Peroxidase-like Active Cu-MOFs Nanozymes for Colorimetric Detection of Total Antioxidant Capacity in Fruits and Vegetables
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Instruments
2.2. Preparation of Nanozyme
2.3. Characterization
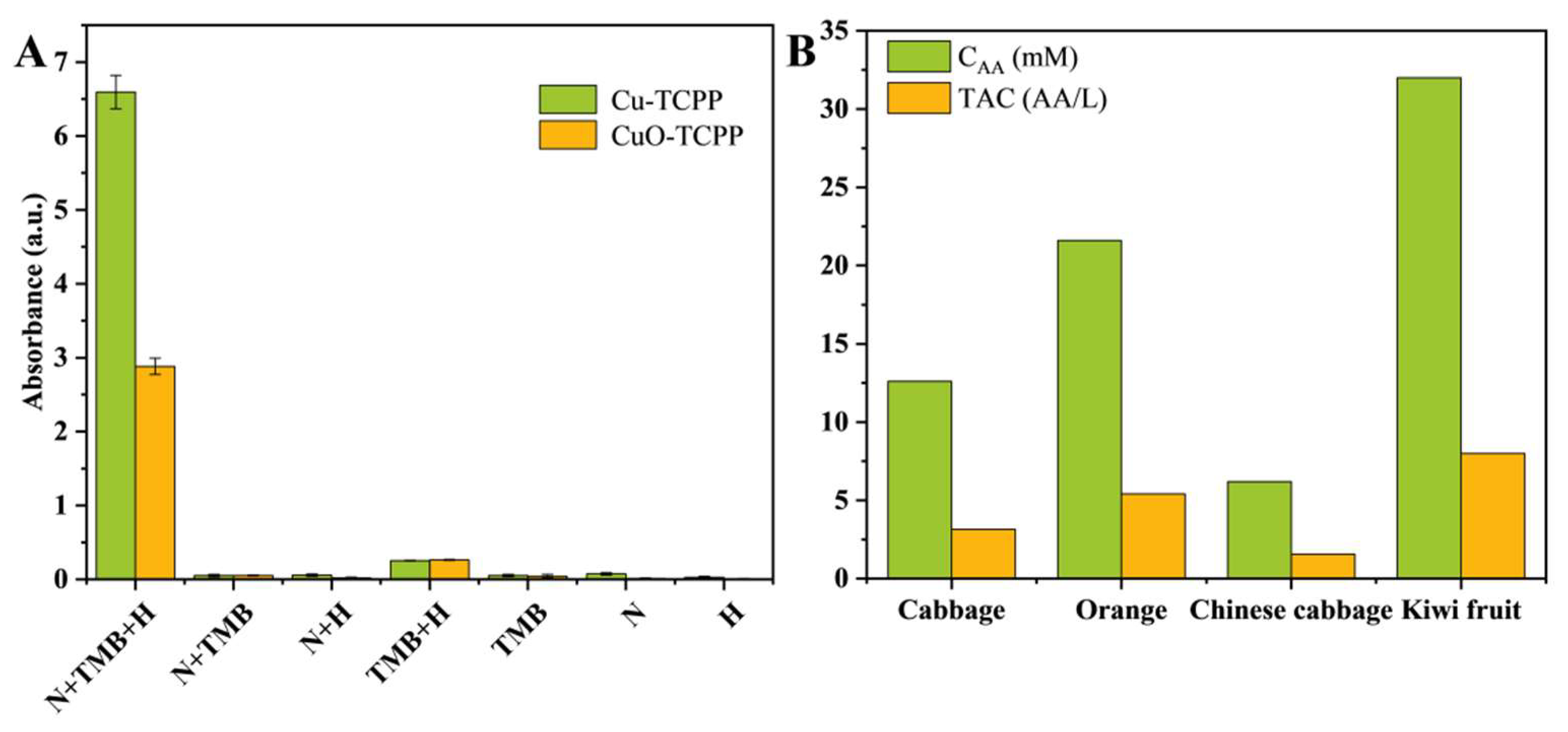
2.4. Peroxide-like Activity of Cu-TCPP and CuO-TCPP
2.5. Colorimetric Detection of Antioxidants
3. Results and Discussion
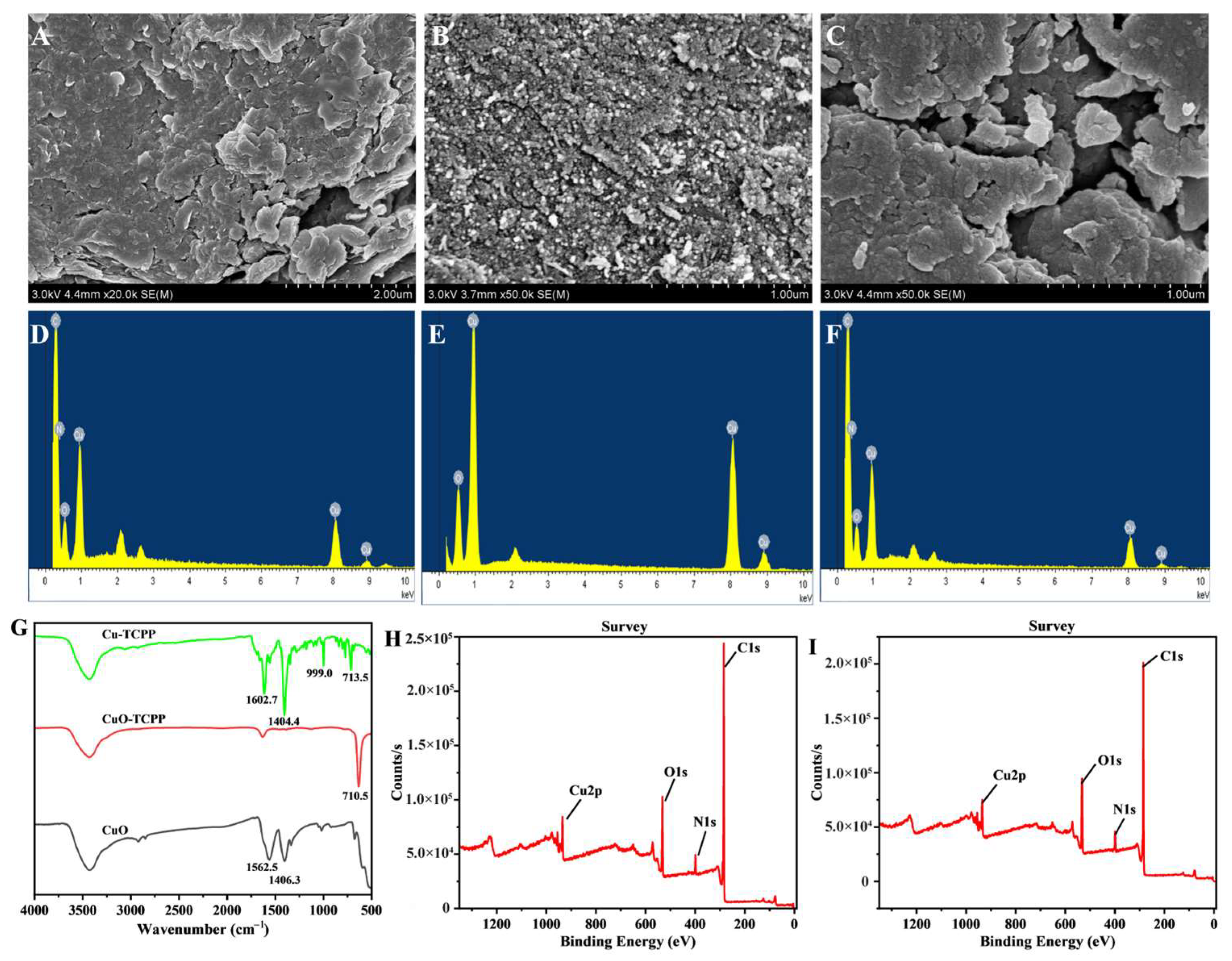
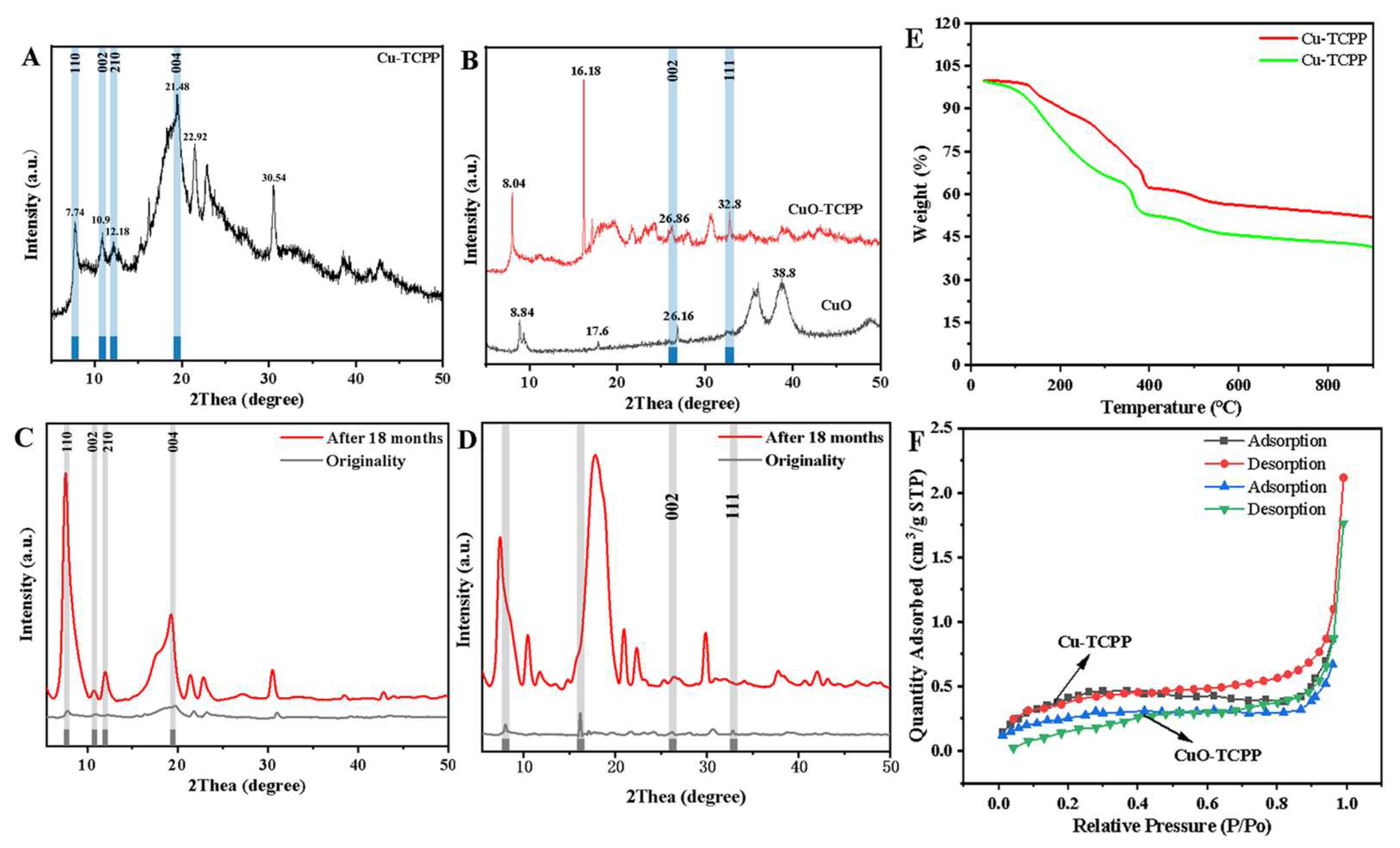
3.1. Characterization of Cu-TCPP and CuO-TCPP
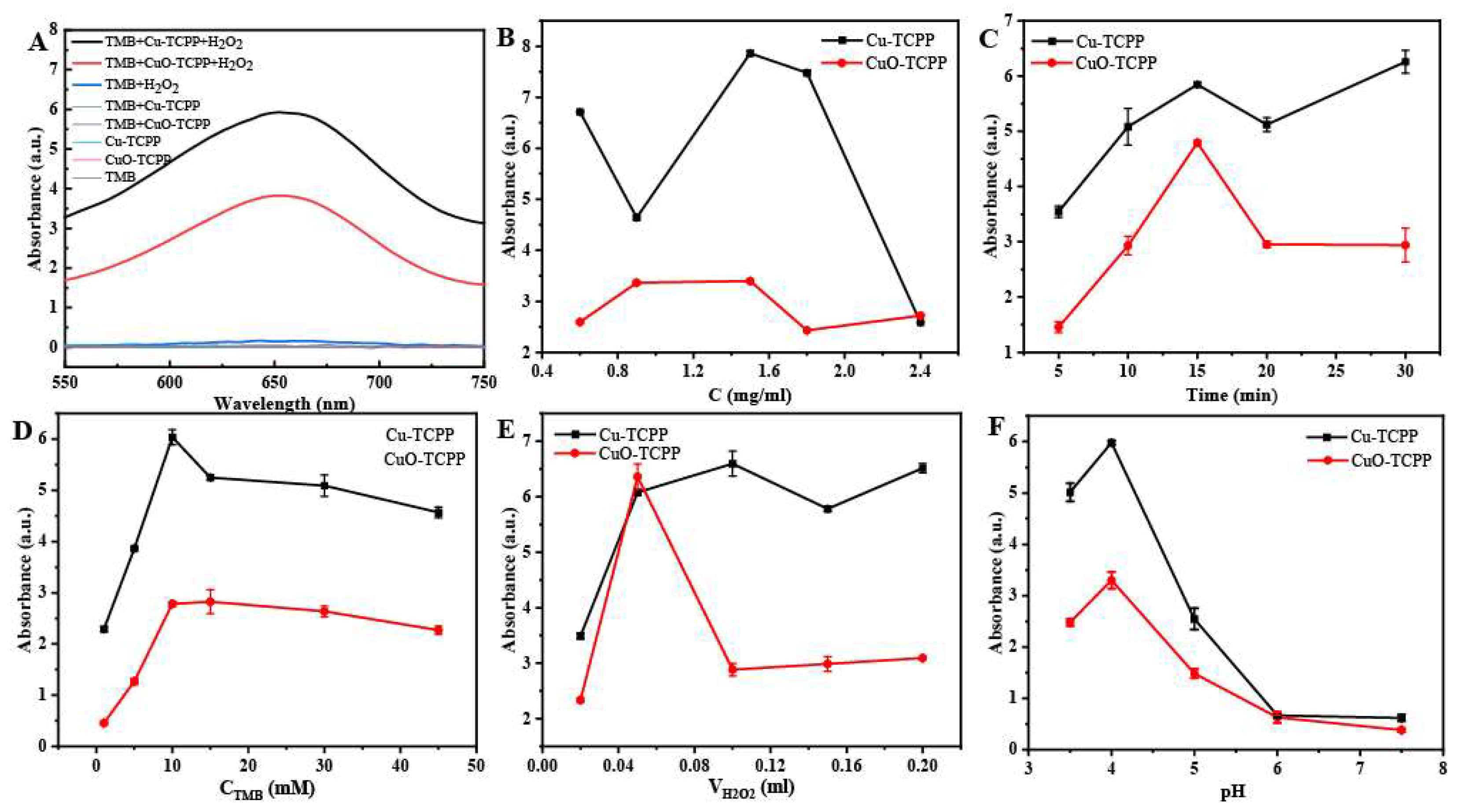
3.2. Optimization of Experimental Conditions and Steady-State Kinetic
3.3. Detection of Antioxidant
3.4. Detection of AA Concentration and TAC in Actual Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barberis, A.; Spissu, Y.; Bazzu, G.; Fadda, A.; Azara, E.; Sanna, D.; Schirra, M.; Serra, P.A. Development and characterization of an ascorbate oxidase-based sensor-biosensor system for telemetric detection of AA and antioxidant capacity in fresh orange juice. Anal. Chem. 2014, 86, 8727–8734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Z.B.; Zuo, X. Chloroauric Acid/Silver Nanoparticle Colorimetric Sensors for Antioxidant Discrimination Based on a Honeycomb Ag-Au Nanostructure. ACS Sustain. Chem. Eng. 2020, 8, 3922–3928. [Google Scholar] [CrossRef]
- Landete, J.M. Dietary Intake of Natural Antioxidants: Vitamins and Polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef]
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Sarker, U.; Islam, M.T.; Rabbani, M.G.; Oba, S. Variability in total antioxidant capacity, antioxidant leaf pigments and foliage yield of vegetable amaranth. J. Integr. Agric. 2018, 17, 1145–1153. [Google Scholar] [CrossRef]
- Ferrari, C.K.; Percário, S.; Silva, J.C.; Da Silva Torres, E.A. An apple plus a Brazil nut a day keeps the doctors away: Antioxidant capacity of foods and their health benefits. Curr. Pharm. Des. 2016, 22, 189–195. [Google Scholar] [CrossRef]
- Xian, Y.; Liang, L.; Qi, S.; Xie, Y.; Song, B.; Ouyang, S.; Xie, Y.; Sun, X.; Wang, W. Antioxidants retard the ageing of mouse oocytes. Mol. Med. Rep. 2018, 18, 1981–1986. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Joshi, M.; Hiremath, P.; John, J.; Ranadive, N.; Nandakumar, K.; Mudgal, J. Modulatory role of vitamins A, B3, C, D, and E on skin health, immunity, microbiome, and diseases. Pharmacol. Rep. 2023, 75, 1096–1114. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Martín-Carro, B.; Cannata-Andía, J.B.; Mora-Fernández, C.; Navarro-González, J.F. Klotho, Oxidative Stress, and Mitochondrial Damage in Kidney Disease. Antioxidants 2023, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- De Bhailís, Á.M.; Chrysochou, C.; Kalra, P.A. Inflammation and Oxidative Damage in Ischaemic Renal Disease. Antioxidants 2021, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.D.; Quadros, A.S.; Weschenfelder, C.; Garofallo, S.B.; Marcadenti, A. Oxidative Stress Biomarkers, Nut-Related Antioxidants, and Cardiovascular Disease. Nutrients 2020, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. [Google Scholar] [CrossRef]
- Mirmiran, P.; Hosseini-Esfahani, F.; Esfandiar, Z.; Hosseinpour-Niazi, S.; Azizi, F. Associations between dietary antioxidant intakes and cardiovascular disease. Sci. Rep. 2022, 12, 1504. [Google Scholar] [CrossRef]
- Labarrere, C.A.; Kassab, G.S. Glutathione: A Samsonian life-sustaining small molecule that protects against oxidative stress, ageing and damaging inflammation. Front. Nutr. 2022, 9, 1007816. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, B.; Ye, B.; Sun, Z.; Qian, Z.; Yu, L.; Bi, Y.; Ma, L.; Ding, Y.; Du, Y.; et al. Mitochondrial-Targeted Delivery of Polyphenol-Mediated Antioxidases Complexes against Pyroptosis and Inflammatory Diseases. Adv. Mater. 2023, 35, e2208571. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Griffiths, K.; Aggarwal, B.B.; Singh, R.B.; Buttar, H.S.; Wilson, D.; De Meester, F. Food Antioxidants and Their Anti-Inflammatory Properties: A Potential Role in Cardiovascular Diseases and Cancer Prevention. Diseases 2016, 4, 28. [Google Scholar] [CrossRef]
- Khadim, R.M.; Al-Fartusie, F.S. Antioxidant vitamins and their effect on immune system. J. Phys. Conf. Ser. 2021, 1853, 012065. [Google Scholar] [CrossRef]
- Ramesh, B.; Isseks, A.; Martin, J.; Mansoori, S.; Browne, A.; Suminski, R.; Chai, S. Vitamin C and Copper Intake Associated with Cognitive Function in Older Adults. Curr. Dev. Nutr. 2022, 6, 164. [Google Scholar] [CrossRef]
- Nilsson, J.; Pillai, D.; Onning, G.; Persson, C.; Nilsson, A.; Akesson, B. Comparison of the 2,2’-azinobis-3-ethylbenzotiazo-line-6-sulfonic acid (ABTS) and ferric reducing anti-oxidant power (FRAP) methods to asses the total antioxidant capacity in extracts of fruit and vegetables. Mol. Nutr. Food Res. 2005, 49, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Liu, D.; Hadiatullah, H.; Guo, T.; Yao, Y.P.; Li, C.M.; Wang, X.G. Evaluating the total antioxidant capacity of processed milk: Utilising applicable antioxidant assays and key antioxidant components. Int. J. Food Sci. Technol. 2024, 59, 1351–1362. [Google Scholar] [CrossRef]
