Ozone-Assisted Green Upgrading of Lactuca sativa Oil: Characterization and Bioactivity for Clean-Label Functional Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Ozonation Process
2.2. GC–MS Analysis
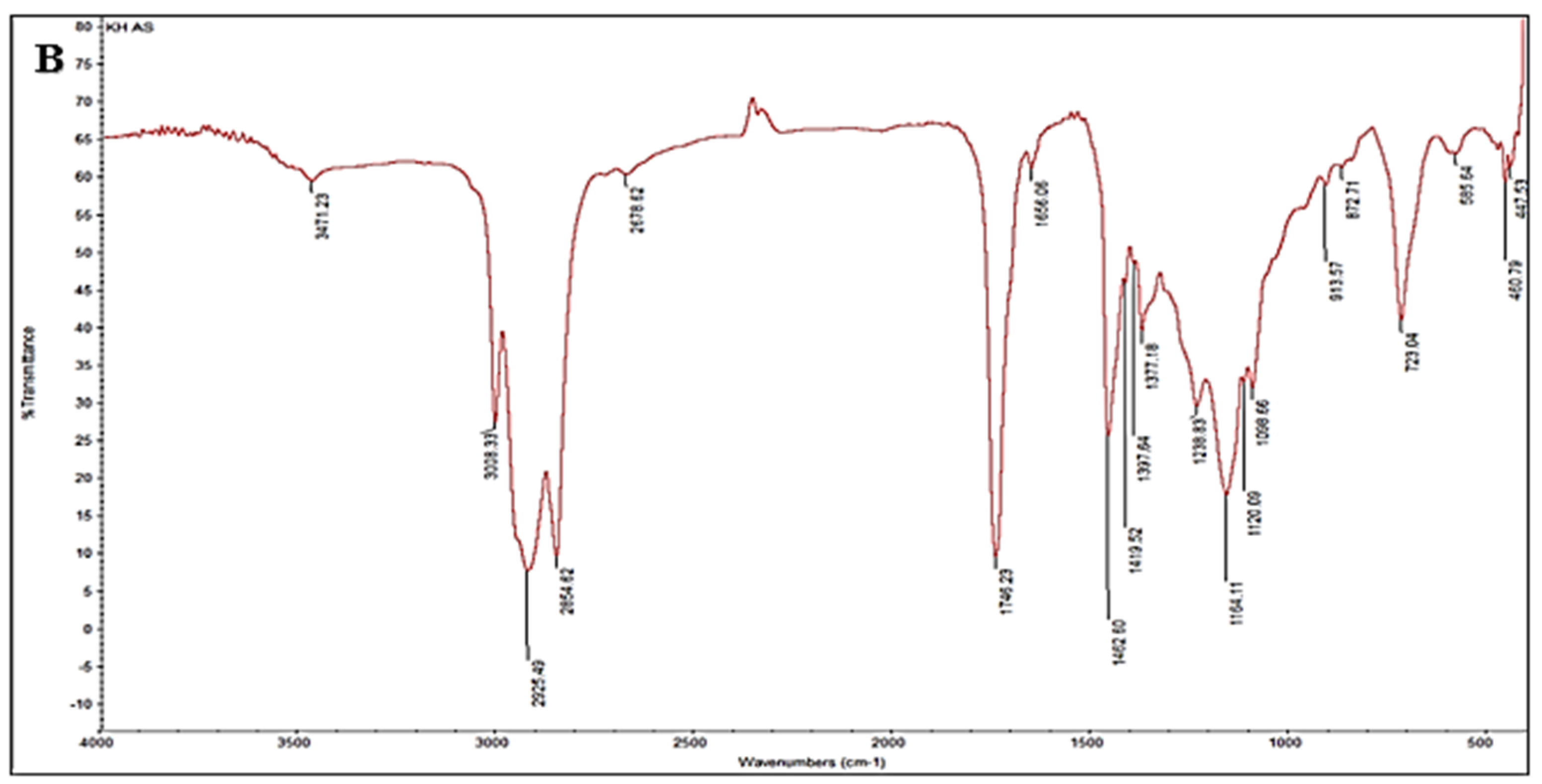
2.3. FTIR Analysis
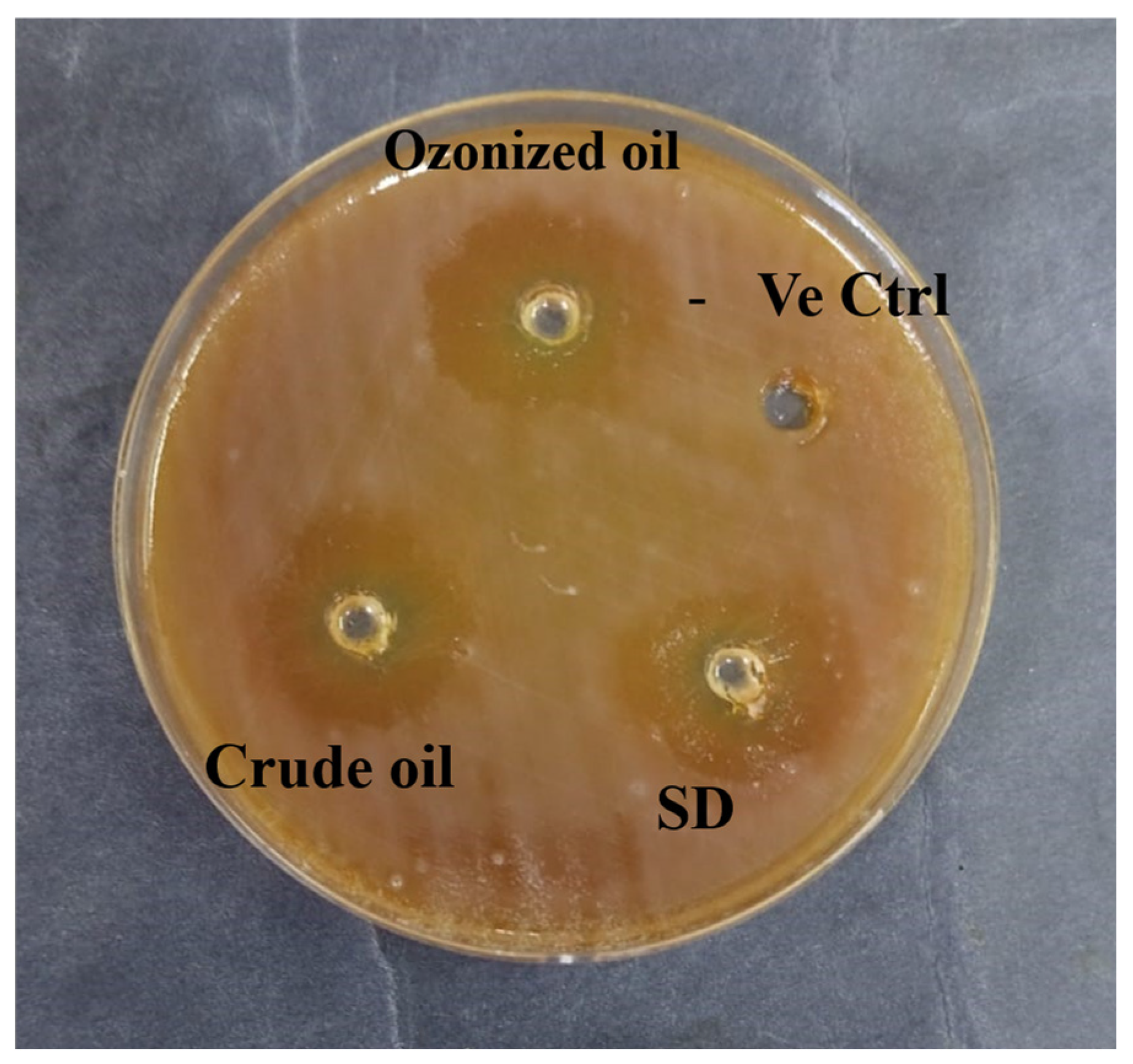
2.4. Anti-H. pylori Assay
2.5. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
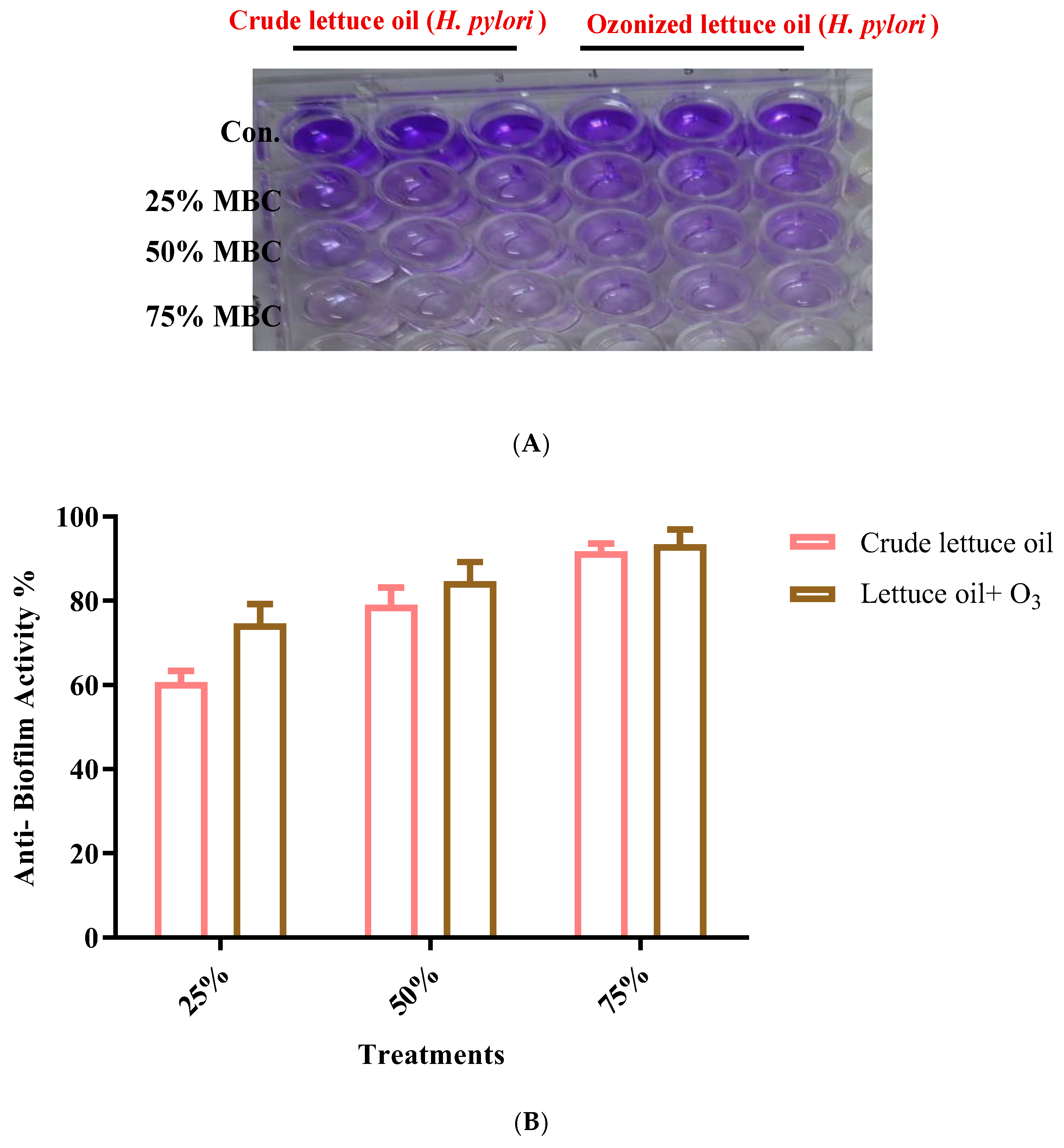
2.6. Anti-Biofilm Activity
2.7. Anti-Hemolytic Assay
2.8. Antioxidant Assay
2.9. Butyrylcholinesterase Inhibition Assay
2.10. Molecular Docking Studies
2.11. Statistical Analysis
3. Results and Discussion
3.1. GC–MS Chemical Profile and Ozonation-Induced Shifts
3.2. FTIR Spectral Signatures and Ozonation Effects
3.3. Anti-H. pylori Activity and Bactericidal Efficacy
3.4. Antibiofilm Activity Against H. pylori
3.5. Antihemolytic Activity
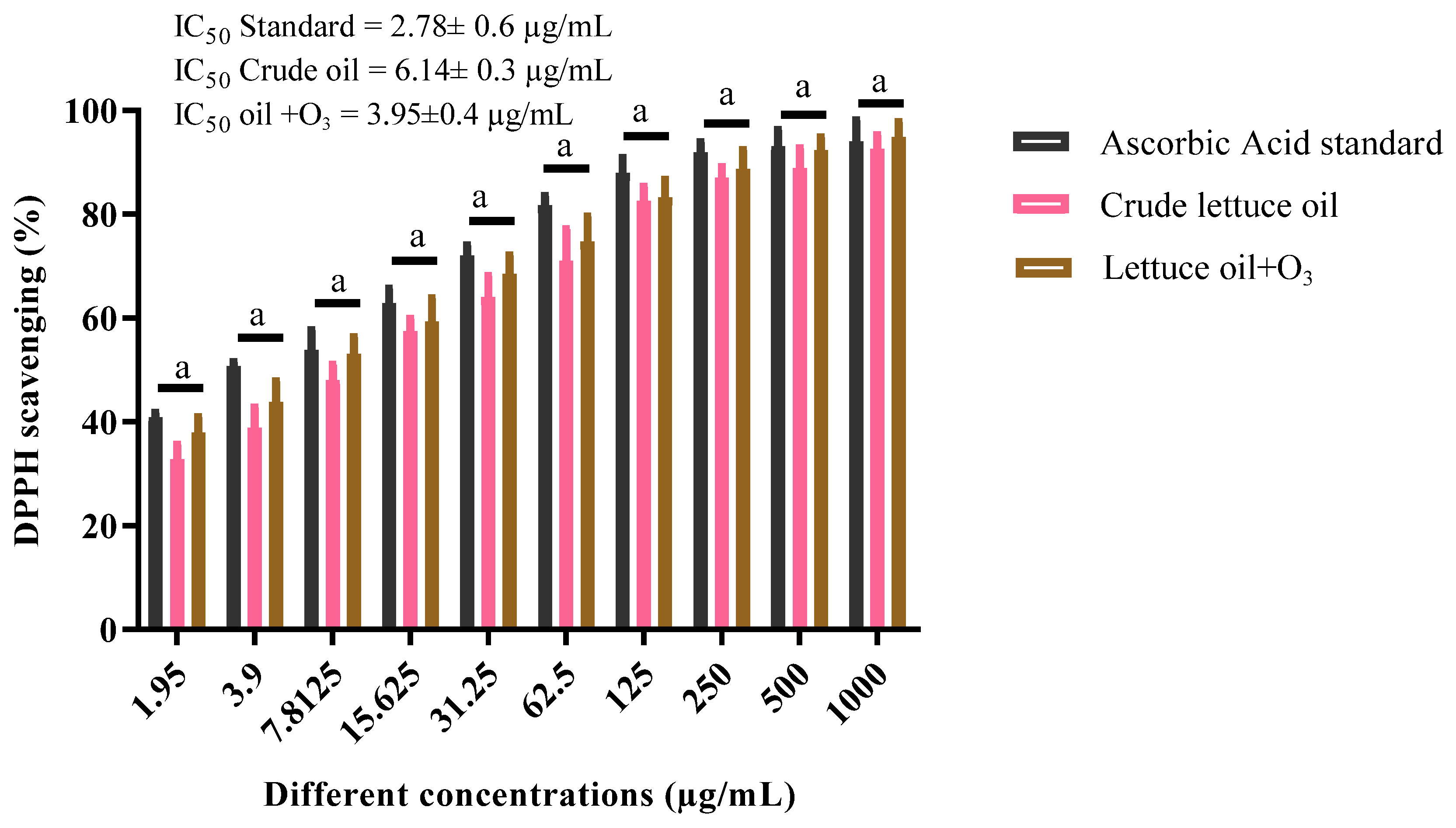
3.6. Antioxidant Capacity (DPPH Assay)
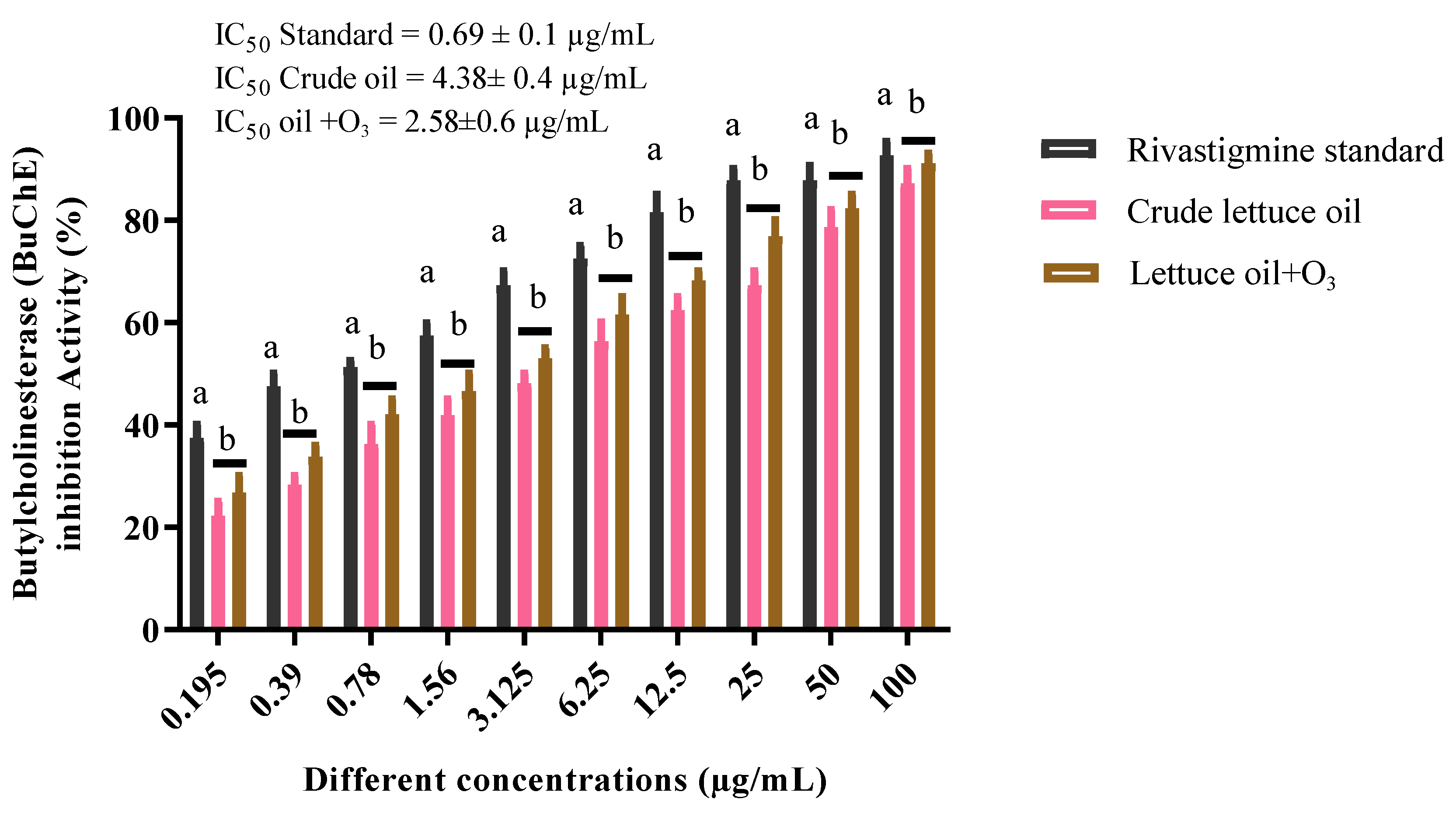
3.7. Butyrylcholinesterase Inhibition
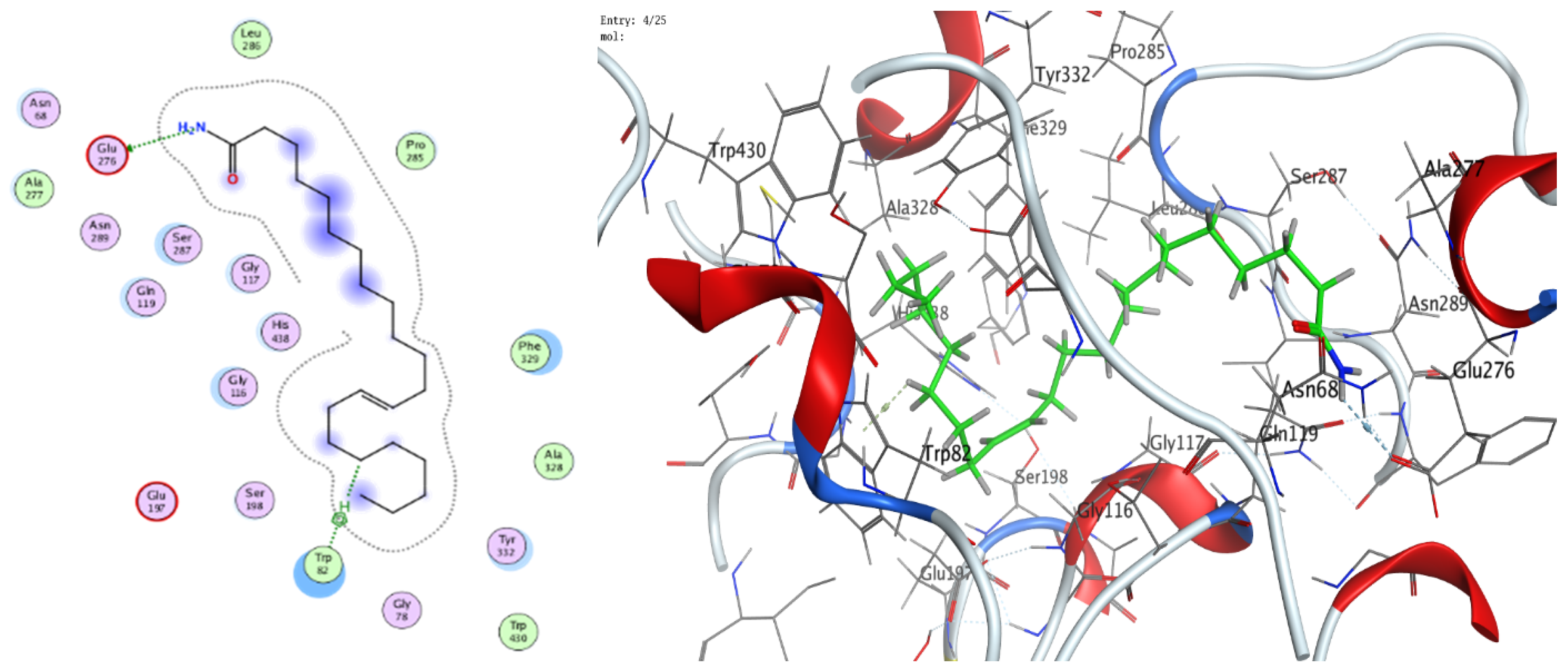
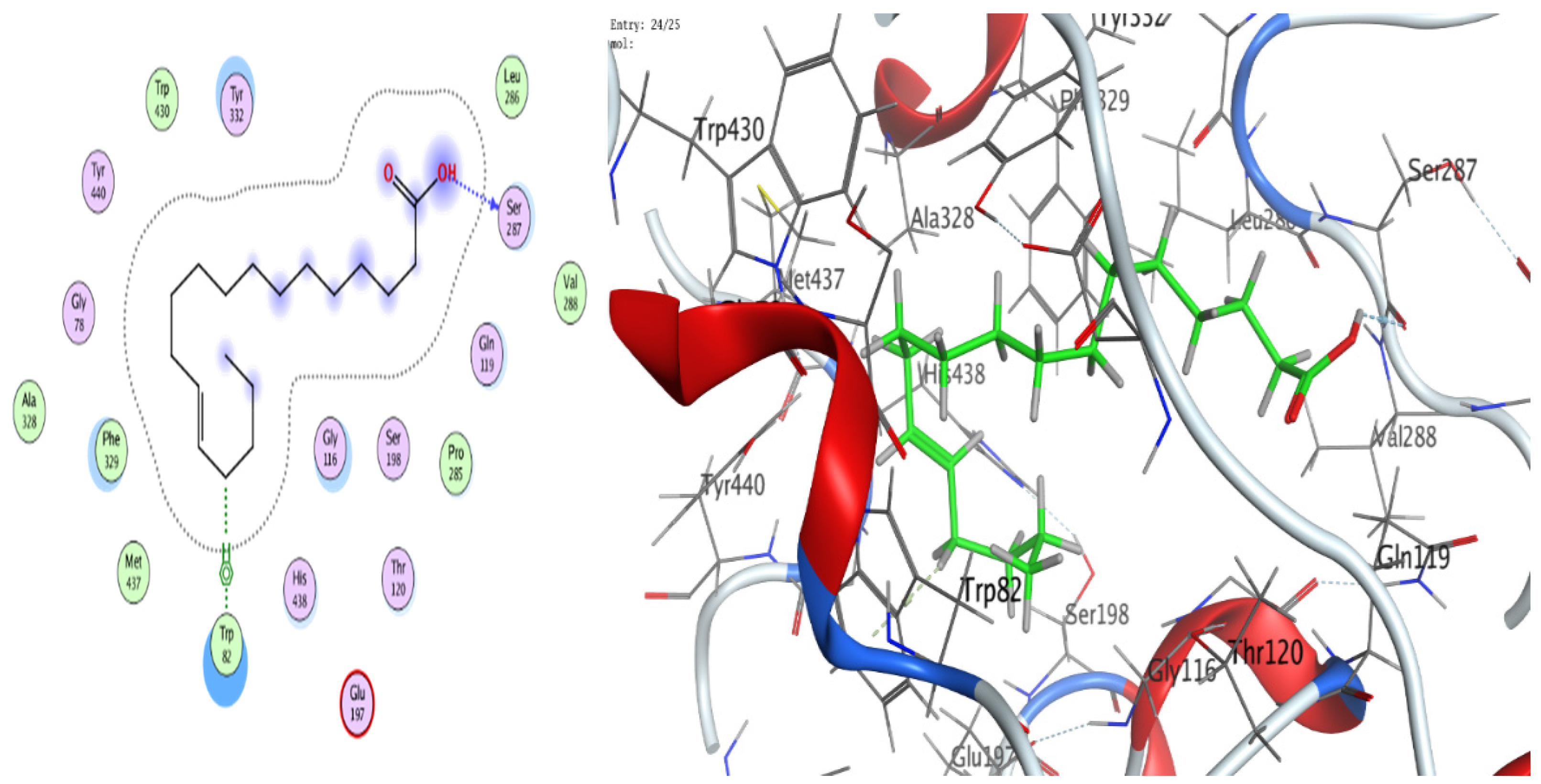
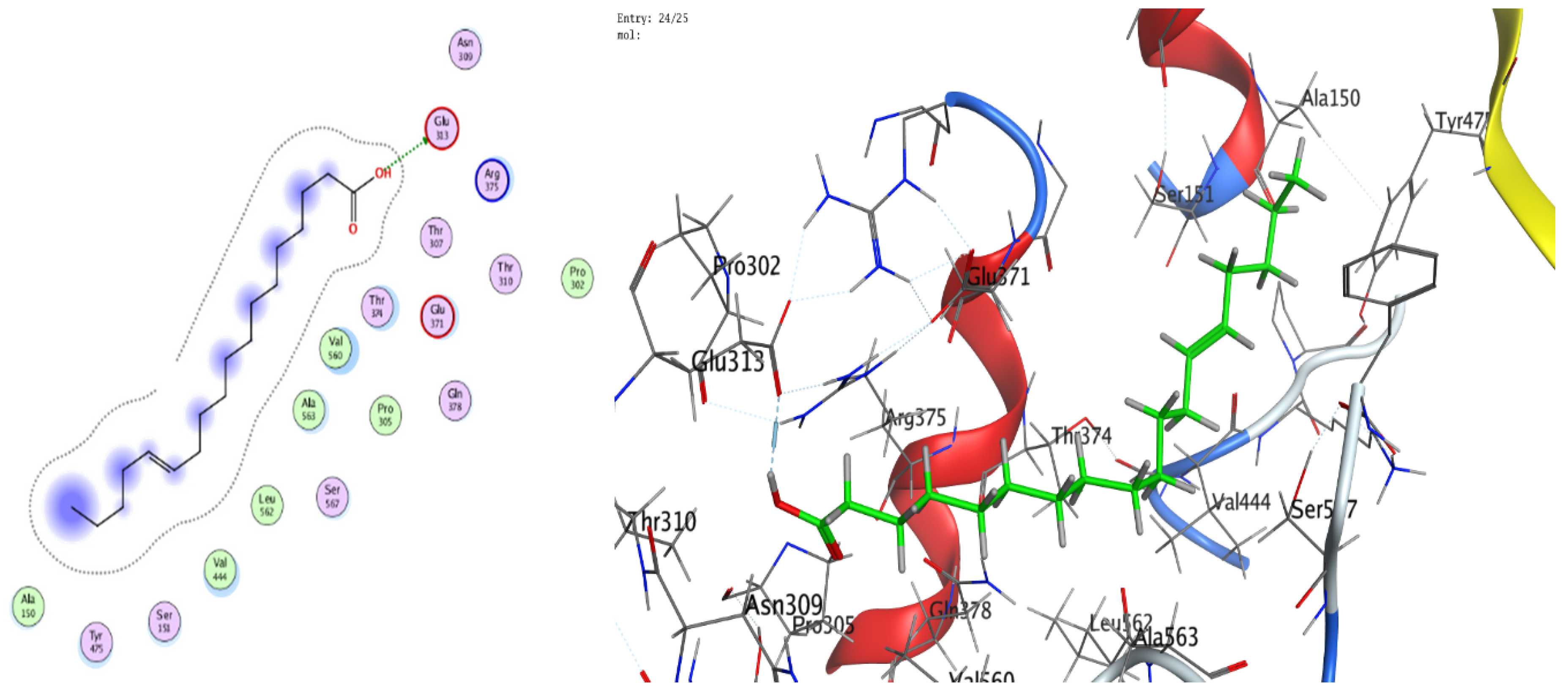
3.8. Molecular Docking (BChE and H. pylori Urease)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel Ghany, T.M.; Ganash, M.; Alawlaqi, M.M.; Al-Rajhi, A.M.H. Antioxidant, Antitumor, Antimicrobial Activities Evaluation of Musa paradisiaca L. Pseudostem Exudate Cultivated in Saudi Arabia. BioNanoScience 2019, 9, 172–178. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Abdel Ghany, T.M. Nanoemulsions of some edible oils and their antimicrobial, antioxidant, and anti-hemolytic activities. BioResources 2023, 18, 1465–1481. [Google Scholar] [CrossRef]
- Selim, S.; Alruwaili, Y.; Ejaz, H.; Abdalla, A.; Almuhayawi, M.; Nagshabandi, M.; Tarabulsi, M.; Al Jaouni, S.; Bazuhair, M.; Abdelghany, T. Estimation and action mechanisms of cinnamon bark via oxidative enzymes and ultrastructures as antimicrobial, anti-biofilm, antioxidant, anti-Diabetic, and anticancer agents. BioResources 2024, 19, 7019–7041. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Hassan, M.M.; El-Naggar, M.A.; El-Mongy, M.A. GC/MS analysis of Juniperus procera extract and its activity with silver nanoparticles against Aspergillus flavus growth and aflatoxins production. Biotechnol. Rep. 2020, 27, e00496. [Google Scholar] [CrossRef]
- Alawlaqi, M.M.; Al-Rajhi, A.M.H.; Abdelghany, T.M.; Ganash, M.; Moawad, H. Evaluation of Biomedical Applications for Linseed Extract: Antimicrobial, Antioxidant, Anti-Diabetic, and Anti-Inflammatory Activities In Vitro. J. Funct. Biomater. 2023, 14, 300. [Google Scholar] [CrossRef]
- Almehayawi, M.; Almuhayawi, M.; El-Fadl, S.; Nagshabandi, M.; Tarabulsi, M.; Selim, S.; Alruwaili, Y.; Mostafa, E.; Al Jaouni, S.; Abdelghany, T. Evaluating the anti-yeast, anti-diabetic, wound healing activities of Moringa oleifera extracted at different conditions of pressure via supercritical fluid extraction. BioResources 2024, 19, 5961–5977. [Google Scholar] [CrossRef]
