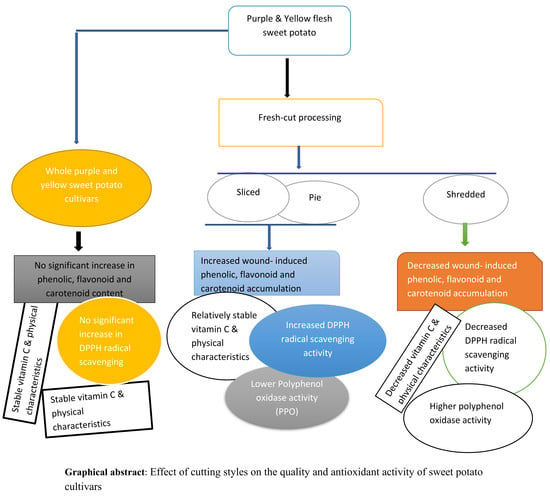
Effect of Cutting Styles on Quality and Antioxidant Activity of Stored Fresh-Cut Sweet Potato (Ipomoea batatas L.) Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. Color Change, Firmness, and Weight Loss Determination
2.3. Total Phenolics and Total Flavonoids Content Determination
2.4. Analysis of Total Carotenoids and Vitamin C Contents
2.5. Antioxidant Determination
2.6. PPO Activity
2.7. Total Aerobic Bacterial Count (TABC)
2.8. Statistical Analysis
3. Results and Discussion
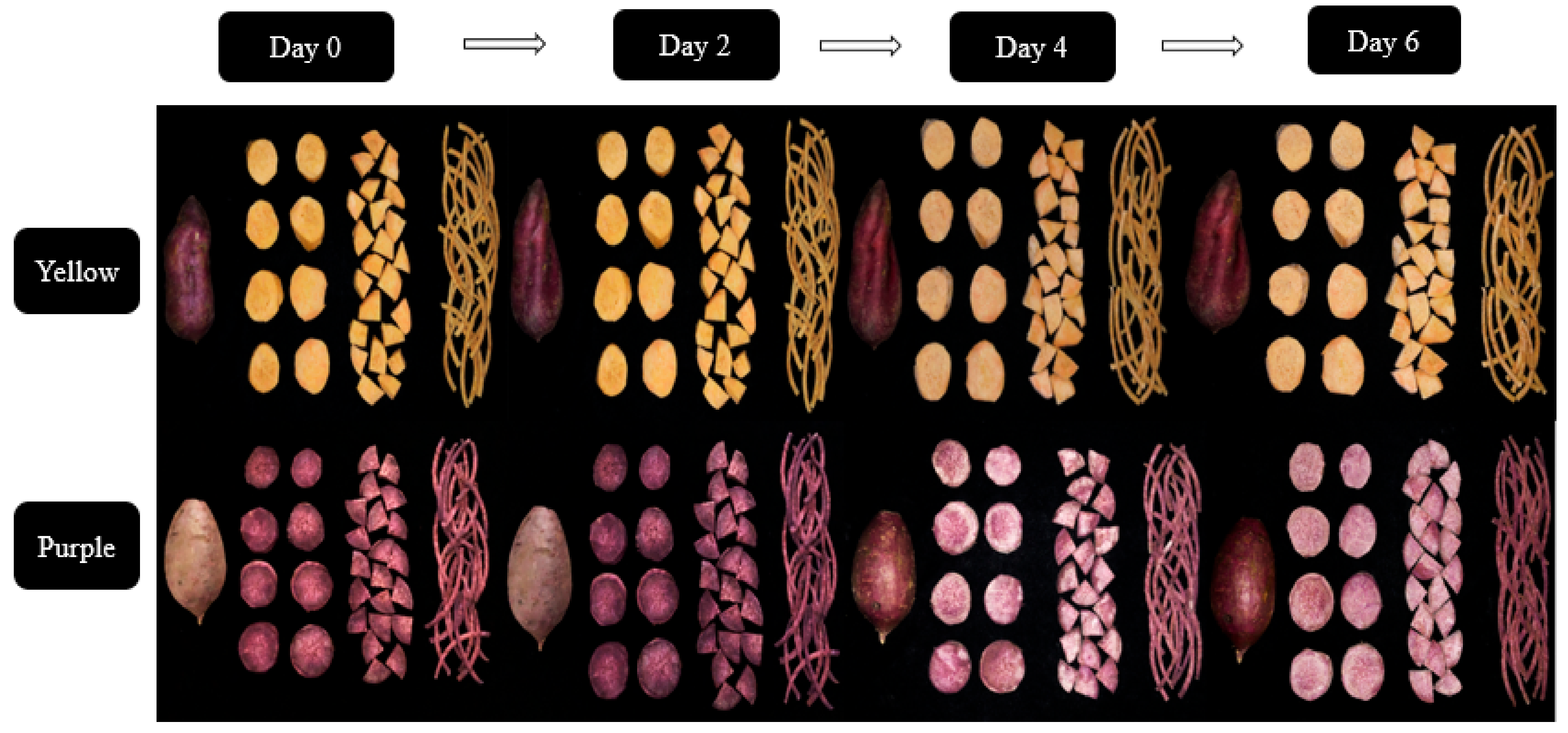
3.1. Whole, Sliced, Pie, and Shredded Purple and Yellow Flesh Sweet Potato Cultivars
3.2. Effect of Cutting Styles on the Color, Firmness, and Weight Loss of the Sweet Potato Cultivars
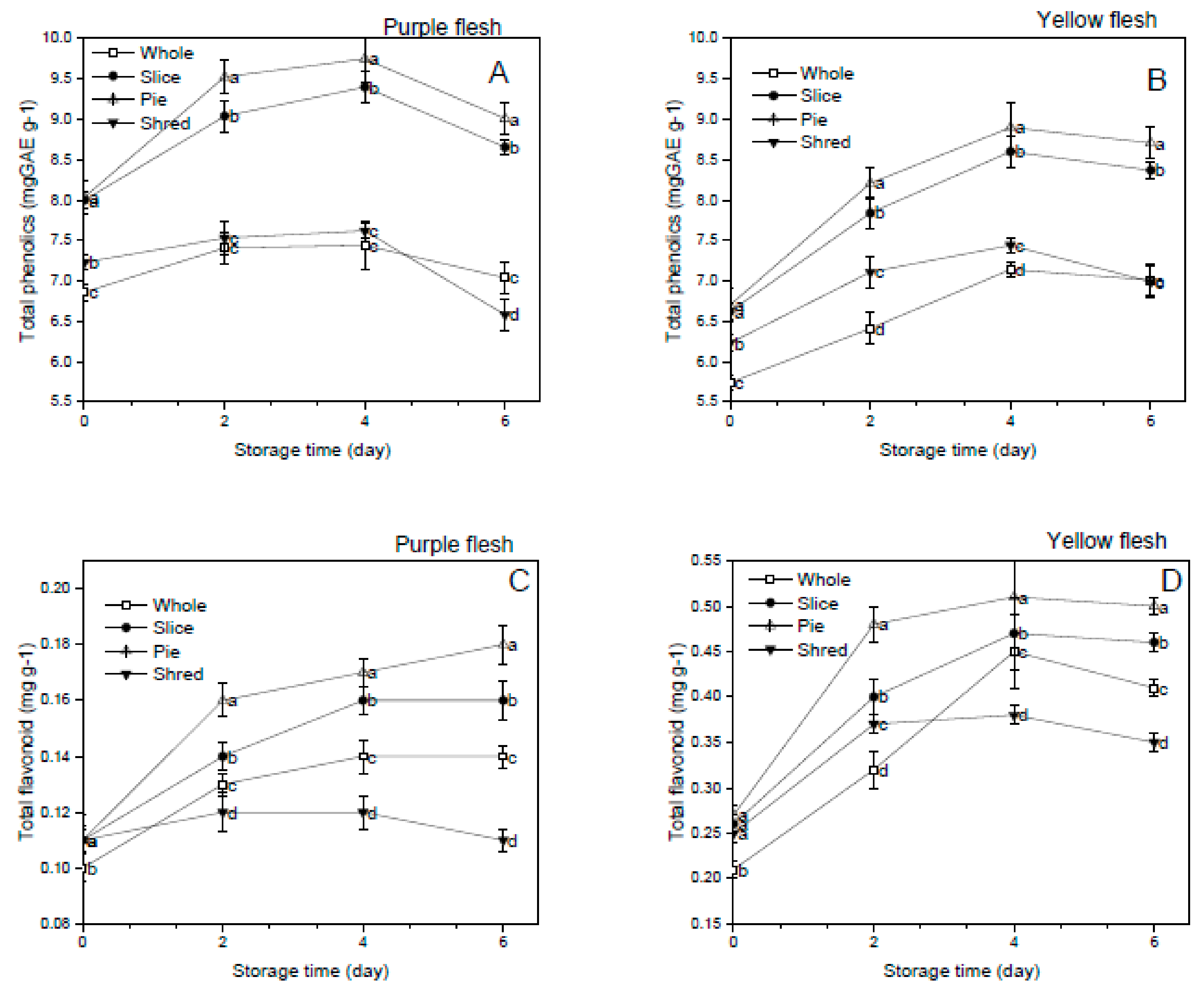
3.3. Effect of Cutting Styles on the Total Phenolics and Flavonoid Content
3.4. Effect of Cutting Styles on the Total Carotenoid and Vitamin C Content
3.5. Effect of Cutting Styles on the DPPH Free Radical Scavenging Activity and Ferric Reducing Antioxidant Power
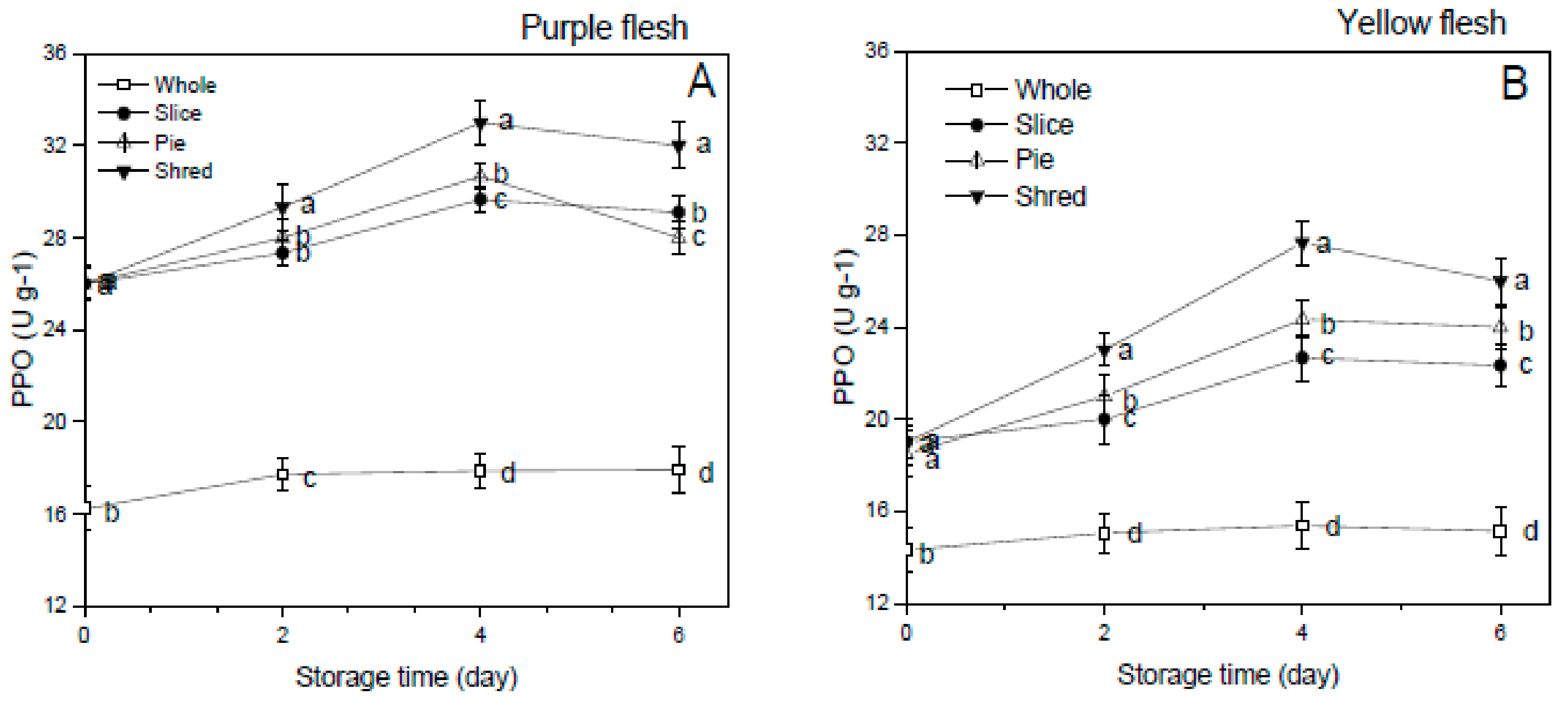
3.6. Polyphenol Oxidase (PPO) Activity
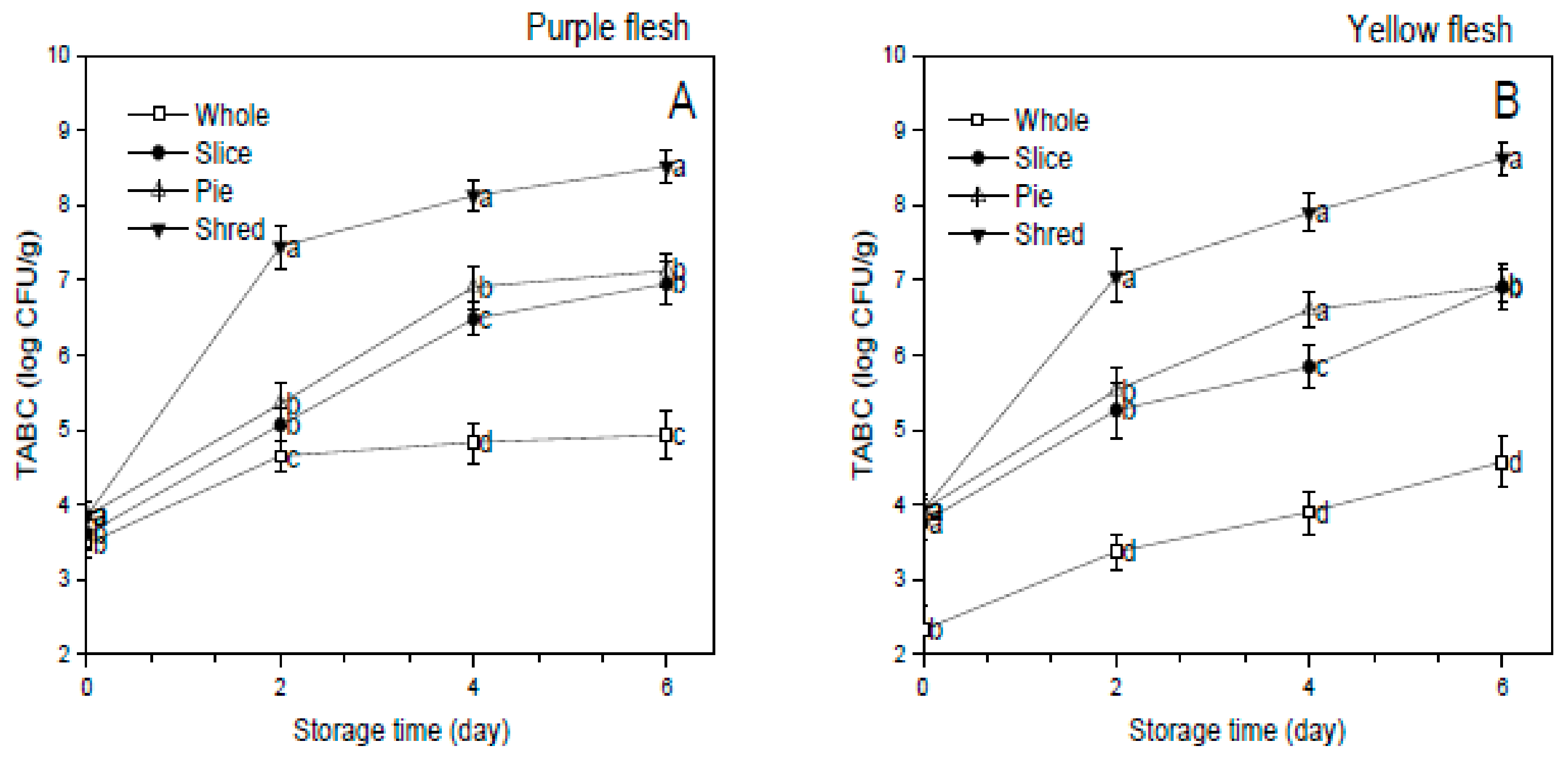
3.7. Total Aerobic Bacterial Count (TABC)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, K.; Liu, T.; Xu, A.; Zhang, L.; Bian, X.; Wei, C. Structural and functional properties of starches from root tubers of white, yellow, and purple sweet potatoes. Food Hydrocoll. 2019, 89, 829–836. [Google Scholar] [CrossRef]
