Advancing Pistacia terebinthus L. (Anacardiaceae) Research: Food Preservation, Functional Foods, and Nutraceutical Potential
Abstract
1. Introduction
2. Bioactive Profile and Nutritional Potential of P. terebinthus L.
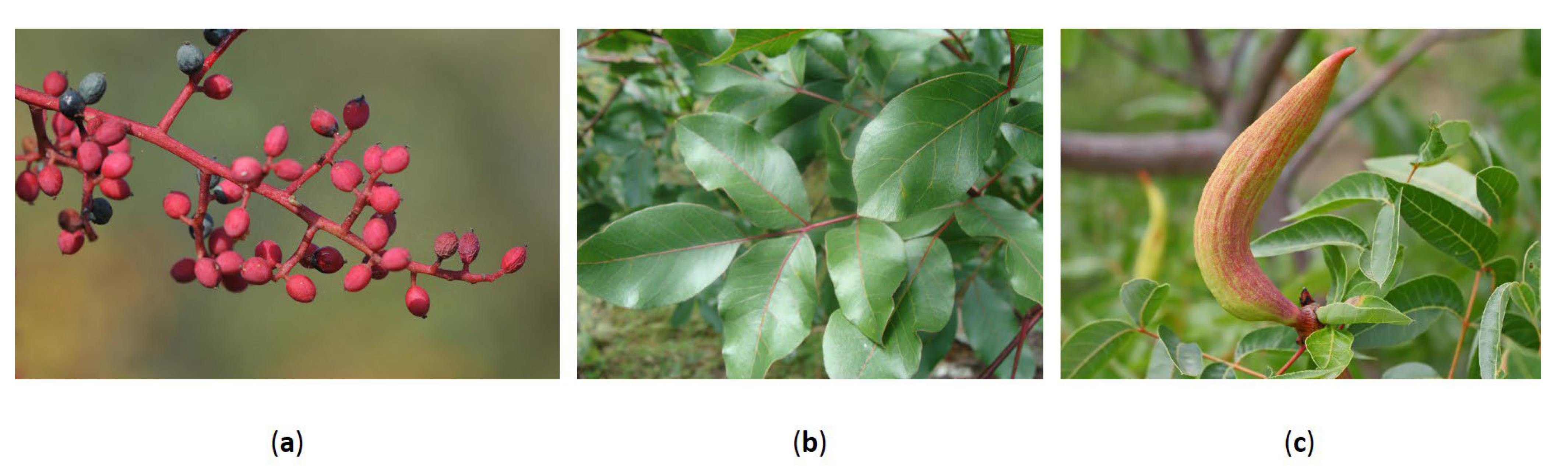
2.1. Fruits and Seeds
2.2. Resin
2.3. Leaves
2.4. Galls
3. Applications in Functional Foods and Nutraceuticals
3.1. Encapsulation for Aroma and Nutrient Retention
3.2. Use of P. terebinthus L. Resin as an Immobilization Support for Lactobacillus casei in Dairy Products
3.3. Applications in Fermentation and Alcoholic Beverages
3.4. Applications in Food Preservation and Antimicrobial Activity
3.5. Functional Foods and Antioxidants
3.6. Applications in Animal Nutrition and Health
4. Future Research Directions
- Optimization of processing techniques: while current studies have explored encapsulation, food packaging, and probiotic immobilization, further research is needed to refine processing parameters—such as carrier materials, drying conditions, and extraction methods—to maximize bioactive compound retention and functional efficacy.
- Standardization and quality control: the phytochemical profile of P. terebinthus L. varies widely depending on geographical origin, plant part, and harvest stage. Establishing standardized protocols for chemical profiling, authentication, and quality control is essential to ensure consistency, reproducibility, and efficacy in commercial applications.
- Yield and extraction efficiency: given that P. terebinthus L. is not yet commercially cultivated, future studies should focus on evaluating the yield variability and extraction efficiency of essential oils and bioactive compounds, particularly from fruits and leaves. This will be crucial for determining the plant’s economic feasibility and scalability for industrial use.
- Long-term stability and shelf-life studies: investigations into the stability of bioactive constituents in different storage conditions and food matrices are needed to validate the long-term effectiveness of P. terebinthus L. in functional food and preservation systems.
- Regulatory and safety assessments: although initial studies support the safety and health benefits of P. terebinthus L. extracts, comprehensive toxicological evaluations and regulatory assessments are required to establish safe usage levels and gain approval for food and nutraceutical applications.
- Allergenicity and oxidative stability of volatile compounds: given that major volatile constituents of P. terebinthus L. essential oils—such as limonene, α-pinene, and β-pinene—are known to oxidize into allergenic compounds, future research should assess their stability under storage and processing conditions relevant to food systems. Although these substances are approved as flavorings, evaluating their allergenic potential, degradation behavior, and safe inclusion levels is critical for ensuring consumer safety, particularly in functional food formulations and edible packaging applications.
