Effect of Glow Discharge Cold Plasma Treatment on the Physicochemical Properties and Antioxidant Capacity of Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Treatment and Maize Storage
2.3. Color Characteristics
2.4. Fatty Acid Value (FAV)
2.5. Malondialdehyde (MDA) Content
2.6. Enzyme Activities of Superoxide Dismutase (SOD) and Catalase (CAT)
2.7. Total Phenol Content (TPC)
2.8. Ascorbic Acid (AA) Content
2.9. Glutathione (GSH) Content
2.10. Antioxidant Capacity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Color Evolution of Maize Kernels
3.2. Fatty Acid Value (FAV) Change and Malondialdehyde (MDA) Accumulation
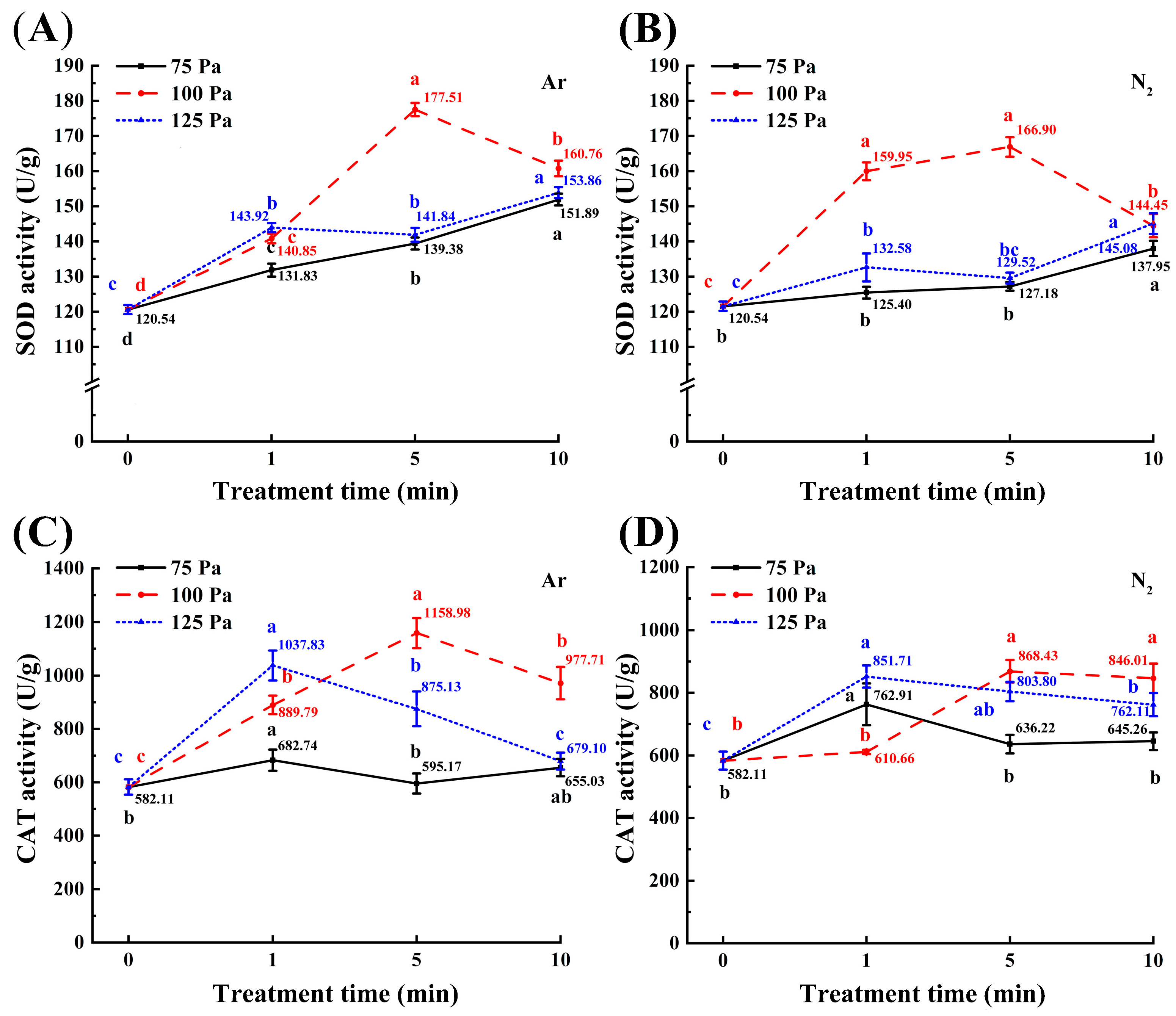
3.3. Superoxide Dismutase (SOD) and Catalase (CAT) Activity Analysis
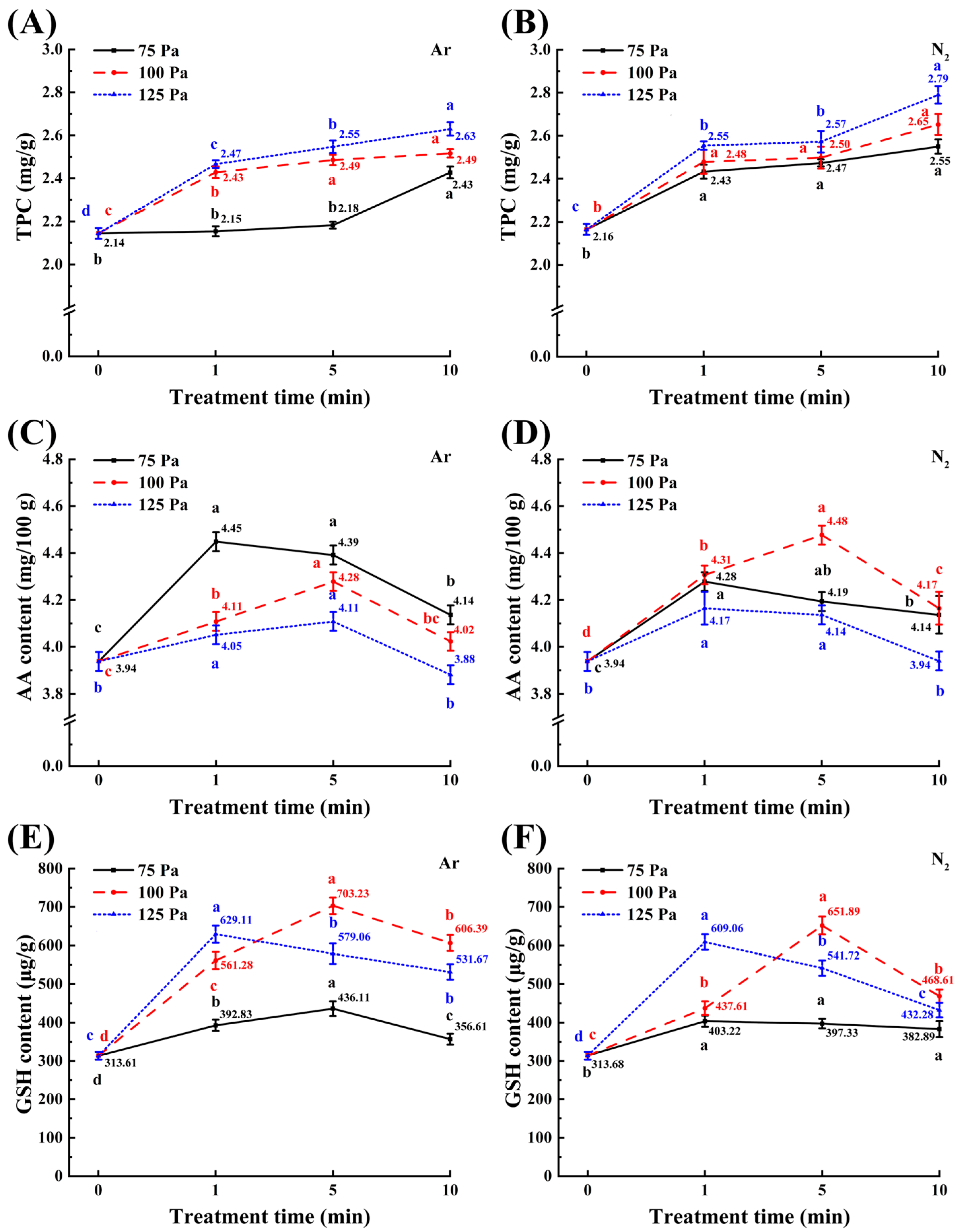
3.4. Changes in Total Phenol, Ascorbic Acid (AA), and Glutathione (GSH) Content
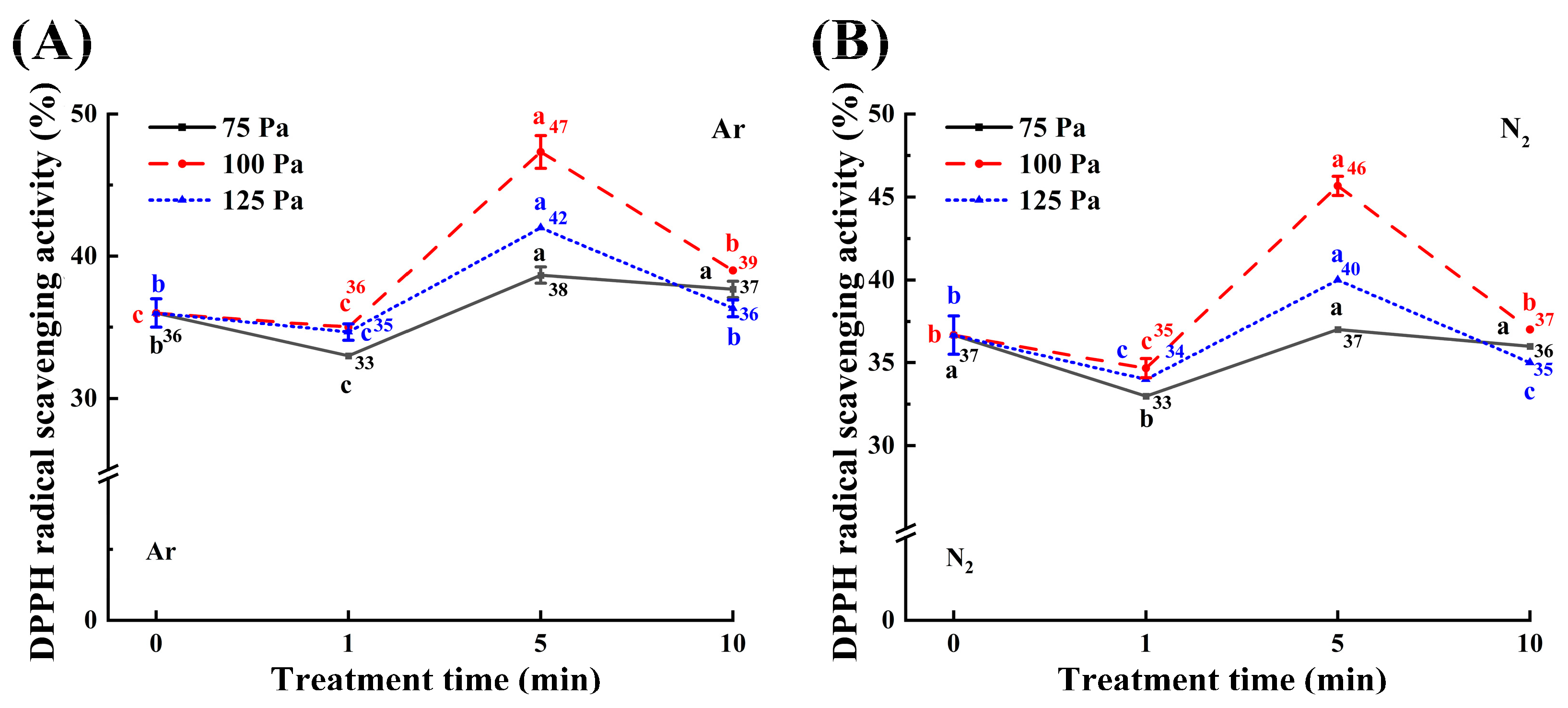
