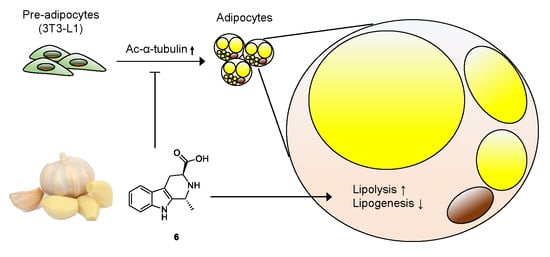
Anti-adipogenic Effect of β-Carboline Alkaloids from Garlic (Allium sativum)
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Garlic Sample and Isolation of Compounds
2.2. Cell Culture and Differentiation
2.3. Cell Counting
2.4. Immunoblotting
2.5. Reverse Transcription and Quantitative Real-Time PCR (RT-qPCR)
2.6. Oil Red O Staining
2.7. Statistical Analysis
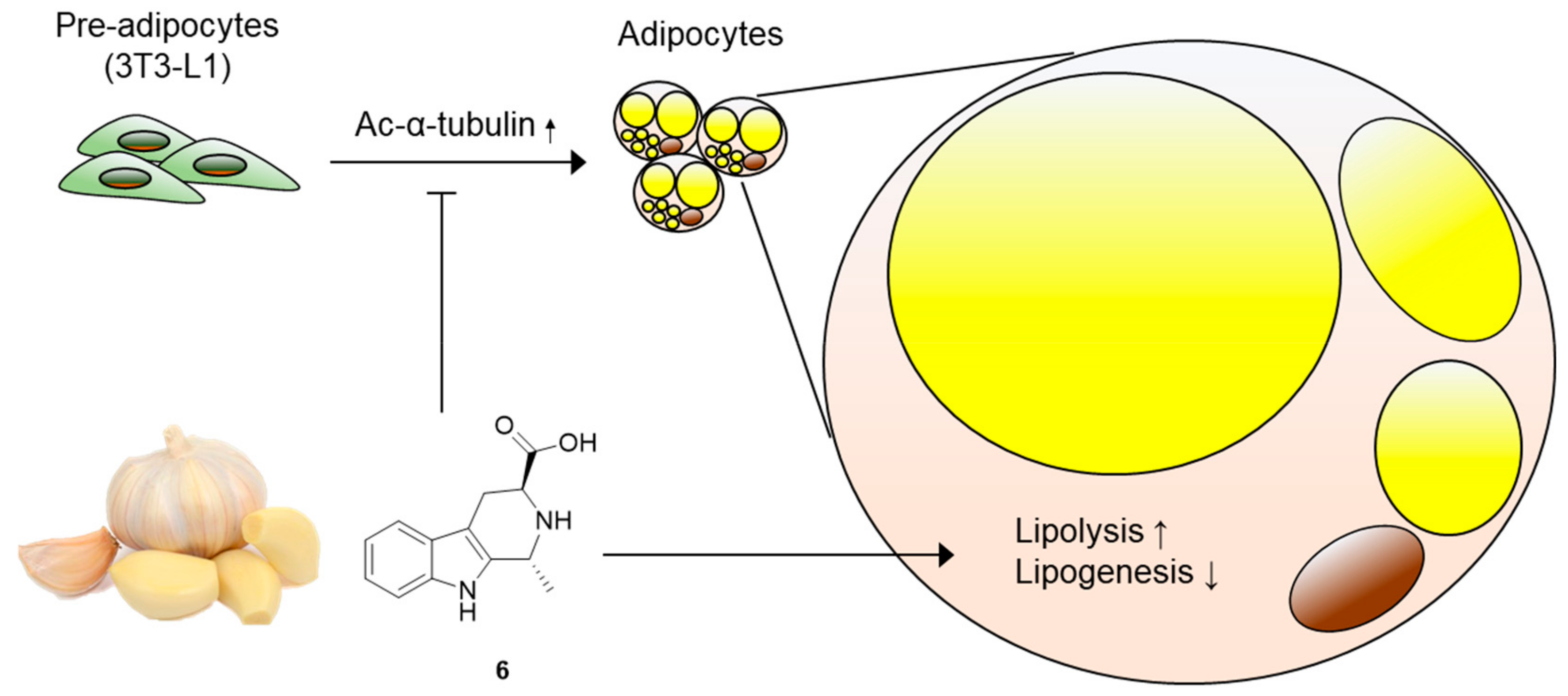
3. Results and Discussion
3.1. Identification of Compounds
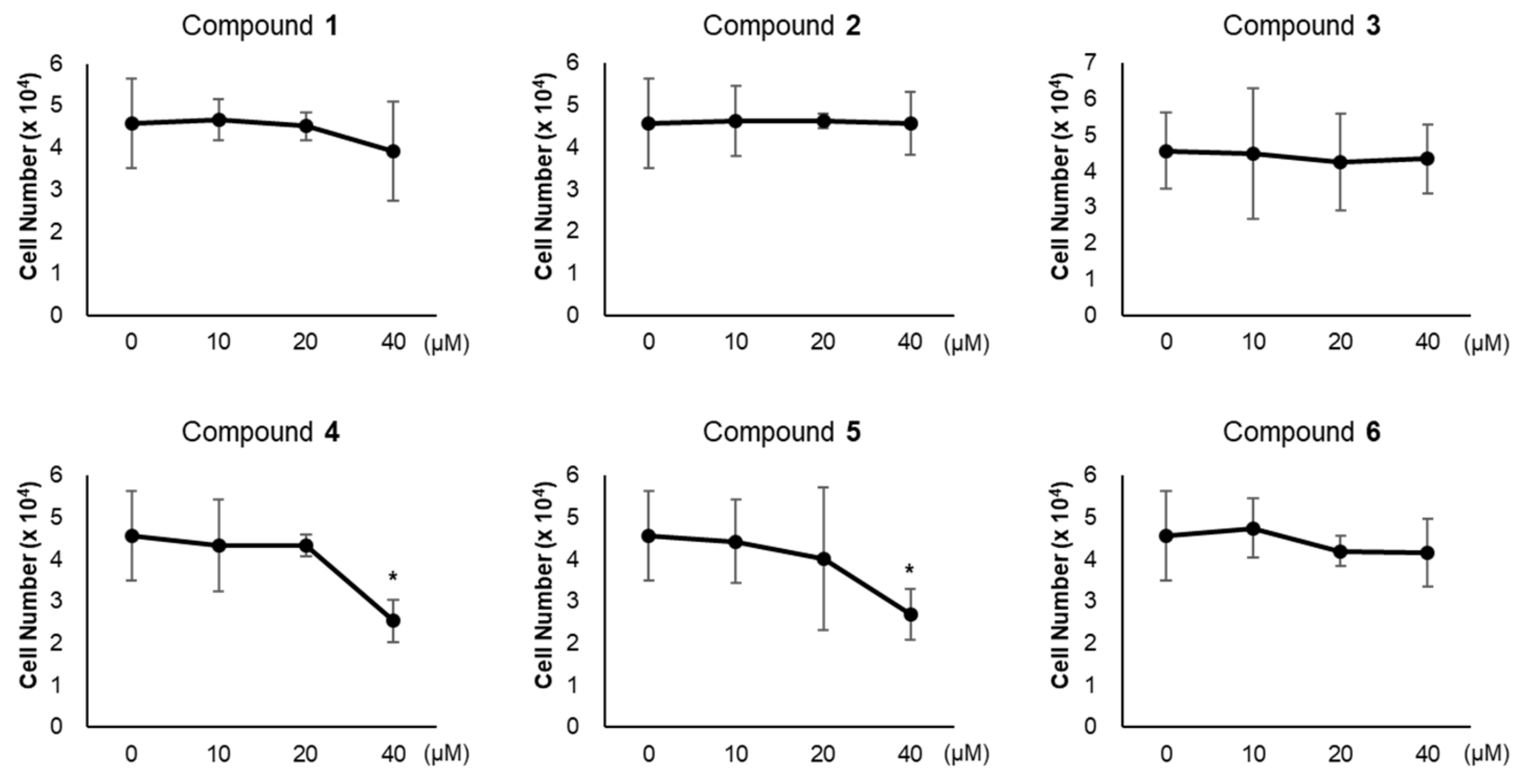
3.2. Effects of Compounds 1–6 on Adipogenesis
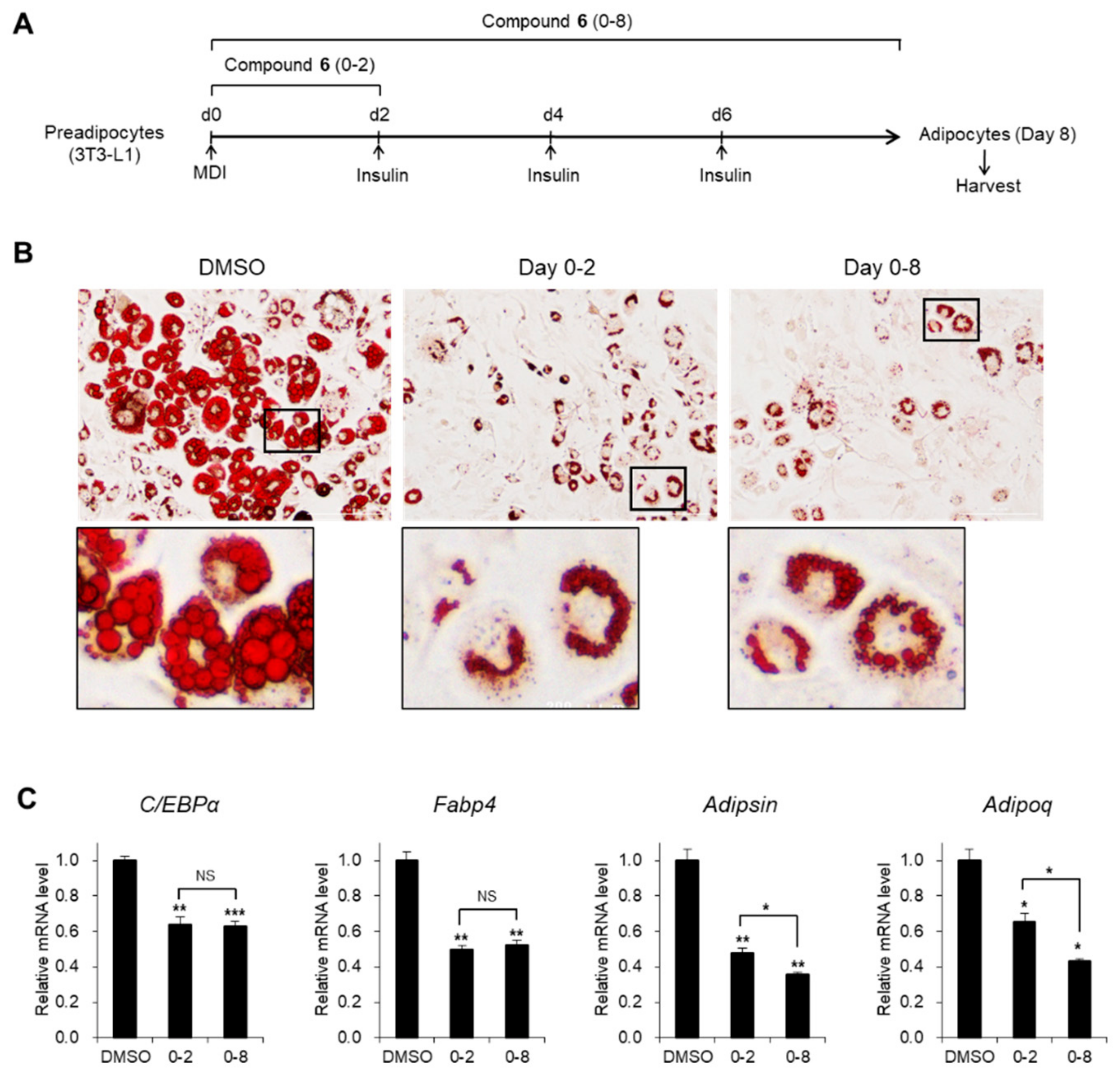
3.3. Effects of Compound 6 on the Early Stages of Adipogenesis
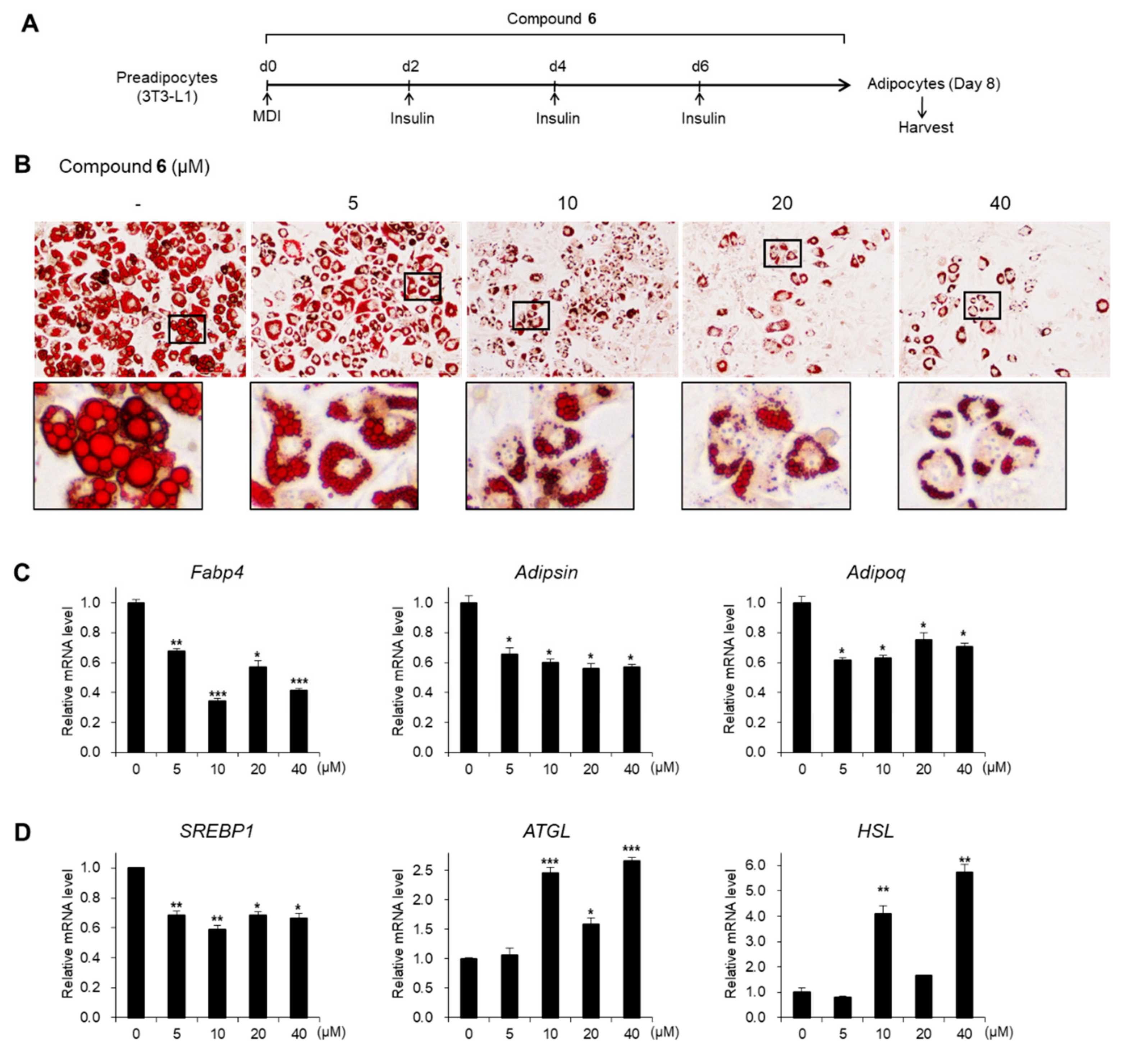
3.4. Effects of Compound 6 on Lipid Metabolism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angiosperm Phylogeny Group. An update of the Angiosperm phylogeny group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar] [CrossRef]
- Rivlin, R.S. Historical perspective on the use of garlic. J. Nutr. 2001, 132, 951S–954S. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Kwiecieri, I.; Wlodek, L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Charrois, T.L.; Vohra, S. Complementary, holistic and integrative medicine: Garlic. Pediatr. Rev. 2006, 27, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Jacob, B.; Narendhirakannan, R.T. Role of medicinal plants in the management of diabetes mellitus: A review. 3 Biotech. 2019, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Pittler, M.H.; Ernst, E. Clinical effectiveness of garlic (Allium sativum). Mol. Nutr. Food Res. 2007, 51, 1382–1385. [Google Scholar] [CrossRef]
