Polydatin and Resveratrol Inhibit the Inflammatory Process Induced by Urate and Pyrophosphate Crystals in THP-1 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Polyphenols
2.3. Cell Culture and Treatment
2.4. Cell Viability, Death and Apoptosis Assays
2.5. IL-1β Measurement
2.6. Gene Expression Analysis
2.7. Assessment of Intracellular Oxidative Stress
2.8. Nitric Oxide Determination
2.9. Evaluation of CPP and MSU Crystal Phagocytosis
2.10. Statistical Analysis
3. Results
3.1. The Effect of PD and RES on Cell Viability, Death and Apoptosis Induced by Crystals
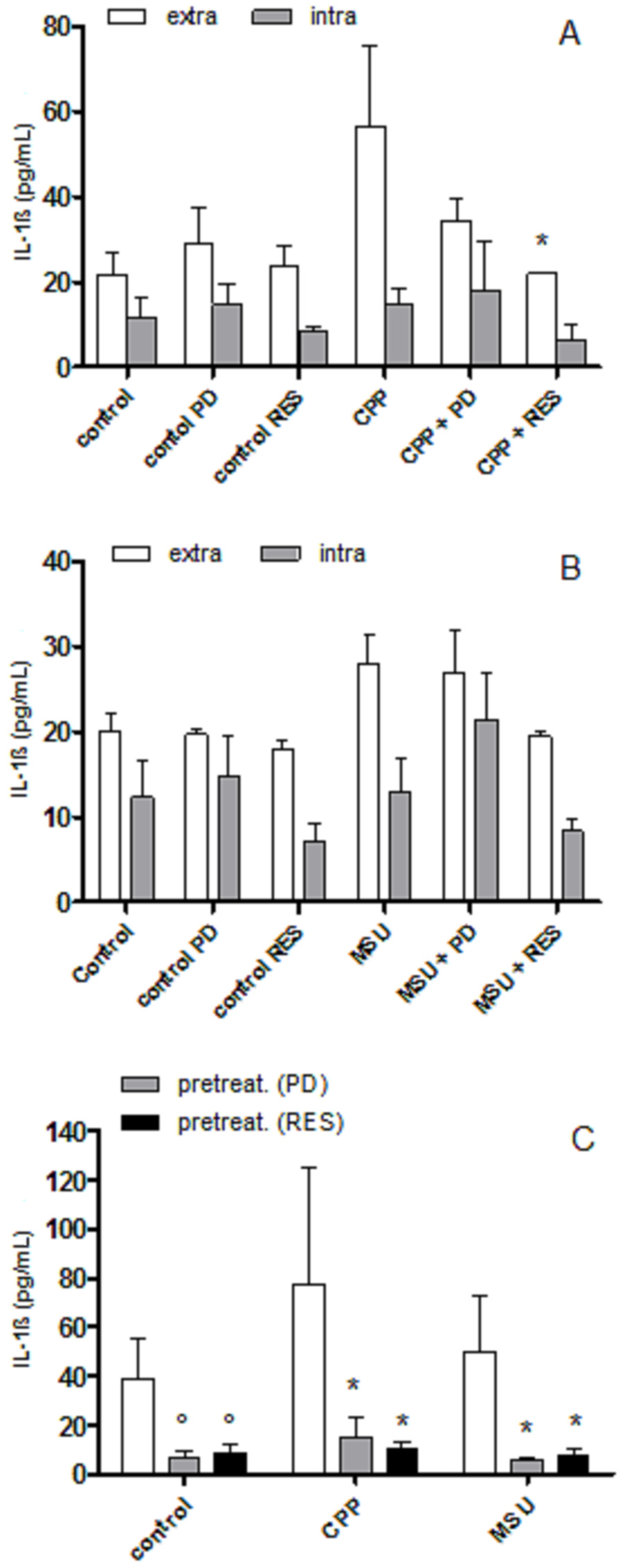
3.2. The Effect of PD and RES on Intra- and Extra-cellular Levels of IL-1β
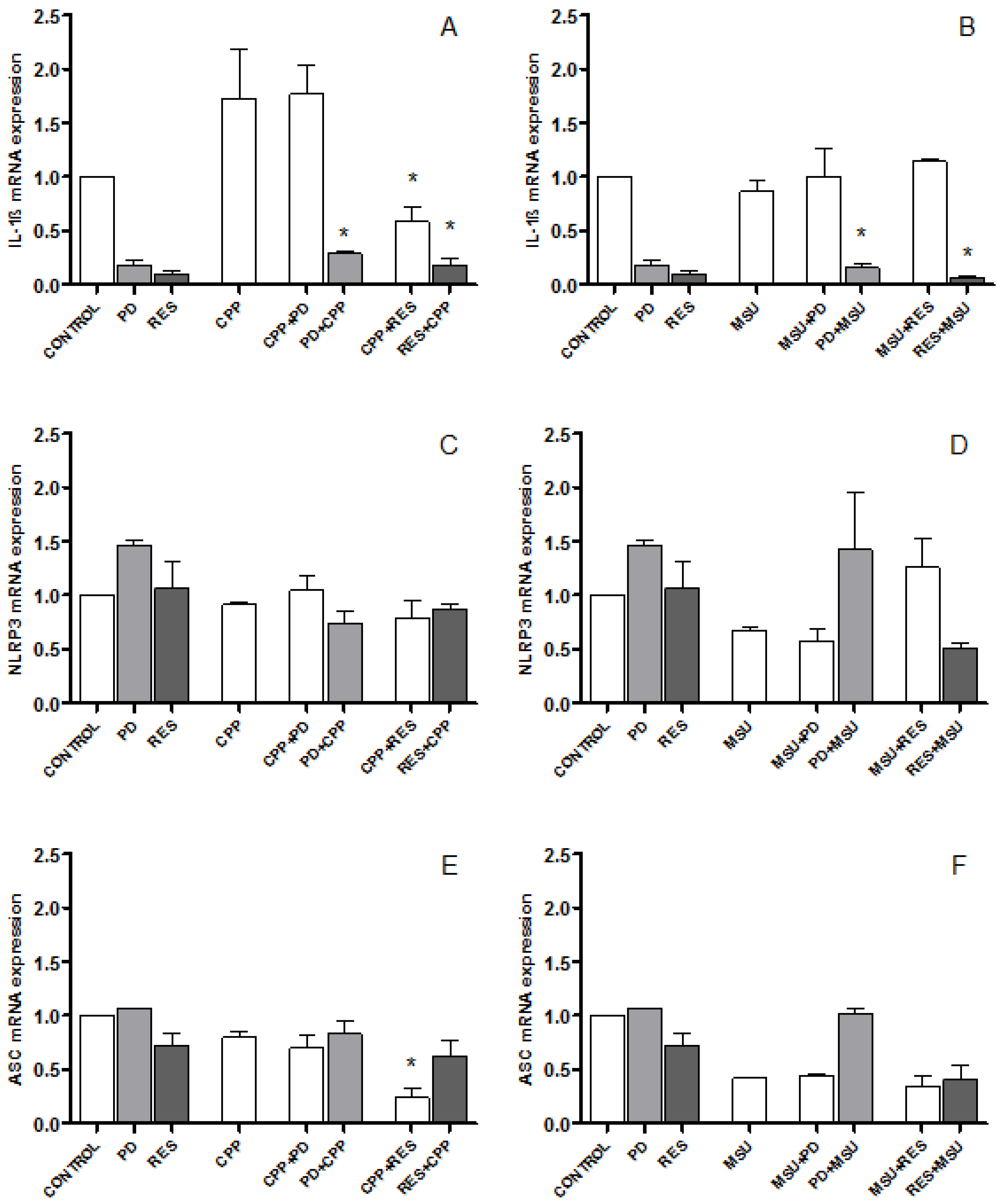
3.3. The Effect of PD and RES on IL-1β, NLRP3 and ASC Expression
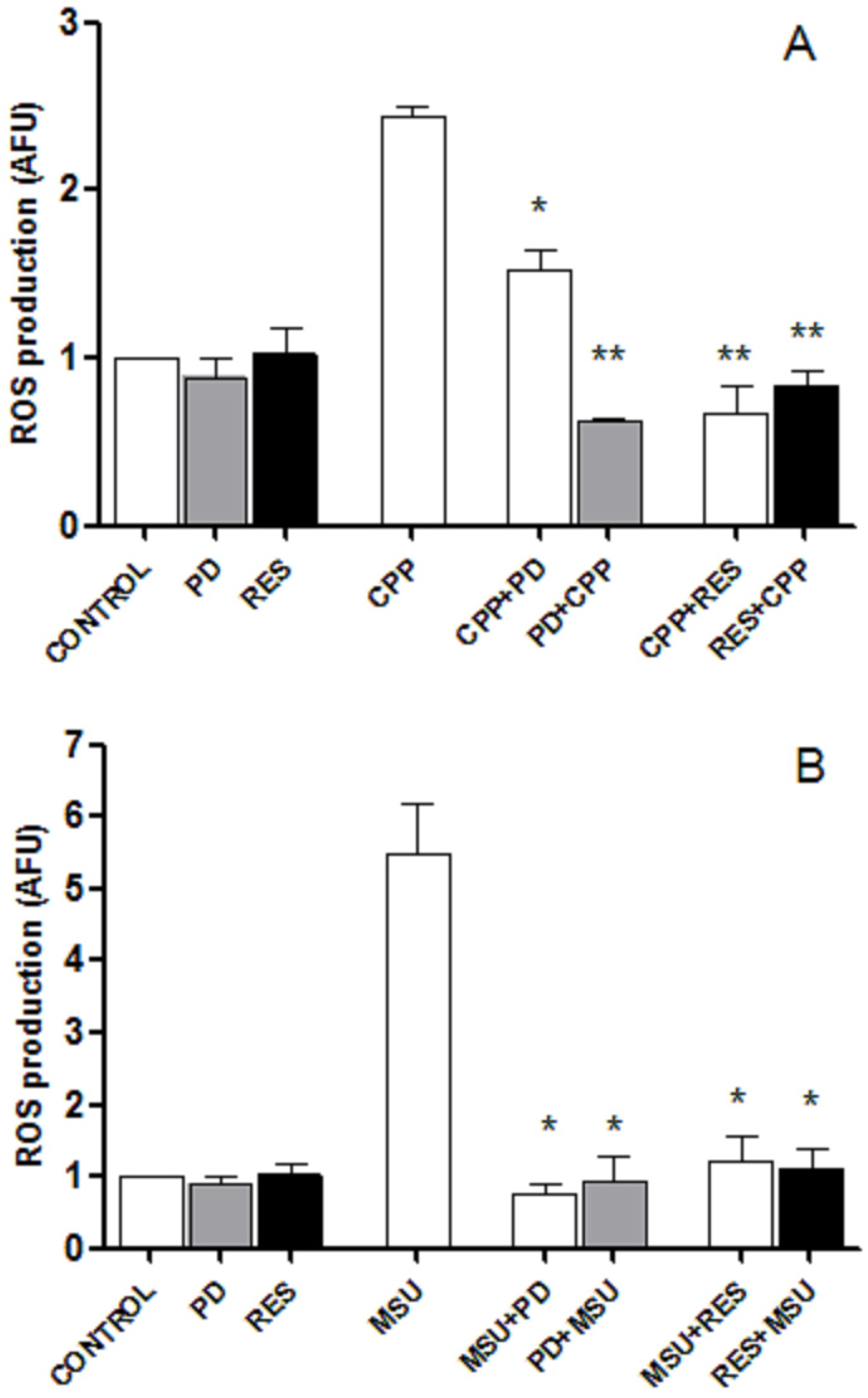
3.4. RES and PD Inhibit ROS Production Induced by Crystals
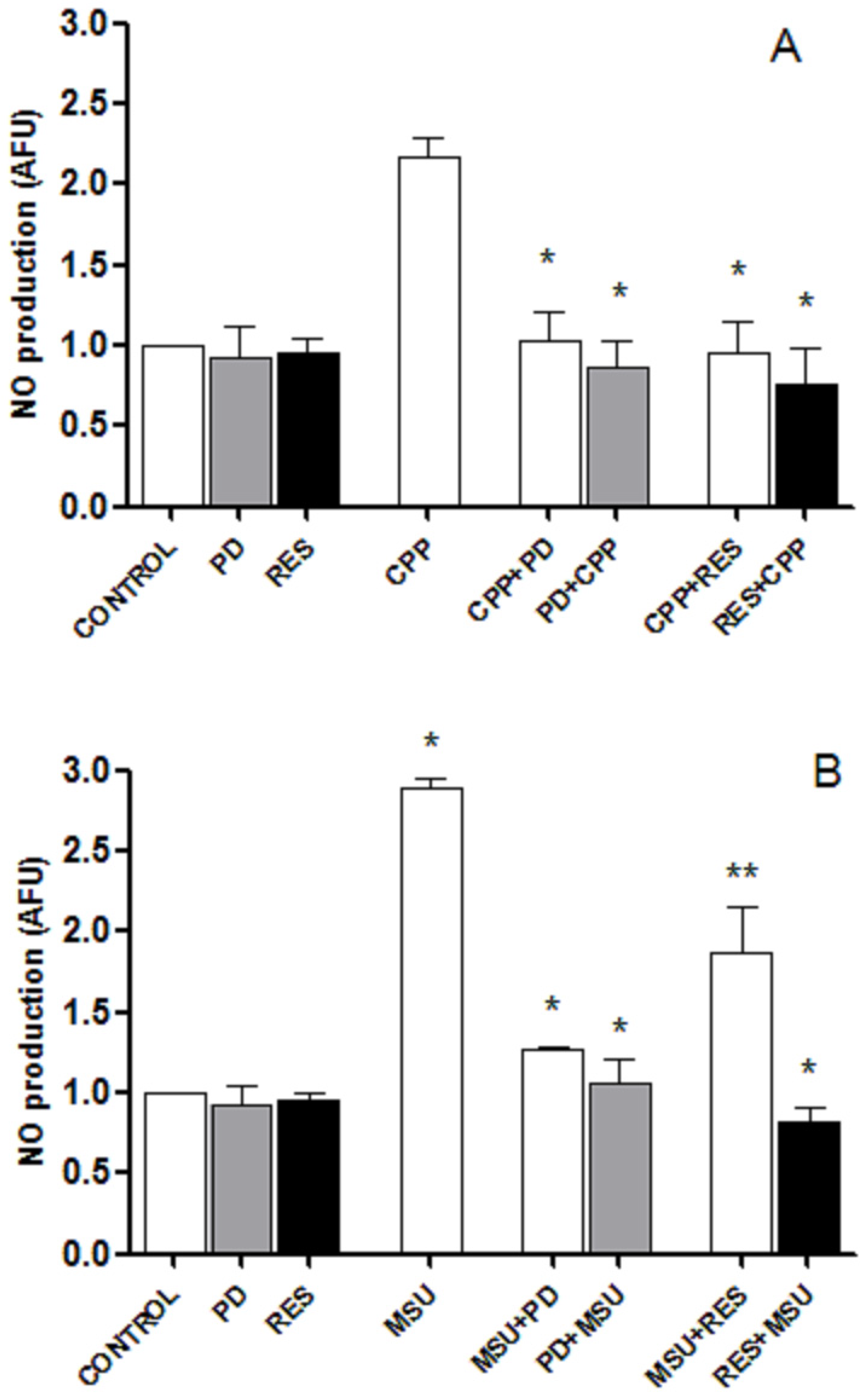
3.5. RES and PD Inhibit Nitric oxide Production Induced by Crystals
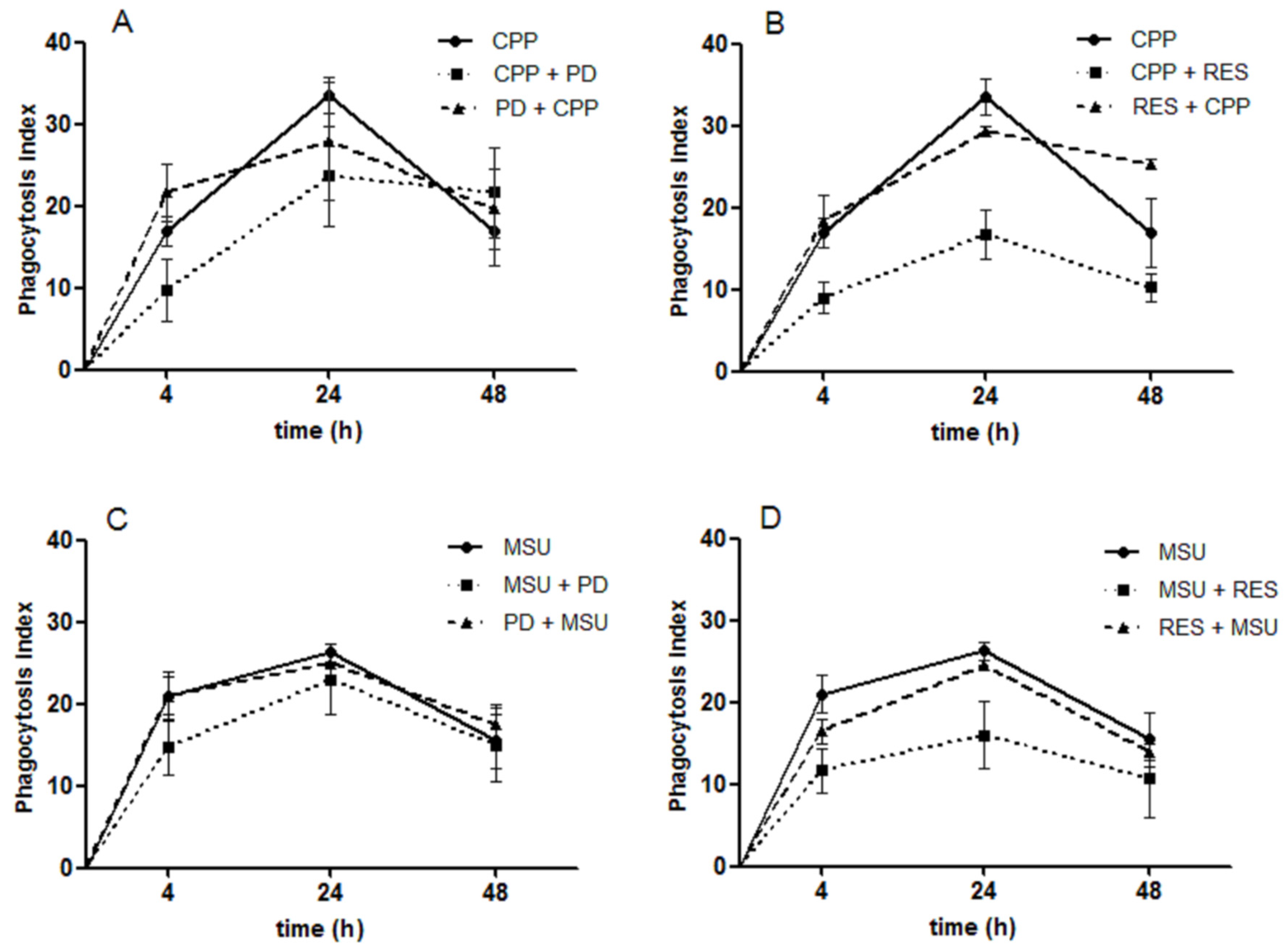
3.6. The Effect of RES and PD on Crystal Phagocytosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mahon, O.R.; Dunne, A. Disease-Associated Particulates and Joint Inflammation; Mechanistic Insights and Potential Therapeutic Targets. Front. Immunol. 2018, 9, 1145. [Google Scholar] [CrossRef]
