A Comparative Study of Essential Oil Constituents and Phenolic Compounds of Arabian Lilac (Vitex Trifolia var. Purpurea): An Evidence of Season Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Essential Oil Extraction
2.3. Essential Oil Composition
2.4. Phenolic Compounds
2.4.1. Preparation of Plant Extracts
2.4.2. Total Phenolic Content
2.4.3. Total Flavonoid Content
2.4.4. Total Flavone and Flavanol Contents
2.5. Total Anthocyanin Content
2.6. Antioxidant Activity
2.7. Statistical Analysis
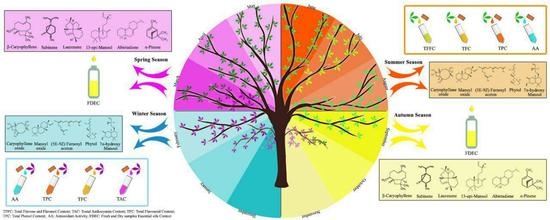
3. Results
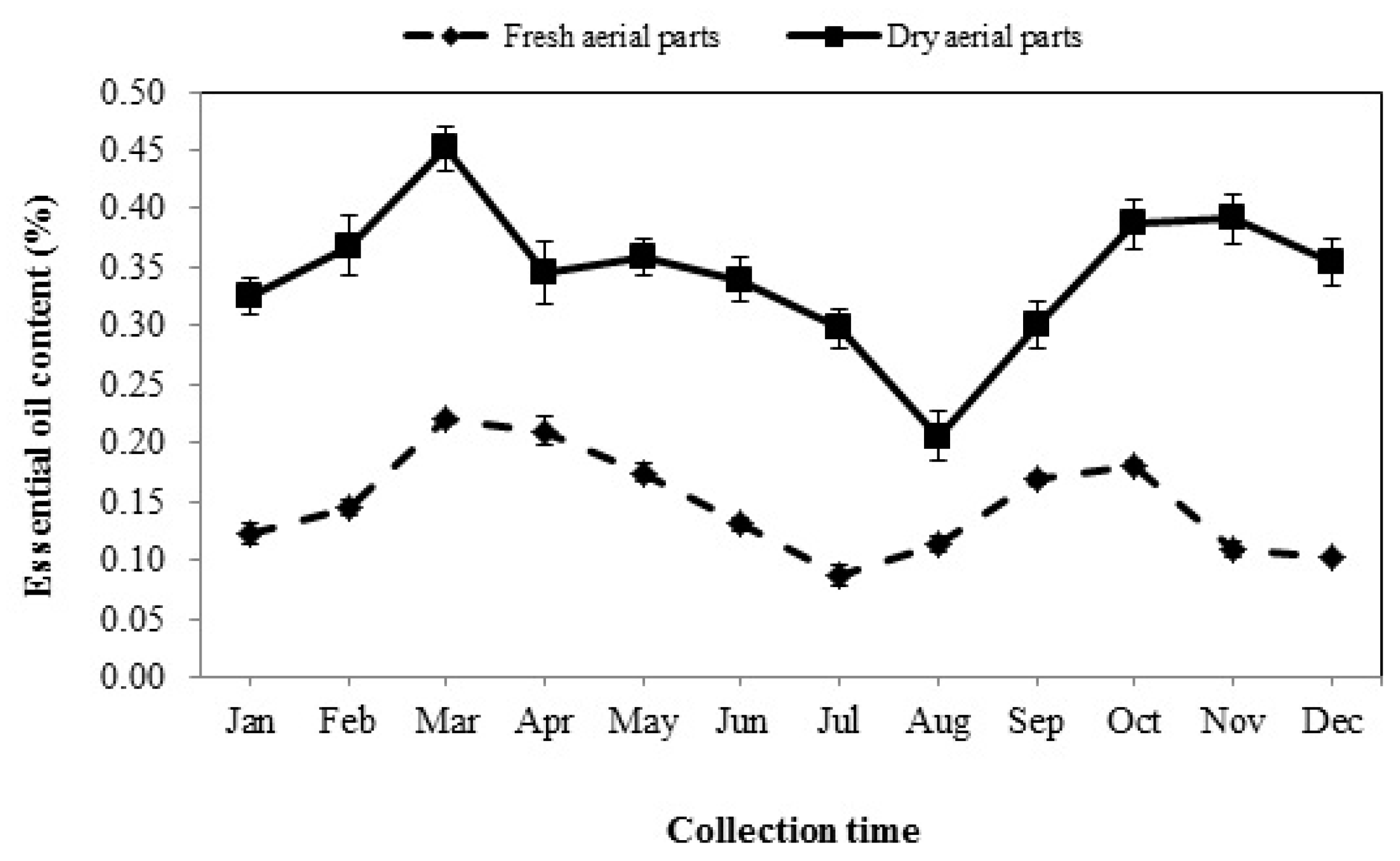
3.1. Essential Oil Content
3.2. Essential Oil Composition
3.3. Total Phenolic Content
3.4. Total Flavonoid Content
3.5. Total Flavone and Flavanol Contents
3.6. Total Anthocyanin Content
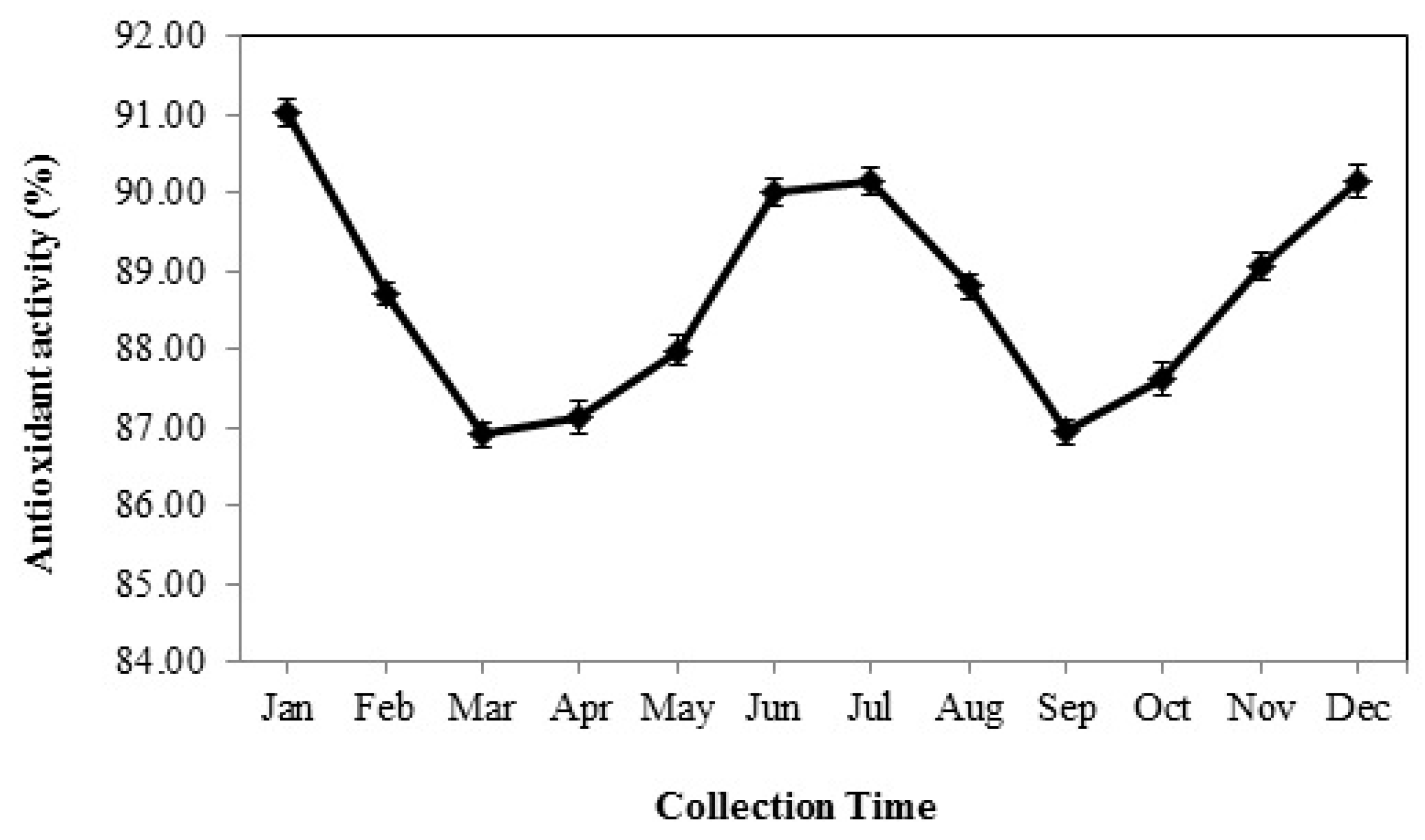
3.7. Total Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Radhapiyari Devi, W.; Singh, C.B. Chemical composition, anti-dermatophytic activity, antioxidant and total phenolic content within the leaves essential oil of Vitex trifolia. Int. J. Phytocos. Nat. Ingred. 2014, 1, 1–5. [Google Scholar] [CrossRef]
- Rani, A.; Sharma, A. The genus Vitex: A review. Pharmacogn. Rev. 2013, 7, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Abdou, A.M.; Hamed, M.M.; Saad, A.M. Characterization of bioactive phytochemical from the leaves of Vitex trifolia. Int. J. Pharm. Appl. 2012, 3, 419–428. [Google Scholar]
- Kannathasan, K.; Senthilkumar, A.; Chandrasekaran, M.; Venkatesalu, V. Differential larvicidal efficacy of four species of Vitex against Culex quinquefasciatus larvae. Parasitol. Res. 2007, 101, 1721–1723. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.L.; Fang, S.M.; Liu, R.; Oppong, M.B.; Liu, E.W.; Fan, G.W.; Zhang, H. A Review on the Terpenes from Genus Vitex. Molecules 2016, 21, 1179. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Thakur, J.; Saikia, D.; Gupta, M.M. Antitubercular diterpenoids from Vitex trifolia. Phytomedicine 2013, 20, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Suksamrarn, A.; Werawattanametin, K.; Brophy, J.J. Variation of essential oil constituents in Vitex trifolia species. Flavour Fragr. J. 1991, 6, 97–99. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef]
- Smitha, G.R.; Tripathy, V. Seasonal variation in the essential oils extracted from leaves and inflorescence of different Ocimum species grown in Western plains of India. Ind. Crop. Prod. 2016, 94, 52–64. [Google Scholar] [CrossRef]