- Štajner, D.; Milić, N.; Čanadanović-Brunet, J.; Kapor, A.; Štajner, M.; Popović, B.M. Exploring Allium species as a source of potential medicinal agents. Phytother. Res. 2006, 20, 581–584. [Google Scholar] [CrossRef]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive components. J. Nutr. 2001, 131, 955S–962S. [Google Scholar] [CrossRef]
- Lawson, L.D.; Ransom, D.K.; Hughes, B.G. Inhibition of whole blood platelet-aggregation by compounds in garlic clove extracts and commercial garlic products. Thromb. Res. 1992, 65, 141–156. [Google Scholar] [CrossRef]
- Hussain, S.P.; Jannu, L.N.; Rao, A.R. Chemopreventive action of garlic on methylcholanthrene-induced carcinogenesis in the uterine cervix of mice. Cancer Lett. 1990, 49, 175–180. [Google Scholar] [CrossRef]
- Pinto, J.T.; Lapsia, S.; Shah, A.; Santiago, H.; Kim, G. Antiproliferative effects of garlic-derived and other allium related compounds. Adv. Exp. Med. Biol. 2001, 492, 83–106. [Google Scholar] [PubMed]
- Key, T.J.; Silcocks, P.B.; Davey, G.K.; Appleby, P.N.; Bishop, D.T. A case-control study of diet and prostate cancer. Br. J. Cancer 1997, 76, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.A. Garlic: Its anticarcinogenic and antitumorigenic properties. Nutr. Rev. 1996, 54, S82–S86. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.C. Therapeutic actions of garlic constituents. Med. Res. Rev. 1996, 16, 111–124. [Google Scholar] [CrossRef]
