Açai (Euterpe oleracea Mart.) Seed Extract Induces Cell Cycle Arrest and Apoptosis in Human Lung Carcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydro-Alcoholic Açai Seed Extract (ASE)
2.2. Antioxidant Activity Assays and Total Phenolic Compounds
2.3. Cell Culture and Treatment Protocol
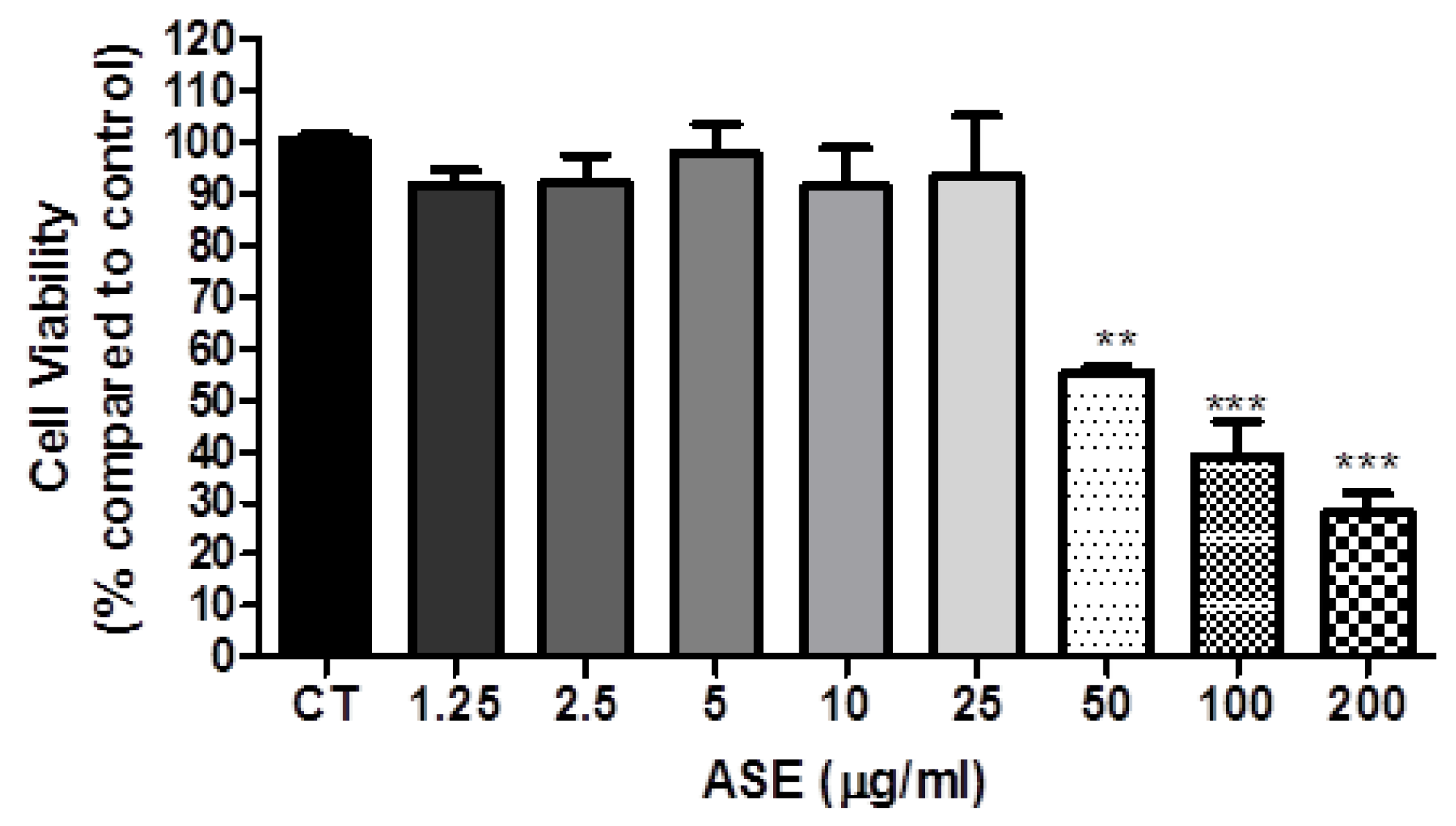
2.4. Cell Viability
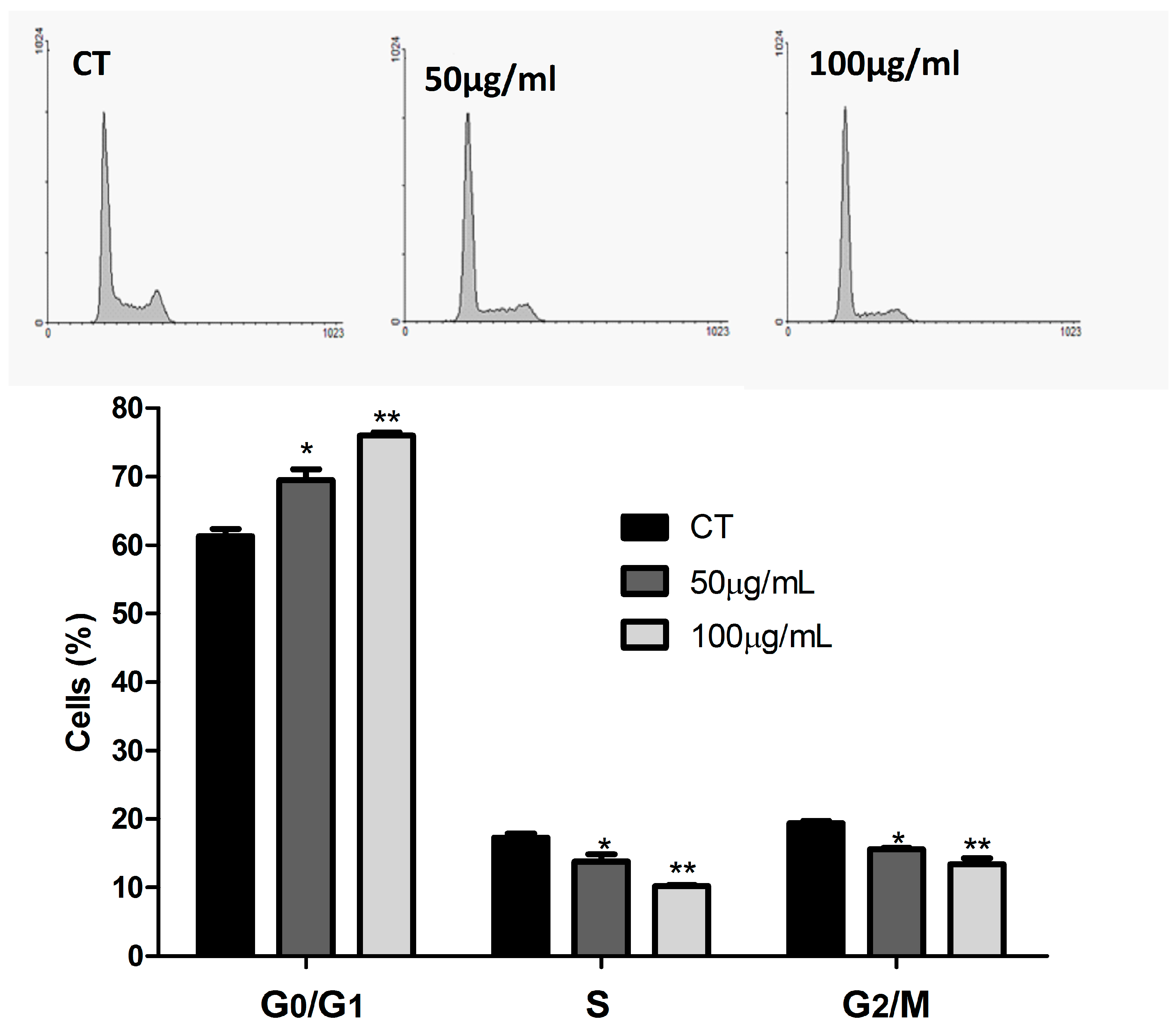
2.5. Cell Cycle Analysis
2.6. Apoptosis Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity and Total Phenolic Compounds
3.2. ASE Effects on Lung Cancer Cells Line (A549)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO—World Health Organization. World Cancer Report 2014; International Agency for Research on Cancer: Lyon, France, 2014; ISBN 978-92-832-0429-9. [Google Scholar]
- Ni, M.; Shi, X.-L.; Qu, Z.-G.; Jiang, H.; Chen, Z.-Q.; Hu, J. Epithelial mesenchymal transition of non-small-cell lung cancer cells A549 induced by SPHK1. Asian Pac. J. Trop. Med. 2015, 8, 142–146. [Google Scholar] [CrossRef]
- Filaire, E.; Dupuis, C.; Galvaing, G.; Aubreton, S.; Laurent, H.; Richard, R.; Filaire, M. Lung cancer: What are the links with oxidative stress, physical activity and nutrition. Lung Cancer 2013, 82, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Folmer, F.; Basavaraju, U.; Jaspars, M.; Hold, G.; El-Omar, E.; Dicato, M.; Diederich, M. Anticancer effects of bioactive berry compounds. Phytochem. Rev. 2014, 13, 295–322. [Google Scholar] [CrossRef]
- Xie, C.; Kang, J.; Li, Z.; Schauss, A.G.; Badger, T.M.; Nagarajan, S.; Wu, T.; Wu, X. The açai flavonoid velutin is a potent anti-inflammatory agent: Blockade of LPS-mediated TNF-α and IL-6 production through inhibiting NF-κB activation and MAPK pathway. J. Nutr. Biochem. 2012, 23, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, M.F.; Prado, M.G.; Barbosa, L.; Rocha, N.S.; Barbisan, L.F. Inhibition of Mouse Urinary Bladder Carcinogenesis by Açai Fruit (Euterpe oleraceae Martius) Intake. Plant Foods Hum. Nutr. 2012, 67, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Choi, Y.J.; Kim, N.K.; Nam, R.H.; Lee, S.; Lee, H.S.; Lee, H.