3.5. Antioxidant Capacity Analysis
3.6. The Recovery of FAV and MDA Content During Storage
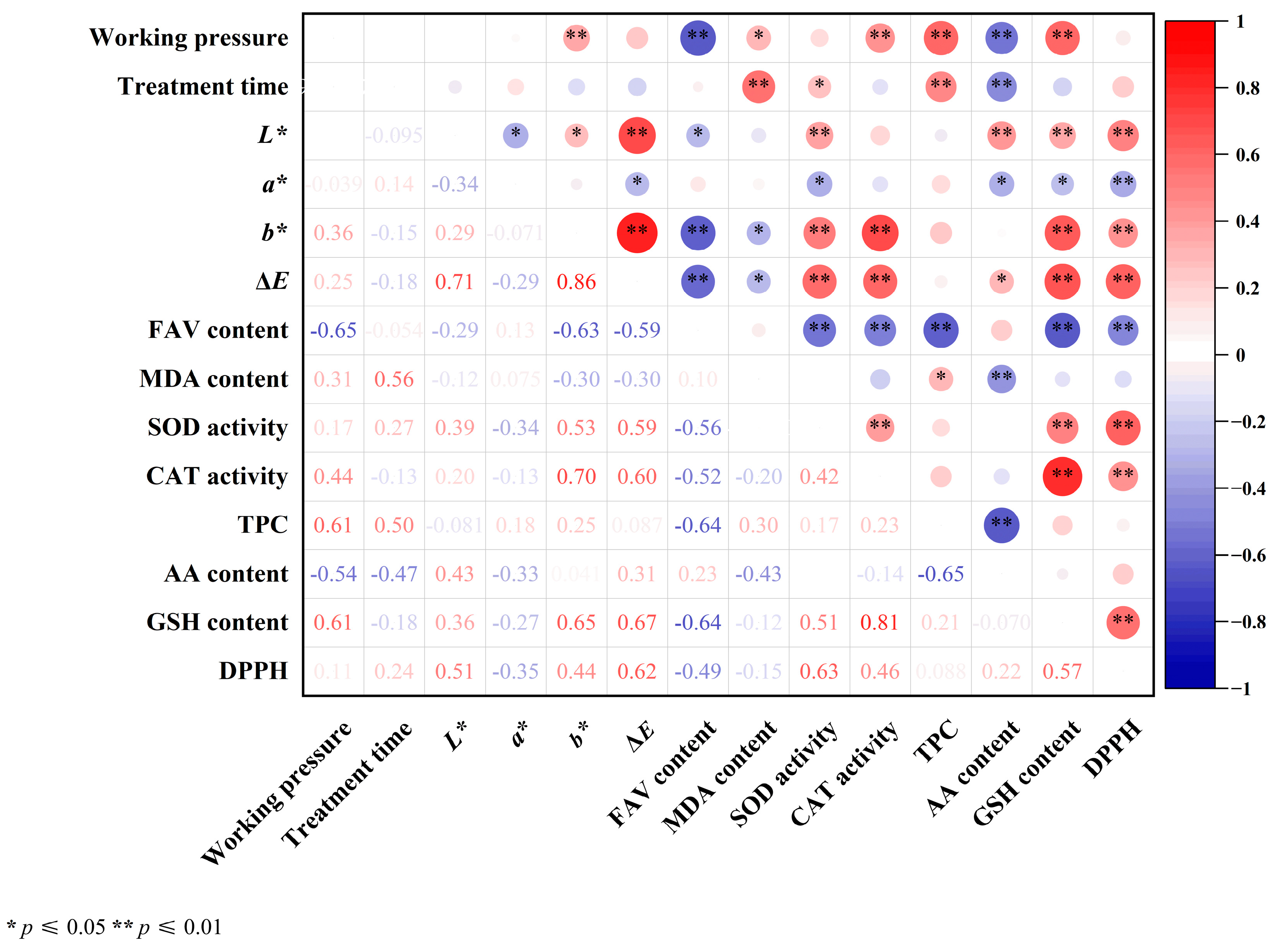
3.7. Correlation Analysis
3.8. Application Potential of Cold Plasma as a Pretreatment Technology for Postharvest Grain Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faostat Online Database. Available online: https://www.fao.org/faostat/zh/#data/QCL/visualize (accessed on 24 February 2025).