- Jones, A.; Acquaviva, A.; Suktham, T.; Dennis, G.R.; Shalliker, R.A.; Soliven, A. Total Antioxidant Capacity with Peak Specificity via Reaction Flow Chromatography and the Ferric Reducing Antioxidant Power Assay. Food Anal. Methods 2020, 13, 608–616. [Google Scholar] [CrossRef]
- Peixoto, C.R.D.; Fraga, S.; Justim, J.D.; Gomes, M.S.; Carvalho, D.G.; Jarenkow, J.A.; De Moura, N.F. Voltammetric determination of total antioxidant capacity of Bunchosia glandulifera tree extracts. J. Electroanal. Chem. 2017, 799, 519–524. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Borahan, T.; Girgin, A.; Atsever, N.; Zaman, B.T.; Chormey, D.S.; Bakırdere, S. Development of a double-monitoring method for the determination of total antioxidant capacity as ascorbic acid equivalent using CUPRAC assay with RP-HPLC and digital image-based colorimetric detection. Eur. Food Res. Technol. 2022, 248, 707–713. [Google Scholar] [CrossRef]
- Berker, K.I.; Demirata, B.; Apak, R. Determination of Total Antioxidant Capacity of Lipophilic and Hydrophilic Antioxidants In the Same Solution by Using Ferric–Ferricyanide Assay. Food Anal. Methods 2012, 5, 1150–1158. [Google Scholar] [CrossRef]
- Kanmaz, N.; Uzer, A.; Hizal, J.; Apak, R. Determination of total antioxidant capacity of Cynara scolymus L. (globe artichoke) by using novel nanoparticle-based ferricyanide/Prussian blue assay. Talanta 2020, 216, 120960. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.B.; Zhang, Y.; Zeng, T.; Aleisa, R.; Qiu, Z.W.; Chen, Y.Z.; Huang, J.K.; Wang, D.W.; Yan, Z.F.; Yin, Y.D. Anisotropic plasmonic nanostructures for colorimetric sensing. Nano Today 2020, 32, 100855. [Google Scholar] [CrossRef]
- Wu, C.M.; Li, J.J.; Song, J.P.; Guo, H.; Bai, S.P.; Lu, C.F.; Peng, H.W.; Wang, X.X. Novel colorimetric detection of oxytetracycline in foods by copper nanozyme. Food Chem. 2024, 430, 137040. [Google Scholar] [CrossRef]
- Ge, J.; Cai, R.; Chen, X.G.; Wu, Q.; Zhang, L.L.; Jiang, Y.; Cui, C.; Wan, S.; Tan, W.H. Facile approach to prepare HSA-templated MnO2 nanosheets as oxidase mimic for colorimetric detection of glutathione. Talanta 2019, 195, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.J.; Xu, C.C.; Huang, X.Y.; Liu, Y.N.; Wan, J. Peroxidase Mimic Activities of Copper Selenide (CuSe) Nanoplates for Sensing H2O2 and L-Cysteine. J. Nanosci. Nanotechnol. 2020, 20, 5369–5375. [Google Scholar]