- Hu, Y.; Deng, L.; Chen, J.; Zhou, S.; Liu, S.; Fu, Y.; Yang, C.; Liao, Z.; Chen, M. An analytical pipeline to compare and characterize the anthocyanin antioxidant activities of purple sweet potato cultivars. Food Chem. 2016, 194, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Qadri, O.S.; Yousuf, B.; Srivastava, A.K. Fresh-cut fruits and vegetables: Critical factors influencing microbiology and novel approaches to prevent microbial risks-A review. Cogent Food Agric. 2015, 1, 1121–1606. [Google Scholar] [CrossRef]
- Li, X.; Long, Q.; Gao, F.; Han, C.; Jin, P.; Zheng, Y. Effect of cutting styles on quality and antioxidant activity in fresh-cut pitaya fruit. Postharvest Biol. Technol. 2017, 124, 1–7. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Cisneros-Zevallos, L. Biosynthesis of phenolic antioxidants in carrot tissue increases with wounding intensity. Food Chem. 2012, 134, 615–624. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Martínez-Hernández, G.B.; Rodríguez, S.D.C.; Cao, C.M.; Cisneros-Zevallos, L. Plants as biofactories: Physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J. Agric. Food Chem. 2011, 59, 6583–6593. [Google Scholar] [CrossRef]
- Jang, J.H.; Moon, K.D. Inhibition of polyphenol oxidase and peroxidase activities on fresh-cut apple by simultaneous treatment of ultrasound and ascorbic acid. Food Chem. 2011, 124, 444–449. [Google Scholar] [CrossRef]
- Han, C.; Li, J.; Jin, P.; Li, X.A.; Wang, L.; Zheng, Y.H. The effect of temperature on phenolic content in wounded carrots. Food Chem. 2017, 215, 116–123. [Google Scholar] [CrossRef]