- Galasso, S.; Pacifico, S.; Kretschmer, N.; Pan, S.P.; Marciano, S.; Piccolella, S.; Bauer, R. Influence of seasonal variation on Thymus longicaulis C. Presl chemical composition and its antioxidant and anti-inflammatory properties. Phytochemistry 2014, 107, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Toncer, O.; Karaman, S.; Diraz, E. An annual variation in essential oil composition of Origanum syriacum from Southeast Anatolia of Turkey. J. Med. Plant Res. 2010, 4, 1059–1064. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Randrianalijaona, J.A.; Ramanoelina, P.A.; Rasoarahona, J.R.; Gaydou, E.M. Seasonal and chemotype influences on the chemical composition of Lantana camara L.: Essential oil from Madagascar. Anal. Chim. Acta 2005, 545, 46–52. [Google Scholar] [CrossRef]
- Muller-Riebau, F.J.; Berger, B.M.; Yegen, O.; Cakir, C. Seasonal variations in the chemical compositions of essential oils of selected aromatic plants growing wild in Turkey. J. Agric. Food Chem. 1997, 45, 4821–4825. [Google Scholar] [CrossRef]
- Bujor, O.C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal variations of the phenolic constituents in bilberry (Vaccinium myrtillus L.) leaves, stems and fruits, and their antioxidant activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, S.A. European Pharmacopoeia, 3rd ed.; Council of Europe: Sainte-Ruffine, France, 1975. [Google Scholar]
- Adams, R.P. Identification of essential oil components by gas chromatography/quadrupole mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 16, 1902–1903. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compound in 32 selected herbs. Food Chem. 2007, 1005, 940–949. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.R.; Conforti, F.; Statti, G.; Di Cindi, B.; Houghton, P.J.; Menichini, F. The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq. cv. Habanero. Food Chem. 2009, 114, 553–560. [Google Scholar] [CrossRef]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef]
- Lako, J.; Trenerry, V.C.; Wahlqvist, M.; Wattanapenpaiboon, N.; Sotheeswaran, S.; Premier, R. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007, 101, 1727–1741. [Google Scholar] [CrossRef]
- Oke, F.; Aslim, B.; Ozturk, S.; Altundag, S. Essential oil composition, antimicrobial and antioxidant activities of Satureja cuneifolia ten. Food Chem. 2009, 112, 874–879. [Google Scholar] [CrossRef]
- Kofidis, G.; Bosabalidis, A.; Kokkini, S. Seasonal variations of essential oils in a linalool-rich chemotype of Mentha spicata grown wild in Greece. J. Essent. Oil Res. 2006, 16, 469–472. [Google Scholar] [CrossRef]
- Chericoni, S.; Flamini, G.; Campeol, E.; Cioni, P.L.; Morelli, I. GC-MS analysis of the essential oil from the aerial parts of Artemisia verlotiorum: Variability during the year. Biochem. Syst. Ecol. 2004, 32, 423–429. [Google Scholar] [CrossRef]