- Ravannakhjavani, F.; Dehghan, S.F.; Panahi, D.; Moradpour, Z.; Zendehdel, R. Multi-metal Nanozyme Properties for Colorimetric Peroxidase Reaction: Overview of an Applicable Method Validation for H2O2 Detection. J. Inorg. Organomet. Polym. Mater. 2024, 34, 818–826. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.M.; Xu, D.; Zheng, X.X.; Huang, Q. A green and facile approach to a graphene-based peroxidase-like nanozyme and its application in sensitive colorimetric detection of l-cysteine. Anal. Bioanal. Chem. 2021, 413, 4013–4022. [Google Scholar] [CrossRef]
- Xiao, F.; Xia, Q.; Zhang, S.; Li, Q.; Chen, D.; Li, H.; Yang, D.; Yang, Y. Ultrasound and defect engineering-enhanced nanozyme with high laccase-like activity for oxidation and detection of phenolic compounds and adrenaline. J. Hazard. Mater. 2024, 465, 133126. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, J.; Zou, J.; Cheng, Z.; Huang, Z.; Gu, L.; Zhong, Z.; Li, S.; Wang, Y.; Li, P. Electrochemical detection of methyl-paraoxon based on bifunctional cerium oxide nanozyme with catalytic activity and signal amplification effect. J. Pharm. Anal. 2021, 11, 653–660. [Google Scholar] [CrossRef]
- Mo, F.; Zhong, S.; You, T.; Lu, J.; Sun, D. Aptamer and DNAzyme-Functionalized Cu-MOF Hybrid Nanozymes for the Monitoring and Management of Bacteria-Infected Wounds. ACS Appl. Mater. Interfaces 2023, 15, 52114–52127. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, B.L.; Pan, X.T.; Wang, H.Y.; Wu, Q.Y.; Li, S.S.; Jiang, B.Y.; Liu, H.Y. Carbon-based nanozymes: Design, catalytic mechanism, and bioapplication. Coord. Chem. Rev. 2023, 475, 214896. [Google Scholar] [CrossRef]
- Gao, W.H.; He, J.Y.; Chen, L.; Meng, X.Q.; Ma, Y.N.; Cheng, L.L.; Tu, K.S.; Gao, X.F.; Liu, C.; Zhang, M.Z.; et al. Deciphering the catalytic mechanism of superoxide dismutase activity of carbon dot nanozyme. Nat. Commun. 2023, 14, 160. [Google Scholar] [CrossRef]
- Chen, J.Q.; Liu, X.Y.; Zheng, G.C.; Feng, W.; Wang, P.; Gao, J.; Liu, J.B.; Wang, M.Z.; Wang, Q.Y. Detection of Glucose Based on Noble Metal Nanozymes: Mechanism, Activity Regulation, and Enantioselective Recognition. Small 2023, 19, e2205924. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, G.H.; Liu, Y.T.; Liu, X.L.; Wang, H.X.; Chen, H.X.; Gao, J.; Jiang, Y.J. Metal Nanoparticles@Covalent Organic Framework@Enzymes: A Universal Platform for Fabricating a Metal-Enzyme Integrated Nanocatalyst. ACS Appl. Mater. Interfaces 2022, 14, 2881–2892. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, L.; Machorro-García, G.; López-Legarrea, A.; Trejo-Ayala, D.; Rostro-Alanis, M.D.; Sánchez-Sánchez, M.; Blanco, R.M.; Rodríguez-Rodríguez, J.; Parra-Saldívar, R. Metal-organic frameworks for enzyme immobilization and nanozymes: A laccase-focused review. Biotechnol. Adv. 2024, 70, 108299. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kolay, S.; Maity, S.; Patra, A. Copper Nanoclusters as Multienzymes Mimic Activities of Oxidase and Ascorbic Acid Oxidase in the Presence of Imidazole. Langmuir 2023, 40, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.K.; Wu, X.; Su, L.; Ma, Y.M.; Graham, N.J.D.; Yu, W.Z. The Fe-N-C oxidase-like nanozyme used for catalytic oxidation of NOM in surface water. Water Res. 2020, 171, 115491. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Q.; Liu, Q.Y.; Zhang, Y.H.; Wang, Q.; Li, S.R.; Zhu, B.J.; Miao, L.Y.; Du, Y.; Zhao, S.; Wei, H. A Dopamine-Enabled Universal Assay for Catalase and Catalase-Like Nanozymes. Anal. Chem. 2022, 94, 10636–10642. [Google Scholar] [CrossRef]
- Chen, Y.J.; Jiang, B.; Hao, H.G.; Li, H.J.; Qiu, C.Y.; Liang, X.; Qu, Q.Y.; Zhang, Z.D.; Gao, R.; Duan, D.M.; et al. Atomic-Level Regulation of Cobalt Single-Atom Nanozymes: Engineering High-Efficiency Catalase Mimics. Angew. Chem.-Int. Ed. 2023, 62, e202301879. [Google Scholar] [CrossRef]
- Liu, C.; Hu, J.; Yang, W.W.; Shi, J.Y.; Chen, Y.M.; Fan, X.; Gao, W.H.; Cheng, L.L.; Luo, Q.Y.; Zhang, M.Z. Carbon dot enhanced peroxidase-like activity of platinum nanozymes. Nanoscale 2024, 16, 4637–4646. [Google Scholar] [CrossRef]
- Wang, Y.F.; Liu, X.; Wang, M.K.; Wang, X.X.; Ma, W.Y.; Li, J.Y. Facile synthesis of CDs@ZIF-8 nanocomposites as excellent peroxidase mimics for colorimetric detection of H2O2 and glutathione. Sens. Actuators B-Chem. 2021, 329, 129115. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Shi, L.; Shi, L.L.; Li, T. Tin Porphyrin-Based Nanozymes with Unprecedented Superoxide Dismutase-Mimicking Activities. Langmuir 2022, 38, 7272–7279. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.L.; Xiong, J.; Yuan, X.; Liu, S.L.; Zong, M.H.; Lou, W.Y. Multivalent Ce-MOFs as biomimetic laccase nanozyme for environmental remediation. Chem. Eng. J. 2022, 450, 138220. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.J.; Du, D.; Ni, L.; Pan, J.M.; Niu, X.H. Emerging applications of nanozymes in environmental analysis: Opportunities and trends. TrAC-Trends Anal. Chem. 2019, 120, 115653. [Google Scholar] [CrossRef]
