Quality Control of Fried Pepper Oils Based on GC-MS Fingerprints and Chemometrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction of Volatile Compounds from Fried Pepper Oils via HS-SPME
2.3. GC-MS Analysis
2.4. Qualitative and Quantitative Analysis
2.5. Methodology Validation
2.6. Similarities Analysis
2.7. Application of Established GC-MS Fingerprints on Quality Control and Discrimination Products from Other Companies
2.8. Statistics Analysis
2.8.1. Hierarchical Cluster Analysis (HCA)
2.8.2. Principal Component Analysis (PCA)
2.8.3. OPLS-DA Analysis
3. Results and Discussion
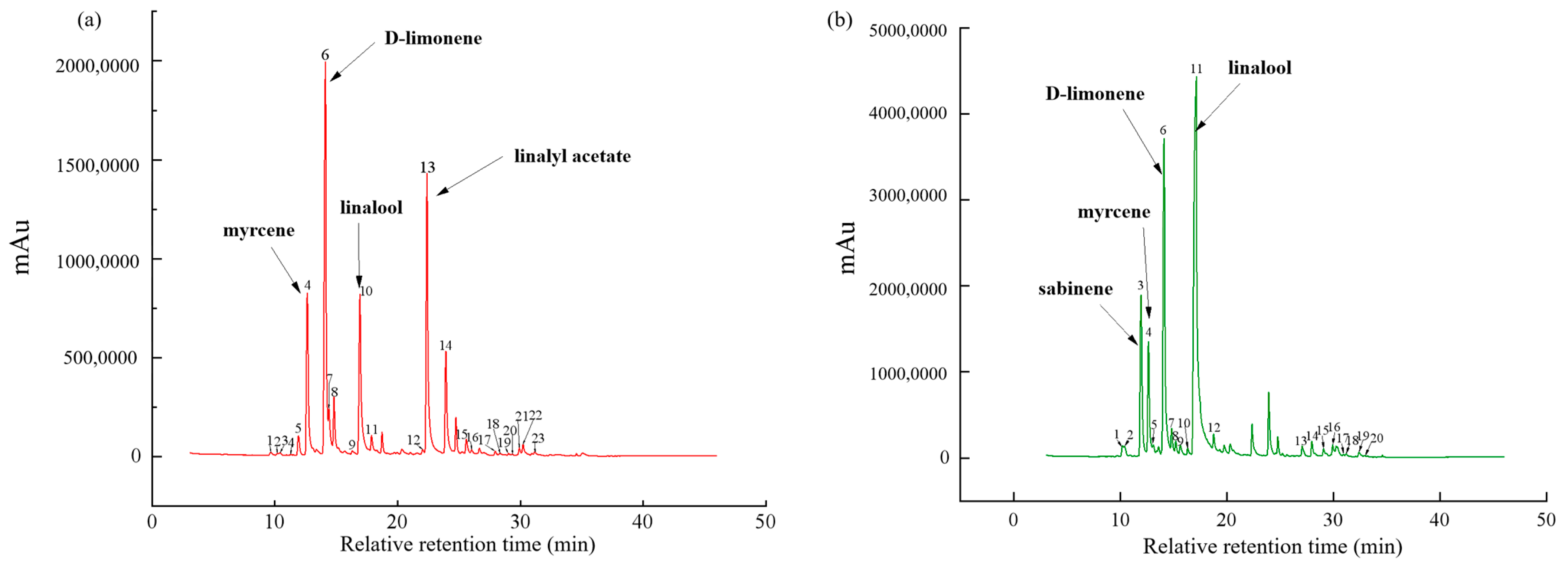
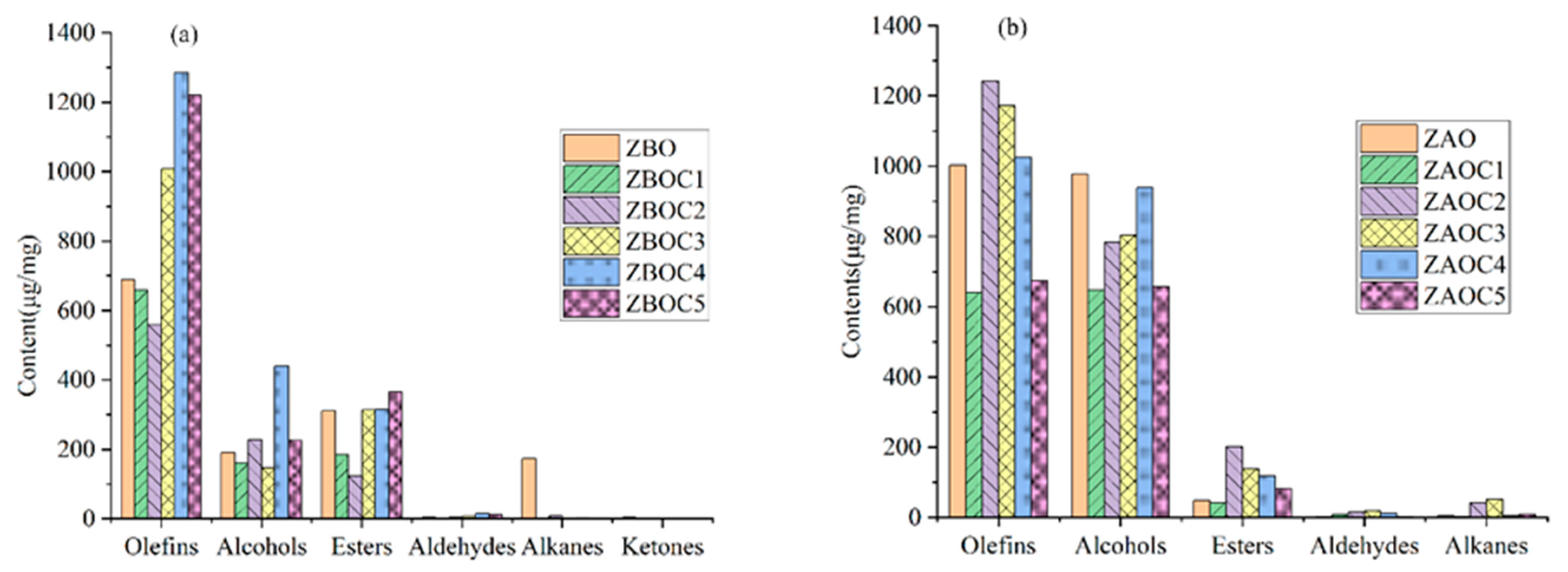
3.1. Volatile Compounds of Huajiao Oils
3.2. Volatile Compounds of Tengjiao Oils
3.3. Identification of the Common Peaks
3.4. Quality Evaluation of Fingerprints
3.5. Determination of Similarity
3.6. Discrimination of Different Fried Pepper Oils Based on Similarity
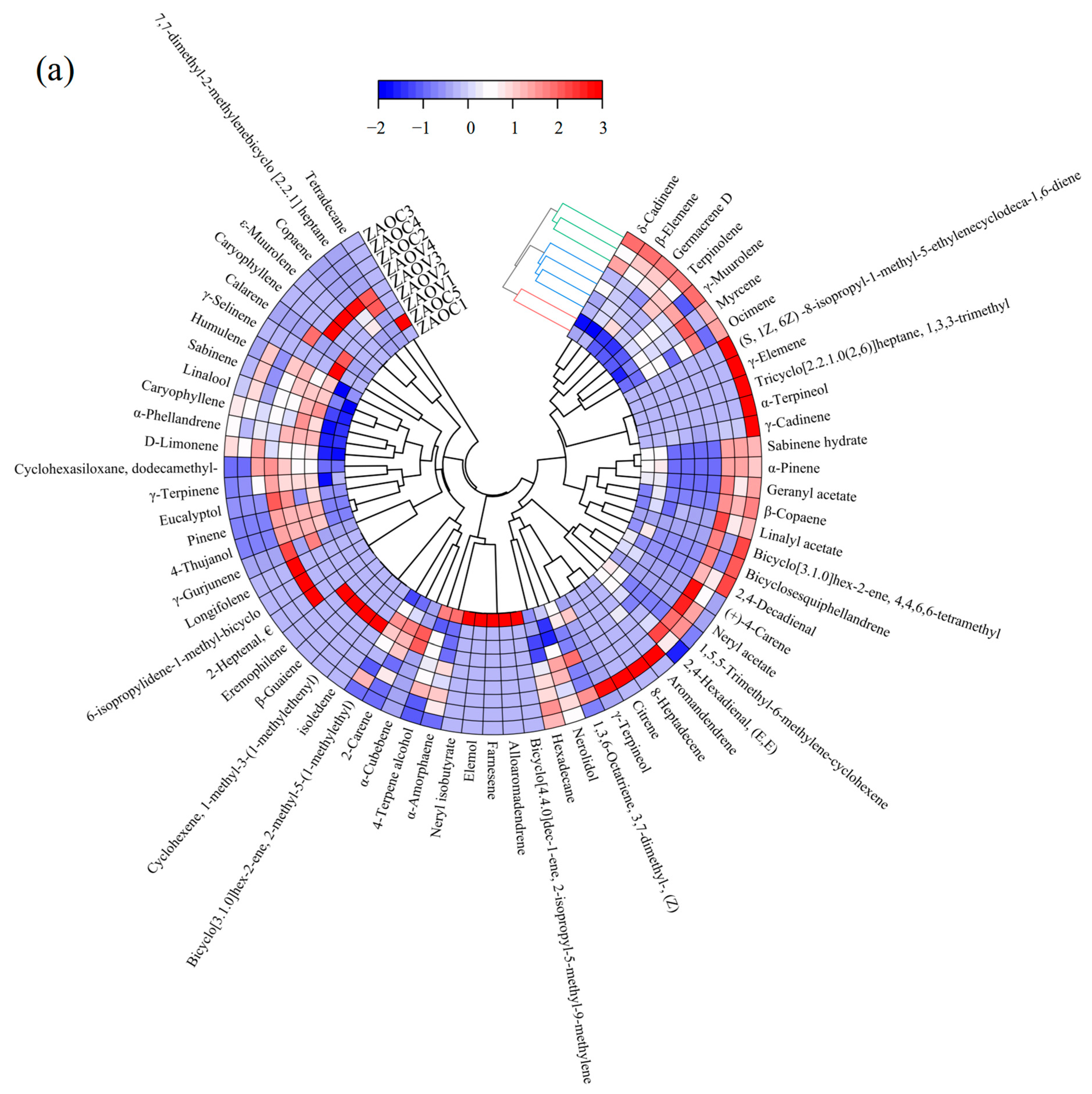
3.7. Hierarchical Clustering Analysis (HCA)
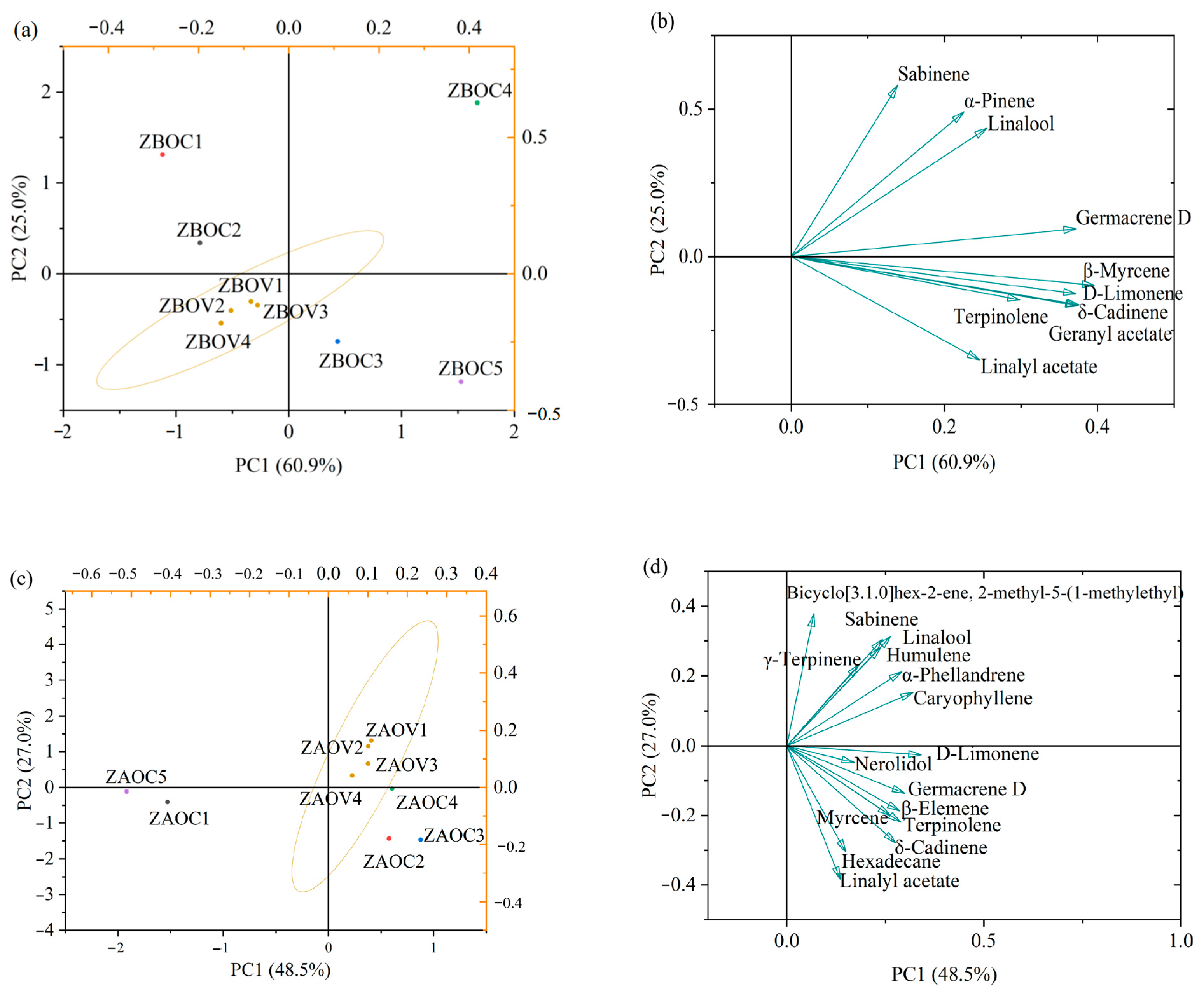
3.8. Principal Component Analysis (PCA)
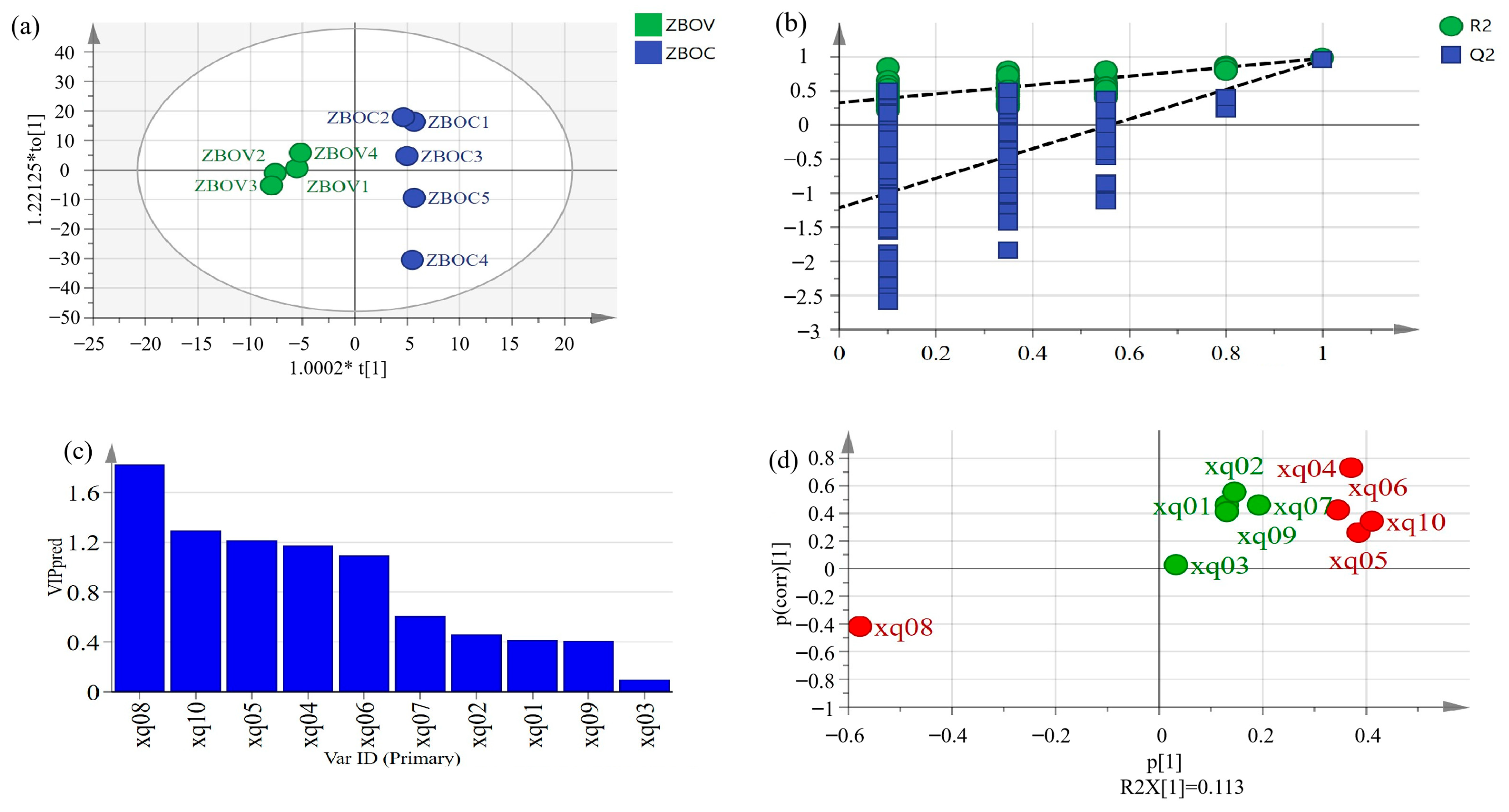
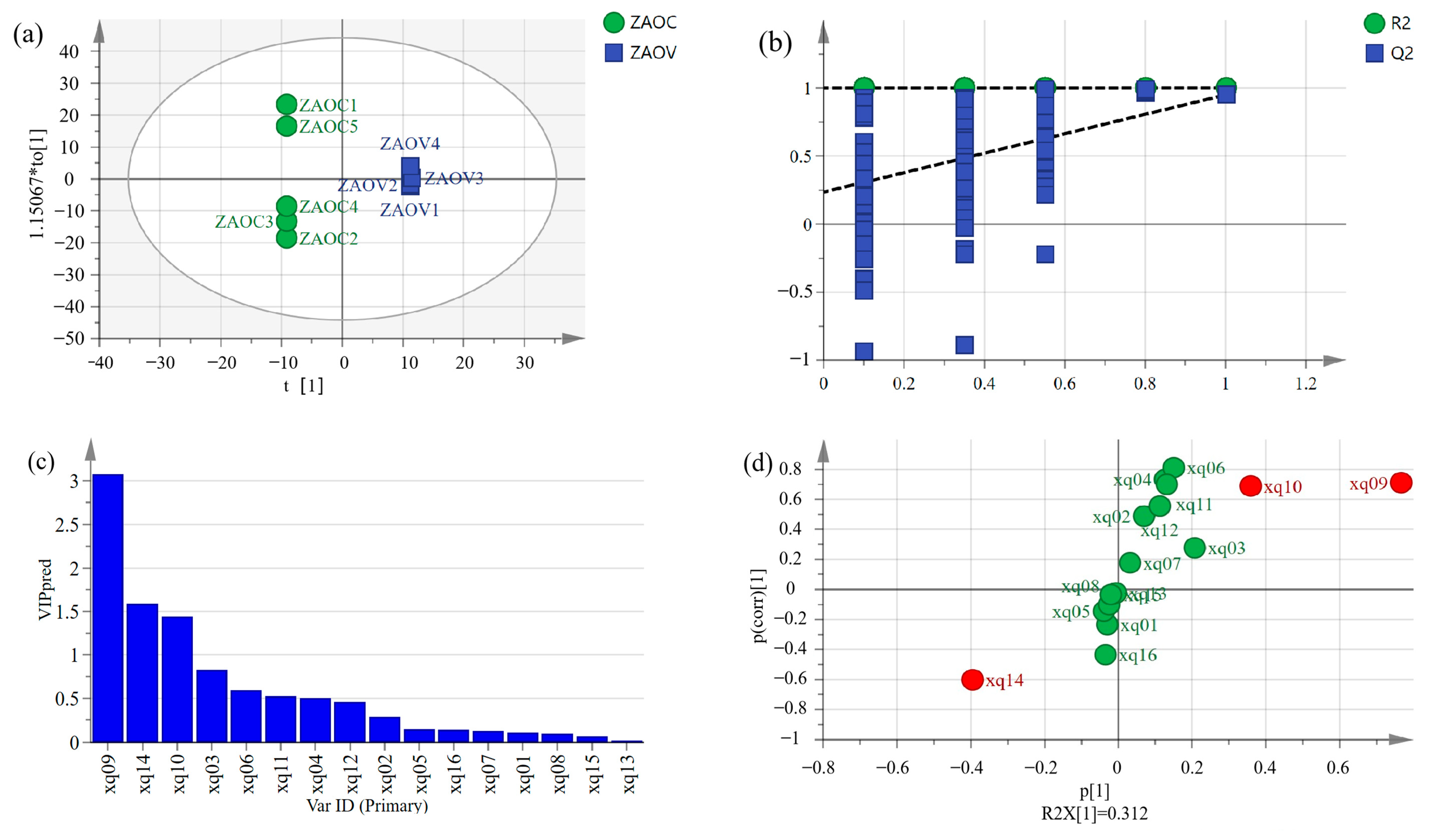
3.9. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPLC | High-performance liquid chromatography |
| GC | Gas chromatography |
| TLC | Thin layer chromatography |
| SPME | Solid-phase microextraction |
| GC-MS | Gas chromatography–mass spectrometry |
| PCA | Principal component analysis |
| HCA | Hierarchical cluster analysis |
| SFDA | State Food and Drug Administration |
| SD | Standard deviation |
| RSD | Relative standard deviation |
References
- Hou, X.; Li, S.; Luo, Q.; Shen, G.; Wu, H.; Li, M.; Liu, X.; Chen, A.; Ye, M.; Zhang, Z. Discovery and identification of antimicrobial peptides in Sichuan pepper (Zanthoxylum bungeanum Maxim) seeds by peptidomics and bioinformatics. Appl. Microbiol. Biotechnol. 2019, 103, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Chaoyang, F.; Shanshan, L.; Qingying, L.; Guanghui, S.; Zhiqing, Z. Mechanism of antimicrobial peptide NP-6 from Sichuan pepper seeds against E. coli and effects of different environmental factors on its activity. Appl. Microbiol. Biotechnol. 2019, 103, 6593–6604. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, Y.; Peng, Z.; Li, C.; Cui, Q.; Chen, A.; Zhang, Z.; Gu, X.; Zhu, X.; Li, J. Fingerprinting of hydroxyl-sanshools in fried pepper oils by HPLC-DAD in combination with chemometrics. LWT 2023, 183, 114935. [Google Scholar] [CrossRef]