- Solon, J.G.; Killeen, S. Decontamination and sterilization. Surgery 2019, 37, 51–57. [Google Scholar]
- Sánchez-Rodríguez, A.R.; Rey, M.D.; Nechate-Drif, H.; Castillejo, M.Á.; Jorrín-Novo, J.V.; Torrent, J.; del Campillo, M.C.; Sacristán, D. Combining P and Zn fertilization to enhance yield and grain quality in maize grown on Mediterranean soils. Sci. Rep. 2021, 11, 7427. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Niu, Y.N.; Bai, X.D.; Mao, T.T. Transcriptomic and metabolic profiling reveals a lignin metabolism network involved in mesocotyl elongation during maize seed germination. Plants 2022, 11, 1034. [Google Scholar] [CrossRef]
- Mannaa, M.; Kim, K.D. Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiology 2017, 45, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Hong, T.N.; Zhao, Z.Y.; Gu, Y.T.; Guo, Y.Z.; Han, J.H. Fatty acid profiles and nutritional evaluation of fresh sweet-waxy corn from three regions of china. Foods 2022, 11, 2636. [Google Scholar] [CrossRef] [PubMed]
- Simões, D.; Carbas, B.; Soares, A.; Freitas, A.; Silva, A.S.; Brites, C.; Andrade, E.D. Assessment of agricultural practices for controlling fusarium and mycotoxins contamination on maize grains: Exploratory study in maize farms. Toxins 2023, 15, 136. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, R.K. Cold plasma treatment of dairy proteins in relation to functionality enhancement. Trends Food Sci. Technol. 2020, 102, 30–36. [Google Scholar] [CrossRef]
- Li, N.; Yu, J.J.; Jin, N.; Chen, Y.; Li, S.H.; Chen, Y. Modification of the physicochemical and structural characteristics of zein suspension by dielectric barrier discharge cold plasma treatment. J. Food Sci. 2020, 85, 2452–2460. [Google Scholar] [CrossRef]
- Zuo, Y.X.; Zou, F.L.; Yang, M.; Xu, G.F.; Wu, J.H.; Wang, L.J.; Wang, H.Y. Effects of plasma-activated water combined with ultrasonic treatment of corn starch on structural, thermal, physicochemical, functional, and pasting properties. Ultrason. Sonochem. 2024, 108, 106963. [Google Scholar] [CrossRef]
- Sruthi, N.U.; Josna, K.; Pandiselvam, R.; Kothakota, A.; Gavahian, M.; Mousavi Khaneghah, A. Impacts of cold plasma treatment on physicochemical, functional, bioactive, textural, and sensory attributes of food: A comprehensive review. Food Chem. 2022, 368, 130809. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Trif, M.; Ozogul, F.; Kumar, M.; Chaudhary, V.; Vukic, M.; Tomar, M.; Changan, S. Recent developments in cold plasma-based enzyme activity (browning, cell wall degradation, and antioxidant) in fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1958–1978. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Chen, Y.K.; Chang, H.C. Evaluation of physicochemical properties of plasma treated brown rice. Food Chem. 2012, 135, 74–79. [Google Scholar] [CrossRef]
- Bozhanova, V.; Marinova, P.; Videva, M.; Nedjalkova, S.; Benova, E. Effect of cold plasma on the germination and seedling growth of durum wheat genotypes. Processes 2024, 12, 544. [Google Scholar] [CrossRef]
- Mravlje, J.; Regvar, M.; Starič, P.; Zaplotnik, R.; Mozetič, M.; Vogel-Mikuš, K. Decontamination and germination of buckwheat grains upon treatment with oxygen plasma glow and afterglow. Plants 2022, 11, 1366. [Google Scholar] [CrossRef] [PubMed]
- Soulier, M.; Maho, T.; Lo, J.; Guillot, P.; Muja, C. Bread wheat (Triticum aestivum L.) fungal and mycotoxin contamination control enhanced by a dual-frequency cold plasma. Food Control 2024, 163, 110477. [Google Scholar] [CrossRef]
- Feizollahi, E.; Iqdiam, B.; Vasanthan, T.; Thilakarathna, M.S.; Roopesh, M.S. Effects of atmospheric-pressure cold plasma treatment on deoxynivalenol degradation, quality parameters, and germination of barley grains. Appl. Sci. 2020, 10, 3530. [Google Scholar] [CrossRef]
- Hossain, M.F.; Sohan, M.S.R.; Hasan, M.; Miah, M.M.; Sajib, S.A.; Karmakar, S.; Khalid-Bin-Ferdaus, K.M.; Kabir, A.H.; Rashid, M.M.; Talukder, M.R.; et al. Enhancement of seed germination rate and growth of maize (Zea mays L.) through LPDBD ar/air plasma. J. Soil Sci. Plant Nutr. 2022, 22, 1778–1791. [Google Scholar] [CrossRef]
- Sohan, M.S.R.; Hasan, M.; Hossain, M.F.; Sajib, S.A.; Khalid-Bin-Ferdaus, K.M.; Kabir, A.H.; Rashid, M.M.; Talukder, M.R.; Elseehy, M.M.; El-Shehawi, A.M.; et al. Low-frequency glow discharge (LFGD) plasma treatment enhances maize (Zea mays L.) seed germination, agronomic traits, enzymatic activities, and nutritional properties. Chem. Biol. Technol. Agric. 2022, 9, 18. [Google Scholar] [CrossRef]