-N.; Surh, Y.-J.; Lee, D.H. Açai Berries Inhibit Colon Tumorigenesis in Azoxymethane/Dextran Sulfate Sodium-Treated Mice. Gut Liver 2017, 11, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.K.D.L.; Pereira, L.F.R.; Lamarão, C.V.; Lima, E.S.; da Veiga-Junior, V.F. Amazon acai: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.B.; Lichtenthäler, R.; Zimmermann, B.F.; Papagiannopoulos, M.; Fabricius, H.; Marx, F. Total oxidant scavenging capacity of Euterpe oleracea Mart. (açai) seeds and identification of their polyphenolic compounds. J. Agric. Food Chem. 2006, 54, 4162–4167. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Santos, E.A.; Regis, W.C.B.; Ferreira, I.C.F.R. The powerful in vitro bioactivity of Euterpe oleracea Mart. seeds and related phenolic compounds. Ind. Crops Prod. 2015, 76, 318–322. [Google Scholar] [CrossRef]
- Wycoff, W.; Luo, R.; Schauss, A.G.; Neal-Kababick, J.; Sabaa-Srur, A.U.O.; Maia, J.G.S.; Tran, K.; Richards, K.M.; Smith, R.E. Chemical and nutritional analysis of seeds from purple and white açai (Euterpe oleracea Mart.). J. Food Compos. Anal. 2015, 41, 181–187. [Google Scholar] [CrossRef]
- Rocha, A.P.M.; Carvalho, L.C.R.M.; Sousa, M.A.V.; Madeira, S.V.F.; Sousa, P.J.C.; Tano, T.; Schini-Kerth, V.B.; Resende, A.C.; De Moura, R.S. Endothelium-dependent vasodilator effect of Euterpe oleracea Mart. (Açai) extracts in mesenteric vascular bed of the rat. Vasc. Pharmacol. 2007, 46, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- De Oliveira, P.R.B.; da Costa, C.A.; de Bem, G.F.; Cordeiro, V.S.C.; Santos, I.B.; de Carvalho, L.C.R.M.; da Conceição, E.P.S.; Lisboa, P.C.; Ognibene, D.T.; Sousa, P.J.C.; et al. Euterpe oleracea Mart.-Derived Polyphenols Protect Mice from Diet-Induced Obesity and Fatty Liver by Regulating Hepatic Lipogenesis and Cholesterol Excretion. PLoS ONE 2015, 10, e0143721. [Google Scholar] [CrossRef] [PubMed]
- De Bem, G.F.; Costa, C.A.; Santos, I.B.; da Cristino Cordeiro, V.S.; de Carvalho, L.C.R.M.; de Souza, M.A.V.; de Soares, R.A.; da Sousa, P.J.C.; Ognibene, D.T.; Resende, A.C.; et al. Antidiabetic effect of Euterpe oleracea Mart. (açai) extract and exercise training on high-fat diet and streptozotocin-induced diabetic rats: A positive interaction. PLoS ONE 2018, 13, e0199207. [Google Scholar] [CrossRef] [PubMed]
