Legume Proteins in Food Products: Extraction Techniques, Functional Properties, and Current Challenges
Abstract
1. Introduction
2. Legume Protein Sources—Functionality, Bioactivity, and Digestibility
2.1. Functional Properties
2.1.1. Solubility
2.1.2. Water- and Oil-Holding Capacity
2.1.3. Emulsifying and Foaming Properties
2.1.4. Gelling Properties
2.1.5. Digestibility
2.1.6. Bioactivity
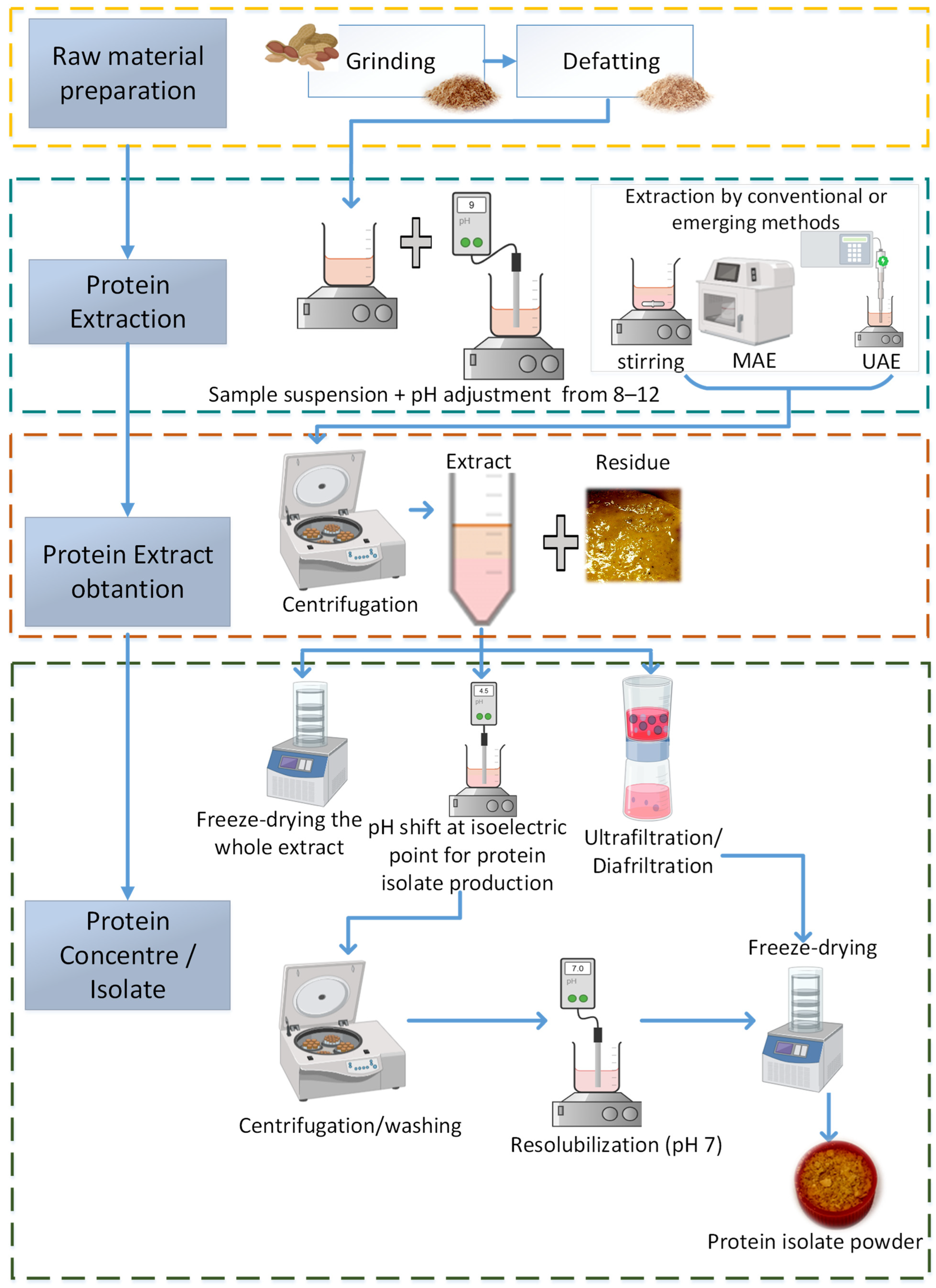
3. Methods to Obtain Legume Proteins
3.1. Conventional Extraction Methods
3.1.1. Water Extraction
3.1.2. Alkaline Extraction
3.1.3. Salt Extraction–Dialysis
3.1.4. Micellization Precipitation
| Sources | Extraction Methods | Protein Yield (%) | Protein Content (%) | Reference |
|---|---|---|---|---|
| Hyacinth bean | Isoelectric precipitation | 68.89 | 84.41 | [26] |
| Hyacinth bean | Micellization precipitation | 72.60 | 87.78 | [26] |
| Hyacinth bean | Salt extraction–dialysis | 66.17 | 71.09 | [26] |
| Acacia coriaceae | Alkaline extraction followed by isoelectric precipitation | 53.4 | 31.7 | [32] |
| Acacia victoriae | Alkaline extraction followed by isoelectric precipitation | 65.2 | 44.6 | [32] |
| Faba bean | Alkaline extraction | 16.41 | 89.88 | [25] |
| Carob | Alkaline extraction | 96 | 46 | [81] |
| Madras thorn | Alkaline extraction | 48.66 | 85.17 | [50] |
| Defatted soy grit | Enzyme-assisted extraction coupled with alkaline extraction | 45.93 | 46.16 | [50] |
| Peanut | Alkaline extraction followed by ultrasound | 50 | 90 | [44] |
| Faba bean | Alkaline extraction | 61.7 | 24.4 | [77] |
| Faba bean | Soaked or water | 43.4 | 21 | [77] |
| Ganxet bean | Alkaline extraction followed by isoelectric precipitation | 51.56 | 50.17 | [42] |
| Lima bean | Water–alkaline extraction | 10.67 | 88.01 | [35] |
| Pea | Micellization precipitation | 31.1 | 87.8 | [19] |
| Pea | Salt extraction–dialysis | 68.2 | 76.1 | [19] |
| Chickpea | Enzyme-assisted extraction | 21.42 | 92.89 | [82] |
3.2. Non-Conventional Extraction Methods
3.2.1. Enzymatic Extraction
3.2.2. Ultrasound-Assisted Extraction (UAE)
3.2.3. Microwave-Assisted Extraction (MAE)
3.2.4. Pulsed Electric Fields (PEF)
3.2.5. Ohmic Heating (OH)
3.2.6. Subcritical Water Extraction (SWE)
3.2.7. Deep Eutectic Solvents (DES)
3.2.8. Dry Fractionation
| Sources | Extraction Methods | Extraction Conditions | Protein Yield (%) | Protein Content (%) | Effect on Functional Properties | Comments | Ref. |
|---|---|---|---|---|---|---|---|
| Black beans | UAE (US bath) 37 kHz, 320 W | Solvent: Tris–HCl buffer pH = 9 t = 20 min S/F = 5:1 T = 25 °C | 9.7 | - | WHC: +297% EAI: +13% Tgel: −3.5 °C | IP: 3.5 No changes in primary structure | [41] |
| Lentils | UAE (US bath) 37 kHz, 320 W | Solvent: Tris–HCl buffer pH = 9 t = 20 min S/F = 10:1 T = 25 °C | 7.6 | - | WHC: +18% FC: +30% EAI: +10% ESI: +163% Tgel: −3.7 °C | IP: 5.0 No changes in primary structure | [41] |
| Faba beans | UAE (probe system) 123 W | Solvent: water pH = 11 t = 41 min S/F = 15/1 T = 20–25 °C | 19.7 (+20%) | 93 (+3%) | WHC: +18% OHC: +20% FC: −7% | IEP = pH 4.0 (1 N HCI) No changes in primary structure; secondary structure modified; and isolates were thermally stable | [25] |
| Faba beans | UAE (probe system) 20 kHz, 100% amplitude, 57.58 W/cm2 power intensity | Solvent: water t = 20 min S/F = 10/1 T = 20–25 °C | 10.9 (ns) | 84 (ns) | - | IEP = pH 4.5 (1 N HCI) SEM showed low adherence and few protein bodies due to greater protein leaching | [38] |
| Faba bean | UAE (probe system) 20 kHz, 100% amplitude, 57.58 W/cm2 power intensity | Solvent: water pH = 10 (0.1 M NaOH) t = 20 min S/F = 10/1 T = 20–25 °C | 12.6 (ns) | 80 (ns) | - | IEP = pH 4.5 (1 N HCI) | [38] |
| Cowpea pulse crop | UAE (probe system) 100 and 200 W | Solvent: water pH = 9 (1 N NaOH) t = 5–20 min S/F = 10/1 T = 25 °C | 59 (+85%), 200 W and 10 min | - | Solubility: +20% (200 w/10 min) FC: +18% (200 w/10 min) FS: +100% (200 w/10 min) WHC: +20% (200 w/10 min) OHC: +58% (200 w/15 min) EAI: +35% (200 w/10 min) ESI: +55% (200 w/10 min) | IEP = pH 4.5 (1 N HCI) Sonicated samples presented higher zeta potential and smaller particles | [45] |
| Raw pea powder | UAE (probe system) 750 W, 30% amplitude | Solvent: water pH = 9 (1 M NaOH) T = 10 min S/F = 9/1 T = 25 °C | 83% (+15%) | 87.5 (+7%) | FC: +19% FS: +23% WHC: +38% OHC: +7% EAI: +11% ESI: +7% LGC: −18% | IEP = pH 4.5 Intact primary structure; secondary structure changed; smaller pea protein particles | [37] |
| Peanut Flour | UAE (probe system) 24 kHz, 20 and 100% amplitude | Solvent: water pH = 9 (50% NaOH) t = 15–40 min S/F = 10/1 | 50–67% (+119%, 100% amplitude and 40 min) | ~90 (ns) | WAI: +700% WSI: −66% FC: +34% FS: −21% EA: −5% In vitro digestibility: +3% | IEP = pH 4.5 (15% HCl) | |
| Pea protein | UAE (US bath), 25 kHz UAE (probe system) 500 and 1000 W | Solvent: water pH = 10 (1 M NaOH) T = 60 min for US bath T = 30 min for US probe S/F = 20/1 | 12% for UAE bath (ns) 14% for US probe (ns) | 79% for UAE bath (ns) 73–75% for US probe (ns) | - | IEP = pH 4.5 No significant primary structure changes; US probe altered secondary structure; tertiary structure changes noted via reduced fluorescence | [89] |
| Lupin | UAE (probe system) 50 and 100 W | Solvent: water pH = 10 (1 M NaOH) t = 10 min S/F = 20/1 | 17.43% for 100 W (+25%) 16.41 for 50 W (+18%) | 82.5% for 100 W (−15%) 85.97% for 50 W (−11%) | No improvements in solubility | IEP = pH 4.5 (1 M HCl) Protein band intensity changed at ~98 kDa; particle size increased | [100] |
| Chickpea | UAE (probe system 325 W | Solvent: water pH = 9 (1 M NaOH) t = 10 min S/F = 10/1 | 11% (+20%) | Solubility: +8% EA: +96% FA: +18% | IEP = pH 4.0 (1 M HCl) Surface morphology looser and fragmented; tertiary structure altered; increased hydrophobicity | [114] | |
| Jack beans | MAE, 400 W, 600 W, and 800 W | Solvent: water pH 9.0, 10.0, and 11.0 (1.0 M NaOH) t = 5 min S/F = 10/1 | 45.7–70% (+up to +53%) Maximum yield at 400 W and pH 10 | 68–80% (up to +17%) | Solubility: −43% at 400 W and pH 10, and increased +3.9% at 400 W and pH 11 EAI = up to +108% (400 W pH 9) ESI = up to +300% (800 W, pH 9) FC = +33% (400 W, pH 9) FS = +8% (400 W, pH 10) WHC = 308% (400 W, pH 9) OHC = +30.6% (400 W, pH 9) | IEP = pH 4.6 (1.0 M HCl) Particle size increased in microwaved samples; FI increased at 400–600 W, then decreased at 800 W | [22] |
| Peanut Flour | MAE, 725 W and 8 min | Solvent: water pH 9.0 (50% M NaOH) t = 2–10 min S/F = 25/1 and 10/1 | 55% (+77%), | 93% | WAI: +900% WSI: −68% FC: +15% FS: −26% EAI: +5% In vitro digestibility: +2% | IEP = pH 4.5 (15% HCl) | [44] |
| Lupin | MAE, 1000 W | Solvent: water pH = 10 (1 M NaOH) t = 10 min S/F = 20/1 | 18.2% (+30%) | 83.57% (−14%) | No improvements in solubility | IEP = pH 4.5 (1 M HCl) Protein bands intensified, likely due to submicron aggregation; particle size increased | [100] |
| Lupin | MAE (500 and 1000 W) + UAE (probe system, 100 and 200 W) | Solvent: water pH = 10 (1 M NaOH) t = 10 min S/F = 20/1 | 22.3% (+60%, 200 W UAE + 1000 W MAE) | 76.4% (−21%) (−21%, 200 W US + 1000 W MW) | Solubility was significantly increased by almost +7% (UAE at 100 W and MW at 500 W MW) | IEP = pH 4.5 (1 M HCl) Particle size increased | [100] |
| Chickpea | PEF (87 s and 0.9 kV/cm) | Solvent: water pH = 9, (1 M NaOH) S/F = 10/1 | 11% (+20%) | - | Solubility = +9% EA = +61% | IEP = pH 4.0 (1 M HCl) Changes in tertiary structure; hydrophobicity increased | [114] |