- Zamudio-Cuevas, Y.; Hernández-Díaz, C.; Pineda, C.; Reginato, A.M.; Cerna-Cortés, J.F.; Ventura-Ríos, L.; López-Reyes, A. Molecular basis of oxidative stress in gouty arthropathy. Clin. Rheumatol. 2015, 34, 1667–1672. [Google Scholar] [CrossRef]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Dinarello, C.A. An expanding role for interleukin-1 blockade from gout to cancer. Mol. Med. 2014, 20, S43–S58. [Google Scholar] [CrossRef] [PubMed]
- So, A.; Dumusc, A.; Nasi, S. The role of IL-1 in gout: From bench to bedside. Rheumatology 2018, 57 (Suppl. 1), i12–i19. [Google Scholar] [PubMed]
- Andrés, M.; Sivera, F.; Pascual, E. Therapy for CPPD: Options and Evidence. Curr. Rheumatol. Rep. 2018, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.K.; Merriman, T.R.; Singh, J.A. Expert opinion on emerging urate-lowering therapies. Expert. Opin. Emerg. Drugs 2018, 23, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef]
- Oliviero, F.; Scanu, A.; Zamudio-Cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food. Agric. 2018, 98, 1653–1659. [Google Scholar] [CrossRef]
- Billingsley, H.E.; Carbone, S. The antioxidant potential of the Mediterranean diet in patients at high cardiovascular risk: An in-depth review of the PREDIMED. Nutr. Diabetes 2018, 8, 13. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, F.; Sfriso, P.; Scanu, A.; Fiocco, U.; Spinella, P.; Punzi, L. Epigallocatechin-3-gallate reduces inflammation induced by calcium pyrophosphate crystals in vitro. Front. Pharmacol. 