- Pazouki, L.; Kanagendran, A.; Li, S.; Kannaste, A.; Memari, H.R.; Bichele, R.; Niinemets, U. Mono-and sesquiterpene release from tomato (Solanum lycopersicum) leaves upon mild and severe heat stress and through recovery: From gene expression to emission responses. Environ. Exp. Bot. 2016, 132, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Celiktas, O.Y.; Kocabas, E.E.H.; Bedir, E.; Sukan, F.V.; Ozek, T.; Baser, K.H.C. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus oficinalis, depending on location and seasonal variations. Food Chem. 2007, 100, 553–559. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Ubi, B.W.; Honda, C.; Bessho, H.; Kondo, S.; Wada, M.; Kobayashi, S.; Moriguchi, T. Expression analysis of anthocyanin biosynthetic genes in apple skin: Effect of UV-B and temperature. Plant Sci. 2006, 170, 571–578. [Google Scholar] [CrossRef]
- Lo Piero, A.R.; Puglisi, I.; Rapisarda, P.; Petrone, G. Anthocyanins accumulation and related gene expression in red orange fruit induced by low temperature storage. J. Agric. Food Chem. 2005, 53, 9083–9088. [Google Scholar] [CrossRef]
- Dela, G.; Or, E.; Ovadia, R.; Nissim-Levi, A.; Weiss, D.; Oren-Shamir, M. Changes in anthocyanin concentration and composition in ‘Jaguar’ rose flowers due to transient high temperature conditions. Plant Sci. 2003, 164, 333–340. [Google Scholar] [CrossRef]
- Shvarts, M.; Borochov, A.; Weiss, D. Low temperature enhances petunia flower pigmentation and induces chalcone synthase gene expression. Physiol. Plant 1997, 99, 67–72. [Google Scholar] [CrossRef]
- Leyva, A.; Jarillo, J.; Salinas, J.; Martinez-Zapater, M. Low temperature induces the accumulation of phenylalanine ammonialyase and chalcone synthase mRNAs of Arabidopsis thaliana in a light-dependent manner. Plant Physiol. 1995, 108, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Gutzeit, H.O.; Ludwig-Muller, J. Plant Natural Products: Synthesis, Biological Functions and Practical Applications; John Wiley and Sons: New York, NY, USA, 2014; pp. 19–21. [Google Scholar]
- Borrelli, G.M.; Trono, D. Molecular Approaches to genetically improve the accumulation of health-promoting secondary metabolites in staple crops-A case study: The lipoxygenase-B1 genes and regulation of the carotenoid content in pasta products. Int. J. Mol. Sci. 2016, 17, 1177. [Google Scholar] [CrossRef] [PubMed]


| Month | Temperature (°C) | Relative Humidity (%) | ||||
|---|---|---|---|---|---|---|
| Minimum | Maximum | Average | Minimum | Maximum | Average | |