- Wei, M.; Lee, J.; Xia, F.; Lin, P.H.; Hu, X.; Li, F.Y.; Ling, D.S. Chemical design of nanozymes for biomedical applications. Acta Biomater. 2021, 126, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.Y.; Chen, D.X.; Wang, Y.; Li, H.F.; Zhang, Y.B.; Chen, H.Y.; Li, X.; Huo, M.F. Nanozymes-recent development and biomedical applications. J. Nanobiotechnol. 2022, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lai, L.X.; Huang, Y.Y.; Su, E.R. Nanozyme: An emerging tool for food packaging. Food Control 2024, 155, 110104. [Google Scholar] [CrossRef]
- Hemalatha, J.; Senthamil, C.; Sakthivel, C.; Nivetha, A.; Umashankar, J.J.; Prabha, I. Efficient transition metal nanozymes as the alternate for natural enzymes in food analysis and environmental remediation. J. Environ. Chem. Eng. 2024, 12, 112575. [Google Scholar] [CrossRef]
- Liang, Y.M.; Li, R.L.; Sun, H.; Dan, J.; Su, Z.H.; Kang, Y.; Zhang, Q.P.; Shi, S.; Wang, J.L.; Zhang, W.T. Functionalized natural melanin nanoparticle mimics natural peroxidase for total antioxidant capacity determination. Sens. Actuators B-Chem. 2022, 359, 131541. [Google Scholar] [CrossRef]
- Wang, T.Y.; Feng, J.X.; Sun, H.; Liang, Y.M.; Du, T.; Dan, J.; Wang, J.L.; Zhang, W.T. CuBi bimetallic aerogel as peroxidase-like nanozyme for total antioxidant capacity colorimetric detection. Sens. Actuators B-Chem. 2023, 379, 133249. [Google Scholar] [CrossRef]
- Dan, J.; Su, Z.H.; Sun, B.Y.; Wang, J.L.; Zhang, W.T. A polymetallic nanozyme with high peroxidase mimetic activity for rapid evaluation of total antioxidant capacity. Microchem. J. 2023, 185, 108302. [Google Scholar] [CrossRef]
- Lan, X.; Zhuo, J.C.; Luo, L.P.; Sun, H.; Liang, Y.M.; Feng, J.X.; Shu, R.; Li, Y.C.; Wang, T.Y.; Zhang, W.T.; et al. Metal-phenolic networks derived CN-FeC hollow nanozyme with robust peroxidase-like activity for total antioxidant capacity detection. Colloids Surf. B-Biointerfaces 2024, 234, 113640. [Google Scholar] [CrossRef]
- Ni, H.H.; Li, X.X.; Yan, G.J.; Huang, C.Z.; Zou, H.Y. Colorimetric evaluation of total antioxidant capacity based on peroxidase-like nonstoichiometric Cu2-xSe nanoparticles. Sens. Actuators B-Chem. 2024, 398, 134794. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Liu, D.; Ma, W.; Dramou, P.; He, H. Nanozymes based on metal-organic frameworks: Construction and prospects. TrAC Trends Anal. Chem. 2020, 133, 116080. [Google Scholar] [CrossRef]