- Bazaid, A.S.; Binsaleh, N.K.; Barnawi, H.; Alharbi, B.; Alsolami, A.; Selim, S.; Al Jaouni, S.K.; Saddiq, A.A.; Ganash, M.; Abdelghany, T.M.; et al. Unveiling the in vitro activity of extracted Euphorbia trigona via Supercritical Fluid Extraction against pathogenic yeasts, obesity, cancer, and its wound healing properties. Bioresour. Bioprocess. 2025, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.F.R.; Alnuaimi, A.K.H.; Askri, A.; Tzortzakis, N. Evaluation of Lettuce (Lactuca sativa L.) Production under Hydroponic System: Nutrient Solution Derived from Fish Waste vs. Inorganic Nutrient Solution. Horticulturae 2021, 7, 292. [Google Scholar] [CrossRef]
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Al-Awthan, Y.S.; et al. Phytochemicals, Nutrition, Metabolism, Bioavailability, and Health Benefits in Lettuce—A Comprehensive Review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef]
- Al Nomaani, R.S.; Hossain, M.A.; Weli, A.M.; Al-Riyami, Q.; Al-Sabahi, J.N. Chemical composition of essential oils and in vitro antioxidant activity of fresh and dry leaves crude extracts of medicinal plant of Lactuca Sativa L. native to Sultanate of Oman. Asian Pac. J. Trop. Biomed. 2013, 3, 353–357. [Google Scholar] [CrossRef]
- Dyląg, A.; Smoleń, S.; Wisła-Świder, A.; Kowalska, I.; Sularz, O.; Krzemińska, J.; Pitala, J.; Koronowicz, A. Evaluation of the chemical composition and nutritional value of lettuce (Lactuca sativa L.) biofortified in hydroponics with iodine in the form of iodoquinolines. Front. Plant Sci. 2023, 14, 1288773. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajhi, A.M.H.; Abdelghany, T.M.; Almuhayawi, M.S.; Alruhaili, M.H.; Saddiq, A.A.; Baghdadi, A.M.; Al Jaouni, S.K.; Albasri, H.M.; Waznah, M.S.; Alraddadi, F.A.; et al. Effect of ozonation on the phytochemicals of black seed oil and its anti-microbial, anti-oxidant, anti-inflammatory, and anti-neoplastic activities in vitro. Sci. Rep. 2024, 14, 30445. [Google Scholar] [CrossRef]
- Alsalamah, S.A.; Alghonaim, M.I.; Mohammad, A.M.; Khalel, A.F.; Alqahtani, F.S.; Abdelghany, T.M. Ozone-modified properties of pumpkin seed oil as anti-H. pylori, anticancer, anti-diabetic and anti-obesity agent. Sci. Rep. 2025, 15, 25959. [Google Scholar] [CrossRef]
- Silva, V.; Peirone, C.; Amaral, J.S.; Capita, R.; Alonso-Calleja, C.; Marques-Magallanes, J.A.; Martins, Â.; Carvalho, Á.; Maltez, L.; Pereira, J.E.; et al. High Efficacy of Ozonated Oils on the Removal of Biofilms Produced by Methicillin-Resistant Staphylococcus aureus (MRSA) from Infected Diabetic Foot Ulcers. Molecules 2020, 25, 3601. [Google Scholar] [CrossRef]
- Repciuc, C.C.; Vișan, G.-A.-M.; Teleky, B.-E.; Pintea, A.; Novac, C.Ș.; Oros, N.V. Physico-Chemical and Antimicrobial Evaluation of Ozonated Olive Oil Produced with a Medical-Grade Generator for Veterinary Purposes. Microorganisms 2025, 13, 1932. [Google Scholar]
- Sathwik, M.; Komarraju, S.; D, S.; Muralidharan, S. Technical Considerations of Ozonated Oils in Medical Applications: A Narrative Review. Cureus 2025, 17, e83185. [Google Scholar] [CrossRef]