- Ji, Y.; Li, S.; Ho, C.T. Chemical composition, sensory properties and application of Sichuan pepper (Zanthoxylum genus). Food Sci. Hum. Wellness 2019, 8, 11. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, L.; Li, R.; Yan, Y.; Yin, J.; Dai, Q.; Guo, X.; Li, W.; Li, Y.; Liu, M. In vitro and in vivo antiviral activity of Maqian (Zanthoxylum myriacanthum var. pubescens) essential oil and its major constituents against strains of influenza virus. Ind. Crop. Prod. 2022, 177, 114524. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, L.; Han, N.; Wang, D.M. Characterization of phenolic chemotypes, anatomy, and histochemistry of Zanthoxylum bungeanum Maxim. Ind. Crop. Prod. 2023, 193, 116149. [Google Scholar] [CrossRef]
- Chang, D.D.; Yang, Y.; Tao, F.Y.; Ding, Y.; Jian, M.L.; Huang, Q.W. Correlation between climatic environment and characteristic components of 14 kinds of huajiao by thermal analysis techniques, GC-MS and HS-IMS. Food Sci. Nutr. 2024, 12, 4783–4799. [Google Scholar] [CrossRef]
- Ni, R.J.; Yan, H.Y.; Tian, H.L.; Zhan, P.; Zhang, Y.Y. Characterization of key odorants in fried red and green huajiao (Zanthoxylum bungeanum maxim. and Zanthoxylum schinifolium sieb. et Zucc.) oils. Food Chem. 2022, 377, 131984. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.Y.; Tian, Z.L.; Wang, S.W.; Sun, J.; Chen, H.T.; Wang, S.Q.; Sun, B.G. Identification of key off-flavor compounds during storage of fried pepper (Zanthoxylum bungeanum Maxim.) oils by sensory-directed flavor analysis and partial least squares regression (PLSR). J. Food Compos. Anal. 2024, 131, 106268. [Google Scholar] [CrossRef]
- Liu, R.J.; Qi, N.; Sun, J.; Chen, H.T.; Zhang, N.; Sun, B.G. Effects of Frying Conditions on Volatile Composition and Odor Characteristics of Fried Pepper (Zanthoxylum bungeanum Maxim.) Oil. Foods 2022, 11, 1661. [Google Scholar] [CrossRef]