- Grace, M.H.; Yousef, G.G.; Gustafson, S.J.; Truong, V.D.; Yencho, G.C.; Lila, M.A. Phytochemical changes in phenolics, anthocyanins, ascorbic acid, and carotenoids associated with sweet potato storage and impacts on bioactive properties. Food Chem. 2014, 145, 717–724. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, J.B.; Cho, S.M.; Chung, M.N.; Lee, Y.M.; Chu, S.M.; Che, J.H.; Kim, S.N.; Kim, S.Y.; Cho, Y.S.; et al. Anthocyanin changes in the Korean purple-fleshed sweet potato, Shinzami, as affected by steaming and baking. Food Chem. 2012, 130, 966–972. [Google Scholar] [CrossRef]
- Tang, Y.; Cai, W.; Xu, B. Profiles of phenolics, carotenoids and antioxidative capacities of thermal processed white, yellow, orange and purple sweet potatoes grown in Guilin, China. Food Sci. Hum. Wellness 2015, 4, 123–132. [Google Scholar] [CrossRef]
- Wall, M.M. Storage quality and composition of sweet potato roots after quarantine treatment using low doses of x-ray irradiation. Hortic. Sci. 2005, 40, 424–427. [Google Scholar]
- Waterhouse, A.L. Determination of Total Phenolics. Curr. Protoc. Food Anal. Chem. 2011, 6, I1.1.1–I1.1.8. [Google Scholar]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chang, Y.H.; Shao, Y.Y. Effects of genotype and treatment on the antioxidant activity of sweet potato in Taiwan. Food Chem. 2006, 98, 529–538. [Google Scholar] [CrossRef]
- Arakawa, N.; Tsutsumi, K.; Sanceda, N.G.; Kurata, T.; Inagaki, C. A rapid and sensitive method for the determination of ascorbic acid using 4,7-diphenyl1,10-phenanthroline. Agric. Biol. Chem. 1981, 45, 1289–1290. [Google Scholar]
- Bae, S.H.; Suh, H.J. Antioxidant activities of five different mulberry cultivars in Korea. LWT-Food Sci. Technol. 2007, 40, 955–962. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, C.; Chen, M.H.; Chiang, P.Y. Stability and Quality of Anthocyanin in Purple Sweet Potato Extracts. Foods 2019, 8, 393. [Google Scholar] [CrossRef]