| January | 4.6 | 21 | 12.8 | 29 | 92 | 61 |
| February | 9.6 | 23.2 | 16.4 | 19 | 64 | 42 |
| March | 15.4 | 30.4 | 22.9 | 27 | 94 | 61 |
| April | 15.2 | 28.2 | 21.7 | 20 | 46 | 33 |
| May | 28 | 37 | 32.5 | 19 | 43 | 31 |
| June | 28.8 | 45.2 | 37 | 8 | 31 | 20 |
| July | 30 | 50.2 | 40.1 | 12 | 62 | 37 |
| August | 30.6 | 48 | 39.3 | 20 | 74 | 47 |
| September | 29.6 | 44.8 | 37.2 | 15 | 46 | 31 |
| October | 24 | 35.6 | 29.8 | 41 | 97 | 69 |
| November | 10.6 | 28.4 | 19.5 | 25 | 73 | 49 |
| December | 14.4 | 17.6 | 16 | 64 | 95 | 80 |
| GC area (%) a | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | RT b | KI c | KI d | January | February | March | April | May | June | July | August | September | October | November | December | ||||||||||||
| FAP | DAP | FAP | DAP | FAP | DAP | FAP | DAP | FAP | DAP | FAP | DAP | FAP | DAP | FAP | DAP | FAP | DAP | FAP | DAP | FAP | DAP | FAP | DAP | ||||
| Thujene | 7.58 | 933 | 924 | 0.15 | 0.37 | 0.18 | 0.38 | 0.23 | 0.36 | 0.29 | 0.36 | 0.48 | 0.60 | 0.23 | 0.51 | 0.17 | 0.34 | 0.16 | 0.27 | 0.21 | 0.20 | 0.26 | 0.42 | 0.14 | 0.35 | 0.11 | 0.20 |
| α-Pinene | 7.79 | 939 | 932 | 3.27 | 1.35 | 4.10 | 4.00 | 4.29 | 4.62 | 4.40 | 5.20 | 2.45 | 3.22 | 1.77 | 2.29 | 2.00 | 2.56 | 2.15 | 2.55 | 3.18 | 5.35 | 3.61 | 4.86 | 2.79 | 4.06 | 2.95 | 3.66 |
| Sabinene | 9.05 | 975 | 969 | 11.71 | 13.03 | 13.23 | 15.1 | 16.73 | 18.14 | 16.38 | 18.17 | 9.58 | 16.45 | 9.11 | 12.49 | 7.24 | 12.08 | 9.22 | 11.04 | 15.13 | 15.09 | 16.02 | 18.38 | 11.63 | 17.94 | 9.68 | 15.47 |
| β-Pinene | 9.09 | 976 | 974 | 1.54 | 0.78 | 2.00 | 0.80 | 1.86 | 1.07 | 0.47 | 1.14 | 0.50 | 0.96 | 0.37 | 0.07 | 0.31 | 0.04 | 0.32 | 0.03 | 0.57 | 0.01 | 0.87 | 0.20 | 1.83 | 0.86 | 1.26 | 0.30 |
| 1-Octen-3-ol | 9.14 | 978 | 974 | 0.64 | 2.61 | 0.71 | 2.14 | - | - | - | - | 2.95 | - | 2.39 | 1.53 | 1.89 | 1.84 | 0.61 | 0.87 | 0.64 | - | - | - | 2.98 | 0.32 | 1.99 | 2.66 |
| Myrcene | 9.43 | 986 | 988 | 0.14 | 0.85 | 0.21 | 0.24 | 0.28 | 0.27 | 0.30 | 0.85 | 0.11 | 0.20 | 0.10 | 0.10 | - | - | - | - | 0.14 | - | 0.30 | 0.42 | 0.13 | 0.32 | - | 0.16 |
| α-Terpinene | 10.16 | 1009 | 1014 | 0.83 | 0.44 | 0.27 | 0.17 | - | 0.14 | - | 0.15 | 0.39 | 0.42 | 0.41 | 0.49 | 0.48 | 0.35 | 0.15 | 0.53 | - | 0.19 | - | 0.27 | 0.21 | 0.45 | 0.20 | - |
| p-Cymene | 10.39 | 1017 | 1020 | - | 0.47 | 0.24 | 0.75 | 0.42 | 0.78 | 0.55 | 0.53 | 0.28 | 0.78 | 0.24 | 0.37 | 0.16 | 0.27 | - | - | 0.21 | - | - | 1.07 | 0.15 | 0.51 | 0.10 | - |
| β-Phellandrene | 10.52 | 1021 | 1025 | - | 0.26 | 0.25 | 0.12 | 0.33 | 0.18 | 0.14 | 0.23 | 0.27 | 0.29 | 0.39 | 0.26 | 0.17 | 0.18 | 0.17 | 0.28 | 0.21 | 0.19 | 0.39 | 0.26 | 0.33 | 0.43 | 0.16 | 0.30 |