2013, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Ravagnan, G.; De Filippis, A.; Cartenì, M.; De Maria, S.; Cozza, V.; Petrazzuolo, M.; Tufano, M.A.; Donnarumma, G. Polydatin, a natural precursor of resveratrol, induces β-defensin production and reduces inflammatory response. Inflammation 2013, 36, 26–34. [Google Scholar] [CrossRef] [PubMed]
- de Sá Coutinho, D.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, A.; Posa, F.; De Maria, S.; Ravagnan, G.; Ballini, A.; Porro, C.; Trotta, T.; Grano, M.; Muzio, L.L.; Mori, G. Polydatin, Natural Precursor of Resveratrol, Promotes Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Med. Sci. 2018, 15, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Martano, M.; Stiuso, P.; Facchiano, A.; De Maria, S.; Vanacore, D.; Restucci, B.; Rubini, C.; Caraglia, M.; Ravagnan, G.; Lo Muzio, L. Aryl hydrocarbon receptor, a tumor grade-associated marker of oral cancer, is directly downregulated by polydatin: A pilot study. Oncol. Rep. 2018, 40, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.W.; Wang, C.P.; Liu, L.; Liu, Y.L.; Wang, X.; Hong, Y.; Li, Z.; Kong, L.D. Antihyperuricemic and nephroprotective effects of resveratrol and its analogues in hyperuricemic mice. Mol. Nutr. Food. Res. 2012, 56, 1433–1444. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, S.; Wang, Y.; Zhu, H.; Liu, Q.; Xue, Y.; Qiu, J.; Zou, H.; Zhu, X. The effect of resveratrol on the recurrent attacks of gouty arthritis. Clin. Rheumatol. 2016, 35, 1189–1195. [Google Scholar] [CrossRef]