- Manohan, D.; Wai, W.C. Characterization of polyphenol oxidase in sweet potato (Ipomoea batatas L.). J. Adv. Sci. Arts 2012, 3, 14–31. [Google Scholar]
- Soison, B.; Jangchud, K.; Jangchud, A.; Harnsilawat, T.; Piyachomkwan, K. Characterization of starch in relation to flesh colors of sweet potato varieties. Int. Food Res. J. 2015, 22, 2302–2308. [Google Scholar]
- Barry-Ryan, C.; Pacussi, J.M.; O’Beirne, D. Quality of shredded carrots as affected by packaging film and storage temperature. J. Food Sci. 2000, 65, 726–730. [Google Scholar] [CrossRef]
- Huang, C.L.; Liao, W.C.; Chan, C.F.; Lai, Y.C. Storage performance of Taiwanese sweet potato cultivars. J. Food Sci. Technol. 2014, 51, 4019–4025. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Stress-induced production of chlorogenic acid isomers in potato tubers as affected by wounding intensity and storage time. Ind. Crop. Prod. 2014, 62, 61–66. [Google Scholar] [CrossRef]
- Hue, S.M.; Boyce, A.N.; Somasundram, C. Antioxidant activity, phenolic and flavonoid contents in the leaves of different varieties of sweet potato (‘ipomoea batatas’). Aust. J. Crop Sci. 2012, 6, 375–380. [Google Scholar]
- Teow, C.C.; Truong, V.D.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, G.C. Antioxidant activities, phenolic, and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Tomlins, K.; Owori, C.; Bechoff, A.; Menya, G.; Westby, A. Relationship among the carotenoid content, dry matter content and sensory attributes of sweet potato. Food Chem. 2012, 131, 14–21. [Google Scholar] [CrossRef]