| 1,8-Cineole | 10.60 | 1024 | 1026 | 0.10 | 0.19 | 0.22 | - | 0.16 | - | - | 0.12 | 0.25 | 0.21 | 0.21 | 0.23 | 0.24 | 0.18 | 0.14 | 0.31 | 0.16 | 0.14 | 0.27 | 0.23 | 0.26 | 0.19 | - | 0.22 |
| γ-Terpinene | 11.42 | 1052 | 1054 | - | 2.12 | 1.39 | 0.88 | - | 0.97 | 0.99 | 0.53 | 2.03 | 1.44 | 1.93 | 0.68 | 1.49 | 0.33 | 0.57 | 0.58 | 0.64 | 0.06 | 1.37 | 2.17 | 1.66 | 2.68 | 1.06 | 2.68 |
| α-Terpinolene | 12.28 | 1081 | 1086 | - | 0.22 | - | 0.26 | - | 0.12 | - | 0.09 | 0.10 | 0.10 | 0.08 | - | - | - | - | - | - | - | 0.11 | 0.18 | 0.35 | 0.24 | - | - |
| Terpinen-4-ol | 14.93 | 1171 | 1174 | 0.75 | - | 0.47 | - | 0.16 | - | 0.16 | - | 0.16 | - | 0.68 | - | 0.70 | - | 0.57 | - | 0.47 | 0.03 | 0.50 | - | 0.62 | - | 0.16 | - |
| Estragol | 15.48 | 1190 | 1195 | - | 2.72 | - | 1.68 | 0.63 | - | - | - | 2.88 | - | 1.33 | - | 1.76 | - | - | 0.98 | 1.68 | - | - | - | 0.98 | - | 0.27 | 2.87 |
| Bornyl acetate | 17.93 | 1280 | 1284 | 0.16 | - | 0.15 | 0.14 | 0.20 | - | - | - | - | - | 0.15 | 0.12 | 0.16 | 0.11 | - | 0.14 | - | 0.09 | - | 0.13 | 0.10 | - | 0.14 | - |
| α-Terpinyl acetate | 19.63 | 1343 | 1346 | 0.30 | 0.51 | 0.42 | 0.33 | 0.18 | - | 0.33 | 0.41 | 0.38 | 0.36 | 0.28 | 0.33 | 0.31 | 0.54 | 0.29 | 0.20 | 0.27 | 0.18 | 0.25 | 0.23 | 0.31 | 0.53 | 0.33 | 0.46 |
| β-Caryophyllene | 21.71 | 1422 | 1418 | 25.76 | 25.29 | 26.33 | 25.14 | 35.03 | 27.51 | 34.41 | 29.39 | 24.32 | 28.80 | 22.6 | 26.03 | 24.01 | 26.71 | 32.49 | 27.46 | 34.16 | 32.43 | 31.81 | 31.99 | 26.44 | 28.76 | 27.36 | 27.03 |
| α-Caryophyllene | 22.37 | 1450 | 1454 | 0.91 | 0.85 | 1.26 | 0.97 | 1.21 | 1.20 | 1.04 | 1.16 | 1.07 | 1.15 | 0.91 | 1.23 | 0.87 | 1.18 | 1.35 | 0.97 | 1.44 | 1.19 | 1.20 | 1.18 | 1.00 | 1.13 | 1.01 | 1.19 |
| Germacrene-D | 23.01 | 1476 | 1484 | - | - | - | - | - | - | 0.16 | - | 0.93 | - | 0.14 | 0.19 | 0.20 | 0.25 | 0.26 | 0.34 | 0.16 | - | 0.12 | - | - | - | - | - |
| β-Selinene | 23.14 | 1481 | 1489 | - | 0.10 | - | - | - | - | - | - | 0.78 | - | 0.53 | 0.21 | 0.23 | 0.29 | 0.42 | 0.38 | 0.25 | - | - | - | - | - | 0.17 | - |
| α-Selinene | 23.35 | 1490 | 1498 | 0.64 | 0.59 | 0.79 | 0.59 | 0.60 | 0.86 | 0.79 | 0.85 | 1.53 | 0.85 | 0.33 | 0.36 | 0.79 | 0.44 | 0.37 | 0.58 | 0.91 | 0.42 | 0.43 | 0.59 | 0.53 | 0.66 | 0.66 | 0.85 |
| E-Nerolidol | 24.91 | 1554 | 1561 | 1.18 | - | - | 0.11 | - | 0.16 | - | - | 0.33 | 0.15 | 0.22 | 0.17 | 0.19 | 0.19 | 0.23 | 0.13 | 0.22 | 0.10 | - | - | 0.14 | - | 0.11 | - |