| Chickpea | PEF (87 s and 0.9 kV/cm) + US (15 min and 325 W) | Solvent: water pH = 9 (1 M NaOH) S/F = 10/1 | 13.52% (+47%) | - | Solubility = +11% EA = +110% FA = 12% | IEP = pH 4.0 (1 M HCl) Changes in tertiary structure; hydrophobicity increased | [114] |
| Lentils | OH, 5 V/cm and 75 V/cm and 80 °C, 20 min | Solvent: water pH = 3 (1 MHCl) S/F = 1/50 | - | - | - | Increased surface hydrophobicity Change in structure depends on the pH and electric field strength | [122] |
| Pea protein | OH, 13 V/cm and 50 V/cm | Solvent: water pH = 7 S/F = 1/50 | |||||
| Soybean meal raw and deoiled | OH, 210 °C, 30 min, (raw); 200 °C (deoiled) | Solvent: water S/F = 1:5 | 44.4% (raw), 33.3% (deoiled) | - | - | - | [151] |
| Soybean flakes | SWE, 66–23 4 °C, 13–47 min | Solvent: water S/F = 1:3.3–1:11.7 | Up to 59.2% at 66 °C, 20–40% at >100 °C | - | - | - | [152] |
| Soybean | Enzyme assisted SWE, 120 °C, 20 min | Solvent: water S/F = 1/10 | 59.3% | >80% | Increased solubility Change in hydrophobicity of the protein Increased emulsifying activity High interfacial activity | - | [153] |
| Defatted soy meal | SWE, 100–250 °C, 5 min | Solvent: water S/F = 1/40 | 52% | - | - | The emulsification and foaming capacity of the extract was highly dependent on the extraction temperature | [154] |
| Faba bean | DES | Solvent: (Choline chloride and glycerol) S/F = 1/10–1/30, 50–90 °C, 1–3 min | 92.33% | 65.42% | Increased secondary structure component, α-helix. A high β-sheet (38.61) was observed | - | [139] |
| Tree bean | NADES | Solvent: (Choline chloride-sorbitol) S/F = 1/20, 80 °C, 20 min | - | - | EA = 50.42% ES = 42.55% | - | [140] |
| Lentils | Dry fractionation | Air flow was set to 52 m3/h, feed rate 1.5–3.5 kg/h, internal pressure of the classifier 30–39 mbar | - | 23.05–54% |
|
| [155] |
| Yellow pea | Dry fractionation | Classifier wheel speed 4000 rpm, airflow 52 m3/h, feed rate 0.75 kg/h | 63% | 67% | - | The gel firmness of the protein-rich fractions was affected by their starch content. | [156] |
| Pea | Dry fractionation | Classifier wheel speed 3166 rpm, air flow 1325 m3/h, feed rate 300 kg/h | 35–43.5% | 85–87% | - | - | [157] |
4. Applications of Legume Protein for Food Development
5. Technological Opportunities and Challenges in Legume Protein Extraction
5.1. Technical, Scalability, and Feasibility Aspects of Emerging Protein Extraction Technologies
5.2. Economic Viability: Market Size and Challenge of Plant-Protein Costs
5.3. Environmental Aspects
5.4. Allergenic Potential
5.5. Sensory Aspects
5.6. Synergistic Potential of Legume Proteins with Other Protein Sources
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duranti, M. Grain Legume Proteins and Nutraceutical Properties. Fitoterapia 2006, 77, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Ramsing, R.; Rahman, N.; Kraemer, K.; Bloem, M.W. Legumes as a Sustainable Source of Protein in Human Diets. Glob. Food Sec. 2021, 28, 100520. [Google Scholar] [CrossRef]
- Sharif, H.R.; Williams, P.A.; Sharif, M.K.; Abbas, S.; Majeed, H.; Masamba, K.G.; Safdar, W.; Zhong, F. Current Progress in the Utilization of Native and Modified Legume Proteins as Emulsifiers and Encapsulants—A Review. Food Hydrocoll. 2018, 76, 2–16. [Google Scholar] [CrossRef]
- Shrestha, S.; van’t Hag, L.; Haritos, V.S.; Dhital, S. Lupin Proteins: Structure, Isolation and Application. Trends Food Sci. Technol. 2021, 116, 928–939. [Google Scholar] [CrossRef]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-Innovative Technologies for Extraction of Proteins for Human Consumption from Renewable Protein Sources of Plant Origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Eze, C.R.; Kwofie, E.M.; Adewale, P.; Lam, E.; Ngadi, M. Advances in Legume Protein Extraction Technologies: A Review. Innov. Food Sci. Emerg. Technol. 2022, 82, 103199. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Getachew, A.T.; Ghelichi, S.; Jacobsen, C. The Use of Green Technologies for Processing Lupin Seeds (Lupinus angustifolius L.): Extraction of Non-Polar and Polar Compounds for Concentrated-Protein Flour Production. Food Res. Int. 2025, 200, 115434. [Google Scholar] [CrossRef]
- Byanju, B.; Lamsal, B. Protein-Rich Pulse Ingredients: Preparation, Modification Technologies and Impact on Important Techno-Functional and Quality Characteristics, and Major Food Applications. Food Rev. Int. 2023, 39, 3314–3343. [Google Scholar] [CrossRef]
- Das, G.; Sharma, A.; Sarkar, P.K. Conventional and Emerging Processing Techniques for the Post-Harvest Reduction of Antinutrients in Edible Legumes. Appl. Food Res. 2022, 2, 100112. [Google Scholar] [CrossRef]
- Lazdiņa, D.; Segliņa, D.; Zvaigzne, Z.A.; Butlers, A.; Ciproviča, I. Effect of Defatting Method on Japanese Quince (Chaenomeles japonica) Fruit Seed Protein Isolate Technological Properties. Foods 2025, 14, 234. [Google Scholar] [CrossRef]
- Nissen, S.H.; Lübeck, M.; Møller, A.H.; Dalsgaard, T.K. Protein Recovery and Quality of Alfalfa Extracts Obtained by Acid Precipitation and Fermentation. Bioresour. Technol. Rep. 2022, 19, 101190. [Google Scholar] [CrossRef]
- Pang, J.; Sha, X.; Chao, Y.; Chen, G.; Han, C.; Zhu, W.; Li, H.; Zhang, Q. Green Aqueous Biphasic Systems Containing Deep Eutectic Solvents and Sodium Salts for the Extraction of Protein. RSC Adv. 2017, 7, 49361–49367. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the Plant Protein Extraction: Mechanism and Recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B. The Functional Modification of Legume Proteins by Ultrasonication: A Review. Trends Food Sci. Technol. 2020, 98, 107–116. [Google Scholar] [CrossRef]
- Detzel, A.; Krüger, M.; Busch, M.; Blanco-Gutiérrez, I.; Varela, C.; Manners, R.; Bez, J.; Zannini, E. Life Cycle Assessment of Animal-Based Foods and Plant-Based Protein-Rich Alternatives: An Environmental Perspective. J. Sci. Food Agric. 2022, 102, 5098–5110. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Zhao, Y.; Jiang, L.; Sui, X. Structural, Extraction and Safety Aspects of Novel Alternative Proteins from Different Sources. Food Chem. 2024, 436, 137712. [Google Scholar] [CrossRef]
- Holzhauser, T.; Wackermann, O.; Ballmer-Weber, B.K.; Bindslev-Jensen, C.; Scibilia, J.; Perono-Garoffo, L.; Utsumi, S.; Poulsen, L.K.; Vieths, S. Soybean (Glycine max) Allergy in Europe: Gly m 5 (β-Conglycinin) and Gly m 6 (Glycinin) Are Potential Diagnostic Markers for Severe Allergic Reactions to Soy. J. Allergy Clin. Immunol. 2009, 123, 452–458.e4. [Google Scholar] [CrossRef]
- Ito, K.; Sjölander, S.; Sato, S.; Movérare, R.; Tanaka, A.; Söderström, L.; Borres, M.; Poorafshar, M.; Ebisawa, M. IgE to Gly m 5 and Gly m 6 Is Associated with Severe Allergic Reactions to Soybean in Japanese Children. J. Allergy Clin. Immunol. 2011, 128, 673–675. [Google Scholar] [CrossRef]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional Attributes of Pea Protein Isolates Prepared Using Different Extraction Methods and Cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Lemus-Conejo, A.; Rivero-Pino, F.; Montserrat-de la Paz, S.; Millan-Linares, M.C. Nutritional Composition and Biological Activity of Narrow-Leafed Lupins (Lupinus angustifolius L.) Hydrolysates and Seeds. Food Chem. 2023, 420, 136104. [Google Scholar] [CrossRef]