- Rabah, I.O.; Hou, D.X.; Komine, S.I.; Fuji, M. Potential chemopreventive properties of extract from baked sweet potato (Ipomoea batatas Lam. Cv. Koganesengan). J. Agric. Food Chem. 2004, 52, 7152–7157. [Google Scholar] [CrossRef]
- Arogundade, L.A.; Mu, T.H. Influence of oxidative browning inhibitors and isolation techniques on sweet potato protein recovery and composition. Food Chem. 2012, 134, 1374–1384. [Google Scholar] [CrossRef]
- Severini, C.; Baiano, A.; De Pilli, T.; Romaniello, R.; Derossi, A. Prevention of enzymatic browning in sliced potatoes by blanching in boiling saline solutions. LWT–Food Sci. Technol. 2003, 36, 657–665. [Google Scholar] [CrossRef]
- Harris, L.J.; Farber, J.N.; Beuchat, L.R.; Parish, M.E.; Suslow, T.V.; Garrett, E.H.; Busta, F.F. Outbreaks associated with fresh produce: Incidence, growth, and survival of pathogens in fresh and fresh-cut produce. Compr. Rev. Food Sci. Food Saf. 2003, 2, 78–141. [Google Scholar] [CrossRef]


| Parameter | Cutting Style | Purple Flesh Sweet Potato | Yellow Flesh Sweet Potato | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Storage Time (Day) | ||||||||||
| 0 | 2 | 4 | 6 | 0 | 2 | 4 | 6 | |||
| Color | L* | Whole | 31.68 ± 1.07 a | 32.45 ± 2.04 c | 32.99 ± 3.01 c | 33.53 ± 1.24 b | 29.24 ± 1.89 b | 67.37 ± 2.48 c | 67.82 ± 2.78 c | 69.71 ± 1.36 b |
| Slice | 31.34 ± 1.51 a | 48.1 ± 2.43 a | 47.77 ± 3.92 b | 47.69 ± 1.65 a | 30.24 ± 0.89 b | 73.37 ± 0.78 a | 74.46 ± 0.78 a | 74.58 ±0.59 a | ||
| Pie | 31.16 ± 1.69 a | 45.74 ± 2.08 b | 49 ± 1.77 a | 46.18 ± 3.56 a | 29.8 ± 0.68 b | 73.28 ± 0.68 a | 75.04 ± 0.46 a | 75.21 ±0.82 a | ||
| Shred | 30.13 ± 1.24 a | 32.95 ± 1.49 c | 31.66 ± 1.21 c | 32.52 ± 1.66 b | 32.03 ± 1.84 a | 69.91 ± 2.99 b | 69.85 ± 1.65 b | 69.93 ±1.68 b | ||