| Caryophyllene oxide | 25.47 | 1578 | 1582 | 6.38 | 6.89 | 4.74 | 6.83 | 3.78 | 6.35 | 3.93 | 6.33 | 4.23 | 7.05 | 5.46 | 7.29 | 6.24 | 6.75 | 5.98 | 4.67 | 3.91 | 4.48 | 3.87 | 4.59 | 4.54 | 4.96 | 4.77 | 5.76 |
| Caryophylladienol II | 26.57 | 1626 | 1631 | 0.61 | 0.66 | - | - | 0.22 | 0.13 | 0.38 | - | 0.14 | 0.13 | 0.40 | 0.43 | 0.31 | 0.36 | 0.30 | 0.70 | - | 0.33 | 0.45 | 0.55 | 0.33 | 0.40 | 0.37 | 0.74 |
| (Z)-14-hydroxy-Caryophyllene | 27.41 | 1664 | 1666 | 0.91 | 1.82 | 0.71 | 0.63 | 0.42 | - | 0.58 | - | 1.81 | 0.75 | 2.46 | 1.34 | 2.75 | 1.25 | 2.63 | 0.87 | 1.85 | 0.62 | 0.45 | 0.52 | 0.39 | 0.72 | 0.55 | 0.71 |
| Phytane | 30.12 | 1789 | 1792 | 2.73 | 3.03 | 2.69 | 2.56 | 3.98 | 2.80 | 3.75 | 2.38 | 4.45 | 2.54 | 3.96 | 3.84 | 4.30 | 3.68 | 4.42 | 3.41 | 4.71 | 3.01 | 3.68 | 2.32 | 3.19 | 2.83 | 3.71 | 3.51 |
| (5E,9Z)-Farnesyl acetone | 31.93 | 1879 | 1886 | 4.76 | 5.01 | 4.49 | 4.10 | 3.12 | 3.22 | 3.41 | 3.29 | 3.23 | 3.37 | 4.25 | 4.11 | 4.61 | 4.42 | 4.49 | 4.88 | 3.55 | 4.28 | 3.03 | 4.11 | 4.09 | 3.49 | 4.49 | 4.04 |
| Laurenene | 32.26 | 1896 | 1887 | 4.07 | 2.80 | 3.71 | 3.92 | 5.26 | 4.42 | 5.46 | 4.56 | 3.29 | 4.10 | 3.56 | 3.57 | 4.06 | 3.67 | 6.88 | 5.89 | 7.32 | 6.90 | 6.23 | 6.89 | 3.68 | 5.77 | 3.90 | 4.00 |
| epi-Laurenene | 32.46 | 1906 | 1901 | - | 0.71 | 0.95 | 0.62 | 1.88 | 0.85 | 1.33 | - | - | 1.46 | 1.23 | - | - | - | - | 1.34 | 2.94 | - | 1.53 | 1.20 | 1.33 | - | 1.15 | 1.08 |
| Phytol | 33.26 | 1946 | 1942 | 4.01 | 3.43 | 3.72 | 4.58 | 3.01 | 3.81 | 2.28 | 3.31 | 2.83 | 3.55 | 2.99 | 3.77 | 3.81 | 4.51 | 3.71 | 4.03 | 2.08 | 3.34 | 2.51 | 3.02 | 2.55 | 3.97 | 3.90 | 3.17 |
| Manool oxide | 33.64 | 1965 | 1987 | 1.99 | 0.10 | 6.93 | 0.11 | 8.34 | 7.50 | 7.11 | 6.03 | 1.05 | 5.35 | 0.10 | - | - | 0.10 | 0.10 | 0.11 | 0.31 | 0.10 | 8.01 | 5.05 | 7.21 | 6.42 | 0.10 | 0.10 |
| (6Z,10Z)-Pseudo phytol | 34.03 | 1985 | 1988 | 10.01 | 0.23 | 0.20 | 0.64 | - | - | 0.10 | 6.72 | 0.11 | 8.34 | 10.92 | 12.67 | 15.02 | 14.12 | 9.93 | 13.54 | 0.10 | 13.63 | 0.27 | 0.34 | 0.40 | 0.28 | 10.5 | 0.15 |
| Manoyl oxide | 34.11 | 1989 | 1989 | 2.98 | 1.99 | 1.96 | 0.98 | - | - | - | - | 2.97 | - | 1.62 | 0.38 | 1.43 | 0.21 | 1.32 | 0.19 | 1.91 | - | 1.54 | - | 1.69 | 0.58 | 2.26 | 1.23 |
| (E,E)-Geranyl linalool | 34.88 | 2031 | 2026 | 0.11 | 1.96 | 1.97 | 1.91 | - | 1.02 | - | - | 2.99 | - | 1.92 | 0.17 | 1.49 | 0.19 | 0.23 | 0.13 | 0.22 | - | - | - | 0.14 | - | 1.21 | 1.09 |
| Manool | 35.37 | 2058 | 2056 | 1.35 | 0.74 | 1.45 | 1.24 | 1.74 | 1.45 | 1.67 | 1.30 | 1.50 | 1.32 | 1.37 | 0.60 | - | 0.90 | 0.10 | 0.89 | 1.36 | 1.09 | 1.30 | 1.26 | 1.44 | 1.19 | 1.62 | 1.12 |