- Misawa, T.; Saitoh, T.; Kozaki, T.; Park, S.; Takahama, M.; Akira, S. Resveratrol inhibits the acetylated α-tubulin-mediated assembly of the NLRP3-inflammasome. Int. Immunol. 2015, 27, 425–434. [Google Scholar] [CrossRef]
- Zamudio-Cuevas, Y.; Martínez-Flores, K.; Fernández-Torres, J.; Loissell-Baltazar, Y.A.; Medina-Luna, D.; López-Macay, A.; Camacho-Galindo, J.; Hernández-Díaz, C.; Santamaría-Olmedo, M.G.; López-Villegas, E.O.; et al. Monosodium urate crystals induce oxidative stress in human synoviocytes. Arthritis Res. Ther. 2016, 18, 117. [Google Scholar] [CrossRef]
- Cleophas, M.C.; Crişan, T.O.; Lemmers, H.; Toenhake-Dijkstra, H.; Fossati, G.; Jansen, T.L.; Dinarello, C.A.; Netea, M.G.; Joosten, L.A. Suppression of monosodium urate crystal-induced cytokine production by butyrate is mediated by the inhibition of class I histone deacetylases. Ann. Rheum. Dis. 2016, 75, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Belluzzi, E.; Olivotto, E.; Toso, G.; Cigolotti, A.; Pozzuoli, A.; Biz, C.; Trisolino, G.; Ruggieri, P.; Grigolo, B.; Ramonda, R.; et al. Conditioned media from human osteoarthritic synovium induces inflammation in a synoviocyte cell line. Connect. Tissue Res. 2019, 60, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Busso, N.; Ea, H.K. The mechanisms of inflammation