- Locali-Pereira, A.R.; Boire, A.; Berton-Carabin, C.; Taboga, S.R.; Solé-Jamault, V.; Nicoletti, V.R. Pigeon Pea, An Emerging Source of Plant-Based Proteins. ACS Food Sci. Technol. 2023, 3, 1777–1799. [Google Scholar] [CrossRef]
- Ajayi, F.F.; Mudgil, P.; Maqsood, S. Unveiling Differential Impact of Heat and Microwave Extraction Treatments on the Structure, Functionality, and Digestibility of Jack Bean Proteins Extracted under Varying Extraction PH. Food Res. Int. 2024, 191, 114686. [Google Scholar] [CrossRef]
- Adiamo, O.Q.; Netzel, M.E.; Hoffman, L.C.; Gidley, M.J.; Osborne, S.; Sultanbawa, Y. Nutritional and Techno-Functional Properties of Australian Acacia Seed Flour: Effects of Roasting on Chemical Composition, Physicochemical Properties, and in Vitro Digestibility and Intestinal Iron Absorption. Food Res. Int. 2023, 164, 112336. [Google Scholar] [CrossRef]
- Bengoechea, C.; Ortiz, S.E.M.; Guerrero, A.; Puppo, M.C. Effect of PH on the Thermal Gelation of Carob Protein Isolate. J. Food Sci. Technol. 2017, 54, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Badjona, A.; Bradshaw, R.; Millman, C.; Howarth, M.; Dubey, B. Optimization of Ultrasound-Assisted Extraction of Faba Bean Protein Isolate: Structural, Functional, and Thermal Properties. Part 2/2. Ultrason. Sonochem. 2024, 110, 107030. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Mellem, J.J. Functional Properties of the Protein Isolates of Hyacinth Bean [Lablab purpureus (L.) Sweet]: An Effect of the Used Procedures. LWT 2020, 129, 109572. [Google Scholar] [CrossRef]
- Keskin, S.O.; Ali, T.M.; Ahmed, J.; Shaikh, M.; Siddiq, M.; Uebersax, M.A. Physico-Chemical and Functional Properties of Legume Protein, Starch, and Dietary Fiber—A Review. Legum. Sci. 2022, 4, e117. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Structural Aspects of Legume Proteins and Nutraceutical Properties. Food Res. Int. 2015, 76, 19–30. [Google Scholar] [CrossRef]
- Huamaní-Perales, C.; Vidaurre-Ruiz, J.; Salas-Valerio, W.; Cabezas, D.M.; Repo-Carrasco-Valencia, R. A Review of Techno-Functional Properties of Legume Proteins and Their Potential for Development of New Products. Eur. Food Res. Technol. 2024, 250, 2069–2092. [Google Scholar] [CrossRef]
- Klupšaitė, D.; Juodeikienė, G. Legume: Composition, Protein Extraction and Functional Properties. A Review. Chem. Technol. 2015, 66, 5–12. [Google Scholar] [CrossRef]
- Ogunniyi, Q.A.; Ogbole, O.O.; Akin-Ajani, O.D.; Ajala, T.O.; Bamidele, O.; Fettke, J.; Odeku, O.A. Medicinal Importance and Phytoconstituents of Underutilized Legumes from the Caesalpinioideae DC Subfamily. Appl. Sci. 2023, 13, 8972. [Google Scholar] [CrossRef]
- Adiamo, O.Q.; Netzel, M.E.; Hoffman, L.C.; Gidley, M.J.; Osborne, S.; Sultanbawa, Y. Structure-Function Relationship of Australian Acacia Seed Protein Concentrates: Amino Acid Composition, in Vitro Protein Digestibility and Molecular Properties. Food Biosci. 2023, 51, 102339. [Google Scholar] [CrossRef]
- Kurek, M.A.; Onopiuk, A.; Pogorzelska-Nowicka, E.; Szpicer, A.; Zalewska, M.; Półtorak, A. Novel Protein Sources for Applications in Meat-Alternative Products—Insight and Challenges. Foods 2022, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Masters, E.T.; Kelly, B.A. Protein Quality of African Locust Bean—A High-Value Gathered Tree Food Contributing Protein and Palatability to Plant-Based Diets. Int. J. Food Sci. 2024, 2024, 1596212. [Google Scholar] [CrossRef]
- Liu, R.; Yan, X.; Liu, R.; Wu, Q.; Gao, Y.; Muhindo, E.M.; Zhi, Z.; Wu, T.; Sui, W.; Zhang, M. Lima Bean (Linn.) Protein Isolate as a Promising Plant Protein Mixed with Xanthan Gum for Stabilizing Oil-in-Water Emulsions. J. Sci. Food Agric. 2024, 104, 818–828. [Google Scholar] [CrossRef]
- Qin, P.; Wang, T.; Luo, Y. A Review on Plant-Based Proteins from Soybean: Health Benefits and Soy Product Development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Xu, L.; Ma, H. An Efficient Ultrasound-Assisted Extraction Method of Pea Protein and Its Effect on Protein Functional Properties and Biological Activities. LWT 2020, 127, 109348. [Google Scholar] [CrossRef]
- Suchintita Das, R.; Zhu, X.; Hannon, S.; Mullins, E.; Alves, S.; Garcia-Vaquero, M.; Tiwari, B.K. Exploring Osborne Fractionation and Laboratory/Pilot Scale Technologies (Conventional Extraction, Ultrasound-Assisted Extraction, High-Pressure Processing and Hydrodynamic Cavitation) for Protein Extraction from Faba Bean (Vicia faba L.). Innov. Food Sci. Emerg. Technol. 2023, 89, 103487. [Google Scholar] [CrossRef]
- Jeong, M.S.; Cho, S.J. Effect of PH-Shifting on the Water Holding Capacity and Gelation Properties of Mung Bean Protein Isolate. Food Res. Int. 2024, 177, 113912. [Google Scholar] [CrossRef]
- Affrifah, N.S.; Uebersax, M.A.; Amin, S. Nutritional Significance, Value-Added Applications, and Consumer Perceptions of Food Legumes: A Review. Legum. Sci. 2023, 5, e192. [Google Scholar] [CrossRef]
- Quintero-Quiroz, J.; Celis-Torres, A.; Ciro-Gómez, G.; Torres, J.; Corrales-García, L.; Rojas, J. Physicochemical Properties and Functional Characteristics of Ultrasound-Assisted Legume-Protein Isolates: A Comparative Study. J. Food Sci. Technol. 2022, 59, 1665–1676. [Google Scholar] [CrossRef]
- Lafarga, T.; Álvarez, C.; Bobo, G.; Aguiló-Aguayo, I. Characterization of Functional Properties of Proteins from Ganxet Beans (Phaseolus vulgaris L. Var. Ganxet) Isolated Using an Ultrasound-Assisted Methodology. LWT 2018, 98, 106–112. [Google Scholar] [CrossRef]
- Byanju, B.; Rahman, M.M.; Hojilla-Evangelista, M.P.; Lamsal, B.P. Effect of High-Power Sonication Pretreatment on Extraction and Some Physicochemical Properties of Proteins from Chickpea, Kidney Bean, and Soybean. Int. J. Biol. Macromol. 2020, 145, 712–721. [Google Scholar] [CrossRef]
- Ochoa-Rivas, A.; Nava-Valdez, Y.; Serna-Saldívar, S.O.; Chuck-Hernández, C. Microwave and Ultrasound to Enhance Protein Extraction from Peanut Flour under Alkaline Conditions: Effects in Yield and Functional Properties of Protein Isolates. Food Bioprocess Tech. 2017, 10, 543–555. [Google Scholar] [CrossRef]
- Loushigam, G.; Shanmugam, A. Modifications to Functional and Biological Properties of Proteins of Cowpea Pulse Crop by Ultrasound-Assisted Extraction. Ultrason. Sonochem. 2023, 97, 106448. [Google Scholar] [CrossRef]
- Bou, R.; Navarro-Vozmediano, P.; Domínguez, R.; López-Gómez, M.; Pinent, M.; Ribas-Agustí, A.; Benedito, J.J.; Lorenzo, J.M.; Terra, X.; García-Pérez, J.V.; et al. Application of Emerging Technologies to Obtain Legume Protein Isolates with Improved Techno-Functional Properties and Health Effects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2200–2232. [Google Scholar] [CrossRef]
- Embaby, H.E.; Swailam, H.M.; Rayan, A.M. Preparation and Physicochemical Properties of Protein Concentrate and Isolate Produced from Acacia tortilis (Forssk.) Hayne Ssp. Raddiana. J. Food Sci. Technol. 2018, 55, 489–495. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, Y.; McClements, D.J.; Liu, X.; Wang, P.; Liu, F. Improving Pea Protein Functionality by Combining High-Pressure Homogenization with an Ultrasound-Assisted Maillard Reaction. Food Hydrocoll. 2022, 126, 107441. [Google Scholar] [CrossRef]
- Seidu, K.T.; Osundahunsi, O.F.; Olaleye, M.T.; Oluwalana, I.B. Amino Acid Composition, Mineral Contents and Protein Solubility of Some Lima Bean (Phaseolus lunatus L. Walp) Seeds Coat. Food Res. Int. 2015, 73, 130–134. [Google Scholar] [CrossRef]