- Mechanistic insights into bioactivity: more in-depth pharmacological and biochemical research is necessary to clarify the specific mechanisms through which P. terebinthus L. compounds exert their antimicrobial, antioxidant, and other health-promoting properties.
- Sustainability and commercial viability: as a wild-growing species, P. terebinthus L. presents challenges related to sustainable harvesting and supply chain development. Future efforts should investigate environmentally responsible collection practices and cost-effective, scalable extraction technologies to support commercialization.
- Expansion into new applications: additional studies are encouraged to explore novel delivery systems and product formats, including incorporation into functional beverages, dietary supplements, edible films, and intelligent food packaging materials.
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaves Lobón, N.; González Félix, M.; Alías Gallego, J.C. Comparison of the Allelopathic Potential of Non-Native and Native Species of Mediterranean Ecosystems. Plants 2023, 12, 972. [Google Scholar] [CrossRef] [PubMed]
- Bozorgi, M.; Memariani, Z.; Mobli, M.; Salehi Surmaghi, M.H.; Shams-Ardekani, M.R.; Rahimi, R. Five Pistacia Species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): A Review of Their Traditional Uses, Phytochemistry, and Pharmacology. Sci. World J. 2013, 2013, 219815. [Google Scholar] [CrossRef] [PubMed]
- Schoina, V.; Terpou, A.; Papadaki, A.; Bosnea, L.; Kopsahelis, N.; Kanellaki, M. Enhanced Aromatic Profile and Functionality of Cheese Whey Beverages by Incorporation of Probiotic Cells Immobilized on Pistacia terebinthus Resin. Foods 2020, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Badem, A. Some Traditional Terebinth Dishes in Turkey and Their Health Effects. J. Gastron. Hosp. Travel 2021, 4, 306–320. [Google Scholar] [CrossRef]
- Fidan, M.S.; Baltacı, C.; Öz, M.; Akar, Z. Chemical Composition of Pistacia terebinthus L. and Its Phytochemical and Biological Properties. BioResources 2023, 18, 6862–6881. [Google Scholar] [CrossRef]
- Yaman, D.M.; Koçak Yanık, D.; Elik Demir, A.; Uzun Karka, H.; Güçlü, G.; Selli, S.; Kelebek, H.; Göğüş, F. Effect of Encapsulation Techniques on Aroma Retention of Pistacia terebinthus L. Fruit Oil: Spray Drying, Spray Freeze Drying, and Freeze Drying. Foods 2023, 12, 3244. [Google Scholar] [CrossRef]
- Batovska, D.; Inbar, M. Beyond the Nut: Pistacia Leaves as Natural Food Preservatives. Foods 2024, 13, 3138. [Google Scholar] [CrossRef]
- Rauf, A.; Patel, S.; Uddin, G.; Siddiqui, B.S.; Ahmad, B.; Muhammad, N.; Mabkhot, Y.N.; Hadda, T.B. Phytochemical, Ethnomedicinal Uses and Pharmacological Profile of Genus Pistacia. Biomed. Pharmacother. 2017, 86, 393–404. [Google Scholar] [CrossRef]
- Bagheri, F.; Hassanshahi, G.; Khanamani Falahatipour, S.; Dini, A.; Mohamadi, M.; Ahmadi, Z.; Noroozi, M.; Khanamani Falahatipour, S. Effects of Pistachios and Their Different Plant Parts on Various Disorders: Evidence about Their Therapeutic Effects on Diabetes Mellitus, Gastrointestinal and Liver Disorders, as well as Blood Pressure. Pistachio Health J. 2021, 4, 28–47. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Ozdal, T.; Bakir, S.; Capanoglu, E. Bioaccessibility of Terebinth (Pistacia terebinthus L.) Coffee Polyphenols: Influence of Milk, Sugar and Sweetener Addition. Food Chem. 2022, 374, 131728. [Google Scholar] [CrossRef]
- Alagöz, E.; Sarıçoban, C. The Effects of Adding Ground Terebinth Fruits on the Emulsification, Microstructural, and Flow Properties of Meat Emulsions. Food Sci. Biotechnol. 2024, 33, 3009–3018. [Google Scholar] [CrossRef] [PubMed]