- Aguilera Matos, I.; Diaz Oliva, S.E.; Escobedo, A.A.; Villa Jiménez, O.M.; Velazco Villaurrutia, Y.d.C. Helicobacter pylori infection in children. BMJ Paediatr. Open 2020, 4, e000679. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.R.; Sachs, G.; Marcus, E.A. The role of acid inhibition in Helicobacter pylori eradication. F1000Research 2016, 5, 1747. [Google Scholar] [CrossRef]
- Elbehiry, A.; Abalkhail, A.; Anajirih, N.; Alkhamisi, F.; Aldamegh, M.; Alramzi, A.; AlShaqi, R.; Alotaibi, N.; Aljuaid, A.; Alzahrani, H.; et al. Helicobacter pylori: Routes of Infection, Antimicrobial Resistance, and Alternative Therapies as a Means to Develop Infection Control. Diseases 2024, 12, 311. [Google Scholar] [CrossRef]
- Cho, J.-H.; Jin, S.-Y. Efficacy and Safety of Modified Bismuth Quadruple Therapy for First-Line Helicobacter pylori Eradication: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Microorganisms 2025, 13, 519. [Google Scholar] [CrossRef]
- Muhammed, M.T.; Aki-Yalcin, E. Molecular Docking: Principles, Advances, and its Applications in Drug Discovery. Lett. Drug Des. Discov. 2024, 21, 480–495. [Google Scholar] [CrossRef]
- Qanash, H.; Alotaibi, K.; Aldarhami, A.; Bazaid, A.S.; Ganash, M.; Saeedi, N.H.; Ghany, T.A. Effectiveness of Oil-based Nanoemulsions with Molecular Docking of its Antimicrobial Potential. BioResources 2023, 18, 1554–1576. [Google Scholar] [CrossRef]
- Ugazio, E.; Tullio, V.; Binello, A.; Tagliapietra, S.; Dosio, F. Ozonated Oils as Antimicrobial Systems in Topical Applications. Their Characterization, Current Applications, and Advances in Improved Delivery Techniques. Molecules 2020, 25, 334. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajhi, A.M.H.; Abdelghany, T.M.; Selim, S.; Almuhayawi, M.S.; Alruhaili, M.H.; Alharbi, M.T.; Al Jaouni, S.K. Phytochemical characterization of peanut oil and its ozonized form to explore biological activities in vitro. AMB Express 2025, 15, 76. [Google Scholar] [CrossRef]
- Muráriková, A.; Ťažký, A.; Neugebauerová, J.; Planková, A.; Jampílek, J.; Mučaji, P.; Mikuš, P. Characterization of Essential Oil Composition in Different Basil Species and Pot Cultures by a GC-MS Method. Molecules 2017, 22, 1221. [Google Scholar] [CrossRef]
- Mao, X.; Chen, W.; Huyan, Z.; Sherazi, S.T.H.; Yu, X. Impact of linolenic acid on oxidative stability of rapeseed oils. J. Food Sci. Technol. 2020, 57, 3184–3192. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Juárez, I.; Rivero-Cruz, F.; Celis, H.; Romero, I. Anti-Helicobacter pylori activity of anacardic acids from Amphipterygium adstringens. J. Ethnopharmacol. 2007, 114, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Shakir, S.M.; Otiso, J.; Keller, G.; Heule, H.V.; Osborn, L.J.; Cole, N.; Schuetz, A.N.; Richter, S.S.; Couturier, M.R. Multicenter Evaluation of a Gradient Diffusion Method for Antimicrobial Susceptibility Testing of Helicobacter pylori. Microbiol. Spectr. 2022, 10, e0211121. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Lin, Z.; Wu, B.; Nong, G.; Chen, Y.; Lu, Y.; Ji, X.; Zhou, X.; Suo, B.; et al. Minimum inhibitory concentrations of commonly used antibiotics against Helicobacter Pylori: A multicenter study in South China. PLoS ONE 2021, 16, e0256225. [Google Scholar] [CrossRef]