in gout and pseudogout (CPP-induced arthritis). Reumatismo 2012, 63, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, F.; Scanu, A.; Dayer, J.M.; Fiocco, U.; Sfriso, P.; Punzi, L. Response to ‘Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4’. Arthritis Res. Ther. 2012, 14, 405. [Google Scholar] [CrossRef][Green Version]
- Scanu, A.; Oliviero, F.; Gruaz, L.; Galozzi, P.; Luisetto, R.; Ramonda, R.; Burger, D.; Punzi, L. Synovial fluid proteins are required for the induction of interleukin-1β production by monosodium urate crystals. Scand. J. Rheumatol. 2016, 45, 384–393. [Google Scholar] [CrossRef]
- Mylona, E.E.; Mouktaroudi, M.; Crisan, T.O.; Makri, S.; Pistiki, A.; Georgitsi, M.; Savva, A.; Netea, M.G.; van der Meer, J.W.; Giamarellos-Bourboulis, E.J.; et al. Enhanced interleukin-1β production of PBMCs from patients with gout after stimulation with Toll-like receptor-2 ligands and urate crystals. Arthritis Res. Ther. 2012, 14, R158. [Google Scholar] [CrossRef]
- Yang, Q.B.; He, Y.L.; Zhong, X.W.; Xie, W.G.; Zhou, J.G. Resveratrol ameliorates gouty inflammation via upregulation of sirtuin 1 to promote autophagy in gout patients. Inflammopharmacology 2019, 27, 47–56. [Google Scholar] [CrossRef]
- Chalons, P.; Amor, S.; Courtaut, F.; Cantos-Villar, E.; Richard, T.; Auger, C.; Chabert, P.; Schni-Kerth, V.; Aires, V.; Delmas, D. Study of Potential Anti-Inflammatory Effects of Red Wine Extract and Resveratrol through a Modulation of Interleukin-1-Beta in Macrophages. Nutrients 2018, 10, 1856. [Google Scholar] [CrossRef]