- Lin, Y.P.; Li, L.; Shi, Z.; Zhang, L.S.; Li, K.; Chen, J.M.; Wang, H.; Lee, J.M. Catalysis with Two-Dimensional Metal-Organic Frameworks: Synthesis, Characterization, and Modulation. Small 2024, 20, 2309841. [Google Scholar] [CrossRef] [PubMed]
- Kute, A.D.; Gaikwad, R.P.; Warkad, I.R.; Gawande, M.B. A review on the synthesis and applications of sustainable copper-based nanomaterials. Green Chem. 2022, 24, 3502–3573. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.W.; Zhang, J.; Liu, J.; Wang, A.Z.; Ding, L.H. Recent Development of Copper-Based Nanozymes for Biomedical Applications. Adv. Healthc. Mater. 2024, 13, 2302023. [Google Scholar] [CrossRef] [PubMed]
- La, D.D.; Thi, H.P.N.; Kim, Y.S.; Rananaware, A.; Bhosale, S.V. Facile fabrication of Cu(II)-porphyrin MOF thin films from tetrakis(4-carboxyphenyl)porphyrin and Cu(OH)2 nanoneedle array. Appl. Surf. Sci. 2017, 424, 145–150. [Google Scholar] [CrossRef]
- Ji, L.D.; Wang, Q.; Peng, L.H.; Li, X.Y.; Zhu, X.M.; Hu, P. Cu-TCPP Nanosheets-Sensitized Electrode for Simultaneous Determination of Hydroquinone and Catechol. Materials 2022, 15, 4625. [Google Scholar] [CrossRef]
- Hussain, S.; Wan, X.Y.; Li, Z.Y.; Peng, X.S. Cu-TCPP nanosheets blended polysulfone ultrafiltration membranes with enhanced antifouling and photo-tunable porosity. Sep. Purif. Technol. 2021, 268, 118688. [Google Scholar] [CrossRef]
- Liu, X.; Wu, F.; Zheng, X.; Liu, H.; Ren, F.; Sun, J.; Ding, H.; Yang, R.; Jin, L. Facile Synthesis of High-Loading Cu Single-Atom Nanozyme for Total Antioxidant Capacity Sensing. ACS Appl. Nano Mater. 2023, 6, 10303–10311. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Li, X.; Liu, R.; Sang, Y.; Wang, X.; Wang, S. Nanozyme-enabled sensing strategies for determining the total antioxidant capacity of food samples. Food Chem. 2022, 384, 132412. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Zhou, Y. Bimetallic MOF-derived three-dimensional nanoflowers PdCoO(x) as peroxidase mimic activity for determining total antioxidant capacity. Food Chem. 2024, 457, 140120. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cheng, Y.; Liang, L.; Zhao, S.; Ye, F. Rational design of ZIF-67 derived hollow nanozyme through a general strategy for biosensing. Microchem. J. 2023, 190, 108601. [Google Scholar] [CrossRef]