- Selim, S.; Abdelghany, T.M.; Almuhayawi, M.S.; Nagshabandi, M.K.; Tarabulsi, M.K.; Elamir, M.Y.M.; Alharbi, A.A.; Al Jaouni, S.K. Biosynthesis and activity of Zn-MnO nanocomposite in vitro with molecular docking studies against multidrug resistance bacteria and inflammatory activators. Sci. Rep. 2025, 15, 2032. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Qanash, H.; Almashjary, M.N.; Hazzazi, M.S.; Felemban, H.R.; Abdelghany, T.M. Anti-Helicobacter pylori, Antioxidant, Antidiabetic, and Anti-Alzheimer’s Activities of Laurel Leaf Extract Treated by Moist Heat and Molecular Docking of Its Flavonoid Constituent, Naringenin, against Acetylcholinesterase and Butyrylcholinesterase. Life 2023, 13, 1512. [Google Scholar] [CrossRef]
- Boittier, E.D.; Tang, Y.Y.; Buckley, M.E.; Schuurs, Z.P.; Richard, D.J.; Gandhi, N.S. Assessing Molecular Docking Tools to Guide Targeted Drug Discovery of CD38 Inhibitors. Int. J. Mol. Sci. 2020, 21, 5183. [Google Scholar] [CrossRef]
- Atatreh, N.; Al Rawashdah, S.; Al Neyadi, S.S.; Abuhamdah, S.M.; Ghattas, M.A. Discovery of new butyrylcholinesterase inhibitors via structure-based virtual screening. J. Enzym. Inhib. Med. Chem. 2019, 34, 1373–1379. [Google Scholar] [CrossRef]
- Masetti, M.; Falchi, F.; Gioia, D.; Recanatini, M.; Ciurli, S.; Musiani, F. Targeting the Protein Tunnels of the Urease Accessory Complex: A Theoretical Investigation. Molecules 2020, 25, 2911. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, D.; Alsaweed, M.; Jamal, Q.M.S.; Asad, M.R.; Rizvi, S.M.D.; Rizvi, M.R.; Albadrani, H.M.; Hamed, M.; Jahan, S.; Alyenbaawi, H. Pharmacophore-Based Screening, Molecular Docking, and Dynamic Simulation of Fungal Metabolites as Inhibitors of Multi-Targets in Neurodegenerative Disorders. Biomolecules 2023, 13, 1613. [Google Scholar] [CrossRef] [PubMed]
- Radzimierska-Kaźmierczak, M.; Śmigielski, K.; Sikora, M.; Nowak, A.; Plucińska, A.; Kunicka-Styczyńska, A.; Czarnecka-Chrebelska, K.H. Olive Oil with Ozone-Modified Properties and Its Application. Molecules 2021, 26, 3074. [Google Scholar] [CrossRef]
- Gutarowska, B.; Szulc, J.; Jastrząbek, K.; Kręgiel, D.; Śmigielski, K.; Cieciura-Włoch, W.; Mroczyńska-Florczak, M.; Liszkowska, W.; Rygała, A.; Berłowska, J. Effectiveness of Ozonation for Improving the Microbiological Safety of Fresh-Cut Parsley (Petroselinum crispum) Leaves. Appl. Sci. 2023, 13, 8946. [Google Scholar] [CrossRef]
- Gardeli, C.; Sykioti, S.; Exarchos, G.; Koliatsou, M.; Andritsos, P.; Panagou, E.Z. The Differentiation of Extra Virgin Olive Oil from Other Olive Oil Categories Based on FTIR Spectroscopy and Random Forest. Appl. Sci. 2025, 15, 1061. [Google Scholar] [CrossRef]