- Peng, Y.J.; Lee, C.H.; Wang, C.C.; Salter, D.M.; Lee, H.S. Pycnogenol attenuates the inflammatory and nitrosative stress on joint inflammation induced by urate crystals. Free Radic. Biol. Med. 2012, 52, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Choe, J.Y.; Park, K.Y. Rebamipide Suppresses Monosodium Urate Crystal-Induced Interleukin-1β Production Through Regulation of Oxidative Stress and Caspase-1 in THP-1 Cells. Inflammation 2016, 39, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Kent, D.; Botello-Smith, W.M.; Nur, F.; Nur, S.; Alsamarah, A.; Chatterjee, P.; Lambros, M.; Luo, Y. Mechanism of Resveratrol’s Lipid Membrane Protection. Sci. Rep. 2018, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Fuggetta, M.P.; Migliorino, M.R.; Ricciardi, S.; Osman, G.; Iacono, D.; Leone, A.; Lombardi, A.; Ravagnan, G.; Greco, S.; Remotti, D.; et al. Prophylactic Dermatologic Treatment of Afatinib-Induced Skin Toxicities in Patients with Metastatic Lung Cancer: A Pilot Study. Scientifica 2019, 2019, 9136249. [Google Scholar] [CrossRef] [PubMed]
- Iyori, M.; Kataoka, H.; Shamsul, H.M.; Kiura, K.; Yasuda, M.; Nakata, T.; Hasebe, A.; Shibata, K. Resveratrol modulates phagocytosis of bacteria through an NF-kappaB-dependent gene program. Antimicrob. Agents Chemother. 2008, 52, 121–127. [Google Scholar] [CrossRef]