- Singh, A.; Sit, N.D. Modification of Manila Tamarind Protein Isolate by Ultrasonication and Autoclaving and Their Characterization. Food Bioprocess Technol. 2023, 16, 2947–2960. [Google Scholar] [CrossRef]
- Tang, H.; Li, X.; Chen, J.; Liu, B.; Tang, R.; Chen, Y.; Li, H.; Zou, L.; Shi, Q. Effects of Dextran on the Gel Properties of Faba Bean Protein Isolates Prepared Using Different Processes. Gels 2023, 9, 972. [Google Scholar] [CrossRef]
- Biswas, B.; Sit, N. Effect of Ultrasonication on Functional Properties of Tamarind Seed Protein Isolates. J. Food Sci. Technol. 2020, 57, 2070–2078. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9780429123894. [Google Scholar]
- Jacobsen, C. Some Strategies for the Stabilization of Long Chain N-3 PUFA-Enriched Foods: A Review. Eur. J. Lipid Sci. Technol. 2015, 117, 1853–1866. [Google Scholar] [CrossRef]
- Zayas, J.F. Gelling Properties of Proteins. In Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997; pp. 310–366. ISBN 978-3-642-59116-7. [Google Scholar]
- Khalesi, M.; Glenn-Davi, K.; Mohammadi, N.; FitzGerald, R.J. Key Factors Influencing Gelation in Plant vs. Animal Proteins: A Comparative Mini-Review. Gels 2024, 10, 575. [Google Scholar] [CrossRef]
- Al-Ali, H.A.; Shah, U.; Hackett, M.J.; Gulzar, M.; Karakyriakos, E.; Johnson, S.K. Technological Strategies to Improve Gelation Properties of Legume Proteins with the Focus on Lupin. Innov. Food Sci. Emerg. Technol. 2021, 68, 102634. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, F. Plant Protein Heat-Induced Gels: Formation Mechanisms and Regulatory Strategies. Coatings 2023, 13, 1899. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Liu, X.; Qiao, M.; Yi, S.; Li, X.; Li, J. Effects of Chickpea and Peanut Protein Isolates on the Gelling Properties of Hairtail (Trichiurus haumela) Myosin. LWT 2022, 163, 113562. [Google Scholar] [CrossRef]
- Moreno, H.M.; Díaz, M.T.; Borderías, A.J.; Domínguez-Timón, F.; Varela, A.; Tovar, C.A.; Pedrosa, M.M. Effect of Different Technological Factors on the Gelation of a Low-Lectin Bean Protein Isolate. Plant Foods Hum. Nutr. 2022, 77, 141–149. [Google Scholar] [CrossRef]
- Ehsani, M.; Westphalen, H.; Doan, H.; Lohi, A.; Abdelrasoul, A. Advancing Faba Bean Protein Purification Using Membrane Technology: Current State and Future Perspectives. J. Compos. Sci. 2024, 8, 15. [Google Scholar] [CrossRef]
- Ohanenye, I.C.; Ekezie, F.-G.C.; Sarteshnizi, R.A.; Boachie, R.T.; Emenike, C.U.; Sun, X.; Nwachukwu, I.D.; Udenigwe, C.C. Legume Seed Protein Digestibility as Influenced by Traditional and Emerging Physical Processing Technologies. Foods 2022, 11, 2299. [Google Scholar] [CrossRef]
- Yakubu, C.M.; Sharma, R.; Sharma, S.; Singh, B. Influence of Alkaline Fermentation Time on in Vitro Nutrient Digestibility, Bio- & Techno-Functionality, Secondary Protein Structure and Macromolecular Morphology of Locust Bean (Parkia biglobosa) Flour. LWT 2022, 161, 113295. [Google Scholar] [CrossRef]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of Whey Hydrolysate, Casein, or Soy Protein Isolate: Effects on Mixed Muscle Protein Synthesis at Rest and Following Resistance Exercise in Young Men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- van Vliet, S.; Burd, N.A.; van Loon, L.J.C. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption1. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- George, K.S.; Muñoz, J.; Akhavan, N.S.; Foley, E.M.; Siebert, S.C.; Tenenbaum, G.; Khalil, D.A.; Chai, S.C.; Arjmandi, B.H. Is Soy Protein Effective in Reducing Cholesterol and Improving Bone Health? Food Funct. 2020, 11, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Perović, M.N.; Pajin, B.S.; Antov, M.G. The Effect of Enzymatic Pretreatment of Chickpea on Functional Properties and Antioxidant Activity of Alkaline Protein Isolate. Food Chem. 2022, 374, 131809. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kadyan, S.; Ukhanov, V.; Cheng, J.; Nagpal, R.; Cui, L. Recent Advances in the Health Benefits of Pea Protein (Pisum sativum): Bioactive Peptides and the Interaction with the Gut Microbiome. Curr. Opin. Food Sci. 2022, 48, 100944. [Google Scholar] [CrossRef]
- Asen, N.D.; Aluko, R.E. Acetylcholinesterase and Butyrylcholinesterase Inhibitory Activities of Antioxidant Peptides Obtained from Enzymatic Pea Protein Hydrolysates and Their Ultrafiltration Peptide Fractions. J. Food Biochem. 2022, 46, e14289. [Google Scholar] [CrossRef]
- Mamoudou, H.; Mune Mune, M.A. Investigating Bambara Bean (Vigna subterranea (Verdc.) L.) Protein and Hydrolysates: A Comprehensive Analysis of Biological and Biochemical Properties. Appl. Food Res. 2024, 4, 100489. [Google Scholar] [CrossRef]
- Liu, L.H.; Hung, T.V.; Bennett, L. Extraction and Characterization of Chickpea (Cicer arietinum) Albumin and Globulin. J. Food Sci. 2008, 73, C299–C305. [Google Scholar] [CrossRef]
- Fonseca-García, C.; Solis-Miranda, J.; Pacheco, R.; Quinto, C. Non-Specific Lipid Transfer Proteins in Legumes and Their Participation During Root-Nodule Symbiosis. Front. Agron. 2021, 3, 660100. [Google Scholar] [CrossRef]
- Ozias-Akins, P.; Breiteneder, H. The Functional Biology of Peanut Allergens and Possible Links to Their Allergenicity. Allergy 2019, 74, 888. [Google Scholar] [CrossRef]
- Plankensteiner, L.; Yang, J.; Bitter, J.H.; Vincken, J.P.; Hennebelle, M.; Nikiforidis, C.V. High Yield Extraction of Oleosins, the Proteins That Plants Developed to Stabilize Oil Droplets. Food Hydrocoll. 2023, 137, 108419. [Google Scholar] [CrossRef]
- Swanson, B.G. Pea and Lentil Protein Extraction and Functionality. J. Am. Oil Chem. Soc. 1990, 67, 276–280. [Google Scholar] [CrossRef]
- Mikkelsen, R.K.; Queiroz, L.; Echers, S.G.; Hobley, T.; Overgaard, M.; Jacobsen, C.; Svensson, B. Extracting Proteins from Brewers’ Spent Grain Using Emerging Technologies: Evaluating Efficiency and Use as Emulsifier. Authorea 2024, Preprints. [Google Scholar] [CrossRef]
- Langton, M.; Ehsanzamir, S.; Karkehabadi, S.; Feng, X.; Johansson, M.; Johansson, D.P. Gelation of Faba Bean Proteins—Effect of Extraction Method, PH and NaCl. Food Hydrocoll. 2020, 103, 105622. [Google Scholar] [CrossRef]
- Peyrano, F.; Speroni, F.; Avanza, M.V. Physicochemical and Functional Properties of Cowpea Protein Isolates Treated with Temperature or High Hydrostatic Pressure. Innov. Food Sci. Emerg. Technol. 2016, 33, 38–46. [Google Scholar] [CrossRef]
- Peyrano, F.; de Lamballerie, M.; Avanza, M.V.; Speroni, F. Gelation of Cowpea Proteins Induced by High Hydrostatic Pressure. Food Hydrocoll. 2021, 111, 106191. [Google Scholar] [CrossRef]
- Wintersohle, C.; Kracke, I.; Ignatzy, L.M.; Etzbach, L.; Schweiggert-Weisz, U. Physicochemical and Chemical Properties of Mung Bean Protein Isolate Affected by the Isolation Procedure. Curr. Res. Food Sci. 2023, 7, 100582. [Google Scholar] [CrossRef]