- Matwijczuk, A.; Oniszczuk, T.; Matwijczuk, A.; Chruściel, E.; Kocira, A.; Niemczynowicz, A.; Wójtowicz, A.; Combrzyński, M.; Wiącek, D. Use of FTIR Spectroscopy and Chemometrics with Respect to Storage Conditions of Moldavian Dragonhead Oil. Sustainability 2019, 11, 6414. [Google Scholar] [CrossRef]
- Koczoń, P.; Hołaj-Krzak, J.T.; Palani, B.K.; Bolewski, T.; Dąbrowski, J.; Bartyzel, B.J.; Gruczyńska-Sękowska, E. The Analytical Possibilities of FT-IR Spectroscopy Powered by Vibrating Molecules. Int. J. Mol. Sci. 2023, 24, 1013. [Google Scholar] [CrossRef]
- Ye, Q.; Meng, X.; Pang, L. D2O assisted FTIR spectroscopic analysis of moisture in edible oil. Food Chem. X 2023, 18, 100679. [Google Scholar] [CrossRef]
- Puxeddu, S.; Scano, A.; Scorciapino, M.A.; Delogu, I.; Vascellari, S.; Ennas, G.; Manzin, A.; Angius, F. Physico-Chemical Investigation and Antimicrobial Efficacy of Ozonated Oils: The Case Study of Commercial Ozonated Olive and Sunflower Seed Refined Oils. Molecules 2024, 29, 679. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Bravo, D.; Soto, C.; Maturana, G.; Cordero-Machuca, J.; Zúñiga-López, M.C.; Oyarzun-Ampuero, F.; Quest, A.F.G. The Anti-Oxidant Curcumin Solubilized as Oil-in-Water Nanoemulsions or Chitosan Nanocapsules Effectively Reduces Helicobacter pylori Growth, Bacterial Biofilm Formation, Gastric Cell Adhesion and Internalization. Antioxidants 2023, 12, 1866. [Google Scholar] [CrossRef]
- Elbestawy, M.K.M.; El-Sherbiny, G.M.; Moghannem, S.A. Antibacterial, Antibiofilm and Anti-Inflammatory Activities of Eugenol Clove Essential Oil against Resistant Helicobacter pylori. Molecules 2023, 28, 2448. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, B.; Qanash, H.; Almashjary, M.N.; Barnawi, H.; Aldarhami, A.; Alsaif, G.; Alsamaan, F.; Monjed, M.K.; Al Shmrany, H.; Bazaid, A.S. Watercress oil loaded with gel: Evaluation of hemolysis inhibition, antioxidant, antimicrobial, and healing properties. Front. Pharmacol. 2024, 15, 1424369. [Google Scholar] [CrossRef]
- Almuhayawi, M.S.; Alruhaili, M.H.; Tarabulsi, M.K.; Al Jaouni, S.K.; Alqurashi, A.A.; Alraddadi, F.A.; Bukhari, D.A.; Albasri, H.M.; Waznah, M.S.; Selim, S. Pharmacological activities and phytochemical evaluation of coconut crude oil and upon exposure to ozone. AMB Express 2025, 15, 3. [Google Scholar] [CrossRef]
- Cho, K.H.; Kang, D.J.; Nam, H.S.; Kim, J.H.; Kim, S.Y.; Lee, J.O.; Kim, B.J. Ozonated Sunflower Oil Exerted Protective Effect for Embryo and Cell Survival via Potent Reduction Power and Antioxidant Activity in HDL with Strong Antimicrobial Activity. Antioxidants 2021, 10, 1651. [Google Scholar] [CrossRef]
- Mohammad, A.A.; Salem, S.H.; Amer, H.M.; Hussein, M.S. Ozone as a postharvest treatment to maintain the quality characteristics of fresh-cut plants. J. Food Meas. Charact. 2025, 19, 4325–4336. [Google Scholar] [CrossRef]
- Calva, J.; Ludeña, C.; Bec, N.; Larroque, C.; Salinas, M.; Vidari, G.; Armijos, C. Constituents and Selective BuChE Inhibitory Activity of the Essential Oil from Hypericum aciculare Kunth. Plants 2023, 12, 2621. [Google Scholar] [CrossRef]
