Characterization of Fulvic Acid Beverages by Mineral Profile and Antioxidant Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Determination of Minerals
2.4. Determination of Total Phenolics
2.5. Determination of Total Flavonoids
2.6. Determination of the Antioxidant Capacity
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ji, F.; McGlone, J.J.; Kim, S.W. Effects of dietary humic substances on pig growth performance, carcass characteristics, and ammonia emission. J. Anim. Sci. 2006, 84, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Łomińska-Płatek, D.; Anielak, A.M. The content of fulvic acids in the primary effluent at the Płaszów WWTP in Kraków, E3S Web of Conferences 17. In Proceedings of the 9th Conference on Interdisciplinary Problems in Environmental Protection and Engineering EKO-DOK, Boguszow-Gorce, Poland, 23–25 April 2017. [Google Scholar] [CrossRef]
- Malan, C. Review: Humic and fulvic acids. A Practical Approach. In Sustainable Soil Management Symposium. Stellenbosch; Agrilibrium Publisher: Cape Town, South Africa, 2015. [Google Scholar]
- Pettit, R.E. Organic Matter, Humus, Humate, Humic Acid, Fulvic Acid and Humin: Their Importance in Soil Fertility and Plant Health. 2004. Available online: https://humates.com (accessed on 19 June 2018).
- Sanmanee, N.; Areekijseree, M. The effects of fulvic acid on copper bioavailability to porcine oviductal epithelial cells. Biol. Trace Elem. Res. 2010, 135, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Reshi, Z.; Tyub, S. Detritus and Decomposition in Ecosystems. Chapter 7: Humus Biosynthesis; New India Publishing Agency: Pitam Pura, India, 2007; pp. 153–176. [Google Scholar]
- Alvarez-Puebla, R.A.; Valenzuela-Calahorro, C.; Garrido, J.J. Theoretical study on fulvic acid structure, confirmation and aggregation, a molecular modelling approach. Sci. Total Environ. 2006, 358, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Kishor, P.; Bimala, S.; Nagendra, T. Shilajit: Humic matter panacea for cancer. Int. J. Toxicol. Pharmacol. Res. 2012, 4, 17–25. [Google Scholar]
- Aydin, S.K.; Dalgic, S.; Karaman, M.; Kirlangic, O.F.; Yildirim, H. Effects of fulvic acid on different cancer cell lines. Proceedings 2017, 1, 1031. [Google Scholar] [CrossRef]
- Christl, I.; Metzger, A.; Heidmann, I.; Kretzschma, R. Effect of humic and fulvic acid concentrations and ionic strength on copper and lead binding. Environ. Sci. Technol. 2005, 39, 5319–5326. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Yang, G.; Dong, Y.; Zhao, Y.-Q.; Sun, X.-R.; Chen, L.; Chen, H.-B. Studies on the binding of fulvic acid with transferrin by spectroscopic analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 1280–1285. [Google Scholar] [CrossRef]