- Park, E.; Kim, D.K.; Chun, H.S. Resveratrol Inhibits Lipopolysaccharide-induced Phagocytotic Activity in BV2 cells. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 803–807. [Google Scholar] [CrossRef]
- Bertelli, A.A.; Ferrara, F.; Diana, G.; Fulgenzi, A.; Corsi, M.; Ponti, W.; Ferrero, M.E.; Bertelli, A. Resveratrol, a natural stilbene in grapes and wine, enhances intraphagocytosis in human promonocytes: A co-factor in antiinflammatory and anticancer chemopreventive activity. Int. J. Tissue React. 1999, 21, 93–104. [Google Scholar]
- Fabris, S.; Momo, F.; Ravagnan, G.; Stevanato, R. Antioxidant properties of resveratrol and piceid on lipid peroxidation in micelles and monolamellar liposomes. Biophys. Chem. 2008, 135, 76–83. [Google Scholar] [CrossRef]
- Romero-Perez, A.I.; Ibern-Gomez, M.; Lamuela-Raventos, R.M.; de La Torre-Boronat, M.C. Piceid, the major resveratrol derivative in grape juices. J. Agric. Food. Chem. 1999, 47, 1533–1536. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites-Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed]
| RNA Template | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| GAPDH | CAAAGTTGTCATGGATGACC | CCATGGAGAAGGCTGGGG |
| IL-1β | CTGTCCTGCGTGTTGAAAGA | TTGGGTAATTTTTGGGATCTACA |
| ASC | CTGTCCATGGACGCCTTGG | CATCCGTCAGGACCTTCCCGT |