- Duran, H.E. Pyrimidines: Molecular docking and inhibition studies on carbonic anhydrase and cholinesterases. Biotechnol. Appl. Biochem. 2023, 70, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, P.; Yang, C.; Yin, Q.; Wang, N.; Liu, Y.; Li, Y. Discovery, Biological Evaluation and Binding Mode Investigation of Novel Butyrylcholinesterase Inhibitors Through Hybrid Virtual Screening. Molecules 2025, 30, 2093. [Google Scholar] [CrossRef] [PubMed]
- Almarmouri, C.; El-Gamal, M.I.; Haider, M.; Hamad, M.; Qumar, S.; Sebastian, M.; Ghemrawi, R.; Muhammad, J.S.; Burucoa, C.; Khoder, G. Anti-urease therapy: A targeted approach to mitigating antibiotic resistance in Helicobacter pylori while preserving the gut microflora. Gut Pathog. 2025, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- El-Gazzar, N.; Said, L.; Al-Otibi, F.O.; AbdelGawwad, M.R.; Rabie, G. Antimicrobial and cytotoxic activities of natural (Z)-13-docosenamide derived from Penicillium chrysogenum. Front. Cell. Infect. Microbiol. 2025, 15, 1529104. [Google Scholar] [CrossRef]
- Sun, Q. The Hydrophobic Effects: Our Current Understanding. Molecules 2022, 27, 7009. [Google Scholar] [CrossRef]
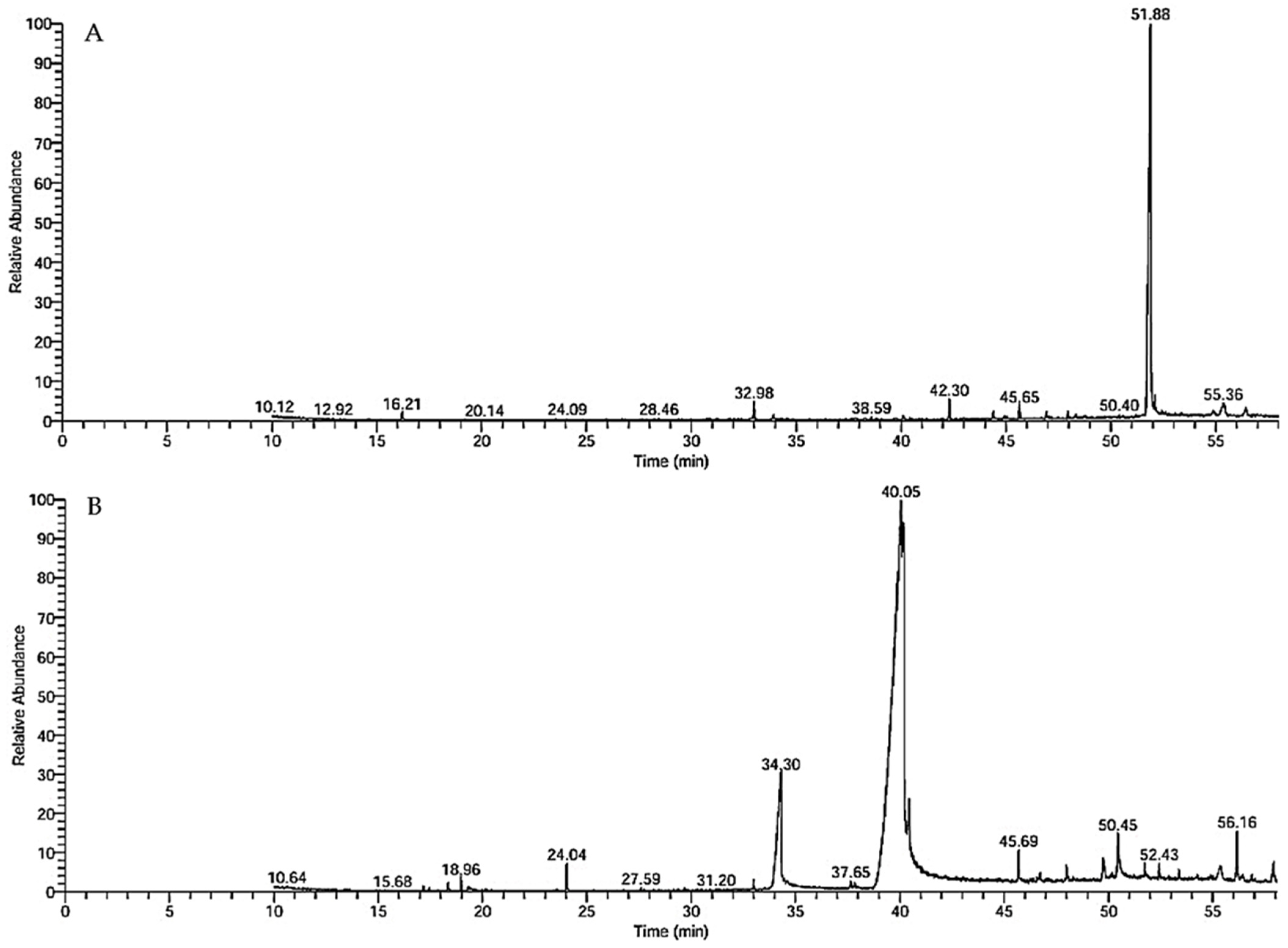



| Lettuce Oils | Ozonated Lettuce Oils | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound Name | Molecular Formula | Molecular Weight | RT (Raw) | Area (%) | Class | Compound Name | Molecular Formula | Molecular Weight | RT (O3) | Area (%) | Class |
| Benzaldehyde, 2,5-dimethyl- | C9H10O | 134 | 16.21 | 0.97 | Aromatic aldehyde | 2-Decenal, (Z)- | C10H18O | 154 | 17.45 | 0.16 | Aldehyde |
| 7,9-Di-tert-butyl-1-ox aspiro(4,5) deca-6,9-d iene-2,8-dione | C17H24O3 | 276 | 32 98 | 2.38 | Spirocyclic diketone | 2,4-Decadienal, (E, E)- | C10H16O | 152 | 18.96 | 1.04 | Aldehyde |
| n-Hexadecanoic acid | C16H32O2 | 256 | 33.90 | 0.94 | Fatty acid | 1,3-Benzodioxol-5-ol | C7H6O3 | 138 | 19.32 | 0.24 | Benzodioxole |
| Heptadecanoic acid, 9-methyl-, methyl ester | C19H38O2 | 298 | 38.58 | 0.44 | Fatty acid ester | Methyl 4,4,7-trimethyl- 4,7-dihydroindan-6-carboxylate | C14H20O2 | 220 | 24.04 | 1.36 | Indane |
| Hexadecanamide | C16H33NO | 255 | 40.11 | 0.57 | Fatty amide | E-7-Tetradecenol | C14H28O | 212 | 27.59 | 0.11 | Fatty alcohol |
| trans-13-Octadecenoic acid | C18H34O2 | 282 | 42.30 | 4.04 | Fatty acid | 7,9-Di-tert-butyl-1-ox aspiro(4,5) deca-6,9-d iene-2,8-dione | C17H24O3 | 276 | 32.98 | 0.53 | Sspirocyclic diketone |
| 9-Octadecenamide, (Z)- | C18H35NO | 281 | 44.39 | 1.4 | Fatty amide | trans-13-Octadecenoic acid | C18H34O2 | 282 | 34.29 | 15.39 | Fatty acid |
| Eicosanoic acid, ethyl ester | C22H44O2 | 340 | 45.05 | 0.36 | Fatty acid ester | 9,12-Octadecadienoic acid, methyl ester, (E, E)- | C19H34O2 | 294 | 37.65 | 4.86 | Fatty acid ester |
| Phenol, 2,2′-methylenebis[6-(1,1-dimethylethyl)-4-methyl-] | C23H32O2 | 340 | 45.64 | 1.94 | Phenol | 9-Octadecenoic acid, methyl ester, (E)- | C19H36O2 | 296 | 37.84 | 0.70 | Fatty acid ester |