- Shi, T.; Wu, G.C.; Jin, Q.Z.; Wang, X.G. Detection of camellia oil adulteration using chemometrics based on fatty acids GC fingerprints and phytosterols GC-MS fingerprints. Food Chem. 2021, 352, 129422. [Google Scholar] [CrossRef]
- Esteki, M.; Shahsavari, Z.; Simal-Gandara, J. Food identification by high performance liquid chromatography fingerprinting and mathematical processing. Food Res. Int. 2019, 122, 303–317. [Google Scholar] [CrossRef]
- Quintanilla-Casas, B.; Bertin, S.; Leik, K.; Bustamante, J.; Guardiola, F.; Valli, E.; Bendini, A.; Gallina Toschi, T.; Tres, A.; Vichi, S. Profiling versus fingerprinting analysis of sesquiterpene hydrocarbons for the geographical authentication of extra virgin olive oils. Riv. Ital. Sostanze Grasse. 2021, 98, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, C.; Rodríguez-Tecedor, S.; Pérez-del-Notario, N.; González-Sáiz, J.M. Recognition of volatile compounds as markers in geographical discrimination of Spanish extra virgin olive oils by chemometric analysis of non-specific chromatography volatile profiles. J. Chromatogr. A 2011, 1218, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, B.G.; Ren, F.Z.; Chen, H.T.; Zhang, N.; Zhang, Y.Y.; Zhang, H.Y. Effects of storage conditions on the flavor stability of fried pepper (Zanthoxylum bungeanum) oil. Foods. 2021, 10, 1292. [Google Scholar] [CrossRef]
- Niu, W.J.; Tian, H.L.; Zhan, P. The Effects of Pepper (Zanthoxylum bungeanum) from different production areas on the volatile flavor compounds of fried pepper oils based on HS-SPME-GC-MS and multivariate statistical method. Molecules 2022, 27, 7760. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Fang, L.; Li, Z.; Xie, X.; Zhang, L. A HPLC fingerprint study on Chaenomelis Fructus. BMC Chem. 2019, 13, 7. [Google Scholar] [CrossRef]
- Xue, Y.; Xie, J.; Xu, X.S.; Yong, L.; Hu, B.; Liang, J.; Li, X.D.; Qing, L.S. UPLC-QqQ/MS combined with similarity assessment of 17 nucleic acid constituents in 147 edible fungi from Sichuan Basin, China. Food Res. Int. 2019, 120, 577–585. [Google Scholar] [CrossRef]
- Gu, H.; Yin, X.; Ma, Y.; Wang, J.; Yang, F.; Sun, W.; Ding, B.; Chen, Y.; Liu, Z. Differentiating grades of Xihu Longjing teas according to the contents of ten major components based on HPLC-DAD in combination with chemometrics. LWT-Food Sci. Technol. 2020, 130, 109688. [Google Scholar] [CrossRef]
