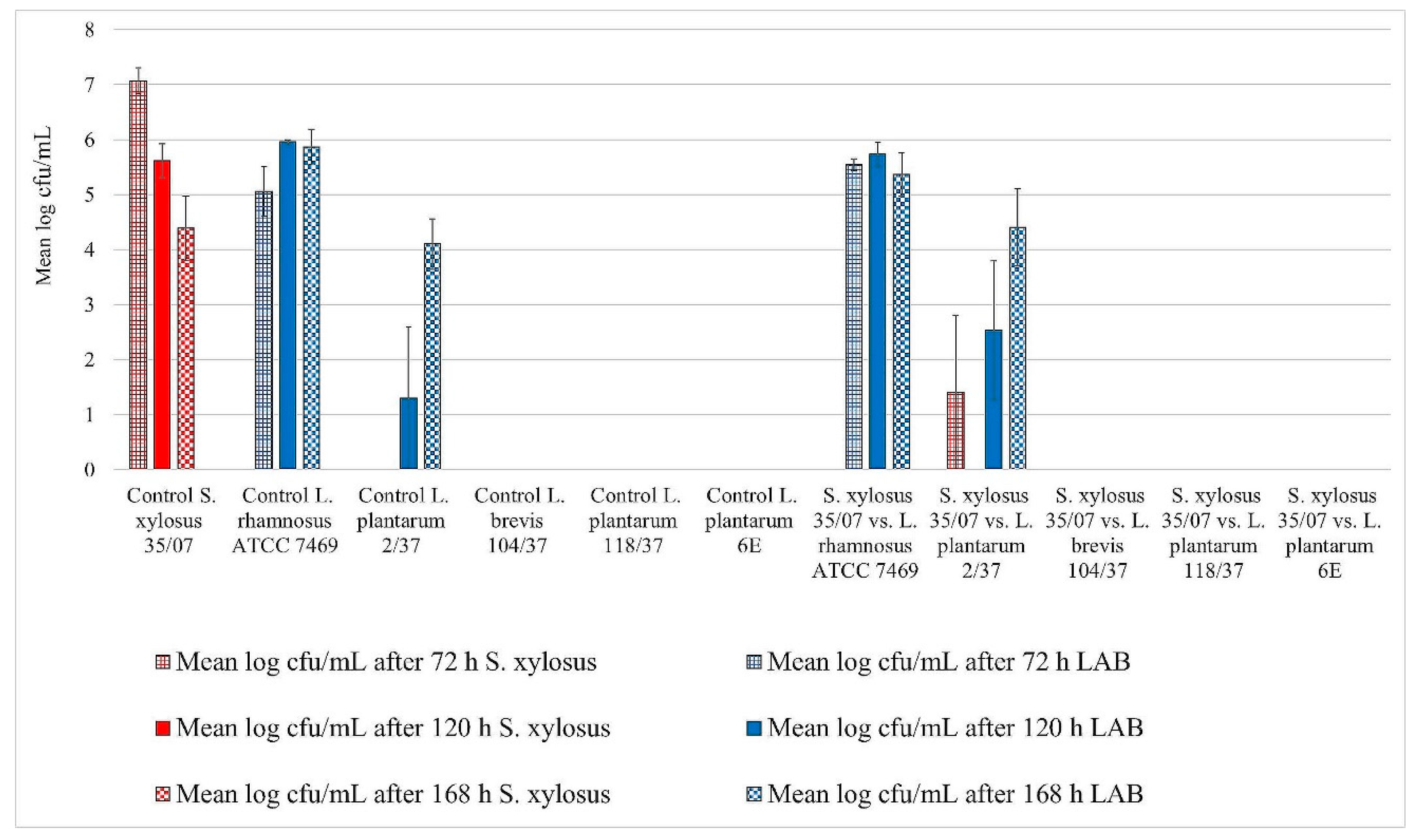
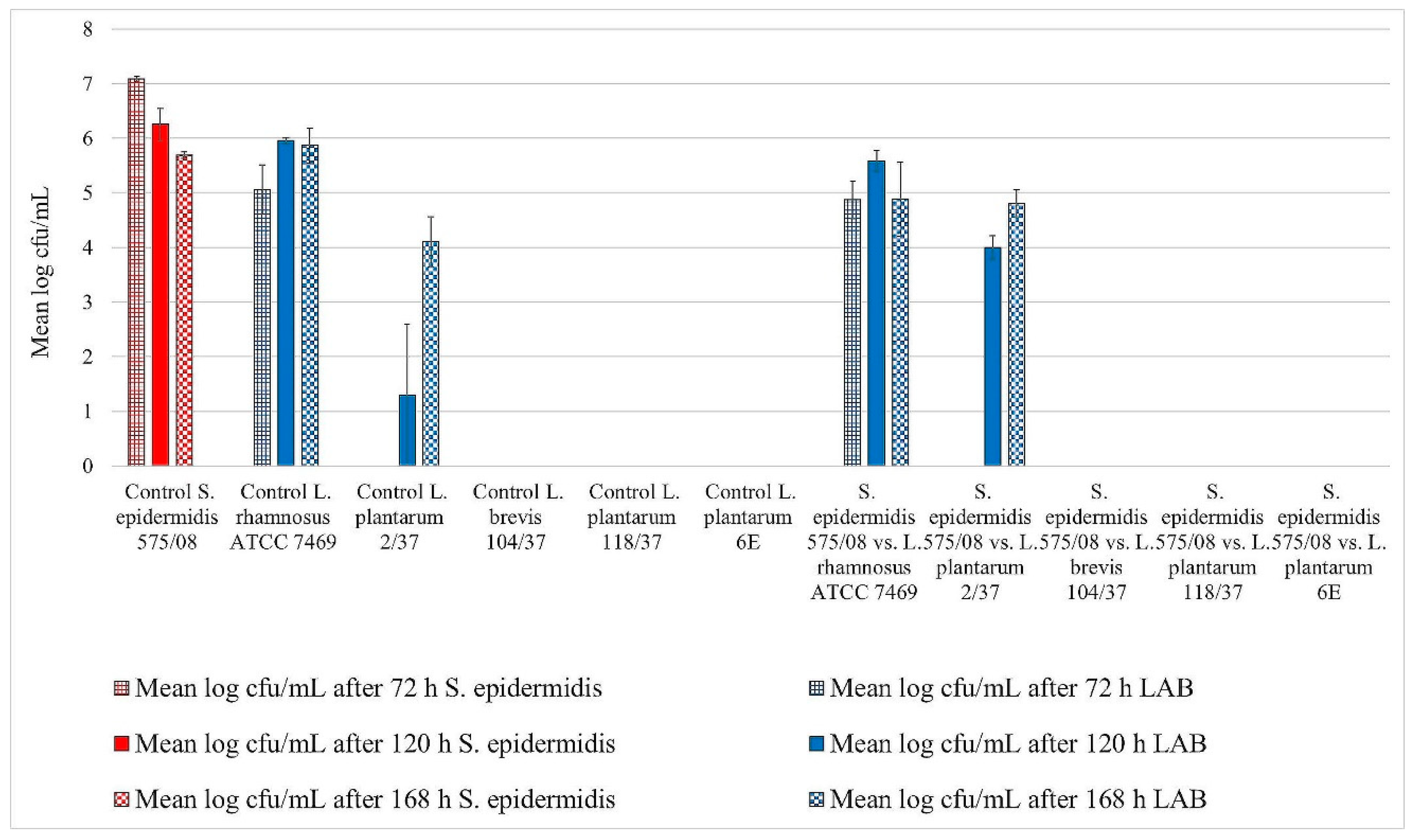
3. Results
3.1. Biofilm Assay
Assessment of Biofilm Formation
3.2. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Halasa, T.; Huijps, K.; Østerås, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet Q. 2007, 29, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.; Paduch, J.H.; Grieger, A.S.; Mansion-de Vries, E.; Knorr, N.; Zinke, C.; Teich, K.; Krömker, V. Cure rates of chronic subclinical Staphylococcus aureus mastitis in lactating dairy cows after antibiotic therapy./Heilungsraten chronischer subklinischer Staphylococcus aureus-Mastitiden nach antibiotischer Therapie bei laktierenden Milchkühen. Berl. Münchener Tierärztliche Wochenschr. 2013, 126, 291–296. [Google Scholar]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Schönborn, S.; Krömker, V. Detection of the biofilm component polysaccharide intercellular adhesin in Staphylococcus aureus infected cow udders. Vet Microbiol. 2016, 196, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Schönborn, S.; Wente, N.; Paduch, J.H.; Krömker, V. In vitro ability of mastitis causing pathogens to form biofilms. J. Dairy Res. 2017, 84, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Beecher, C.; Daly, M.; Berry, D.P.; Klostermann, K.; Flynn, J.; Meaney, W.; Hill, C.; Mc Carthy, T.V.; Ross, R.P.; Giblin, L. Administration of a live culture of Lactococcus lactis DPC 3147 into the bovine mammary gland stimulates the local host immune response, particularly IL-1β and IL-8 gene expression. J. Dairy Res. 2009, 76, 340. [Google Scholar] [CrossRef] [PubMed]
- Crispie, F.; Alonso-Gomez, M.; O’Loughlin, C.; Klostermann, K.; Flynn, J.; Arkins, S.; Meaney, W.; Ross, R.P.; Hill, C. Intramammary infusion of a live culture for treatment of bovine mastitis: Effect of live lactococci on the mammary immune response. J. Dairy Res. 2008, 75, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Klostermann, K.; Crispie, F.; Flynn, J.; Ross, R.P.; Hill, C.; Meaney, W. Intramammary infusion of a live culture of Lactococcus lactis for treatment of bovine mastitis: Comparison with antibiotic treatment in field trials. J. Dairy Res. 2008, 75, 365–373. [Google Scholar] [CrossRef]