| a* | Whole | 17.29 ± 1.05 c | 17.8 ±1.5 b | 17.41 ± 0.96 a | 16.48 ± 1.03 a | 8.167 ± 0.72 b | 8.8 ± 0.79 c | 8.78 ± 0.99 b | 8.63 ± 0.52 b | |
| Slice | 19.29 ± 1.4 b | 17.4 ± 1.08 b | 16.46 ± 0.96 b | 13.66 ± 1.58 b | 8.37 ± 0.98 b | 9.5 ± 0.88 a | 9.47 ± 0.99 a | 9.37 ± 0.52 a | ||
| Pie | 21.72 ± 0.77 a | 18.65 ± 0.59 a | 17.24 ± 5.14 a | 14.22 ± 1.91 b | 9.73 ± 0.74 a | 9.30 ± 0.91 b | 8.98 ± 0.91 b | 8.27 ± 0.6 b | ||
| Shred | 16.45 ± 0.51 c | 17.61 ± 0.52 b | 16.88 ± 0.4 b | 15.94 ± 0.85 a | 9.44 ± 0.94 a | 10.14 ± 0.58 a | 9.7 ± 0.84 a | 9.5 ± 0.88 a | ||
| b* | Whole | 1.5 ± 0.28 b | 3.98 ± 0.66 b | 4.31 ± 0.67 b | 4.8 ± 0.69 b | 28.72 ± 0.67 a | 27.8 ± 0.84 c | 26.45 ± 0.89 b | 25.3 ± 0.61 a | |
| Slice | 1.4 ± 0.28 b | 4.47 ± 0.46 a | 4.58 ± 0.47 a | 4.7 ± 0.86 b | 28.98 ± 0.67 a | 25.7 ± 0.44 b | 24.67 ± 0.59 c | 24.3 ± 0.34 ac | ||
| Pie | 1.4 ± 0.67 b | 3.8 ± 0.35 b | 4.46 ± 0.46 a | 4.9 ± 1.09 b | 29.89 ± 0.48 a | 28.18 ± 0.45 a | 25.78 ± 0.52 b | 23.84 ± 0.53 c | ||
| Shred | 2.37 ± 0.21 a | 3.2 ± 0.57 c | 3.98 ± 0.27 c | 5.22 ± 0.3 a | 28.47 ± 0.76 a | 28.85 ± 0.42 a | 28.79 ± 0.65 a | 28.77 ± 0.8 a | ||
| Firmness (N) | Whole | 198.7 ± 5.67 a | 197.16 ± 4.8 a | 195.76 ± 2.56 a | 183.22 ± 2.91 a | 170.06 ± 1.79 a | 169.74 ± 2.64 a | 168.38 ± 2.05 a | 167.05 ± 2.1 a | |
| Slice | 196.3 ± 3.67 a | 196.16 ± 1.7 a | 195.38 ± 1.56 a | 175.52 ± 2.91 b | 169.95 ± 0.93 a | 169.53 ± 0.98 a | 168.45 ± 0.89 a | 167.55 ± 0.8 a | ||
| Pie | 196.95 ± 1.71 a | 196.76 ± 1.48 a | 194.9 ± 0.66 a | 151.38 ± 2.61 c | 169.71 ± 0.95 a | 168.48 ± 0.8 a | 165.08 ± 0.71 b | 159.67 ± 0.9 b | ||