- Bhumireddy, S.R.; Rocchetti, G.; Pallerla, P.; Lucini, L.; Sripadi, P. A combined targeted/untargeted screening based on GC/MS to detect low-molecular-weight compounds in different milk samples of different species and as affected by processing. Int. Dairy J. 2021, 118, 105045. [Google Scholar] [CrossRef]
- Sun, J.B.F.H. Characterization of key odorants in Hanyuan and Hancheng fried pepper (Zanthoxylum bungeanum) oil. J. Agric. Food. Chem. 2020, 68, 6403–6411. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Chen, K.; Bhandari, B.; Deng, D.W. Impact of cooking methods on the quality, sensory and flavor compounds of Sichuan pepper oleoresin. Food Chem. 2023, 427, 136639. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jeon, J.; Kwon, H.H. Characterization and evaluation of changes in the aroma-active components in Szechuan pepper (Zanthoxylum bungeanum Maxim) under different cooking temperatures using Gas Chromatography-Olfactometry. Chemosens. Percept. 2019, 12, 32–39. [Google Scholar] [CrossRef]
- Zhao, M.; Li, T.; Yang, F.; Cui, X.; Zou, T.; Song, H.; Liu, Y. Characterization of key aroma-active compounds in Hanyuan Zanthoxylum bungeanum by GC-O-MS and switchable GC×GC-O-MS. Food Chem. 2022, 385, 132659. [Google Scholar] [CrossRef]
- Liu, Y.P.; Li, Q.R.; Yang, W.X.; Sun, B.G.; Zhou, Y.; Zheng, Y.; Huang, M.Q.; Yang, W.J. Characterization of the potent odorants in Zanthoxylum armatum DC Prodr. pericarp oil by application of gas chromatography-mass spectrometry-olfactometry and odor activity value. Food Chem. 2020, 319, 126564. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, B.; An, Y.Z.; Yang, G.; Liao, S.L.; Kan, J.Q. Comprehensive study on nutritional, flavour and metabolite characteristics of green prickly ash (Zanthoxylum schinifolium) at different picking stages. Int. J. Food Sci. Technol. 2022, 57, 7961–7973. [Google Scholar] [CrossRef]
- Zeng, C.Y.; Wei, Q.M.; Pu, F.L.; Liu, Y.; Sun, W.F.; Che, Z.M.; Huang, Y.K. Discrimination of Zanthoxylum bungeanum Maxim through volatile aroma compounds analysis with artificial neural network. J. Food Biochem. 2021, 45, e13621. [Google Scholar] [CrossRef]