- Zárate-Ramírez, L.S.; Bengoechea, C.; Cordobés, F.; Guerrero, A. Linear Viscoelasticity of Carob Protein Isolate/Locust Bean Gum Blends. J. Food Eng. 2010, 100, 435–445. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of Ultrasound on the Structure and Physical Properties of Black Bean Protein Isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, Q.; Xiao, L.; Huang, Y.; Yang, Z.; Wu, Z.; Tu, Z.; Qin, W.; Chen, H.; Wu, D.; et al. Effects of Ultrasound on Functional Properties, Structure and Glycation Properties of Proteins: A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Perović, M.N.; Knežević Jugović, Z.D.; Antov, M.G. Improved Recovery of Protein from Soy Grit by Enzyme-Assisted Alkaline Extraction. J. Food Eng. 2020, 276, 109894. [Google Scholar] [CrossRef]
- Sridhar, K.; Bouhallab, S.; Croguennec, T.; Renard, D.; Lechevalier, V. Application of High-Pressure and Ultrasound Technologies for Legume Proteins as Wall Material in Microencapsulation: New Insights and Advances. Trends Food Sci. Technol. 2022, 127, 49–62. [Google Scholar] [CrossRef]
- Prado, J.M.; Veggi, P.C.; Náthia-Neves, G.; Meireles, M.A.A. Extraction Methods for Obtaining Natural Blue Colorants. Curr. Anal. Chem. 2020, 16, 504–532. [Google Scholar] [CrossRef]
- Chandran, A.S.; Suri, S.; Choudhary, P. Sustainable Plant Protein: An up-to-Date Overview of Sources, Extraction Techniques and Utilization. Sustain. Food Technol. 2023, 1, 466–483. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; Álvarez, C.; O’Donnell, C.P. Ultrasound Applications for the Extraction, Identification and Delivery of Food Proteins and Bioactive Peptides. Trends Food Sci. Technol. 2015, 46, 60–67. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, X.; Dong, G.; Hannon, S.; Santos, H.M.; Sun, D.W.; Tiwari, B.K. Comparative Studies on Enhancing Pea Protein Extraction Recovery Rates and Structural Integrity Using Ultrasonic and Hydrodynamic Cavitation Technologies. LWT 2024, 200, 116130. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Guo, Y.; Liu, C.; Liu, J.; Tan, B.; Guo, Z.; Wang, Z.; Jiang, L. Effects of Ultrasound on the Structural and Emulsifying Properties and Interfacial Properties of Oxidized Soybean Protein Aggregates. Ultrason. Sonochem. 2022, 87, 106046. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, W.; Yi, J.; Liu, N.; Cao, Y.; Lu, J.; Decker, E.A.; McClements, D.J. Effects of Sonication on the Physicochemical and Functional Properties of Walnut Protein Isolate. Food Res. Int. 2018, 106, 853–861. [Google Scholar] [CrossRef]
- Hu, H.; Fan, X.; Zhou, Z.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S.; Zhu, L. Acid-Induced Gelation Behavior of Soybean Protein Isolate with High Intensity Ultrasonic Pre-Treatments. Ultrason. Sonochem. 2013, 20, 187–195. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lamsal, B.P. Ultrasound-Assisted Extraction and Modification of Plant-Based Proteins: Impact on Physicochemical, Functional, and Nutritional Properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1457–1480. [Google Scholar] [CrossRef]
- Hernández-Álvarez, A.J.; Mondor, M.; Nosworthy, M.G. Green Protein Processing Technologies from Plants: Novel Extraction and Purification Methods for Product Development; Springer International Publishing: Cham, Switzerland, 2023; ISBN 9783031169687. [Google Scholar]
- Náthia-Neves, G.; Alonso, E. Valorization of Sunflower By-Product Using Microwave-Assisted Extraction to Obtain a Rich Protein Flour: Recovery of Chlorogenic Acid, Phenolic Content and Antioxidant Capacity. Food Bioprod. Process. 2021, 125, 57–67. [Google Scholar] [CrossRef]
- Kala, H.K.; Mehta, R.; Sen, K.K.; Tandey, R.; Mandal, V. Critical Analysis of Research Trends and Issues in Microwave Assisted Extraction of Phenolics: Have We Really Done Enough. TrAC Trends Anal. Chem. 2016, 85, 140–152. [Google Scholar] [CrossRef]
- Nonglait, D.L.; Gokhale, J.S. Review Insights on the Demand for Natural Pigments and Their Recovery by Emerging Microwave-Assisted Extraction (MAE). Food Bioproc. Tech. 2024, 17, 1681–1705. [Google Scholar] [CrossRef]
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, Antiradical, and Antimicrobial Activities of Polysaccharides Obtained by Microwave-Assisted Extraction Method: A Review. Carbohydr. Polym. 2020, 229, 115421. [Google Scholar] [CrossRef] [PubMed]
- Kargar, Z.; Hematian Sourki, A. Physicochemical, Techno-Functional, and Thermal Properties of Kabuli Chickpea’s Aquafaba Extracted by Optimized Microwave-Assistive Process. Appl. Food Res. 2024, 4, 100589. [Google Scholar] [CrossRef]
- Tang, J.; Cases, L.; Alves, S.; Sun, D.-W.; Tiwari, B.K. Protein Extraction from Lupin (Lupinus angustifolius L.) Using Combined Ultrasound and Microwave Techniques: Impact on Protein Recovery, Structure, and Functional Properties. Ultrason. Sonochem. 2025, 115, 107232. [Google Scholar] [CrossRef]
- Han, Z.; Cai, M.-J.; Cheng, J.H.; Sun, D.W. Effects of Electric Fields and Electromagnetic Wave on Food Protein Structure and Functionality: A Review. Trends Food Sci. Technol. 2018, 75, 1–9. [Google Scholar] [CrossRef]
- Qin, X.S.; Luo, S.Z.; Cai, J.; Zhong, X.Y.; Jiang, S.T.; Zheng, Z.; Zhao, Y.Y. Effects of Microwave Pretreatment and Transglutaminase Crosslinking on the Gelation Properties of Soybean Protein Isolate and Wheat Gluten Mixtures. J. Sci. Food Agric. 2016, 96, 3559–3566. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, T.; Zhang, L.; Xu, H.; Xu, J.; Wang, J. Impact of Microwave Time on the Structure and Functional Properties of Glycosylated Soy 7S Globulins. Foods 2025, 14, 151. [Google Scholar] [CrossRef]
- Li, H.; Zhu, K.; Zhou, H.; Peng, W.; Guo, X. Comparative Study of Four Physical Approaches about Allergenicity of Soybean Protein Isolate for Infant Formula. Food Agric. Immunol. 2016, 27, 604–623. [Google Scholar] [CrossRef]
- Phongthai, S.; Lim, S.T.; Rawdkuen, S. Optimization of Microwave-Assisted Extraction of Rice Bran Protein and Its Hydrolysates Properties. J. Cereal Sci. 2016, 70, 146–154. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, L.; Ju, H.; Bao, Z.; Zeng, X.-A.; Lin, S. Research Advances and Application of Pulsed Electric Field on Proteins and Peptides in Food. Food Res. Int. 2021, 139, 109914. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Avelar, Z.; Machado, L.; Pereira, R.N.; Vicente, A.A. Electric Field Effects on Proteins—Novel Perspectives on Food and Potential Health Implications. Food Res. Int. 2020, 137, 109709. [Google Scholar] [CrossRef] [PubMed]
- Giteru, S.G.; Oey, I.; Ali, M.A. Feasibility of Using Pulsed Electric Fields to Modify Biomacromolecules: A Review. Trends Food Sci. Technol. 2018, 72, 91–113. [Google Scholar] [CrossRef]
- Castagnini, M.; Carbó, J.M.; Ferrer, E.; Berrada, E.; Barba, H.; Calleja-Gómez, M.; Castagnini, J.M.; Carbó, E.; Ferrer, E.; Berrada, H.; et al. Evaluation of Pulsed Electric Field-Assisted Extraction on the Microstructure and Recovery of Nutrients and Bioactive Compounds from Mushroom Evaluation of Pulsed Electric Field-Assisted Extraction on the Microstructure and Recovery of Nutrients and Bioactive Compounds from Mushroom (Agaricus bisporus). Separations 2022, 9, 302. [Google Scholar] [CrossRef]
- Iqbal, A.; Khalil, I.A.; Ateeq, N.; Sayyar Khan, M. Nutritional Quality of Important Food Legumes. Food Chem. 2006, 97, 331–335. [Google Scholar] [CrossRef]
- Erbersdobler, H.F.; Jahreis, G.; Erbersdobler, H.F.; Barth, C.A. Legumes in Human Nutrition Nutrient Content and Protein Quality of Pulses. Ernahr. Umsch. 2018, 64, 134–139. [Google Scholar] [CrossRef]
- Naliyadhara, N.; Kumar, A.; Girisa, S.; Daimary, U.D.; Hegde, M.; Kunnumakkara, A.B. Pulsed Electric Field (PEF): Avant-Garde Extraction Escalation Technology in Food Industry. Trends Food Sci. Technol. 2022, 122, 238–255. [Google Scholar] [CrossRef]