- Yeh, Y.Y. Garlic phytochemicals in disease prevention and health promotion: An overview. New Drug Clin. 1996, 45, 441–450. [Google Scholar]
- Hirsch, K.; Danilenko, M.; Giat, J.; Miron, T.; Rabinkov, A.; Wilchek, M.; Mirelman, D.; Levy, H.; Sharoni, Y. Effect of purified allicin, the major ingredient of freshly crushed garlic, on cancer cell proliferation. Nutr. Cancer 2000, 38, 245–254. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.; Xu, X.; Gan, R.; Tang, G.; Corke, H.; Mavumengwana, V.; Li, H. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Corzo-Martınez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Lanzotti, V.; Barile, E.; Antignani, V.; Bonanomi, G.; Scala, F. Antifungal saponins from bulbs of garlic, Allium sativum L. var. Voghiera. Phytochemistry 2012, 78, 126–134. [Google Scholar] [CrossRef]
- Ichikawa, M.; Yoshida, J.; Ide, N.; Sasaoka, T.; Yamaguchi, H.; Ono, K. Tetrahydro-beta-carboline derivatives in aged garlic extract show antioxidant properties. J. Nutr. 2006, 136, 726S–731S. [Google Scholar] [CrossRef] [PubMed]
- So, H.M.; Eom, H.J.; Lee, D.; Kim, S.; Kang, K.S.; Lee, I.K.; Baek, K.-H.; Park, J.Y.; Kim, K.H. Bioactivity evaluations of betulin identified from the bark of Betula platyphylla var. japonica for cancer therapy. Arch. Pharm. Res. 2018, 41, 815–822. [Google Scholar] [PubMed]