| Methyl erucate | C23H44O2 | 352 | 46.93 | 1.71 | Fatty acid ester | 13-Docosenamide, (Z) | C22H43NO | 282 | 40.05 | 55.96 | Fatty amide |
| Isochiapin B | C19H22O6 | 346 | 47.03 | 1.10 | Sesquiterpene lactone | Octadecanoic acid | C18H36O2 | 284 | 40.5 | 3.74 | Fatty acid |
| 1,2-Benzenedicarboxylic acid | C24H38O4 | 390 | 47.96 | 0.83 | Carboxylic acid | Phenol, 2,2′-methylenebis[6-(1,1-dimethylethyl)-4-methyl-] | C23H32O2 | 340 | 45.69 | 1.83 | Phenol |
| 13-Docosenamide, (Z)- | C22H43NO | 337 | 51.86 | 78.41 | Fatty amide | Linoleic acid ethyl ester | C20H36O2 | 308 | 46.59 | 0.81 | Fatty acid ester |
| Ethyl iso-allocholate | C26H44O5 | 436 | 52.43 | 0.44 | Steroid | 12-Methyl-E, E-2,13 octadecadien-1-ol | C19H36O | 280 | 47.06 | 0.17 | Fatty alcohol |
| Oleic acid, 3-(octadecyloxy)prop yl ester | C39H76O3 | 592 | 54.88 | 2.89 | Fatty acid ester | n-Propyl 9,12-octadecadienoate | C21H38O2 | 322 | 48.01 | 0.68 | Fatty acid ester |
| Dotriacontane | C32H66 | 450 | 56.43 | 1.58 | Alkane | Oleic Acid | C18H34O2 | 282 | 49.73 | 1.48 | Fatty acid |
| trans-9-Octadecenoic acid, pentyl ester | C23H44O2 | 352 | 50.45 | 3.74 | Fatty acid ester | ||||||
| Ethyl iso-allocholate | C26H44O5 | 436 | 51.73 | 1.03 | Steroid | ||||||
| n-Hexadecanoic acid | C16H32O2 | 256 | 52.43 | 0.76 | Fatty acid | ||||||
| 9-Octadecenoic acid (Z)-, oxiranylmethyl ester | C21H38O3 | 338 | 54.27 | 0.95 | Fatty acid ester | ||||||
| Dotriacontane | C32H66 | 450 | 55.73 | 2.57 | Alkane | ||||||
| ç-Sitosterol | C29H50O | 414 | 57.01 | 1.89 | Phytosterol | ||||||
| Sample Code | Inhibition Zone (mm) | MIC µg/mL | MBC µg/mL | MBC/MIC |
|---|---|---|---|---|
| Crude lettuce oil | 13.7 ± 0.6 a | 62.5 ± 0.3 a | 125 ± 0.8 a | 2 a |
| Lettuce oil + O3 | 21.3 ± 0.3 a | 31.25 ± 0.5 b | 62.5 ± 0.2 b | 2 a |
| Standard drug | 15.3 ± 0.4 a | 31.25 ± 0.6 b | 13.25 ± 0.6 c | 1 b |
| Mol | S | rmsd_refine | E_conf | E_place | E_score1 | E_refine | E_score2 |
|---|---|---|---|---|---|---|---|
| 13-Docosenamide | −8.13092 | 1.5926156 | −19.6867 | −72.8444 | −9.60851 | −39.1181 | −8.13092 |
| 13-Docosenamide | −8.04835 | 1.7194235 | −20.1995 | −74.5038 | −9.65519 | −41.7513 | −8.04835 |
| 13-Docosenamide | −7.62981 | 2.3539269 | −19.1456 | −40.5661 | −10.4494 | −41.0256 | −7.62981 |
| 13-Docosenamide | −7.54724 | 1.1664486 | −7.69996 | −69.2003 | −9.77358 | −36.6556 | −7.54724 |
| 13-Docosenamide | −7.52264 | 1.743559 | −12.6639 | −74.552 | −9.41631 | −32.9138 | −7.52264 |
| trans-13-Octadecenoic acid | −7.31024 | 2.458077 | −13.897 | −58.9793 | −9.9934 | −40.7344 | −7.31024 |
| trans-13-Octadecenoic acid | −7.19911 | 1.197924 | −13.8079 | −67.8339 | −9.94211 | −32.6767 | −7.19911 |
| trans-13-Octadecenoic acid | −7.19094 | 1.1504909 | −16.0386 | −58.7043 | −9.75688 | −39.1273 | −7.19094 |
| trans-13-Octadecenoic acid | −7.10033 | 3.2766879 | −16.3373 | −62.915 | −10.9963 | −36.7538 | −7.10033 |