- FAO. Probiotics in animal nutrition: Production, impact and regulation. In Animal Production and Health Paper; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2016. [Google Scholar]
- Hagi, T.; Sasaki, K.; Aso, H.; Nomura, M. Adhesive properties of predominant bacteria in raw cow’s milk to bovine mammary gland epithelial cells. Folia Microbiol. 2013, 58, 515–522. [Google Scholar] [CrossRef]
- Frola, I.D.; Pellegrino, M.S.; Espeche, M.C.; Giraudo, J.A.; Nader-Macias, M.E.F.; Bogni, C.I. Effects of intramammary inoculation of Lactobacillus perolens CRL1724 in lactating cows’ udders. J. Dairy Res. 2012, 79, 84–92. [Google Scholar] [CrossRef]
- Diepers, A.-C.; Krömker, V.; Zinke, C.; Wente, N.; Pan, L.; Paulsen, K.; Paduch, J.H. In vitro ability of lactic acid bacteria to inhibit mastitis-causing pathogens. Sustain. Chem. Pharm. 2017, 5, 84–92. [Google Scholar] [CrossRef]
- Wallis, J.K.; Krömker, V.; Paduch, J.H. Biofilm formation and adhesion to bovine udder epithelium of potentially probiotic lactic acid bacteria. AIMS Microbiol. 2018, 4, 209–224. [Google Scholar] [CrossRef]
- Artursson, K.; Soderlund, R.; Liu, L.; Monecke, S.; Schelin, J. Genotyping of Staphylococcus aureus in bovine mastitis and correlation to phenotypic characteristics. Vet Microbiol. 2016, 193, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, B.E.; Headrick, S.I.; Boonyayatra, S.; Oliver, S.P. Prevalence and persistence of coagulase-negative Staphylococcus species in three dairy research herds. Vet Microbiol. 2009, 134, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Krömker, V. Kurzes Lehrbuch Milchkunde und Milchhygiene, 1st ed.; Parey: Stuttgart, Germany, 2007; p. 60. [Google Scholar]