- Man, D.; Pisarek, I.; Braczkowski, M.; Pytel, B.; Olchawa, R. The impact of humic and fulvic acids on the dynamic properties of liposome membranes: The ESR method. J. Liposome Res. 2014, 24, 106–112. [Google Scholar] [CrossRef]
- Cárdenas Rodríguez, N.; Coballase Urrutia, E.; Huerta Gertrudis, B.; Pedraza Chaverri, J.; Barragán Mejía, G. Antioxidant activity of fulvic acid: A living matter-derived bioactive compound. J. Food, Agric. Environ. 2011, 9, 123–127. [Google Scholar]
- Carrasco-Gallardo, C.; Farías, G.A.; Fuentes, P.; Crespo, F.; Maccioni, R.B. Can nutraceuticals prevent Alzheimer’s disease? Potential therapeutic role of a formulation containing shilajit and complex B vitamins. Arch. Med. Res. 2012, 43, 699–704. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X. Antioxidant Therapies for Alzheimer’s Disease. Oxid. Med. Cell Longev. 2012, 17. [Google Scholar] [CrossRef] [PubMed]
- Meena, H.; Pandey, H.K.; Arya, M.C.; Ahmed, Z. Shilajit: A panacea for high-altitude problems. Int. J. Ayurveda Res. 2010, 1, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Van Rensburg, C. The antiinflammatory properties of humic substances: A mini review. Phytother. Res. 2015, 29, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Ghosh, S. Therapeutic potential of fulvic acid in chronic inflammatory diseases and diabetes. J. Diabetes Res. 2018, 2018, 7. [Google Scholar] [CrossRef]
- Baigorri, R.; Fuentes, M.; González-Gaitano, G.; García-Mina, J.M.; Almendros, G.; González-Vila, F.J. Complementary multianalytical approach to study the distinctive structural features of the main humic fractions in solution: Gray humic acid, brown humic acid, and fulvic acid. J. Agric. Food Chem. 2009, 57, 3266–3272. [Google Scholar] [CrossRef]
- Shailesh, K.B.; Aswin, M.T.; Jitendra, K.M. Shilajit. In Nutraceuticals Efficacy, Safety and Toxicity; Academic Press: Cambridge, MA, USA, 2016; pp. 707–716. [Google Scholar]
- Wilson, E.; Rajamanickam, G.V.; Dubey, G.P.; Klose, P.; Musial, F.; Saha, F.J.; Rampp, T.; Michalsen, A.; Dobosa, G.J. Review on shilajit used in traditional Indian medicine. J. Ethnopharmacol. 2011, 136, 1–9. [Google Scholar] [CrossRef]
- Ozbek, N.; Akman, S. Method development for the determination of calcium, copper, magnesium, manganese, iron, potassium, phosphorus and zinc in different types of breads by microwave induced plasma-atomic emission spectrometry. Food Chem. 2016, 200, 245–248. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Karadeniz, F.; Burdurlu, H.S.; Koca, N.; Soyer, Y. Antioxidant activity of selected fruits and vegetables grown in Turkey. Turk. J. Agric. For. 2005, 29, 297–303. [Google Scholar]