- Gong, F.; Liang, Y.; Xie, P.; Chau, F. Information theory applied to chromatographic fingerprint of herbal medicine for quality control. J. Chromatogr. A 2003, 1002, 25–40. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, S.; Xie, M.; Nie, S.; Liu, W.; Li, C.; Gong, X.; Wang, Y. Quality control and original discrimination of Ganoderma lucidum based on high-performance liquid chromatographic fingerprints and combined chemometrics methods. Anal. Chim. Acta. 2008, 623, 146–156. [Google Scholar] [CrossRef]
- Dai, W.; Hu, L.; Ji, L.; Li, J.; Bi, K.; Li, Q. A Comprehensive Method for Quality Evaluation of Houttuyniae Herba by a Single Standard to Determine Multi-components, Fingerprint and HPTLC Method. Anal. Sci. 2015, 31, 535–541. [Google Scholar] [CrossRef]
- Tomar, M.; Bhardwaj, R.; Kumar, M.; Pal Singh, S.; Krishnan, V.; Kansal, R.; Verma, R.; Yadav, V.K.; Dahuja, A.; Ahlawat, S.P.; et al. Nutritional composition patterns and application of multivariate analysis to evaluate indigenous Pearl millet (Pennisetum glaucum (L.) R. Br.) germplasm. J. Food Compos. Anal. 2021, 103, 104086. [Google Scholar] [CrossRef]
- Florentino-Ramos, E.; Villa-Ruano, N.; Hidalgo-Martinez, D.; Ramirez-Meraz, M.; Mendez-Aguilar, R.; Velasquez-Valle, R.; Gerardo Zepeda-Vallejo, L.; Perez-Hernandez, N.; Becerra-Martinez, E. 1H NMR-based fingerprinting of eleven Mexican Capsicum annuum cultivars. Food Res. Int. 2019, 121, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zhang, M.; Zhang, Y.; Wang, H.; Li, S.; Dai, Z.; Shen, Q. In Situ method for real-time discriminating salmon and rainbow trout without sample preparation using iKnife and rapid evaporative ionization mass spectrometry-based lipidomics. J. Agric. Food. Chem. 2019, 67, 4679–4688. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Zhang, Y.; Zhang, M.; Qi, J.; Zhao, W.; Gu, J.; Guo, W.; Li, Y. Screening of specific quantitative peptides of beef by LC—MS/MS coupled with OPLS-DA. Food Chem. 2022, 387, 132932. [Google Scholar] [CrossRef]

| Huajiao Oils | Tengjiao Oils | ||
|---|---|---|---|
| Common Peaks | Relative Retention Time (min, RRT) | Common Peaks | Relative Retention Time (min, RRT) |