- Shi, Z.Q.; Liu, Y.; Hu, Z.M.; Liu, L.; Yan, Q.H.; Geng, D.D.; Wei, M.; Wan, Y.; Fan, G.Q.; Yang, H.K.; et al. Effect of radiation processing on phenolic antioxidants in cereal and legume seeds: A review. Food Chem. 2022, 396, 133661. [Google Scholar] [CrossRef]
- Öztürk, S.; Cerit, İ.; Mutlu, S.; Demirkol, O. Enrichment of cookies with glutathione by inactive yeast cells (Saccharomyces cerevisiae): Physicochemical and functional properties. J. Cereal Sci. 2017, 78, 19–24. [Google Scholar] [CrossRef]
- Pitkänen, O.; Hallman, M.; Andersson, S. Generation of free radicals in lipid emulsion used in parenteral nutrition. Pediatr. Res. 1991, 29, 56–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barro, F.; Barceló, P.; Lazzeri, P.A.; Shewry, P.R.; Martín, A.; Ballesteros, J. Functional properties and agronomic performance of transgenic tritordeum expressing high molecular weight glutenin subunit genes 1ax1 and 1dx5. J. Cereal Sci. 2003, 37, 65–70. [Google Scholar] [CrossRef]
- Chen, Z.T.; Wang, R.L.; Li, X.J.; Zhu, J.; Xu, Y.N.; Liu, J.J. Sorption equilibrium moisture and isosteric heat of cold plasma treated milled rice. Innov. Food Sci. Emerg. Technol. 2019, 55, 35–47. [Google Scholar] [CrossRef]
- Puač, N.; Petrović, Z.L.; Živković, S.; Giba, Z.; Grubišić, D.; Đorđević, A.R. Low-temperature plasma treatment of dry empress-tree seeds. In Plasma Processes and Polymers; d’Agostino, R., Favia, P., Oehr, C., Wertheimer, M.R., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 193–203. [Google Scholar]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A. Colour measurement and analysis in fresh and processed foods: A review. Food Bioproc. Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Bohlooli, S.; Ramezan, Y.; Esfarjani, F.; Hosseini, H.; Eskandari, S. Effect of soaking in plasma-activated liquids (PALS) on heavy metals and other physicochemical properties of contaminated rice. Food Chem. X 2024, 24, 101788. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Liu, Y.; Chen, F.S. Effect of gamma irradiation on the physicochemical properties and nutrient contents of peanut. LWT—Food Sci. Technol. 2018, 96, 535–542. [Google Scholar] [CrossRef]
- Wu, Q.; Li, C.J.; Zhang, D.D.; Tian, Q.S.; Tao, X.Y.; Luo, Z.S.; Fu, X.Z.; Zhang, Y.R. Nitrogen modified atmosphere packaging maintains the bioactive compounds and antioxidant capacity of postharvest fresh edible peanuts. Postharvest Biol. Technol. 2022, 190, 111957. [Google Scholar] [CrossRef]
- Spanos, G.A.; Wrolstad, R.E. Influence of processing and storage on the phenolic composition of Thompson Seedless grape juice. J. Agric. Food Chem. 1990, 38, 1565–1571. [Google Scholar] [CrossRef]
- Han, S.J.; Zhao, J.F.; Liu, Y.; Xi, L.Q.; Liao, J.A.; Liu, X.Y.; Su, G.D. Effects of green manure planting mode on the quality of korla fragrant pears (Pyrus sinkiangensis Yu). Front. Plant Sci. 2022, 13, 1027595. [Google Scholar] [CrossRef]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione s-transferase activities in rat lung and liver. Biochim. Biophys. Acta Gen. Subj. 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Choi, D.S.; Park, S.H.; Choi, S.R.; Kim, J.S.; Chun, H.H. The combined effects of ultraviolet-c irradiation and modified atmosphere packaging for inactivating Salmonella enterica serovar Typhimurium and extending the shelf life of cherry tomatoes during cold storage. Food Packag. Shelf Life 2015, 3, 19–30. [Google Scholar] [CrossRef]
- Calvo-Brenes, P.; O’Hare, T. Effect of freezing and cool storage on carotenoid content and quality of zeaxanthin-biofortified and standard yellow sweet-corn (Zea mays L.). J. Food Compos. Anal. 2020, 86, 103353. [Google Scholar] [CrossRef]
- Kljak, K.; Grbeša, D.; Karolyi, D. Reflectance colorimetry as a simple method for estimating carotenoid content in maize grain. J. Cereal Sci. 2014, 59, 109–111. [Google Scholar] [CrossRef]
- Saenz, E.; Abdala, L.J.; Borrás, L.; Gerde, J.A. Maize kernel color depends on the interaction between hardness and carotenoid concentration. J. Cereal Sci. 2020, 91, 102901. [Google Scholar] [CrossRef]
- Santos, L.C.O.; Cubas, A.L.V.; Moecke, E.H.S.; Ribeiro, D.H.B.; Amante, E.R. Use of cold plasma to inactivate escherichia coli and physicochemical evaluation in pumpkin puree. J. Food Prot. 2018, 81, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Darvish, H.; Ramezan, Y.; Khani, M.R.; Kamkari, A. Effect of low-pressure cold plasma processing on decontamination and quality attributes of saffron (Crocus sativus L.). Food Sci. Nutr. 2022, 10, 2082–2090. [Google Scholar] [CrossRef]