- Re, R.; Pellergini, N.; Proteggente, A.; Pannala, A.S.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘’antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Enko, J.; Gliszczyńska-Świgło, A. Influence of the interactions between tea (Camellia sinensis) extracts and ascorbic acid on their antioxidant activity: Analysis with interaction indexes and isobolograms. Food Addit. Contam. Part A 2015, 32, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M. (Ed.) Normy Żywienia dla Populacji Polskiej–Nowelizacja [Nutritional Requirements for the Polish Population–Upgrade]; National Food and Nutrition Institute: Warsaw, Poland, 2017; p. 223. [Google Scholar]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2015.
- Adani, F.; Genevini, P.; Zaccheo, P.; Zocchi, G. The effect of commercial humic acid on tomato plant growth and mineral nutrition. J. Plant Nutr. 1998, 21, 561–575. [Google Scholar] [CrossRef]
- Bocanegra, M.P.; Lobartini, J.C.; Orioli, G.A. Plant uptake of iron chelated by humic acids of different molecular weights. Commun. Soil. Sci. Plant Anal. 2006, 37, 1–2. [Google Scholar] [CrossRef]
- Canellas, L.P.; Dantas, D.J.; Aguiar, N.O.; Peres, L.E.P.; Zsögön, A.; Olivares, F.L.; Dobbss, L.B.; Façanha, A.R.; Nebbioso, A.; Piccolo, A. Probing the hormonal activity of fractionated molecular humic components in tomato auxin mutants. Ann. Appl. Biol. 2011, 159, 202–211. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, A.; Sánchez-Andreu, J.; Juárez, M.; Jordá, J.; Bermúdez, D. Humic substances and amino acids improve effectiveness of chelate FeEDDHA in lemon trees. J. Plant Nutr. 2002, 25, 2433–2442. [Google Scholar] [CrossRef]
- Grant, T.D.; Wuilloud, R.G.; Wuilloud, J.C.; Caruso, J.A. Investigation of the elemental composition and chemical association of several elements in fulvic acids dietary supplements by size-exclusion chromatography UV inductively coupled plasma mass spectrometric. J. Chrom. A 2004, 1054, 313–319. [Google Scholar] [CrossRef]
- Abountiolas, M.; Nascimento Nunes, C.D. Polyphenols, ascorbic acid and antioxidant capacity of commercial nutritional drinks, fruit juices, smoothies and teas. Int. J. Food Sci. Technol. 2018, 53, 188–198. [Google Scholar] [CrossRef]
- Aguiilar, T.; de Bruijn, J.; Loyola, C.; Bustamante, L.; Vergara, C.; von Baer, D.; Mardne, C.; Serra, I. Characterization of an Antioxidant-Enriched Beverage from Grape Musts and Extracts of Winery and Grapevine By-Products. Beverages 2018, 4, 4. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef]
- Stella, S.P.; Ferrarezi, A.C.; dos Santos, K.O.; Monteiro, M. Antioxidant activity of commercial ready to drink orange juice and nectar. J. Food Sci. 2011, 76, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Lal, G.G. Processing of beverages for the health food market consumer. In Nutraceutical and Functional Food Processing Technology; Boye, J.I., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 189–208. [Google Scholar]
| Category of Product | Ca | K | Mg | Na | |||||