- Bocker, R.; Silva, E.K. Pulsed Electric Field Assisted Extraction of Natural Food Pigments and Colorings from Plant Matrices. Food Chem. X 2022, 15, 100398. [Google Scholar] [CrossRef]
- Lai, X.J.; Chen, J.Q.; Nie, J.; Guo, P.F.; Faisal Manzoor, M.; Huang, Y.Y.; Li, J.; Lin, S.Y.; Zeng, X.A.; Wang, R. Enhancement of Extraction Efficiency and Functional Properties of Chickpea Protein Isolate Using Pulsed Electric Field Combined with Ultrasound Treatment. Ultrason. Sonochem. 2024, 111, 107089. [Google Scholar] [CrossRef]
- Yan, L.G.; He, L.; Xi, J. High Intensity Pulsed Electric Field as an Innovative Technique for Extraction of Bioactive Compounds—A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2877–2888. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed Electric Field-Assisted Extraction of Valuable Compounds from Microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef]
- Li, Y.-Q. Structure Changes of Soybean Protein Isolates by Pulsed Electric Fields. Phys. Procedia 2012, 33, 132–137. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, A.K. Ohmic Heating: Concept and Applications—A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2338–2351. [Google Scholar] [CrossRef] [PubMed]
- Avelar, Z.; Monge-Morera, M.; Delcour, J.A.; Saraiva, J.A.; Vicente, A.A.; Rodrigues, R.M. Ohmic Heating as an Innovative Strategy to Modulate Protein Fibrillation. Innov. Food Sci. Emerg. Technol. 2024, 92, 103587. [Google Scholar] [CrossRef]
- Talha, M.; Khalid, S.; Maan, A.A.; Tanveer, N.; Khan, M.K.I.; Asif, M.; Arif, S.; Sarwar, A. Ohmic Assisted Extraction: A Sustainable and Environment Friendly Approach to Substitute Conventional Extraction Methods. Food Rev. Int. 2024, 40, 3508–3529. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A.; Vicente, A.A.; Pereira, R.N. Ohmic Heating for Preservation, Transformation, and Extraction. In Green Food Processing Techniques: Preservation, Transformation and Extraction; Academic Press: Cambridge, MA, USA, 2019; pp. 159–191. [Google Scholar] [CrossRef]
- Miranda, C.G.; Rodrigues, R.M.; Pereira, R.N.; Speranza, P.; Kurozawa, L.E.; Vicente, A.A.; Sato, A.C.K. Influence of Ohmic Heating on Lentil Protein Structure and Protein-Pectin Interactions. Innov. Food Sci. Emerg. Technol. 2023, 87, 103413. [Google Scholar] [CrossRef]
- dos Santos, I.F.; Pimentel, T.C.; da Cruz, A.G.; Stringheta, P.C.; Martins, E.; Campelo, P.H. Ohmic Heating in Food Processing: An Overview of Plant-Based Protein Modification. Processes 2024, 12, 1800. [Google Scholar] [CrossRef]
- Avelar, Z.; Saraiva, J.A.; Vicente, A.A.; Rodrigues, R.M. Unravelling the Impact of Ohmic Heating on Commercial Pea Protein Structure. Food Hydrocoll. 2024, 150, 109748. [Google Scholar] [CrossRef]
- Sousa, V.; Loureiro, L.; Carvalho, G.; Pereira, R.N. Extraction of Biomolecules from Coelastrella Sp. LRF1 Biomass Using Ohmic Heating Technology. Innov. Food Sci. Emerg. Technol. 2022, 80, 103059. [Google Scholar] [CrossRef]
- Getachew, A.T.; Chun, B.S. Influence of Pretreatment and Modifiers on Subcritical Water Liquefaction of Spent Coffee Grounds: A Green Waste Valorization Approach. J. Clean. Prod. 2017, 142, 3719–3727. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent Advances in the Extraction of Bioactive Compounds with Subcritical Water: A Review. Trends Food Sci. Technol. 2019, 95, 183–195. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. High Antioxidant Activity of Coffee Silverskin Extracts Obtained by the Treatment of Coffee Silverskin with Subcritical Water. Food Chem. 2012, 135, 943–949. [Google Scholar] [CrossRef]
- Barea, P.; Melgosa, R.; Benito-Román, Ó.; Illera, A.E.; Beltrán, S.; Sanz, M.T. Green Fractionation and Hydrolysis of Fish Meal to Improve Their Techno-Functional Properties. Food Chem. 2024, 452, 139550. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Tu, Z.C.; Wang, H.; Huang, X.Q.; Fan, L.L.; Bao, Z.Y.; Xiao, H. Functional Properties and Structure Changes of Soybean Protein Isolate after Subcritical Water Treatment. J. Food Sci. Technol. 2015, 52, 3412–3421. [Google Scholar] [CrossRef] [PubMed]
- Ramachandraiah, K.; Koh, B.B.; Davaatseren, M.; Hong, G.P. Characterization of Soy Protein Hydrolysates Produced by Varying Subcritical Water Processing Temperature. Innov. Food Sci. Emerg. Technol. 2017, 43, 201–206. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Database of Deep Eutectic Solvents and Their Physical Properties: A Review. J. Mol. Liq. 2023, 384, 121899. [Google Scholar] [CrossRef]
- Chen, Q.; Chaihu, L.; Yao, X.; Cao, X.; Bi, W.; Lin, J.; Chen, D.D.Y. Molecular Property-Tailored Soy Protein Extraction Process Using a Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2021, 9, 10083–10092. [Google Scholar] [CrossRef]
- Ali Redha, A. Review on Extraction of Phenolic Compounds from Natural Sources Using Green Deep Eutectic Solvents. J. Agric. Food Chem. 2021, 69, 878–912. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Wang, J.; Teng, C.; Yan, L. Applications of Deep Eutectic Solvents in the Extraction, Dissolution, and Functional Materials of Chitin: Research Progress and Prospects. Green Chem. 2022, 24, 552–564. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, W.; Zhang, N.; Soladoye, O.P.; Zhang, Y.; Fu, Y. Deep Eutectic Solvents as New Media for Green Extraction of Food Proteins: Opportunity and Challenges. Food Res. Int. 2022, 161, 111842. [Google Scholar] [CrossRef] [PubMed]
- Hewage, A.; Olatunde, O.O.; Nimalaratne, C.; House, J.D.; Aluko, R.E.; Bandara, N. Improved Protein Extraction Technology Using Deep Eutectic Solvent System for Producing High Purity Fava Bean Protein Isolates at Mild Conditions. Food Hydrocoll. 2024, 147, 109283. [Google Scholar] [CrossRef]
- Chakravorty, P.; Das, A.B. Impact of Choline Choride and Sugar Natural Deep Eutectic Solvents on Structure and Functionality of Treebean (Parkia timoriana) Seed Protein. Sci. Rep. 2025, 15, 1–10. [Google Scholar] [CrossRef]
- Xing, Q.; Utami, D.P.; Demattey, M.B.; Kyriakopoulou, K.; de Wit, M.; Boom, R.M.; Schutyser, M.A.I. A Two-Step Air Classification and Electrostatic Separation Process for Protein Enrichment of Starch-Containing Legumes. Innov. Food Sci. Emerg. Technol. 2020, 66, 102480. [Google Scholar] [CrossRef]
- Tabtabaei, S.; Kuspangaliyeva, B.; Legge, R.L.; Rajabzadeh, A.R. Air Classification of Plant Proteins. In Green Protein Processing Technologies from Plants: Novel Extraction and Purification Methods for Product Development; Springer: Cham, Switzerland, 2023; pp. 31–59. [Google Scholar] [CrossRef]
- Dumoulin, L.; Jacquet, N.; Malumba, P.; Richel, A.; Blecker, C. Dry and Wet Fractionation of Plant Proteins: How a Hybrid Process Increases Yield and Impacts Nutritional Value of Faba Beans Proteins. Innov. Food Sci. Emerg. Technol. 2021, 72, 102747. [Google Scholar] [CrossRef]
- Schutyser, M.A.I.; Pelgrom, P.J.M.; van der Goot, A.J.; Boom, R.M. Dry Fractionation for Sustainable Production of Functional Legume Protein Concentrates. Trends Food Sci. Technol. 2015, 45, 327–335. [Google Scholar] [CrossRef]
- Schlangen, M.; Taghian Dinani, S.; Schutyser, M.A.I.; van der Goot, A.J. Dry Fractionation to Produce Functional Fractions from Mung Bean, Yellow Pea and Cowpea Flour. Innov. Food Sci. Emerg. Technol. 2022, 78, 103018. [Google Scholar] [CrossRef]