- Thirumdas, R.; Saragapani, C.; Ajinkya, M.T.; Deshmukh, R.R.; Annapure, U.S. Influence of low pressure cold plasma on cooking and textural properties of brown rice. Innov. Food Sci. Emerg. Technol. 2016, 37, 53–60. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, H.J.; Woo, K.S.; Jo, C.; Kim, J.K.; Kim, S.H.; Park, H.Y.; Oh, S.K.; Kim, W.H. Evaluation of cold plasma treatments for improved microbial and physicochemical qualities of brown rice. LWT—Food Sci. Technol. 2016, 73, 442–447. [Google Scholar] [CrossRef]
- Shen, C.; Chen, W.Q.; Li, C.Z.; Cui, H.Y.; Lin, L. The effects of cold plasma (cp) treatment on the inactivation of yam peroxidase and characteristics of yam slices. J. Food Eng. 2023, 359, 111693. [Google Scholar] [CrossRef]
- Chaple, S.; Sarangapani, C.; Jones, J.; Carey, E.; Causeret, L.; Genson, A.; Duffy, B.; Bourke, P. Effect of atmospheric cold plasma on the functional properties of whole wheat (Triticum aestivum L.) grain and wheat flour. Innov. Food Sci. Emerg. Technol. 2020, 66, 102529. [Google Scholar] [CrossRef]
- An, N.N.; Lv, W.Q.; Li, D.; Wang, L.J.; Wang, Y. Effects of hot-air microwave rolling blanching pretreatment on the drying of turmeric (Curcuma longa L.): Physiochemical properties and microstructure evaluation. Food Chem. 2023, 398, 133925. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.W.; Li, D.D.; Abulaiti, R.; Wang, M.Q.; Wu, X.Z.; Feng, Z.W.; Zhu, Y.T.; Cai, J.R. Cold plasma as a novel pretreatment to improve the drying kinetics and quality of green peas. Foods 2025, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Kalinger, R.S.; Pulsifer, I.P.; Hepworth, S.R.; Rowland, O. Fatty acyl synthetases and thioesterases in plant lipid metabolism: Diverse functions and biotechnological applications. Lipids 2020, 55, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.P.; Lv, Y.Y.; Yuan, W.J.; Zhang, S.B.; Hu, Y.S. Effect of artificial aging on wheat quality deterioration during storage. J. Stored Prod. Res. 2019, 80, 50–56. [Google Scholar] [CrossRef]
- Ebone, L.A.; Caverzan, A.; Chavarria, G. Physiologic alterations in orthodox seeds due to deterioration processes. Plant Physiol. Biochem. 2019, 145, 34–42. [Google Scholar] [CrossRef]
- Cao, Z.Y.; Li, X.J.; Song, H.D.; Jie, Y.; Liu, C. Effect of intermittent low-pressure radiofrequency helium cold plasma treatments on rice gelatinization, fatty acid, and hygroscopicity. Foods 2024, 13, 1056. [Google Scholar] [CrossRef]
- Rajarammanna, R.; Jayas, D.; White, N.D.G. Comparison of deterioration of rye under two different storage regimes. J. Stored Prod. Res. 2010, 46, 87–92. [Google Scholar] [CrossRef]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kalináková, B.; Kovácik, D.; Medvecká, V.; Cernák, M. Effect of cold atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seeds surface. Plasma Chem. Plasma Process. 2016, 36, 397–414. [Google Scholar] [CrossRef]
- Ghodsimaab, S.P.; Makarian, H.; Hagh, Z.G.; Gholipoor, M. Scanning electron microscopy, biochemical and enzymatic studies to evaluate hydro-priming and cold plasma treatment effects on the germination of Salvia leriifolia Benth. Seeds. Front. Plant Sci. 2023, 13, 1035296. [Google Scholar] [CrossRef]
- Mathavaraj, P.; Muthusamy, V.; Katral, A.; Mandal, P.; Zunjare, R.U.; Hossain, F. Lipoxygenases (loxs): Will turning off this genetic switch help safeguard the flavor and nutritional quality of stored lipid-rich staple foods? Food Chem. 2025, 470, 142637. [Google Scholar] [CrossRef]
- Schiller, D.; Contreras, C.; Vogt, J.; Dunemann, F.; Defilippi, B.G.; Beaudry, R.; Schwab, W. A dual positional specific lipoxygenase functions in the generation of flavor compounds during climacteric ripening of apple. Hortic. Res. 2015, 2, 15003. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.; Srivastav, P. Effect of cold plasma on the technological and functional modification of plant proteins and enzymes. Innov. Food Sci. Emerg. Technol. 2023, 88, 103447. [Google Scholar] [CrossRef]
- Mayookha, V.P.; Pandiselvam, R.; Kothakota, A.; Padma Ishwarya, S.; Chandra Khanashyam, A.; Kutlu, N.; Rifna, E.J.; Kumar, M.; Panesar, P.S.; Abd El-Maksoud, A.A. Ozone and cold plasma: Emerging oxidation technologies for inactivation of enzymes in fruits, vegetables, and fruit juices. Food Control 2023, 144, 109399. [Google Scholar] [CrossRef]