- Baek, S.C.; Choi, E.; Eom, H.J.; Jo, M.S.; Kim, S.; So, H.M.; Kim, S.H.; Kang, K.S.; Kim, K.H. LC/MS-based analysis of bioactive compounds from the bark of Betula platyphylla var japonica and their effects on regulation of adipocyte and osteoblast differentiation. Nat. Prod. Sci. 2018, 24, 235–240. [Google Scholar]
- Yu, J.S.; Roh, H.-S.; Baek, K.-H.; Lee, S.; Kim, S.; So, H.M.; Moon, E.; Pang, C.; Jang, T.S.; Kim, K.H. Bioactivity-guided isolation of ginsenosides from Korean Red Ginsengwith cytotoxic activity against human lung adenocarcinoma cells. J. Ginseng Res. 2018, 42, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Ambati, S.; Yang, J.Y.; Rayalam, S.; Park, H.J.; Della-Fera, M.A.; Baile, C.A. Ajoene exerts potent effects in 3T3-L1 adipocytes by inhibiting adipogenesis and inducing apoptosis. Phytother. Res. 2009, 23, 513–518. [Google Scholar] [CrossRef]
- Lii, C.K.; Huang, C.Y.; Chen, H.W.; Chow, M.Y.; Lin, Y.R.; Huang, C.S.; Tsai, C.W. Diallyl trisulfide suppresses the adipogenesis of 3T3-L1 preadipocytes through ERK activation. Food Chem. Toxicol. 2012, 50, 478–484. [Google Scholar] [CrossRef]
- Ban, J.O.; Lee, D.H.; Kim, E.J.; Kang, J.W.; Kim, M.S.; Cho, M.C.; Jeong, H.S.; Kim, J.W.; Yang, Y.; Hong, J.T.; et al. Antiobesity effects of a sulfur compound thiacremonone mediated via down-regulation of serum triglyceride and glucose levels and lipid accumulation in the liver of db/db mice. Phytother. Res. 2012, 26, 1265–1271. [Google Scholar] [CrossRef]