|---|---|---|---|---|---|---|---|---|---|
| I 1 | II 1 | I | II | I | II | I | II | ||
| CONCENTRATE | |||||||||
| 1 | mg/mL mg/daily portion 2 %RDA/AI 3 | 0.99 ± 0.01 c | 2.09 ± 0.11 b | 24.0 ± 0.2 a | 24.1 ± 0.6 a | 1.09 ± 0.02 c | 1.05 ± 0.02 c | 3.76 ± 0.14 a | 3.62 ± 0.15 a,b |
| 1.4 | 3.0 | 34 | 35 | 1.6 | 1.5 | 5.5 | 5.3 | ||
| 0.1% | 0.3% | 0.7% | 0.7% | 0.5% | 0.5% | 0.4% | 0.4% | ||
| 2 | 0.92 ± 0.05 c,d | 0.91 ± 0.03 d | 23.0 ± 0.4 b | 23.0 ± 0.4 b | 1.46 ± 0.02 b | 1.46 ± 0.03 b | 3.50 ± 0.21 a,b | 3.32 ± 0.16 b | |
| 0.6 | 0.6 | 16 | 16 | 1.0 | 1.0 | 2.5 | 2.3 | ||
| <0.1% | <0.1% | 0.3% | 0.3% | 0.3% | 0.3% | 0.2% | 0.2% | ||
| 3 | 2.57 ± 0.17 a | 2.62 ± 0.09 a | 1.82 ± 0.17 c | 1.98 ± 0.07 c | 50 ± 2.3 a | 48 ± 2.2 a | 2.12 ± 0.06 c | 2.05 ± 0.05 c | |
| 7.2 | 7.3 | 5.1 | 5.5 | 139 | 135 | 5.9 | 5.7 | ||
| 0.7% | 0.7% | 0.1% | 0.1% | 43% | 42% | 0.4% | 0.4% | ||
| READY-TO-DRINK | |||||||||
| 4 | mg/mL mg/daily portion %RDA/AI | 0.20 ± 0.01 c | 0.33 ± 0.01 b | 0.28 ± 0.01 b | 0.18 ± 0.00 c | 0.06 ± 0.01 b | 0.07 ± 0.01 a | 0.58 ± 0.02 a | 0.57 ± 0.05 a,b |
| 5.6 | 9.2 | 7.8 | 5.0 | 1.7 | 2.0 | 16 | 16 | ||
| 0.6% | 0.9% | 0.2% | 0.1% | 0.5% | 0.6% | 1.1% | 1.1% | ||
| 5 | 0.12 ± 0.01 d | 0.20 ± 0.00 c | <0.01 | <0.01 | <0.01 | <0.01 | 0.53 ± 0.04 a,b | 0.52 ± 0.03 b | |
| 3.4 | 5.6 | 0 | 0 | 0.3 | 0.3 | 15 | 15 | ||
| 0.3% | 0.6% | 0 | 0 | 0.1% | 0.1% | 1% | 1% | ||
| 6 | 0.38 ± 0.03 a | 0.37 ± 0.01 a | 2.46 ± 0.06 a | 2.56 ± 0.07 a | 0.10 ± 0.00 a | 0.10 ± 0.00 a | 0.58 ± 0.04 a | 0.62 ± 0.06 a | |
| 6.1 | 5.9 | 40 | 41 | 1.6 | 1.6 | 9.3 | 9.9 | ||
| 0.6% | 0.6% | 0.8% | 0.9% | 0.5% | 0.5% | 0.6% | 0.7% | ||
| 7 | 0.02 ± 0.00 e | 0.02 ± 0.00 e | 0.04 ± 0.00 d | 0.04 ± 0.00 d | <0.01 | <0.01 | 0.53 ± 0.01 b | 0.54 ± 0.01 b | |
| 10 | 10 | 20 | 20 | 0 | 0 | 265 | 270 | ||
| 1% | 1% | 0.4% | 0.4% | 0 | 0 | 18% | 18% | ||
| Category of Product | Cu | Fe | Mn | Zn | |||||
|---|---|---|---|---|---|---|---|---|---|
| I 1 | II 1 | I | II | I | II | I | II | ||
| CONCENTRATE | |||||||||
| 1 | mg/mL mg/daily portion 2 %RDA 3 | <0.01 | <0.01 | 16.0 ± 1.5 a | 14.0 ± 1.0 a,b | 0.16 ± 0.00 b | 0.16 ± 0.00 b | 0.06 ± 0.00 a | 0.05 ± 0.00 a,b |
| 0 | 0 | 23 | 20 | 0.23 | 0.23 | 0.09 | 0.07 | ||
| 0 | 0 | 129% | 113% | 13% | 13% | 1.1% | 1.0% | ||
| 2 | <0.01 | <0.01 | 13.0 ± 1.1 b | 12.0 ± 1.1 b | 0.20 ± 0.01 a | 0.21 ± 0.00 a | 0.04 ± 0.00 b | 0.04 ± 0.00 b | |
| 0 | 0 | 9.0 | 8.1 | 0.14 | 0.15 | 0.03 | 0.03 | ||
| 0 | 0 | 50% | 45% | 7.8% | 8.2% | 0.4% | 0.4% | ||
| 3 | <0.01 | <0.01 | 0.04 ± 0.00 c | 0.04 ± 0.00 c | <0.01 | <0.01 | 0.02 ± 0.00 c | 0.02 ± 0.00 c | |
| 0 | 0 | 0.11 | 0.11 | 0 | 0 | 0.06 | 0.06 | ||
| 0 | 0 | 0.6% | 0.6% | 0 | 0 | 0.7% | 0.7% | ||
| READY-TO-DRINK | |||||||||
| 4 | mg/mL mg/daily portion %RDA | <0.01 | <0.01 | 0.83 ± 0.04 b | 0.87 ± 0.04 b | <0.01 | <0.01 | 0.01 ± 0.00 b | 0.01 ± 0.00 b |