- Misra, N.N.; Pankaj, S.K.; Segat, A.; Ishikawa, K. Cold plasma interactions with enzymes in foods and model systems. Trends Food Sci. Technol. 2016, 55, 39–47. [Google Scholar] [CrossRef]
- Wang, C.T.; Wang, C.T.; Cao, Y.P.; Robert Nout, M.J.; Sun, B.G.; Liu, L. Effect of modified atmosphere packaging (MAP) with low and superatmospheric oxygen on the quality and antioxidant enzyme system of golden needle mushrooms (Flammulina velutipes) during postharvest storage. Eur. Food Res. Technol. 2011, 232, 851–860. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Cheng, J.H.; Sun, D.W. Chemical, physical and physiological quality attributes of fruit and vegetables induced by cold plasma treatment: Mechanisms and application advances. Crit. Rev. Food Sci. Nutr. 2019, 60, 2676–2690. [Google Scholar] [CrossRef]
- Dong, X.Y.; Yang, Y. A novel approach to enhance blueberry quality during storage using cold plasma at atmospheric air pressure. Food Bioproc. Technol. 2019, 12, 1409–1421. [Google Scholar] [CrossRef]
- Rasooli, Z.; Barzin, G.; Davari Mahabadi, T.; Entezari, M. Stimulating effects of cold plasma seed priming on germination and seedling growth of cumin plant. S. Afr. J. Bot. 2021, 142, 106–113. [Google Scholar] [CrossRef]
- Wei, S.; Zhao, L.; Liu, Z.; Zhang, X.; Hui, Y.; Wang, D.; Liu, Z.; Liu, G. Cold plasma treatment for Astragalus membranaceus seed germination and seedling growth improvement. Legume Res. 2023, 46, 609–615. [Google Scholar] [CrossRef]
- Li, Y.J.; Wang, T.C.; Meng, Y.R.; Qu, G.Z.; Sun, Q.H.; Liang, D.L.; Hu, S.B. Air atmospheric dielectric barrier discharge plasma induced germination and growth enhancement of wheat seed. Plasma Chem. Plasma Process. 2017, 37, 1621–1634. [Google Scholar] [CrossRef]
- Surovy, M.Z.; Mahmud, N.U.; Bhattacharjee, P.; Hossain, M.S.; Mehebub, M.S.; Rahman, M.; Majumdar, B.C.; Gupta, D.R.; Islam, T. Modulation of nutritional and biochemical properties of wheat grains infected by blast fungus Magnaporthe oryzae Triticum pathotype. Front. Microbiol. 2020, 11, 1174. [Google Scholar] [CrossRef]
- Tahir, H.; Sajjad, M.; Qian, M.J.; Zeeshan Ul Haq, M.Z.; Tahir, A.; Farooq, M.A.; Wei, L.; Shi, S.P.; Zhou, K.B.; Yao, Q.S. Glutathione and ascorbic acid accumulation in mango pulp under enhanced UV-B based on transcriptome. Antioxidants 2024, 13, 1429. [Google Scholar] [CrossRef] [PubMed]
- Keutgen, A.J.; Pawelzik, E. Modifications of strawberry fruit antioxidant pools and fruit quality under NaCl stress. J. Agric. Food Chem. 2007, 55, 4066–4072. [Google Scholar] [CrossRef]
- Wang, K.; Shao, X.; Gong, Y.; Xu, F.; Wang, H. Effects of postharvest hot air treatment on gene expression associated with ascorbic acid metabolism in peach fruit. Plant Mol. Biol. Rep. 2014, 32, 881–887. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Farias, T.R.B.; Rodrigues, S.; Fernandes, F.A.N. Effect of dielectric barrier discharge plasma excitation frequency on the enzymatic activity, antioxidant capacity and phenolic content of apple cubes and apple juice. Food Res. Int. 2020, 136, 109617. [Google Scholar] [CrossRef]
- Cui, D.J.; Yin, Y.; Li, H.D.; Hu, X.X.; Zhuang, J.; Ma, R.N.; Jiao, Z. Comparative transcriptome analysis of atmospheric pressure cold plasma enhanced early seedling growth in Arabidopsis thaliana. Plasma Sci. Technol. 2021, 23, 95–117. [Google Scholar] [CrossRef]
- Mellidou, I.; Keulemans, J.; Kanellis, A.K.; Davey, M.W. Regulation of fruit ascorbic acid concentrations during ripening in high and low vitamin c tomato cultivars. BMC Plant Biol. 2012, 12, 239. [Google Scholar] [CrossRef]
- Arya, A.; Azarmehr, N.; Mansourian, M.; Doustimotlagh, A.H. Inactivation of the superoxide dismutase by malondialdehyde in the nonalcoholic fatty liver disease: A combined molecular docking approach to clinical studies. Arch. Physiol. Biochem. 2021, 127, 557–564. [Google Scholar] [CrossRef]
- Chen, H.H.; Hung, C.L.; Lin, S.Y.; Liou, G.J. Effect of low-pressure plasma exposure on the storage characteristics of brown rice. Food Bioproc. Technol. 2015, 8, 471–477. [Google Scholar] [CrossRef]
- Wang, L.H.; Li, Z.; Qin, J.; Huang, Y.; Zeng, X.A.; Aadil, R.M. Investigation on the impact of quality characteristics and storage stability of foxtail millet induced by air cold plasma. Front. Nutr. 2022, 9, 1064812. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, V.; Garavand, F.; Goudarzi, M.; Sarlak, Z.; Cacciotti, I.; Tiwari, B. Cold atmospheric-pressure plasma treatment of turmeric powder: Microbial load, essential oil profile, bioactivity and microstructure analyses. Int. J. Food Sci. Technol. 2021, 56, 2224–2232. [Google Scholar] [CrossRef]