- De Angelis, D.; Pasqualone, A.; Manfredi, L.; Allegretta, I.; Terzano, R.; Summo, C. Dry Fractionation as a Promising Technology to Reuse the Physically Defected Legume-Based Gluten-Free Pasta. Int. J. Food Sci. Technol. 2022, 57, 4816–4824. [Google Scholar] [CrossRef]
- Li, Z.; Messina, V.; Skylas, D.J.; Valtchev, P.; Whiteway, C.; Cheng, S.; Langrish, T.A.G.; Quail, K.J.; Dehghani, F. Effect of Dry and Wet Fractionation on Nutritional and Physicochemical Properties of Faba Bean and Yellow Pea Protein. Legum. Sci. 2024, 6, e244. [Google Scholar] [CrossRef]
- Schutyser, M.; Novoa, S.C.; Wetterauw, K.; Politiek, R.; Wilms, P. Dry Fractionation for Sustainable Production of Functional, Nutritional and Palatable Grain Legume Protein Ingredients. Food Eng. Rev. 2025, 1–15. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Boom, R.M.; Schutyser, M.A.I. Method Development to Increase Protein Enrichment During Dry Fractionation of Starch-Rich Legumes. Food Bioproc. Tech. 2015, 8, 1495–1502. [Google Scholar] [CrossRef]
- Hopf, A.; Agarwal, D.; Skylas, D.J.; Whiteway, C.; Buckow, R.; Dehghani, F. Techno-Functional Properties of Dry and Wet Fractionated Pulse Protein Ingredients. Legum. Sci. 2024, 6, e70005. [Google Scholar] [CrossRef]
- Watchararuji, K.; Goto, M.; Sasaki, M.; Shotipruk, A. Value-Added Subcritical Water Hydrolysate from Rice Bran and Soybean Meal. Bioresour. Technol. 2008, 99, 6207–6213. [Google Scholar] [CrossRef]
- Ndlela, S.C.; De Moura, J.M.L.N.; Olson, N.K.; Johnson, L.A. Aqueous Extraction of Oil and Protein from Soybeans with Subcritical Water. JAOCS J. Am. Oil Chem. Soc. 2012, 89, 1145–1153. [Google Scholar] [CrossRef]
- Lu, W.; Chen, X.W.; Wang, J.M.; Yang, X.Q.; Qi, J.R. Enzyme-Assisted Subcritical Water Extraction and Characterization of Soy Protein from Heat-Denatured Meal. J. Food Eng. 2016, 169, 250–258. [Google Scholar] [CrossRef]
- Khuwijitjaru, P.; Anantanasuwong, S.; Adachi, S. Emulsifying and Foaming Properties of Defatted Soy Meal Extracts Obtained by Subcritical Water Treatment. Int. J. Food Prop. 2011, 14, 9–16. [Google Scholar] [CrossRef]
- Funke, M.; Boom, R.; Weiss, J. Dry Fractionation of Lentils by Air Classification—Composition, Interfacial Properties and Behavior in Concentrated O/W Emulsions. LWT 2022, 154, 112718. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Boom, R.M.; Schutyser, M.A.I. Functional Analysis of Mildly Refined Fractions from Yellow Pea. Food Hydrocoll. 2015, 44, 12–22. [Google Scholar] [CrossRef]
- Rempel, C.; Geng, X.; Zhang, Y. Industrial Scale Preparation of Pea Flour Fractions with Enhanced Nutritive Composition by Dry Fractionation. Food Chem. 2019, 276, 119–128. [Google Scholar] [CrossRef]
- Tan, M.; Nawaz, M.A.; Buckow, R. Functional and Food Application of Plant Proteins—A Review. Food Rev. Int. 2023, 39, 2428–2456. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Shen, A.; Zhang, T.; Jiang, L.; El-Seedi, H.; Zhang, G.; Sui, X. Legumes as an Alternative Protein Source in Plant-Based Foods: Applications, Challenges, and Strategies. Curr. Res. Food Sci. 2024, 9, 100876. [Google Scholar] [CrossRef] [PubMed]
- Saran, V.; Pavithra, R.; Koli, V.; Dattatrya, P.A.; Nikashini, T.; Ashika, R.; Nanje Gowda, N.A.; Sunil, C.K. Ultrasound Modification of Techno-Functional, Structural, and Physico-Chemical Properties of Legume Proteins: A Review. Food Biosci. 2024, 60, 104456. [Google Scholar] [CrossRef]
- Jiang, W.; Feng, J.; Yang, X.; Li, L. Structure of Pea Protein-Based Complexes on High-Moisture Extrusion: Raw Materials and Extrusion Zones. LWT 2024, 194, 115823. [Google Scholar] [CrossRef]
- Hu, X.; McClements, D.J. Construction of Plant-Based Adipose Tissue Using High Internal Phase Emulsions and Emulsion Gels. Innov. Food Sci. Emerg. Technol. 2022, 78, 103016. [Google Scholar] [CrossRef]
- Mecha, E.; Alves, M.L.; Bento da Silva, A.; Pereira, A.B.; Rubiales, D.; Vaz Patto, M.C.; Bronze, M.R. High Inter- and Intra-Diversity of Amino Acid Content and Protein Digestibility Disclosed in Five Cool Season Legume Species with a Growing Market Demand. Foods 2023, 12, 1383. [Google Scholar] [CrossRef]
- Matemu, A.; Nakamura, S.; Katayama, S. Health Benefits of Antioxidative Peptides Derived from Legume Proteins with a High Amino Acid Score. Antioxidants 2021, 10, 316. [Google Scholar] [CrossRef]
- Tawalbeh, D.; Al-U’datt, M.H.; Wan Ahmad, W.A.N.; Ahmad, F.; Sarbon, N.M. Recent Advances in In Vitro and In Vivo Studies of Antioxidant, ACE-Inhibitory and Anti-Inflammatory Peptides from Legume Protein Hydrolysates. Molecules 2023, 28, 2423. [Google Scholar] [CrossRef]
- Caporaso, N. The Impact of Molecular Gastronomy within the Food Science Community. In Gastronomy and Food Science; Academic Press: Cambridge, MA, USA, 2021; pp. 1–18. [Google Scholar] [CrossRef]
- McCormick, C.; Prokes, S. Molecular Gastronomy: Materials Science in the Kitchen. MRS Bull. 2009, 34, 802–803. [Google Scholar] [CrossRef]
- Gu, J.; Bk, A.; Wu, H.; Lu, P.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Impact of Processing and Storage on Protein Digestibility and Bioavailability of Legumes. Food Rev. Int. 2023, 39, 4697–4724. [Google Scholar] [CrossRef]
- Kamal, H.; Le, C.F.; Salter, A.M.; Ali, A. Extraction of Protein from Food Waste: An Overview of Current Status and Opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2455–2475. [Google Scholar] [CrossRef]
- Ganjeh, A.M.; Saraiva, J.A.; Pinto, C.A.; Casal, S.; Silva, A.M.S. Emergent Technologies to Improve Protein Extraction from Fish and Seafood By-Products: An Overview. Appl. Food Res. 2023, 3, 100339. [Google Scholar] [CrossRef]
- Jain, I.; Kaur, R.; Kumar, A.; Paul, M.; Singh, N. Emerging Protein Sources and Novel Extraction Techniques: A Systematic Review on Sustainable Approaches. Int. J. Food Sci. Technol. 2024, 59, 6797–6820. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Vardanega, R.; Hatami, T.; Meireles, M.A.A. Process Integration for Recovering High Added-Value Products from Genipa americana L.: Process Optimization and Economic Evaluation. J. Supercrit. Fluids 2020, 164, 104897. [Google Scholar] [CrossRef]
- Ciriminna, R.; Carnaroglio, D.; Delisi, R.; Arvati, S.; Tamburino, A.; Pagliaro, M. Industrial Feasibility of Natural Products Extraction with Microwave Technology. ChemistrySelect 2016, 1, 549–555. [Google Scholar] [CrossRef]
- Plant-Based Protein Market. Available online: https://www.marketsandmarkets.com/Market-Reports/plant-based-protein-market-14715651.html (accessed on 20 December 2024).
- Talwar, R.; Freymond, M.; Beesabathuni, K.; Lingala, S. Current and Future Market Opportunities for Alternative Proteins in Low- and Middle-Income Countries. Curr. Dev. Nutr. 2024, 8, 102035. [Google Scholar] [CrossRef]
- Azarkamand, S.; Fernández Ríos, A.; Batlle-Bayer, L.; Bala, A.; Sazdovski, I.; Roca, M.; Margallo, M.; Aldaco, R.; Laso, J.; Puig, R.; et al. Calculating the True Costs of Protein Sources by Integrating Environmental Costs and Market Prices. Sustain. Prod. Consum. 2024, 49, 28–41. [Google Scholar] [CrossRef]
- Harris, R. Plant Versus Animal: Which One Wins the Protein Price War? Available online: https://canadiangrocer.com/plant-versus-animal-which-one-wins-protein-price-war (accessed on 20 December 2024).