- Ameen, S.S.M.; Omer, K.M. Temperature-resilient and sustainable Mn-MOF oxidase-like nanozyme (UoZ-4) for total antioxidant capacity sensing in some citrus fruits: Breaking the temperature barrier. Food Chem. 2024, 448, 139170. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Xiao, Y.; Cai, S.; Huang, C.; Guo, S.; Sun, Y.; Song, R.-B.; Li, Z. Carbon dots as light-responsive oxidase-like nanozyme for colorimetric detection of total antioxidant capacity in fruits. Food Chem. 2023, 405, 134749. [Google Scholar] [CrossRef]
- Kathiravan, S.; Sundaram, E.; Paulraj, B.A.; Johnson, P.M.; Huang, S.T.; Mani, V.; Vasantha, V.S. Simple and selective optical biosensor using Ultrasonicator synthesis of 5-((anthracen-9-ylmethylene) amino)-2,3-dihydrophthalazine-1,4-dione for direct detection of ascorbic acid in vegetables and fruits. Food Chem. 2020, 332, 127150. [Google Scholar] [CrossRef]

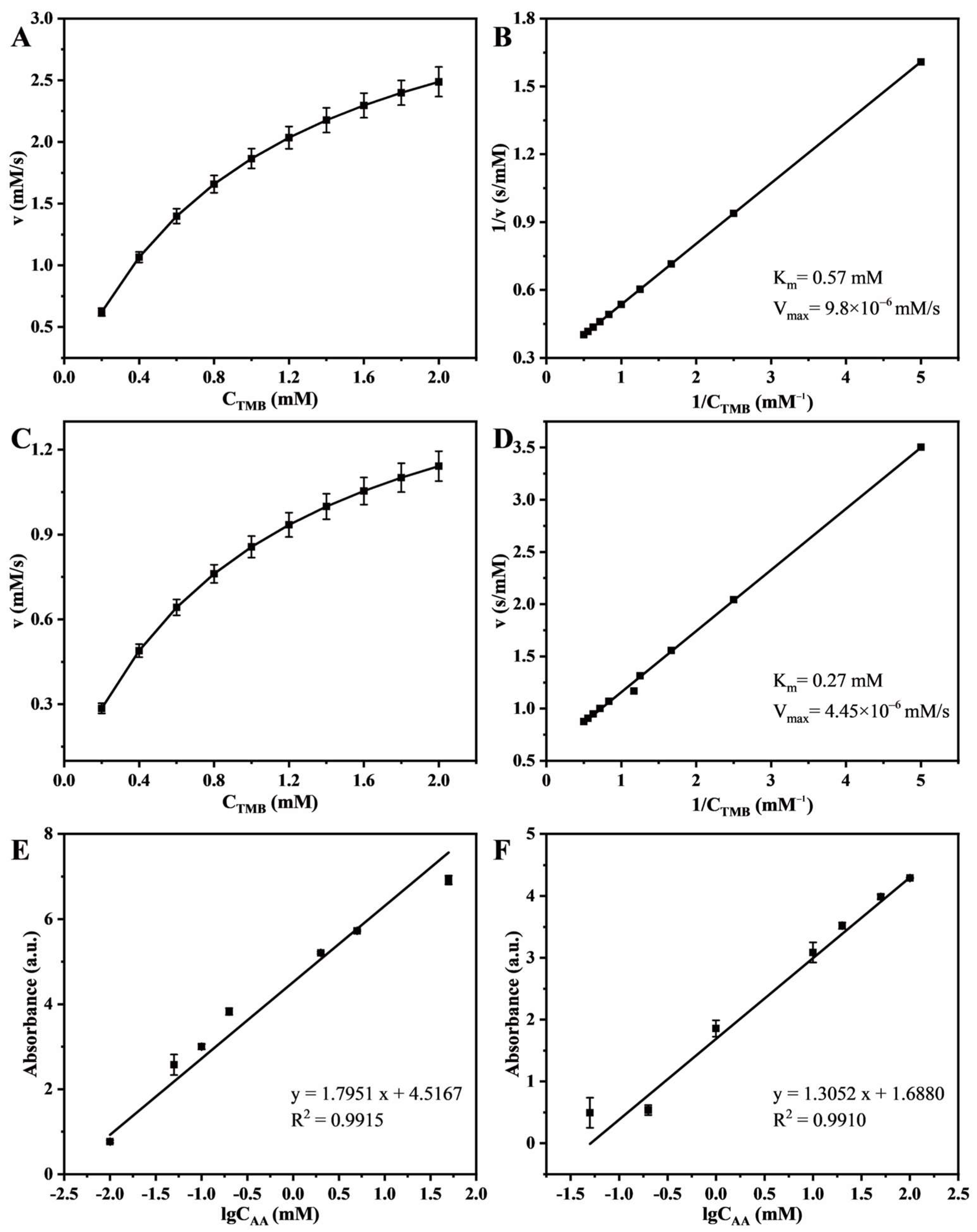
| Nanozyme | Substrate | Km (mM) | Linear Range (µM) | Ref. |
|---|---|---|---|---|
| Cu-TCPP | TMB | 0.57 | 10~1 × 105 | This work |
| CuO-TCPP | TMB | 0.27 | 50~1 × 105 | This work |
| Cu-SAC | TMB | 0.077 | 1~30 | [72] |
| Au@Cu-HCF | TMB | - | 7~250 | [73] |
| CuBi aerogel | TMB | 2.7 | 300~900 | [60] |
| Cu2-xSe | TMB | 0.457 | 0.5~50 | [63] |
| PdCoOx | TMB | 0.16 | 0~700 | [74] |
| Fe3+-PA | TMB | 1.15 | 2~40 | [75] |
| UoZ-4 | TMB | 0.071 | 3~25 | [76] |
| CDs | TMB | - | 1~30 | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Han, J.; Ping, Y.; Chen, X.; Zhao, Y.; Chen, G.; Lv, J.; Xu, D.; Zhang, Y.; Chen, J.; et al. Peroxidase-like Active Cu-MOFs Nanozymes for Colorimetric Detection of Total Antioxidant Capacity in Fruits and Vegetables. Foods 2025, 14, 1311. https://doi.org/10.3390/foods14081311
Huang Y, Han J, Ping Y, Chen X, Zhao Y, Chen G, Lv J, Xu D, Zhang Y, Chen J, et al. Peroxidase-like Active Cu-MOFs Nanozymes for Colorimetric Detection of Total Antioxidant Capacity in Fruits and Vegetables. Foods. 2025; 14(8):1311. https://doi.org/10.3390/foods14081311
Chicago/Turabian StyleHuang, Yanyan, Jiatong Han, Yi Ping, Xin Chen, Yiming Zhao, Ge Chen, Jun Lv, Donghui Xu, Yanguo Zhang, Jing Chen, and et al. 2025. "Peroxidase-like Active Cu-MOFs Nanozymes for Colorimetric Detection of Total Antioxidant Capacity in Fruits and Vegetables" Foods 14, no. 8: 1311. https://doi.org/10.3390/foods14081311
APA StyleHuang, Y., Han, J., Ping, Y., Chen, X., Zhao, Y., Chen, G., Lv, J., Xu, D., Zhang, Y., Chen, J., & Liu, G. (2025). Peroxidase-like Active Cu-MOFs Nanozymes for Colorimetric Detection of Total Antioxidant Capacity in Fruits and Vegetables. Foods, 14(8), 1311. https://doi.org/10.3390/foods14081311