- Genkawa, T.; Uchino, T.; Inoue, A.; Tanaka, F.; Hamanaka, D. Development of a low-moisture-content storage system for brown rice: Storability at decreased moisture contents. Biosys. Eng. 2008, 99, 515–522. [Google Scholar] [CrossRef]
- Park, C.E.; Kim, Y.S.; Park, K.J.; Kim, B.K. Changes in physicochemical characteristics of rice during storage at different temperatures. J. Stored Prod. Res. 2012, 48, 25–29. [Google Scholar] [CrossRef]
- Zhao, Y.; Qi, T.J.; Cui, C.Y.; Liu, X.G.; Liu, R.Y.; Liu, S.C.; Song, Y.L.; Li, Y.F.; Lv, H.X. Effects of different storage temperatures on the quality and metabolome of maize with high moisture content. LWT Food Sci. Technol. 2024, 214, 117117. [Google Scholar] [CrossRef]
- Qu, C.L.; Xia, Y.Z.; Yang, Q.K.; Li, W.H.; Hu, M.; Lu, P. Novel insights into rice deterioration for nitrogen controlled atmosphere and re-aeration storage based on no-targeted metabolomics. LWT Food Sci. Technol. 2023, 178, 114631. [Google Scholar] [CrossRef]
- Turner, M. Chapter 2—Physics of cold plasma. In Cold Plasma in Food and Agriculture; Misra, N.N., Schlüter, O., Cullen, P.J., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 17–51. [Google Scholar]



| Gas | Samples | L* | a* | b* | ΔE | C* | h* |
|---|---|---|---|---|---|---|---|
| Ar | Untreated (0 min) | 57.74 ± 0.75 b | 9.00 ± 0.21 a | 20.91 ± 0.54 e | - | 22.77 ± 0.53 d | 1.16 ± 0.02 a |
| 75-1 m | 59.29 ± 0.73 ab | 8.19 ± 0.12 bcd | 22.80 ± 0.30 cd | 2.66 | 24.22 ± 0.35 c | 1.23 ± 0.01 a | |
| 75-5 m | 59.08 ± 0.76 ab | 7.87 ± 0.16 cd | 21.97 ± 0.49 d | 2.17 | 23.44 ± 0.56 d | 1.23 ± 0.01 a | |
| 75-10 m | 59.03 ± 0.81 ab | 8.29 ± 0.21 bc | 23.34 ± 0.46 bc | 2.94 | 24.77 ± 0.45 bc | 1.23 ± 0.02 a | |
| 100-1 m | 58.66 ± 0.74 ab | 8.11 ± 0.04 bcd | 23.90 ± 0.37 b | 3.33 | 25.24 ± 0.44 ab | 1.24 ± 0.00 a | |
| 100-5 m | 60.05 ± 0.79 a | 7.77 ± 0.32 d | 24.92 ± 0.51 a | 4.87 | 26.10 ± 0.71 a | 1.27 ± 0.01 a | |
| 100-10 m | 58.63 ± 0.56 ab | 8.49 ± 0.27 b | 23.92 ± 0.48 b | 3.23 | 25.39 ± 0.58 ab | 1.23 ± 0.01 a | |
| 125-1 m | 58.76 ± 0.60 ab | 8.19 ± 0.13 bcd | 23.70 ± 0.40 bc | 3.13 | 25.07 ± 0.51 bc | 1.24 ± 0.00 a | |
| 125-5 m | 59.83 ± 0.36 a | 8.05 ± 0.24 bcd | 23.41 ± 0.51 bc | 3.45 | 24.75 ± 0.68 bc | 1.24 ± 0.00 a | |
| 125-10 m | 58.84 ± 0.68 ab | 8.36 ± 0.27 bc | 23.22 ± 0.05 bc | 2.73 | 24.68 ± 0.13 bc | 1.23 ± 0.01 a | |
| N2 | Untreated (0 min) | 57.74 ± 0.75 b | 9.00 ± 0.21 a | 20.91 ± 0.54 e | - | 22.77 ± 0.53 e | 1.16 ± 0.02 e |
| 75-1 m | 58.65 ± 0.75 b | 8.51 ± 0.32 ab | 23.98 ± 0.29 b | 3.35 | 25.45 ± 0.46 ab | 1.23 ± 0.01 bc | |
| 75-5 m | 58.87 ± 0.62 b | 8.47 ± 0.20 ab | 22.06 ± 0.39 d | 1.82 | 23.63 ± 0.53 d | 1.20 ± 0.00 d | |
| 75-10 m | 58.55 ± 0.79 b | 8.39 ± 0.19 b | 22.35 ± 0.07 d | 1.91 | 23.88 ± 0.16 d | 1.21 ± 0.01 cd | |
| 100-1 m | 59.04 ± 0.66 b | 8.14 ± 0.22 b | 23.51 ± 0.34 bc | 3.12 | 24.88 ± 0.48 bc | 1.24 ± 0.01 b | |
| 100-5 m | 61.58 ± 0.66 a | 7.94 ± 0.27 b | 24.80 ± 0.16 a | 5.61 | 26.05 ± 0.19 a | 1.26 ± 0.01 a | |
| 100-10 m | 58.70 ± 0.61 b | 8.06 ± 0.26 b | 23.61 ± 0.26 bc | 3.06 | 24.95 ± 0.19 bc | 1.24 ± 0.02 ab | |
| 125-1 m | 59.22 ± 0.48 b | 8.15 ± 0.33 b | 23.65 ± 0.05 bc | 3.27 | 25.02 ± 0.08 bc | 1.24 ± 0.02 ab | |
| 125-5 m | 58.24 ± 0.77 b | 8.47 ± 0.28 ab | 23.85 ± 0.27 bc | 3.14 | 25.31 ± 0.39 bc | 1.23 ± 0.01 bc | |
| 125-10 m | 58.79 ± 0.61 b | 8.32 ± 0.23 b | 23.21 ± 0.17 c | 2.68 | 24.66 ± 0.23 c | 1.23 ± 0.01 bcd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Ren, C.; Li, C.; Fan, Z.; Zhu, J.; Qu, C. Effect of Glow Discharge Cold Plasma Treatment on the Physicochemical Properties and Antioxidant Capacity of Maize. Foods 2025, 14, 1312. https://doi.org/10.3390/foods14081312
Li M, Ren C, Li C, Fan Z, Zhu J, Qu C. Effect of Glow Discharge Cold Plasma Treatment on the Physicochemical Properties and Antioxidant Capacity of Maize. Foods. 2025; 14(8):1312. https://doi.org/10.3390/foods14081312
Chicago/Turabian StyleLi, Miao, Chengcheng Ren, Caihong Li, Zengxuan Fan, Jiayin Zhu, and Chenling Qu. 2025. "Effect of Glow Discharge Cold Plasma Treatment on the Physicochemical Properties and Antioxidant Capacity of Maize" Foods 14, no. 8: 1312. https://doi.org/10.3390/foods14081312
APA StyleLi, M., Ren, C., Li, C., Fan, Z., Zhu, J., & Qu, C. (2025). Effect of Glow Discharge Cold Plasma Treatment on the Physicochemical Properties and Antioxidant Capacity of Maize. Foods, 14(8), 1312. https://doi.org/10.3390/foods14081312