- Kim, E.J.; Lee, D.H.; Kim, H.J.; Lee, S.J.; Ban, J.O.; Cho, M.C.; Jeong, H.S.; Yang, Y.; Hong, J.T.; Yoon, D.Y. Thiacremonone, a sulfur compound isolated from garlic, attenuates lipid accumulation partially mediated via AMPK activation in 3T3-L1 adipocytes. J. Nutr. Biochem. 2012, 23, 1552–1558. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yahara, S.; Kato, K.; Nohara, T. Studies on the constituents of the water soluble portion in Asiasari radix. Shoyakugaku Zasshi 1990, 44, 331–334. [Google Scholar]
- Orihara, Y.; Furuya, T.; Hashimoto, N.; Deguchi, Y.; Tokoro, K.; Kanisawa, T. Biotransformation of isoeugenol and eugenol by cultured cells of Eucalyptus perriniana. Phytochemistry 1992, 31, 827–831. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Yang, Y.; Zhang, M. Isolation, purification and identification of antioxidants in an aqueous aged garlic extract. Food Chem. 2015, 187, 37–43. [Google Scholar] [CrossRef]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef]
- Yang, W.; Guo, X.; Thein, S.; Xu, F.; Sugii, S.; Baas, P.W.; Radda, G.K.; Han, W. Regulation of adipogenesis by cytoskeleton remodelling is facilitated by acetyltransferase MEC-17-dependent acetylation of α-tubulin. Biochem. J. 2013, 449, 605–612. [Google Scholar] [CrossRef]
- Spiegelman, B.M.; Farmer, S.R. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell 1982, 29, 53–60. [Google Scholar] [CrossRef]
- Spiegelman, B.M.; Flier, J.S. Obesity and the Regulation of Energy Balance. Cell 2001, 104, 531–543. [Google Scholar] [CrossRef]
- Smith, U.; Kahn, B.B. Adipose tissue regulates insulin sensitivity: Role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med. 2016, 280, 465–475. [Google Scholar] [CrossRef]