| Sclareolide | 35.48 | 2064 | 2065 | - | 2.69 | 3.21 | 2.03 | - | 1.97 | 3.87 | - | 5.08 | 0.15 | 1.27 | 0.96 | 1.33 | 1.13 | - | 1.07 | - | 2.02 | 1.88 | 1.60 | 3.44 | 2.94 | - | 2.83 |
| 1-Octadecanol | 35.64 | 2073 | 2077 | 0.56 | 2.86 | 0.84 | 1.98 | - | - | - | - | 2.99 | - | 2.95 | 1.43 | 2.73 | 1.92 | 2.29 | 1.89 | 0.55 | - | - | 0.88 | 1.99 | 0.49 | 0.93 | 2.48 |
| Abietadiene | 35.82 | 2082 | 2087 | 4.12 | - | 1.63 | 3.56 | 2.97 | 4.59 | 2.80 | 4.36 | 2.93 | 4.29 | - | 3.23 | 1.88 | 0.10 | 2.01 | 1.11 | 3.61 | 2.81 | 5.04 | 2.03 | 4.88 | 3.17 | 4.96 | 4.36 |
| Laurenan-2-one | 36.40 | 2114 | 2115 | 0.79 | 1.97 | 1.63 | 0.94 | - | - | - | - | 2.04 | - | 3.08 | 0.20 | 0.75 | 0.26 | 0.58 | 0.29 | 0.52 | - | 0.37 | - | 0.47 | - | 0.57 | 0.26 |
| 7α-hydroxy-Manool | 38.62 | 2240 | 2237 | 1.58 | 3.06 | 0.53 | 1.73 | - | 0.31 | 0.11 | 1.58 | 2.25 | 1.58 | 2.92 | 4.18 | 1.63 | 2.80 | 0.93 | 2.48 | 0.36 | 1.66 | 0.31 | 1.97 | 0.35 | 1.28 | 1.42 | 2.07 |
| Dehydroabietal | 39.22 | 2276 | 2263 | 2.04 | 4.52 | 3.76 | 4.87 | - | 3.17 | - | 0.40 | 2.94 | - | 2.16 | 1.90 | 1.43 | 2.83 | 1.72 | 1.91 | 1.07 | - | 0.94 | - | 2.90 | 0.51 | 2.92 | 0.38 |
| Chemical class | |||||||||||||||||||||||||||
| Monoterpene hydrocarbons | 17.64 | 19.89 | 21.87 | 22.70 | 24.14 | 26.65 | 23.52 | 27.25 | 16.19 | 24.46 | 14.63 | 17.26 | 12.02 | 16.15 | 12.74 | 15.28 | 20.29 | 21.09 | 22.93 | 28.23 | 19.22 | 27.84 | 15.52 | 22.77 | |||
| Oxygenated monoterpenes | 1.31 | 0.70 | 1.26 | 0.47 | 0.70 | - | 0.49 | 0.53 | 0.79 | 0.57 | 1.32 | 0.68 | 1.41 | 0.83 | 1.00 | 0.65 | 0.90 | 0.44 | 1.02 | 0.59 | 1.29 | 0.72 | 0.63 | 0.68 | |||
| Total monoterpenes | 18.95 | 20.59 | 23.13 | 23.17 | 24.84 | 26.65 | 24.01 | 27.78 | 16.98 | 25.03 | 15.95 | 17.94 | 13.43 | 16.98 | 13.74 | 15.93 | 21.19 | 21.53 | 23.95 | 28.82 | 20.51 | 28.56 | 16.15 | 23.45 | |||
| Sesquiterpene hydrocarbons | 31.38 | 30.34 | 33.04 | 31.24 | 43.98 | 34.84 | 43.19 | 35.96 | 31.92 | 36.36 | 29.30 | 31.59 | 30.16 | 32.54 | 41.77 | 36.96 | 47.18 | 40.94 | 41.32 | 41.85 | 32.98 | 36.32 | 34.25 | 34.15 | |||
| Oxygenated sesquiterpenes | 14.63 | 19.04 | 14.78 | 14.64 | 7.54 | 11.83 | 12.17 | 9.62 | 16.86 | 11.60 | 17.14 | 14.50 | 16.18 | 14.36 | 14.21 | 12.61 | 10.05 | 11.83 | 10.05 | 11.37 | 13.40 | 12.51 | 10.86 | 14.34 | |||
| Total sesquiterpenes | 46.01 | 49.38 | 47.82 | 45.88 | 51.52 | 46.67 | 55.36 | 45.58 | 48.78 | 47.96 | 46.44 | 46.09 | 46.34 | 46.90 | 55.98 | 49.57 | 57.23 | 52.77 | 51.37 | 53.22 | 46.38 | 48.83 | 45.11 | 48.49 | |||
| Diterpene hydrocarbons | 6.85 | 3.03 | 4.32 | 6.12 | 6.95 | 7.39 | 6.55 | 6.74 | 7.38 | 6.83 | 3.96 | 7.07 | 6.18 | 3.78 | 6.43 | 4.52 | 8.32 | 5.82 | 8.72 | 4.35 | 8.07 | 6.00 | 8.67 | 7.87 | |||