- De Moura, R.S.; Resende, Â.C. Cardiovascular and Metabolic Effects of Açai, an Amazon Plant. J. Cardiovasc. Pharmacol. 2016, 68, 19–26. [Google Scholar] [CrossRef] [PubMed]
- De Moura, R.S.; Ferreira, T.S.; Lopes, A.A.; Pires, K.M.P.; Nesi, R.T.; Resende, A.C.; Souza, P.J.C.; da Silva, A.J.R.; Borges, R.M.; Porto, L.C.; et al. Effects of Euterpe oleracea Mart. (AÇAI) extract in acute lung inflammation induced by cigarette smoke in the mouse. Phytomedicine 2012, 19, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Marques Cardoso, L.; Dias Novaes, R.; Aparecida de Castro, C.; Azevedo Novello, A.; Vilela Gonçalves, R.; Ricci-Silva, M.E.; de Oliveira Ramos, H.J.; Gouveia Peluzio, M.d.C.; Viana Leite, J.P. Chemical composition, characterization of anthocyanins and antioxidant potential of euterpe edulis fruits: applicability on genetic dyslipidemia and hepatic steatosis in mice. Nutr. Hosp. 2015, 32, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Cedrim, P.C.A.S.; Barros, E.M.A.; do Nascimento, T.G.; Cedrim, P.C.A.S.; Barros, E.M.A.; do Nascimento, T.G. Antioxidant properties of acai (Euterpe oleracea) in the metabolic syndrome. Braz. J. Food Technol. 2018, 21. [Google Scholar] [CrossRef]
- Fragoso, M.F.; Romualdo, G.R.; Ribeiro, D.A.; Barbisan, L.F. Açai (Euterpe oleracea Mart.) feeding attenuates dimethylhydrazine-induced rat colon carcinogenesis. Food Chem. Toxicol. 2013, 58, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Mei, J.; Yin, J.; Wang, H.; Wang, J.; Xie, M. Correlation analysis between expression of PCNA, Ki-67 and COX-2 and X-ray features in mammography in breast cancer. Oncol. Lett. 2017, 14, 2912–2918. [Google Scholar] [CrossRef] [PubMed]
- Guzińska-Ustymowicz, K.; Pryczynicz, A.; Kemona, A.; Czyzewska, J. Correlation between proliferation markers: PCNA, Ki-67, MCM-2 and antiapoptotic protein Bcl-2 in colorectal cancer. Anticancer Res. 2009, 29, 3049–3052. [Google Scholar] [PubMed]