- Piechowska, P.; Zawirska-Wojtasiak, R.; Mildner-Szkudlarz, S. Bioactive β-Carbolines in Food: A Review. Nutrients 2019, 11, 814. [Google Scholar] [CrossRef]
- Ferraz, C.A.A.; de Oliveira Júnior, R.G.; Picot, L.; da Silva Almeida, J.R.G.; Nunes, X.P. Pre-clinical investigations of β-carboline alkaloids as antidepressant agents: A systematic review. Fitoterapia 2019, 137, 104196. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Zhou, W.Y.; Chen, J.J.; Yao, G.D.; Lin, B.; Wang, X.B.; Huang, X.X.; Song, S.J. Enantiomeric β-carboline dimers from Picrasma quassioides and their anti-hepatoma potential. Phytochemistry 2019, 159, 39–45. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, D. Recent Advances of Natural and Synthetic β-Carbolines as Anticancer Agents. Anticancer Agents Med. Chem. 2015, 15, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Forcioli-Conti, N.; Estève, D.; Bouloumié, A.; Dani, C.; Peraldi, P. The size of the primary cilium and acetylated tubulin are modulated during adipocyte differentiation: Analysis of HDAC6 functions in these processes. Biochimie 2016, 124, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Shi, S.; Wang, H.; Liao, K. Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. J. Cell Sci. 2009, 122, 2760–2768. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward | Reverse |
|---|---|---|
| β-Actin | 5′-ACGGCCAGGTCATCACTATTG-3′ | 5′-TGGATGCCACAGGATTCCA-3′ |
| Adipsin | 5‘-CATGCTCGGCCCTACATG-3′ | 5‘-CACAGAGTCGTCATCCGTCAC-3′ |