- Fry, P.R.; Middleton, J.R.; Dufour, S.; Perry, J.; Scholl, D.; Dohoo, I. Association of coagulase-negative staphylococcal species, mammary quarter milk somatic cell count, and persistence of intramammary infection in dairy cattle. J. Dairy Sci. 2014, 97, 4876–4885. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, E.; de Niederhausern, S.; Messi, P.; Sabia, C.; Iseppi, R.; Anacarso, I.; Bondi, M. Use of lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes in a small-scale model. Food Control. 2009, 20, 861–865. [Google Scholar] [CrossRef]
- Leccese Terraf, M.C.; Juárez Tomás, M.S.; Nader-Macías, M.E.F.; Silva, C. Screening of biofilm formation by beneficial vaginal lactobacilli and influence of culture media components. J. Appl. Microbiol. 2012, 113, 1517–1529. [Google Scholar] [CrossRef] [PubMed]
- Jahid, I.K.; Ha, S.D. The Paradox of Mixed-Species Biofilms in the Context of Food Safety. Compr. Rev. Food Sci. Food Saf. 2014, 13, 990–1011. [Google Scholar] [CrossRef]
- Gonzalez, S.; Fernandez, L.; Campelo, A.B.; Gutierrez, D.; Martinez, B.; Rodriguez, A.; García, P. The Behavior of Staphylococcus aureus Dual-Species Biofilms Treated with Bacteriophage phiIPLA-RODI Depends on the Accompanying Microorganism. Appl. Environ. Microbiol. 2017, 83, 14. [Google Scholar] [CrossRef]
- Fernández Ramírez, M.D.; Smid, E.J.; Abee, T.; Nierop Groot, M.N. Characterisation of biofilms formed by Lactobacillus plantarum WCFS1 and food spoilage isolates. Int. J. Food Microbiol. 2015, 207, 23–29. [Google Scholar] [CrossRef]
- Klinger-Strobel, M.; Suesse, H.; Fischer, D.; Pletz, M.W.; Makarewicz, O. A Novel Computerized Cell Count Algorithm for Biofilm Analysis. PLoS ONE 2016, 11, e0154937. [Google Scholar] [CrossRef] [PubMed]
- James, G.A.; Beaudette, L.; Costerton, J.W. Interspecies bacterial interactions in biofilms. J. Indust. Microbiol. 1995, 15, 257–262. [Google Scholar] [CrossRef]