- Graziano, V.; De Laurenzi, V. Role of p63 in cancer development. Biochim. Biophys. Acta Rev. Cancer 2011, 1816, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Alessandra-Perini, J.; Rodrigues-Baptista, K.C.; Machado, D.E.; Nasciutti, L.E.; Perini, J.A. Anticancer potential, molecular mechanisms and toxicity of Euterpe oleracea extract (açai): A systematic review. PLoS ONE 2018, 13, e0200101. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.; Antunes, L.M.G.; Aissa, A.F.; Darin, J.D.C.; De Rosso, V.V.; Mercadante, A.Z.; Bianchi, M.D.L.P. Evaluation of the genotoxic and antigenotoxic effects after acute and subacute treatments with açai pulp (Euterpe oleracea Mart.) on mice using the erythrocytes micronucleus test and the comet assay. Mutat. Res. Toxicol. Environ. Mutagen. 2010, 695, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.S.; Froder, J.G.; Carvalho, J.C.T.; Rosa, P.C.P.; Perazzo, F.F.; Maistro, E.L. Evaluation of the genotoxicity of Euterpe oleraceae Mart. (Arecaceae) fruit oil (açai), in mammalian cells in vivo. Food Chem. Toxicol. 2016, 93, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Schauss, A.G.; Clewell, A.; Balogh, L.; Szakonyi, I.P.; Financsek, I.; Horváth, J.; Thuroczy, J.; Béres, E.; Vértesi, A.; Hirka, G. Safety evaluation of an açai-fortified fruit and berry functional juice beverage (MonaVie Active®). Toxicology 2010, 278, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.; De Abreu, J.P.; Oliveira, H.S.S.; Goes-Neto, A.; Koblitz, M.G.B.; Teodoro, A.J. Antioxidant Activity and Cytotoxicity Effect of Cocoa Beans Subjected to Different Processing Conditions in Human Lung Carcinoma Cells. Oxid. Med. Cell. Longev. 2016, 2016, 7428515. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.A.; Schwartz, G.K. Development of cell-cycle inhibitors for cancer therapy. Curr. Oncol. 