| Adipoq | 5′-TGTTCCTCTTAATCCTGCCCA-3′ | 5′-CCAACCTGCACAAGTTCCCTT-3′ |
| ATGL | 5′-TTCACCATCCGCTTGTTGGAG-3′ | 5′-AGATGGTCACCCAATTTCCTC-3′ |
| C/EBPα | 5′-CTCCCAGAGGACCAATGAAA-3′ | 5′-AAGTCTTAGCCGGAGGAAGC-3′ |
| C/EBPβ | 5′-GGACAAGCTGAGCGACGAGTA-3′ | 5′-CAGCTGCTCCACCTTCTTCTG-3′ |
| Fabp4 | 5‘-AAGGTGAAGAGCATCATAACCCT-3′ | 5‘-TCACGCCTTTCATAACACATTCC-3′ |
| HSL | 5′-CACAAAGGCTGCTTCTACGG-3′ | 5′-GGAGAGAGTCTGCAGGAACG-3′ |
| PPARγ | 5‘-GCATGGTGCCTTCGCTGA-3′ | 5‘-TGGCATCTCTGTGTCAACCATG-3′ |
| SREBP1 | 5′-AACGTCACTTCCAGCTAGAC-3′ | 5′-CCACTAAGGTGCCTACAGAGC-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.C.; Nam, K.H.; Yi, S.A.; Jo, M.S.; Lee, K.H.; Lee, Y.H.; Lee, J.; Kim, K.H. Anti-adipogenic Effect of β-Carboline Alkaloids from Garlic (Allium sativum). Foods 2019, 8, 673. https://doi.org/10.3390/foods8120673
Baek SC, Nam KH, Yi SA, Jo MS, Lee KH, Lee YH, Lee J, Kim KH. Anti-adipogenic Effect of β-Carboline Alkaloids from Garlic (Allium sativum). Foods. 2019; 8(12):673. https://doi.org/10.3390/foods8120673
Chicago/Turabian StyleBaek, Su Cheol, Ki Hong Nam, Sang Ah Yi, Mun Seok Jo, Kwang Ho Lee, Yong Hoon Lee, Jaecheol Lee, and Ki Hyun Kim. 2019. "Anti-adipogenic Effect of β-Carboline Alkaloids from Garlic (Allium sativum)" Foods 8, no. 12: 673. https://doi.org/10.3390/foods8120673
APA StyleBaek, S. C., Nam, K. H., Yi, S. A., Jo, M. S., Lee, K. H., Lee, Y. H., Lee, J., & Kim, K. H. (2019). Anti-adipogenic Effect of β-Carboline Alkaloids from Garlic (Allium sativum). Foods, 8(12), 673. https://doi.org/10.3390/foods8120673