| Shred | 196.34 ± 1.84 b | 159.91 ± 2.09 b | 154.67 ± 2.77 b | 134.24 ± 0.75 d | 164.62 ± 0.85 b | 162.52 ± 0.8 b | 159.01± 0.84 c | 156.96 ± 0.8 c | ||
| Weight loss (%) | Whole | 171.17 ± 6.15 a | 171.06 ± 4.66 a | 170.95 ± 3.08 a | 170.15 ± 4.56 a | 171.27 ± 1.25 a | 171.02 ± 1.63 a | 170.01 ± 2.8 a | 169.99 ± 4.6 a | |
| Slice | 171.17 ± 2.315 a | 170.37 ± 1.56 a | 170.11 ± 2.08 a | 169.07 ± 3.07 a | 171.17 ± 1.25 a | 170.64 ± 1.63 a | 170.17 ± 2.8 a | 169.87 ± 2.0 a | ||
| Pie | 171.17 ± 2.25 a | 169.67 ± 1.8 a | 168.86 ± 2.13 a | 168.8 ± 2.15 a | 171.57 ± 2.18 a | 170.36 ± 3.5 a | 169.71 ± 1.6 a | 169.39 ± 1.6 a | ||
| Shred | 171.17 ± 3.11 a | 167.12 ± 1.91 b | 165.59 ± 1.45 b | 165.46 ± 0.98 b | 171.37 ± 1.82 a | 169.72 ± 2.11 a | 166.89 ± 3.2 b | 166.25 ± 2.5 b | ||
| Purple Flesh Sweet Potato | Yellow Flesh Sweet Potato | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | Total Phenolics | Total Flavonoids | Total Carotenoids | Vitamin C | DPPH | Total Phenolics | Total Flavonoids | Total Carotenoids | Vitamin C | ||
| DPPH | 1 | DPPH | 1 | ||||||||
| Total phenolics | 0.89898 | 1 | Total phenolics | 0.73097 | 1 | ||||||
| Total flavonoids | 0.91732 | 0.96331 * | 1 | Total flavonoids | 0.96981 | 0.86526 | 1 | ||||
| Total carotenoids | 0.86532 | 0.99568 * | 0.96644 * | 1 | Total carotenoids | 0.86865 | 0.952 * | 0.96324 * | 1 | ||
| Vitamin C | 0.65444 | 0.26038 | 0.33792 | 0.18736 | 1 | Vitamin C | 0.71281 | 0.0704 | 0.52025 | 0.27173 | 1 |
| Purple Flesh Sweet Potato | Yellow Flesh Sweet Potato | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FRAP | Total Phenolics | Total Flavonoids | Total Carotenoids | Vitamin C | FRAP | Total Phenolics | Total Flavonoids | Total Carotenoids | Vitamin C | ||