| trans-13-Octadecenoic acid | −7.0948 | 1.0196184 | −11.9226 | −62.6675 | −10.2318 | −34.4483 | −7.0948 |
| Mol | Ligand | Receptor | Interaction | Distance (Å) | E (kcal/mol) |
|---|---|---|---|---|---|
| 13-Docosenamide | N 2 | OE1 GLU 276 (A) | H-donor | 3.42 | −0.6 |
| C 35 | 5-ring TRP 82 (A) | H-pi | 3.72 | −0.7 | |
| trans-13-Octadecenoic acid | O 1 | O SER 287 (A) | H-donor | 2.97 | −1.3 |
| C 39 | 6-ring TRP 82 (A) | H-pi | 3.69 | −0.8 |
| Mol | S | rmsd_refine | E_conf | E_place | E_score1 | E_refine | E_score2 |
|---|---|---|---|---|---|---|---|
| 13-Docosenamide | −6.00058 | 2.5410826 | −21.2019 | −42.7297 | −9.22741 | −32.8763 | −6.00058 |
| 13-Docosenamide | −5.9666 | 2.395309 | −19.3768 | −88.8088 | −9.60097 | −32.495 | −5.9666 |
| 13-Docosenamide | −5.9415 | 1.8721349 | −17.2656 | −71.2884 | −9.55546 | −31.9705 | −5.9415 |
| 13-Docosenamide | −5.88933 | 2.118561 | −21.9907 | −57.6622 | −9.80536 | −31.4298 | −5.88933 |
| 13-Docosenamide | −5.88896 | 2.0570378 | −15.985 | −69.9133 | −8.96797 | −29.6238 | −5.88896 |
| trans-13-Octadecenoic acid | −7.33015 | 1.6620129 | −19.1551 | −54.2166 | −9.41694 | −36.3137 | −7.33015 |
| trans-13-Octadecenoic acid | −7.15466 | 1.249053 | −20.4189 | −52.7527 | −9.34551 | −35.3725 | −7.15466 |
| trans-13-Octadecenoic acid | −7.02536 | 1.0522252 | −13.2661 | −42.4143 | −9.99574 | −26.2253 | −7.02536 |
| trans-13-Octadecenoic acid | −6.93917 | 1.3046346 | −18.3703 | −59.747 | −9.90554 | −33.8415 | −6.93917 |
| trans-13-Octadecenoic acid | −6.86541 | 2.0998428 | −19.6161 | −42.7127 | −9.0778 | −34.6881 | −6.86541 |
| Mol | Ligand | Receptor | Interaction | Distance | E (kcal/mol) |
|---|---|---|---|---|---|
| 13-Docosenamide, (z) | O 1 | NH1 ARG 375 (B) | H-acceptor | 3.05 | −0.7 |
| trans-13-Octadecenoic acid | O 1 | OE1 GLU 313 (B) | H-donor | 2.83 | −6.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazaid, A.S.; Alsalamah, S.A.; Hakami, W.; Alghonaim, M.I.; Duhduh, A.; Qanash, H. Ozone-Assisted Green Upgrading of Lactuca sativa Oil: Characterization and Bioactivity for Clean-Label Functional Applications. Foods 2025, 14, 3458. https://doi.org/10.3390/foods14203458
Bazaid AS, Alsalamah SA, Hakami W, Alghonaim MI, Duhduh A, Qanash H. Ozone-Assisted Green Upgrading of Lactuca sativa Oil: Characterization and Bioactivity for Clean-Label Functional Applications. Foods. 2025; 14(20):3458. https://doi.org/10.3390/foods14203458
Chicago/Turabian StyleBazaid, Abdulrahman S., Sulaiman A. Alsalamah, Waleed Hakami, Mohammed Ibrahim Alghonaim, Amro Duhduh, and Husam Qanash. 2025. "Ozone-Assisted Green Upgrading of Lactuca sativa Oil: Characterization and Bioactivity for Clean-Label Functional Applications" Foods 14, no. 20: 3458. https://doi.org/10.3390/foods14203458
APA StyleBazaid, A. S., Alsalamah, S. A., Hakami, W., Alghonaim, M. I., Duhduh, A., & Qanash, H. (2025). Ozone-Assisted Green Upgrading of Lactuca sativa Oil: Characterization and Bioactivity for Clean-Label Functional Applications. Foods, 14(20), 3458. https://doi.org/10.3390/foods14203458