| 1 | 9.68 | 1 | 10.23 |
| 2 | 10.21 | 2 | 10.41 |
| 3 | 10.40 | 3 | 11.94 |
| 4 | 11.93 | 4 | 12.64 |
| 5 | 12.65 | 5 | 13.10 |
| 6 | 14.11 | 6 | 14.10 |
| 7 | 14.41 | 7 | 14.81 |
| 8 | 14.82 | 8 | 15.20 |
| 9 | 16.31 | 9 | 15.62 |
| 10 | 16.93 | 10 | 16.32 |
| 11 | 17.88 | 11 | 17.14 |
| 12 | 22.05 | 12 | 22.35 |
| 13 | 22.40 | 13 | 27.05 |
| 14 | 25.60 | 14 | 27.95 |
| 15 | 26.03 | 15 | 29.05 |
| 16 | 26.66 | 16 | 29.91 |
| 17 | 27.96 | 17 | 30.92 |
| 18 | 28.35 | 18 | 31.16 |
| 19 | 29.05 | 19 | 32.38 |
| 20 | 29.34 | 20 | 33.19 |
| 21 | 29.92 | ||
| 22 | 30.94 | ||
| 23 | 31.17 | ||
| Peak No. | Precision (RSD, %) | Repeatability (RSD, %) | Stability (RSD, %) | |||
|---|---|---|---|---|---|---|
| RRT | RPA | RRT | RPA | RRT | RPA | |
| 1 | 1.81 | 0.32 | 1.05 | 0.28 | 0.99 | 0.35 |
| 2 | 1.66 | 0.66 | 2.21 | 0.33 | 1.45 | 0.27 |
| 3 | 1.58 | 0.34 | 1.98 | 0.56 | 1.88 | 0.36 |
| 4 | 1.33 | 0.32 | 1.66 | 0.92 | 2.01 | 0.15 |
| 5 | 2.56 | 0.21 | 1.88 | 0.88 | 2.13 | 0.22 |
| 6 | 2.62 | 0.69 | 2.05 | 0.52 | 1.77 | 0.37 |
| 7 | 1.88 | 0.36 | 1.96 | 0.49 | 2.05 | 0.29 |
| 8 | 2.79 | 0.29 | 1.85 | 0.66 | 2.13 | 0.48 |
| 9 | 1.92 | 0.38 | 1.66 | 1.02 | 1.97 | 0.55 |
| 10 | 1.45 | 0.41 | 1.79 | 0.76 | 1.96 | 0.29 |
| 11 | 2.06 | 0.36 | 1.91 | 0.68 | 2.45 | 0.37 |
| 12 | 2.33 | 0.55 | 2.05 | 0.81 | 2.85 | 0.36 |
| 13 | 1.96 | 0.38 | 2.11 | 0.91 | 2.46 | 0.39 |
| 14 | 1.46 | 0.62 | 1.91 | 0.53 | 2.11 | 0.41 |
| 15 | 1.88 | 0.56 | 1.88 | 0.68 | 1.98 | 0.32 |
| 16 | 2.76 | 0.76 | 1.67 | 0.99 | 2.03 | 0.26 |
| 17 | 2.08 | 0.55 | 2.06 | 0.76 | 2.66 | 0.33 |
| 18 | 2.11 | 0.62 | 2.13 | 0.62 | 2.25 | 0.51 |
| 19 | 1.99 | 0.39 | 1.98 | 0.77 | 2.20 | 0.33 |
| 20 | 2.33 | 0.31 | 2.14 | 0.72 | 2.08 | 0.27 |
| Similarity | ZBO1 | ZBO2 | ZBO3 | ZBO4 | ZBO5 | ZBO6 | ZBO7 | ZBO8 | ZBO9 | ZBO10 | ZBO11 | ZBO12 | ZBO13 | ZBO14 | ZBO15 | ZBO16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZBO1 | 1.000 | |||||||||||||||
| ZBO2 | 0.998 | 1.000 | ||||||||||||||
| ZBO3 | 0.998 | 0.999 | 1.000 | |||||||||||||
| ZBO4 | 0.993 | 0.998 | 0.996 | 1.000 | ||||||||||||
| ZBO5 | 0.995 | 0.998 | 0.998 | 0.998 | 1.000 | |||||||||||
| ZBO6 | 0.995 | 0.996 | 0.995 | 0.996 | 0.996 | 1.000 | ||||||||||