| FRAP | 1 | FRAP | 1 | ||||||||
| Total phenolics | 0.9857 * | 1 | Total phenolics | 0.97686 * | 1 | ||||||
| Total flavonoids | 0.97557 * | 0.96331 * | 1 | Total flavonoids | 0.94999 * | 0.86526 | 1 | ||||
| Total carotenoids | 0.99631 ** | 0.99568 ** | 0.96644 * | 1 | Total carotenoids | 0.99393 ** | 0.952 * | 0.96324 * | 1 | ||
| Vitamin C | 0.16286 | 0.26038 | 0.33792 | 0.18736 | 1 | Vitamin C | 0.24224 | 0.0704 | 0.5202 *5 | 0.27173 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dovene, A.K.; Wang, L.; Bokhary, S.U.F.; Madebo, M.P.; Zheng, Y.; Jin, P. Effect of Cutting Styles on Quality and Antioxidant Activity of Stored Fresh-Cut Sweet Potato (Ipomoea batatas L.) Cultivars. Foods 2019, 8, 674. https://doi.org/10.3390/foods8120674
Dovene AK, Wang L, Bokhary SUF, Madebo MP, Zheng Y, Jin P. Effect of Cutting Styles on Quality and Antioxidant Activity of Stored Fresh-Cut Sweet Potato (Ipomoea batatas L.) Cultivars. Foods. 2019; 8(12):674. https://doi.org/10.3390/foods8120674
Chicago/Turabian StyleDovene, Atigan Komlan, Li Wang, Syed Umar Farooq Bokhary, Miilion Paulos Madebo, Yonghua Zheng, and Peng Jin. 2019. "Effect of Cutting Styles on Quality and Antioxidant Activity of Stored Fresh-Cut Sweet Potato (Ipomoea batatas L.) Cultivars" Foods 8, no. 12: 674. https://doi.org/10.3390/foods8120674
APA StyleDovene, A. K., Wang, L., Bokhary, S. U. F., Madebo, M. P., Zheng, Y., & Jin, P. (2019). Effect of Cutting Styles on Quality and Antioxidant Activity of Stored Fresh-Cut Sweet Potato (Ipomoea batatas L.) Cultivars. Foods, 8(12), 674. https://doi.org/10.3390/foods8120674