| Oxygenated diterpenes | 24.07 | 16.03 | 20.52 | 16.06 | 13.09 | 17.26 | 11.27 | 19.34 | 16.64 | 20.14 | 24.00 | 23.67 | 24.81 | 25.66 | 18.04 | 23.27 | 7.41 | 19.82 | 14.88 | 11.64 | 16.68 | 14.23 | 23.93 | 9.31 | |||
| Total diterpenes | 30.92 | 19.06 | 24.84 | 22.18 | 20.04 | 24.65 | 17.82 | 26.08 | 24.02 | 26.97 | 27.96 | 30.74 | 30.99 | 29.44 | 24.47 | 27.79 | 15.73 | 25.64 | 23.6 | 15.99 | 24.75 | 20.23 | 32.60 | 17.18 | |||
| Phenylpropanoids | - | 2.72 | - | 1.68 | 0.63 | - | - | - | 2.88 | - | 1.33 | - | 1.76 | - | - | 0.98 | 1.68 | - | - | - | 0.98 | - | 0.27 | 2.87 | |||
| Alcohols | 1.20 | 5.47 | 1.55 | 4.12 | - | - | - | - | 5.94 | - | 5.34 | 2.96 | 4.62 | 3.76 | 2.90 | 2.76 | 1.19 | - | - | 0.88 | 4.97 | 0.81 | 2.92 | 5.14 | |||
| Total Identified | 97.08 | 97.22 | 97.34 | 97.03 | 97.03 | 97.97 | 97.19 | 99.44 | 98.6 | 99.96 | 97.02 | 97.73 | 97.14 | 97.08 | 97.09 | 97.03 | 97.02 | 99.94 | 98.92 | 98.91 | 97.59 | 98.43 | 97.05 | 97.13 | |||
| Not-identified | 2.92 | 2.78 | 2.66 | 2.97 | 2.97 | 2.03 | 2.81 | 0.56 | 1.40 | 0.04 | 2.98 | 2.27 | 2.86 | 2.92 | 2.91 | 2.97 | 2.98 | 0.06 | 1.08 | 1.09 | 2.41 | 1.57 | 2.95 | 2.87 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boveiri Dehsheikh, A.; Mahmoodi Sourestani, M.; Boveiri Dehsheikh, P.; Vitalini, S.; Iriti, M.; Mottaghipisheh, J. A Comparative Study of Essential Oil Constituents and Phenolic Compounds of Arabian Lilac (Vitex Trifolia var. Purpurea): An Evidence of Season Effects. Foods 2019, 8, 52. https://doi.org/10.3390/foods8020052
Boveiri Dehsheikh A, Mahmoodi Sourestani M, Boveiri Dehsheikh P, Vitalini S, Iriti M, Mottaghipisheh J. A Comparative Study of Essential Oil Constituents and Phenolic Compounds of Arabian Lilac (Vitex Trifolia var. Purpurea): An Evidence of Season Effects. Foods. 2019; 8(2):52. https://doi.org/10.3390/foods8020052
Chicago/Turabian StyleBoveiri Dehsheikh, Anahita, Mohammad Mahmoodi Sourestani, Paria Boveiri Dehsheikh, Sara Vitalini, Marcello Iriti, and Javad Mottaghipisheh. 2019. "A Comparative Study of Essential Oil Constituents and Phenolic Compounds of Arabian Lilac (Vitex Trifolia var. Purpurea): An Evidence of Season Effects" Foods 8, no. 2: 52. https://doi.org/10.3390/foods8020052
APA StyleBoveiri Dehsheikh, A., Mahmoodi Sourestani, M., Boveiri Dehsheikh, P., Vitalini, S., Iriti, M., & Mottaghipisheh, J. (2019). A Comparative Study of Essential Oil Constituents and Phenolic Compounds of Arabian Lilac (Vitex Trifolia var. Purpurea): An Evidence of Season Effects. Foods, 8(2), 52. https://doi.org/10.3390/foods8020052