| ZBO7 | 0.978 | 0.979 | 0.977 | 0.980 | 0.979 | 0.986 | 1.000 | |||||||||
| ZBO8 | 0.984 | 0.982 | 0.981 | 0.981 | 0.977 | 0.985 | 0.992 | 1.000 | ||||||||
| ZBO9 | 0.971 | 0.976 | 0.972 | 0.981 | 0.975 | 0.981 | 0.985 | 0.981 | 1.000 | |||||||
| ZBO10 | 0.983 | 0.983 | 0.982 | 0.982 | 0.983 | 0.992 | 0.994 | 0.989 | 0.981 | 1.000 | ||||||
| ZBO11 | 0.989 | 0.988 | 0.989 | 0.987 | 0.988 | 0.994 | 0.992 | 0.993 | 0.982 | 0.998 | 1.000 | |||||
| ZBO12 | 0.985 | 0.987 | 0.986 | 0.987 | 0.989 | 0.995 | 0.990 | 0.985 | 0.982 | 0.998 | 0.998 | 1.000 | ||||
| ZBO13 | 0.992 | 0.995 | 0.994 | 0.994 | 0.996 | 0.998 | 0.986 | 0.982 | 0.981 | 0.993 | 0.995 | 0.997 | 1.000 | |||
| ZBO14 | 0.996 | 0.997 | 0.997 | 0.995 | 0.997 | 0.998 | 0.983 | 0.984 | 0.978 | 0.991 | 0.995 | 0.994 | 0.998 | 1.000 | ||
| ZBO15 | 0.993 | 0.995 | 0.995 | 0.994 | 0.997 | 0.998 | 0.985 | 0.983 | 0.980 | 0.993 | 0.995 | 0.997 | 1.000 | 0.999 | 1.000 | |
| ZBO16 | 0.992 | 0.995 | 0.994 | 0.994 | 0.997 | 0.998 | 0.986 | 0.982 | 0.981 | 0.993 | 0.995 | 0.997 | 1.000 | 0.999 | 1.000 | 1.000 |
| R1 | 0.995 | 0.998 | 0.996 | 0.998 | 0.997 | 0.999 | 0.989 | 0.989 | 0.984 | 0.992 | 0.995 | 0.994 | 0.998 | 0.998 | 0.998 | 0.998 |
| Similarity | ZAO1 | ZAO2 | ZAO3 | ZAO4 | ZAO5 | ZAO6 | ZAO7 | ZAO8 | ZAO9 | ZAO10 | ZAO11 | ZAO12 | ZAO13 | ZAO14 | ZAO15 | ZAO16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZAO1 | 1.000 | |||||||||||||||
| ZAO2 | 0.999 | 1.000 | ||||||||||||||
| ZAO3 | 0.999 | 0.999 | 1.000 | |||||||||||||
| ZAO4 | 0.999 | 0.998 | 0.999 | 1.000 | ||||||||||||
| ZAO5 | 0.998 | 0.998 | 0.998 | 0.999 | 1.000 | |||||||||||
| ZAO6 | 0.998 | 0.998 | 0.998 | 0.998 | 0.999 | 1.000 | ||||||||||
| ZAO7 | 0.998 | 0.997 | 0.997 | 0.997 | 0.998 | 0.998 | 1.000 | |||||||||
| ZAO8 | 0.998 | 0.998 | 0.997 | 0.997 | 0.998 | 0.998 | 1.000 | 1.000 | ||||||||
| ZAO9 | 0.998 | 0.998 | 0.997 | 0.997 | 0.998 | 0.998 | 0.999 | 0.999 | 1.000 | |||||||
| ZAO10 | 0.998 | 0.998 | 0.997 | 0.997 | 0.998 | 0.998 | 0.999 | 0.999 | 0.999 | 1.000 | ||||||
| ZAO11 | 0.998 | 0.998 | 0.997 | 0.997 | 0.998 | 0.998 | 0.999 | 0.999 | 0.999 | 1.000 | 1.000 | |||||
| ZAO12 | 0.997 | 0.997 | 0.996 | 0.996 | 0.997 | 0.998 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 1.000 | ||||