- Tallon, R.; Arias, S.; Bressollier, P.; Urdaci, M.C. Strain- and matrix-dependent adhesion of Lactobacillus plantarum is mediated by proteinaceous bacterial compounds. J. Appl. Microbiol. 2007, 102, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Varhimo, E.; Varmanen, P.; Fallarero, A.; Skogman, M.; Pyorala, S.; Iivanainen, A.; Sukura, A.; Vuorela, P.; Savijoki, K. Alpha- and b-casein components of host milk induce biofilm formation in the mastitis bacterium Streptococcus uberis. Vet Microbiol. 2011, 149, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Plumed-Ferrer, C.; Uusikyla, K.; Korhonen, J.; von Wright, A. Characterization of Lactococcus lactis isolates from bovine mastitis. Vet Microbiol. 2013, 167, 592–599. [Google Scholar] [CrossRef] [PubMed]
| Strain | Origin |
|---|---|
| L. rhamnosus ATCC 7469 | American Type Culture Collection |
| L. plantarum 2/37 | Quarter milk samples with normal secretion (somatic cell count <100,000/mL, no pathogen detected) |
| L. brevis 104/37 | |
| L. plantarum 118/37 | |
| L. plantarum 6E | Bedding sample |
| S. aureus ATCC 12,600 | American Type Culture Collection |
| S. xylosus (35/07) | Quarter milk sample from udders of infected cows |
| S. epidermidis (575/08) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallis, J.K.; Krömker, V.; Paduch, J.-H. Biofilm Challenge: Lactic Acid Bacteria Isolated from Bovine Udders versus Staphylococci. Foods 2019, 8, 79. https://doi.org/10.3390/foods8020079
Wallis JK, Krömker V, Paduch J-H. Biofilm Challenge: Lactic Acid Bacteria Isolated from Bovine Udders versus Staphylococci. Foods. 2019; 8(2):79. https://doi.org/10.3390/foods8020079
Chicago/Turabian StyleWallis, Jonathan K., Volker Krömker, and Jan-Hendrik Paduch. 2019. "Biofilm Challenge: Lactic Acid Bacteria Isolated from Bovine Udders versus Staphylococci" Foods 8, no. 2: 79. https://doi.org/10.3390/foods8020079
APA StyleWallis, J. K., Krömker, V., & Paduch, J.-H. (2019). Biofilm Challenge: Lactic Acid Bacteria Isolated from Bovine Udders versus Staphylococci. Foods, 8(2), 79. https://doi.org/10.3390/foods8020079