2009, 16, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo-Insfran, D.; Percival, S.S.; Talcott, S.T. Açai (Euterpe oleracea Mart.) polyphenolics in their glycoside and aglycone forms induce apoptosis of HL-60 leukemia cells. J. Agric. Food Chem. 2006, 54, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.M.D.S.; Noratto, G.; Martino, H.S.D.; Arbizu, S.; Peluzio, M.D.C.G.; Talcott, S.; Ramos, A.M.; Mertens-Talcott, S.U. Pro-Apoptotic Activities of Polyphenolics from Açai (Euterpe oleracea Martius) in Human SW-480 Colon Cancer Cells. Nutr. Cancer 2014, 66, 1394–1405. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Açai Seed Extract (ASE) |
|---|---|
| Total phenolic compound (acid gallic equivalent g/100 g of ASE) | 37.08 ± 8.56 |
| DPPH (% of reduction) | 92.05 ± 2.56 |
| ABTS (μM trolox equivalent antioxidant capacity/μg of ASE) | 566.01 ± 43.24 |
| FRAP (mmol Fe2 + Eq/g of ASE) | 8.98 ± 0.35 |
| ORAC (μM equivalent of Trolox) | 16679.17 ± 4879.81 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, R.M.; Guimarães, D.d.A.B.; Berniz, C.R.; Abreu, J.P.d.; Rocha, A.P.M.d.; Moura, R.S.d.; Resende, A.C.; Teodoro, A.J. Açai (Euterpe oleracea Mart.) Seed Extract Induces Cell Cycle Arrest and Apoptosis in Human Lung Carcinoma Cells. Foods 2018, 7, 178. https://doi.org/10.3390/foods7110178
Martinez RM, Guimarães DdAB, Berniz CR, Abreu JPd, Rocha APMd, Moura RSd, Resende AC, Teodoro AJ. Açai (Euterpe oleracea Mart.) Seed Extract Induces Cell Cycle Arrest and Apoptosis in Human Lung Carcinoma Cells. Foods. 2018; 7(11):178. https://doi.org/10.3390/foods7110178
Chicago/Turabian StyleMartinez, Raquel Martins, Deborah de Almeida Bauer Guimarães, Camila Ramos Berniz, Joel Pimentel de Abreu, Ana Paula Machado da Rocha, Roberto Soares de Moura, Angela Castro Resende, and Anderson Junger Teodoro. 2018. "Açai (Euterpe oleracea Mart.) Seed Extract Induces Cell Cycle Arrest and Apoptosis in Human Lung Carcinoma Cells" Foods 7, no. 11: 178. https://doi.org/10.3390/foods7110178
APA StyleMartinez, R. M., Guimarães, D. d. A. B., Berniz, C. R., Abreu, J. P. d., Rocha, A. P. M. d., Moura, R. S. d., Resende, A. C., & Teodoro, A. J. (2018). Açai (Euterpe oleracea Mart.) Seed Extract Induces Cell Cycle Arrest and Apoptosis in Human Lung Carcinoma Cells. Foods, 7(11), 178. https://doi.org/10.3390/foods7110178