| ZAO13 | 0.997 | 0.997 | 0.996 | 0.996 | 0.997 | 0.998 | 0.999 | 0.999 | 0.998 | 0.998 | 0.998 | 0.998 | 1.000 | |||
| ZAO14 | 0.995 | 0.996 | 0.994 | 0.993 | 0.996 | 0.996 | 0.997 | 0.997 | 0.997 | 0.997 | 0.997 | 0.998 | 0.997 | 1.000 | ||
| ZAO15 | 0.996 | 0.997 | 0.995 | 0.995 | 0.997 | 0.998 | 0.997 | 0.998 | 0.997 | 0.998 | 0.998 | 0.999 | 0.997 | 0.999 | 1.000 | |
| ZAO16 | 0.995 | 0.996 | 0.994 | 0.994 | 0.997 | 0.996 | 0.998 | 0.998 | 0.997 | 0.998 | 0.998 | 0.999 | 0.998 | 0.999 | 0.999 | 1.000 |
| R2 | 0.999 | 0.999 | 0.998 | 0.998 | 0.999 | 0.999 | 0.999 | 1.000 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.998 | 0.999 | 0.998 |
| Samples | R1 | ZBOC1 | ZBOC2 | ZBOC3 | ZBOC4 | ZBOC5 |
|---|---|---|---|---|---|---|
| R1 | 1.000 | |||||
| ZBOC1 | 0.958 | 1.000 | ||||
| ZBOC2 | 0.987 | 0.976 | 1.000 | |||
| ZBOC3 | 0.946 | 0.983 | 0.962 | 1.000 | ||
| ZBOC4 | 0.982 | 0.961 | 0.991 | 0.929 | 1.000 | |
| ZBOC5 | 0.980 | 0.944 | 0.987 | 0.915 | 0.997 | 1.000 |
| Samples | R2 | ZAOC1 | ZAOC2 | ZAOC3 | ZAOC4 | ZAOC5 |
|---|---|---|---|---|---|---|
| R2 | 1.000 | |||||
| ZAOC1 | 0.996 | 1.000 | ||||
| ZAOC2 | 0.989 | 0.991 | 1.000 | |||
| ZAOC3 | 0.996 | 0.995 | 0.993 | 1.000 | ||
| ZAOC4 | 0.997 | 0.995 | 0.991 | 0.998 | 1.000 | |
| ZAOC5 | 0.983 | 0.983 | 0.998 | 0.988 | 0.987 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, Y.; Cui, Q.; Zhang, Z.; Hou, X. Quality Control of Fried Pepper Oils Based on GC-MS Fingerprints and Chemometrics. Foods 2025, 14, 1624. https://doi.org/10.3390/foods14091624
Li J, Zhang Y, Cui Q, Zhang Z, Hou X. Quality Control of Fried Pepper Oils Based on GC-MS Fingerprints and Chemometrics. Foods. 2025; 14(9):1624. https://doi.org/10.3390/foods14091624
Chicago/Turabian StyleLi, Jianlong, Yu Zhang, Qiang Cui, Zhiqing Zhang, and Xiaoyan Hou. 2025. "Quality Control of Fried Pepper Oils Based on GC-MS Fingerprints and Chemometrics" Foods 14, no. 9: 1624. https://doi.org/10.3390/foods14091624
APA StyleLi, J., Zhang, Y., Cui, Q., Zhang, Z., & Hou, X. (2025). Quality Control of Fried Pepper Oils Based on GC-MS Fingerprints and Chemometrics. Foods, 14(9), 1624. https://doi.org/10.3390/foods14091624