- Verza, M.; Ceccacci, A.; Frigo, G.; Mulazzani, L.; Chatzinikolaou, P. Legumes on the Rise: The Impact of Sustainability Attributes on Market Prices. Sustainability 2024, 16, 2644. [Google Scholar] [CrossRef]
- Rogers, H.; Dora, M.; Tsolakis, N.; Kumar, M. Plant-Based Food Supply Chains: Recognising Market Opportunities and Industry Challenges of Pea Protein. Appl. Food Res. 2024, 4, 100440. [Google Scholar] [CrossRef]
- Reducing the Price of Alternative Proteins. Available online: https://gfi.org/wp-content/uploads/2021/12/Reducing-the-price-of-alternative-proteins_GFI_2022.pdf (accessed on 1 October 2024).
- Varela-Ortega, C.; Blanco-Gutiérrez, I.; Manners, R.; Detzel, A. Life Cycle Assessment of Animal-Based Foods and Plant-Based Protein-Rich Alternatives: A Socio-Economic Perspective. J. Sci. Food Agric. 2022, 102, 5111–5120. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, E.H.-J.; Serventi, L. Evaluation of the Protein Profile and Emulsifying Properties of Legume Wastewater as Emulsifier in Circular Food Applications. LWT 2024, 202, 116320. [Google Scholar] [CrossRef]
- Plazzotta, S.; Nicoli, M.C.; Manzocco, L. Upcycling Soy Processing Waste (Okara) into Structured Emulsions for Fat Replacement in Sweet Bread. J. Sci. Food Agric. 2023, 103, 4025–4033. [Google Scholar] [CrossRef]
- Svanes, E.; Waalen, W.; Uhlen, A.K. Environmental Impacts of Field Peas and Faba Beans Grown in Norway and Derived Products, Compared to Other Food Protein Sources. Sustain. Prod. Consum. 2022, 33, 756–766. [Google Scholar] [CrossRef]
- Hueppe, R.; Zander, K. Legume Consumption and Sustainability—The Minority Goes Green. Appetite 2025, 206, 107831. [Google Scholar] [CrossRef]
- Goldstein, N.; Reifen, R. The Potential of Legume-Derived Proteins in the Food Industry. Grain Oil Sci. Technol. 2022, 5, 167–178. [Google Scholar] [CrossRef]
- Pérez, R.; Fernández, C.; Laca, A.; Laca, A. Evaluation of Environmental Impacts in Legume Crops: A Case Study of PGI White Bean Production in Southern Europe. Sustainability 2024, 16, 8024. [Google Scholar] [CrossRef]
- Heusala, H.; Sinkko, T.; Sözer, N.; Hytönen, E.; Mogensen, L.; Knudsen, M.T. Carbon Footprint and Land Use of Oat and Faba Bean Protein Concentrates Using a Life Cycle Assessment Approach. J. Clean. Prod. 2020, 242, 118376. [Google Scholar] [CrossRef]
- L’Hocine, L.; Pitre, M.; Achouri, A. Allergenicity of Plant Proteins. In Functionality of Plant Proteins: Properties, Methods of Assessment, Modifications and Applications; Elsevier: Amsterdam, The Netherlands, 2024; pp. 429–461. ISBN 9780323917216. [Google Scholar]
- Kopko, C.; Garthoff, J.A.; Zhou, K.; Meunier, L.; O’Sullivan, A.J.; Fattori, V. Are Alternative Proteins Increasing Food Allergies? Trends, Drivers and Future Perspectives. Trends Food Sci. Technol. 2022, 129, 126–133. [Google Scholar] [CrossRef]
- Kasera, R.; Singh, A.B.; Kumar, R.; Lavasa, S.; Prasad, K.N.; Arora, N. Effect of Thermal Processing and γ-Irradiation on Allergenicity of Legume Proteins. Food Chem. Toxicol. 2012, 50, 3456–3461. [Google Scholar] [CrossRef]
- Calcinai, L.; Bonomini, M.G.; Leni, G.; Faccini, A.; Puxeddu, I.; Giannini, D.; Petrelli, F.; Prandi, B.; Sforza, S.; Tedeschi, T. Effectiveness of Enzymatic Hydrolysis for Reducing the Allergenic Potential of Legume By-Products. Sci. Rep. 2022, 12, 16902. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, R.; Bala Krishnan, S. Pulsed Electric Field Treatment in Extracting Proteins from Legumes: A Review. Processes 2024, 12, 2667. [Google Scholar] [CrossRef]
- El Mecherfi, K.E.; Todorov, S.D.; De Albuquerque, M.A.C.; Denery-Papini, S.; Lupi, R.; Haertlé, T.; De Melo Franco, B.D.G.; Larre, C. Allergenicity of Fermented Foods: Emphasis on Seeds Protein-Based Products. Foods 2020, 9, 792. [Google Scholar] [CrossRef]
- Duque-Estrada, P.; Kyriakopoulou, K.; de Groot, W.; van der Goot, A.J.; Berton-Carabin, C.C. Oxidative Stability of Soy Proteins: From Ground Soybeans to Structured Products. Food Chem. 2020, 318, 126499. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, W.; Bi, S.; Liu, Y. Application of Legumes in Plant-Based Milk Alternatives: A Review of Limitations and Solutions. Crit. Rev. Food Sci. Nutr. 2024, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rehman, Z.U.; Shah, W.H. Thermal Heat Processing Effects on Antinutrients, Protein and Starch Digestibility of Food Legumes. Food Chem. 2005, 91, 327–331. [Google Scholar] [CrossRef]
- Pokharel, U.; Adhikari, N.; Gautam, N.; Poudel, R.; Timsina, P.; Dangal, A.; Giuffrè, A.M. Effects of Different Processing Methods on the Antinutritional Factors Present in Mungbean (Vigna radiata L.). Analytica 2024, 5, 414–429. [Google Scholar] [CrossRef]
- Adebo, J.A.; Njobeh, P.B.; Gbashi, S.; Oyedeji, A.B.; Ogundele, O.M.; Oyeyinka, S.A.; Adebo, O.A. Fermentation of Cereals and Legumes: Impact on Nutritional Constituents and Nutrient Bioavailability. Fermentation 2022, 8, 63. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic Hydrolysis and Microbial Fermentation: The Most Favorable Biotechnological Methods for the Release of Bioactive Peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Mubarak, A.E. Chemical, Nutritional and Sensory Properties of Bread Supplemented with Lupin Seed (Lupinus albus) Products. Mol. Nutr. Food Res. 2001, 45, 241–245. [Google Scholar] [CrossRef]
- Boukid, F.; Rosell, C.M.; Castellari, M. Pea Protein Ingredients: A Mainstream Ingredient to (Re) Formulate Innovative Foods and Beverages. Trends Food Sci. Technol. 2021, 110, 729–742. [Google Scholar] [CrossRef]
- Jiménez-Munoz, L.M.; Tavares, G.M.; Corredig, M. Design Future Foods Using Plant Protein Blends for Best Nutritional and Technological Functionality. Trends Food Sci. Technol. 2021, 113, 139–150. [Google Scholar] [CrossRef]
- Muhu-Din Ahmed, H.G.; Naeem, M.; Faisal, A.; Fatima, N.; Tariq, S.; Owais, M. Enriching the Content of Proteins and Essential Amino Acids in Legumes. In Legumes Biofortification; Springer: Berlin/Heidelberg, Germany, 2023; pp. 417–447. [Google Scholar] [CrossRef]
- Žugčić, T.; Abdelkebir, R.; Barba, F.J.; Rezek-Jambrak, A.; Gálvez, F.; Zamuz, S.; Granato, D.; Lorenzo, J.M. Effects of Pulses and Microalgal Proteins on Quality Traits of Beef Patties. J. Food Sci. Technol. 2018, 55, 4544–4553. [Google Scholar] [CrossRef]

| Sources | Protein Content (%) | Reference |
|---|---|---|
| Soybean | 35–40 | [36] |
| Pea | 23–27 | [37] |
| Beans: | ||
| Faba bean | 20–41 | [38] |
| Mung bean | 21–31 | [39] |
| Green bean | [40] | |
| Black bean | 20–30 | [41] |
| Azuki bean | 19.90 | [40] |
| Cranberry bean | 23.00 | [40] |
| Ganxet bean | 24–29 | [42] |
| Kidney bean | 22.53 | [40] |
| Jack bean | 23–35 | [22] |
| Pigeon pea | 18–28 | [21] |
| Lentil | 22–30 | [41] |
| Hyacinth bean | 22–25 | [26] |
| Chickpea | 20–24 | [40,43] |
| Lupins | 29–55 | [7] |
| Acacia | 18–36 | [32] |
| Peanut | 26–29 | [44] |
| Cowpea | 23–32 | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Náthia-Neves, G.; Getachew, A.T.; Santana, Á.L.; Jacobsen, C. Legume Proteins in Food Products: Extraction Techniques, Functional Properties, and Current Challenges. Foods 2025, 14, 1626. https://doi.org/10.3390/foods14091626
Náthia-Neves G, Getachew AT, Santana ÁL, Jacobsen C. Legume Proteins in Food Products: Extraction Techniques, Functional Properties, and Current Challenges. Foods. 2025; 14(9):1626. https://doi.org/10.3390/foods14091626
Chicago/Turabian StyleNáthia-Neves, Grazielle, Adane Tilahun Getachew, Ádina L. Santana, and Charlotte Jacobsen. 2025. "Legume Proteins in Food Products: Extraction Techniques, Functional Properties, and Current Challenges" Foods 14, no. 9: 1626. https://doi.org/10.3390/foods14091626
APA StyleNáthia-Neves, G., Getachew, A. T., Santana, Á. L., & Jacobsen, C. (2025). Legume Proteins in Food Products: Extraction Techniques, Functional Properties, and Current Challenges. Foods, 14(9), 1626. https://doi.org/10.3390/foods14091626