| NLRP3 | CACCTGTTGTGCAATCTGAAG | GCAAGATCCTGACAACATGC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliviero, F.; Zamudio-Cuevas, Y.; Belluzzi, E.; Andretto, L.; Scanu, A.; Favero, M.; Ramonda, R.; Ravagnan, G.; López-Reyes, A.; Spinella, P.; et al. Polydatin and Resveratrol Inhibit the Inflammatory Process Induced by Urate and Pyrophosphate Crystals in THP-1 Cells. Foods 2019, 8, 560. https://doi.org/10.3390/foods8110560
Oliviero F, Zamudio-Cuevas Y, Belluzzi E, Andretto L, Scanu A, Favero M, Ramonda R, Ravagnan G, López-Reyes A, Spinella P, et al. Polydatin and Resveratrol Inhibit the Inflammatory Process Induced by Urate and Pyrophosphate Crystals in THP-1 Cells. Foods. 2019; 8(11):560. https://doi.org/10.3390/foods8110560
Chicago/Turabian StyleOliviero, Francesca, Yessica Zamudio-Cuevas, Elisa Belluzzi, Lisa Andretto, Anna Scanu, Marta Favero, Roberta Ramonda, Giampietro Ravagnan, Alberto López-Reyes, Paolo Spinella, and et al. 2019. "Polydatin and Resveratrol Inhibit the Inflammatory Process Induced by Urate and Pyrophosphate Crystals in THP-1 Cells" Foods 8, no. 11: 560. https://doi.org/10.3390/foods8110560
APA StyleOliviero, F., Zamudio-Cuevas, Y., Belluzzi, E., Andretto, L., Scanu, A., Favero, M., Ramonda, R., Ravagnan, G., López-Reyes, A., Spinella, P., & Punzi, L. (2019). Polydatin and Resveratrol Inhibit the Inflammatory Process Induced by Urate and Pyrophosphate Crystals in THP-1 Cells. Foods, 8(11), 560. https://doi.org/10.3390/foods8110560