| 0 | 0 | 23 | 24 | 0 | 0 | 0.28 | 0.28 | ||
| 0 | 0 | 129% | 135% | 0 | 0 | 3.5% | 3.5% | ||
| 5 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 ± 0.00 a | 0.01 ± 0.00 b | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0.56 | 0.28 | ||
| 0 | 0 | 0 | 0 | 0 | 0 | 7% | 3.5% | ||
| 6 | <0.01 | <0.01 | 1.19 ± 0.01 a | 1.20 ± 0.01 a | 0.08± 0.00 | 0.08 ± 0.00 | 0.01 ± 0.00 b | 0.01 ± 0.00 b | |
| 0 | 0 | 19 | 19 | 1.28 | 1.28 | 0.16 | 0.16 | ||
| 0 | 0 | 106% | 107% | 71% | 71% | 2% | 2% | ||
| 7 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Category of Product | TP | TF | FRAP | TEAC | |||||
|---|---|---|---|---|---|---|---|---|---|
| I 1 | II 1 | I | II | I | II | I | II | ||
| CONCENTRATE | |||||||||
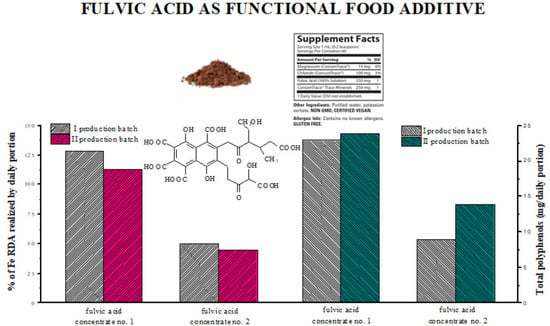
| 1 | µg/mL µg/daily portion 2 | 15,762 ± 140 c | 16,457 ± 442 b | 7321 ± 152 a | 5886 ± 228 b | 309 ± 15 a | 235 ± 6 b | 141 ± 5 b | 117 ± 13 c |
| 22,855 | 23,863 | 10,615 | 8535 | 448 | 341 | 204 | 170 | ||
| 2 | 12,814 ± 175 d | 19,844 ± 231 a | 6167 ± 163 b | 7113 ± 270 a | 309 ± 16 a | 338 ± 18 a | 150 ± 10 b | 172 ± 9 a | |
| 8970 | 13,891 | 4317 | 4979 | 216 | 237 | 105 | 120 | ||
| 3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||
| READY-TO-DRINK | |||||||||
| 4 | µg/mL µg/daily portion | 44.7 ± 1.3 b | 67.3 ± 2.8 a | 17.1 ± 0.4 e | 24.4 ± 0.6 d | n.d. | n.d. | n.d. | n.d. |
| 1252 | 1884 | 479 | 683 | n.d. | n.d. | n.d. | n.d. | ||
| 5 | 12.0 ± 2.6 e | 12.0 ± 0.5 e | 6.5 ± 0.5 g | 8.1 ± 0.3 f | n.d. | n.d. | n.d. | n.d. | |
| 336 | 336 | 182 | 227 | n.d. | n.d. | n.d. | n.d. | ||
| 6 | 22.0 ± 1.7 c | 16.0 ± 0.5 d | 187 ± 10 a | 135 ± 20 b | 4.8 ± 0.7 | 4.5 ± 0.1 | n.d. | n.d. | |
| 352 | 256 | 2992 | 2160 | 77 | 72 | n.d. | n.d. | ||
| 7 | 22.2 ± 0.8 c | 21.4 ± 1.0 c | 28.4 ± 0.6 c | 29.3 ± 0.8 c | n.d. | n.d. | n.d. | n.d. | |
| 11,100 | 10,700 | 14,200 | 14,650 | n.d. | n.d. | n.d. | n.d. | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swat, M.; Rybicka, I.; Gliszczyńska-Świgło, A. Characterization of Fulvic Acid Beverages by Mineral Profile and Antioxidant Capacity. Foods 2019, 8, 605. https://doi.org/10.3390/foods8120605
Swat M, Rybicka I, Gliszczyńska-Świgło A. Characterization of Fulvic Acid Beverages by Mineral Profile and Antioxidant Capacity. Foods. 2019; 8(12):605. https://doi.org/10.3390/foods8120605
Chicago/Turabian StyleSwat, Monika, Iga Rybicka, and Anna Gliszczyńska-Świgło. 2019. "Characterization of Fulvic Acid Beverages by Mineral Profile and Antioxidant Capacity" Foods 8, no. 12: 605. https://doi.org/10.3390/foods8120605
APA StyleSwat, M., Rybicka, I., & Gliszczyńska-Świgło, A. (2019). Characterization of Fulvic Acid Beverages by Mineral Profile and Antioxidant Capacity. Foods, 8(12), 605. https://doi.org/10.3390/foods8120605