- Ozgolet, M.; Cakmak, Z.H.T.; Bozkurt, F.; Sagdic, O.; Karasu, S. Response Surface Optimization of Protein Extraction from Cold-Pressed Terebinth (Pistacia terebinthus L.) Oil Byproducts: Physicochemical and Functional Characteristics. J. Food Sci. 2024, 89, 7380–7396. [Google Scholar] [CrossRef] [PubMed]
- Schoina, V.; Terpou, A.; Angelika-Ioanna, G.; Koutinas, A.; Kanellaki, M.; Bosnea, L. Use of Pistacia terebinthus Resin as Immobilization Support for Lactobacillus casei Cells and Application in Selected Dairy Products. J. Food Sci. Technol. 2015, 52, 5700–5708. [Google Scholar] [CrossRef] [PubMed]
- Kallis, M.; Sideris, K.; Kopsahelis, N.; Bosnea, L.; Kourkoutas, Y.; Terpou, A.; Kanellaki, M. Pistacia terebinthus Resin as Yeast Immobilization Support for Alcoholic Fermentation. Foods 2019, 8, 127. [Google Scholar] [CrossRef]
- Kallis, M.; Boura, K.; Karabagias, I.K.; Kanellaki, M.; Koutinas, A.A. Beneficial Effects of Pistacia terebinthus Resin on Wine Making. Appl. Sci. 2022, 12, 9097. [Google Scholar] [CrossRef]
- Akpulat, S.; Tiras, M.; Sahinkaya, M.S.; Akpulat, H.A. Antimicrobial Effect of Pistacia terebinthus (Turpentine Tree) Galls and GC/MS Analysis. Turk. J. Biodivers. 2021, 4, 98–104. [Google Scholar] [CrossRef]
- Nazım, B.; Ismaıl, B.; Houari, T.; Zakaria, N. In Vitro Antimitotic Activity of Gall Extract of Pistacia terebinthus. Curr. Perspect. Med. Aromat. Plants 2020, 3, 128–134. [Google Scholar] [CrossRef]
- Mandalari, G.; Barreca, D.; Gervasi, T.; Roussell, M.A.; Klein, B.; Feeney, M.J.; Carughi, A. Pistachio Nuts (Pistacia vera L.): Production, Nutrients, Bioactives and Novel Health Effects. Plants 2022, 11, 18. [Google Scholar] [CrossRef]
- Soulaidopoulos, S.; Tsiogka, A.; Chrysohoou, C.; Lazarou, E.; Aznaouridis, K.; Doundoulakis, I.; Tyrovola, D.; Tousoulis, D.; Tsioufis, K.; Vlachopoulos, C.; et al. Overview of Chios Mastic Gum (Pistacia lentiscus) Effects on Human Health. Nutrients 2022, 14, 590. [Google Scholar] [CrossRef]
- Ben Ahmed, Z.; Yousfi, M.; Viaene, J.; Dejaegher, B.; Demeyer, K.; Vander Heyden, Y. Four Pistacia atlantica Subspecies (atlantica, cabulica, kurdica and mutica): A Review of Their Botany, Ethnobotany, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2021, 265, 113329. [Google Scholar] [CrossRef]
- Benmahieddine, A.; Belyagoubi-Benhammou, N.; Belyagoubi, L.; Amari, N.O.; Zerey-Belaskri, A.E.; Gismondi, A.; Di Marco, G.; Canini, A.; Habi, S.; Atik Bekkara, F.; et al. Leaf-Buds of Pistacia atlantica: A Novel Source of Bioactive Molecules with High Anti-Inflammatory, Antioxidant, Anti-Tyrosinase, and Antimicrobial Properties. Physiol. Mol. Biol. Plants 2023, 29, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Belyagoubi-Benhammou, N.; Belyagoubi, L.; Benmahieddine, A.; El Zerey-Belaskri, A.; Di Marco, G.; D’Agostino, A.; Canini, A.; Gismondi, A. Nutraceutical Content and Biological Properties of Lipophilic and Hydrophilic Fractions of the Phytocomplex from Pistacia atlantica Desf. Buds, Roots, and Fruits. Plants 2024, 13, 611. [Google Scholar] [CrossRef] [PubMed]
- Tufan, T.; Arslan, C.; Daş, A. Effects of Terebinth (Pistacia terebinthus L.) Fruit Oil Supplementation to Diets on Fattening Performance, Carcass Characteristics, Blood Parameters, and Breast Meat Fatty Acid Composition in Japanese Quails (Coturnix coturnix japonica). Kafkas Universitesi Vet. Fakultesi Derg. 2017, 23, 289–295. [Google Scholar] [CrossRef]
- Özcan, M.M.; Al Juhaimi, F.; Uslu, N.; Ahmed, I.A.M.; Babiker, E.E.; Osman, M.A.; Gassem, M.A.; Alqah, H.A.S.; Ghafoor, K. Effect of Sonication Process of Terebinth (Pistacia terebinthus L.) Fruits on Antioxidant Activity, Phenolic Compounds, Fatty Acids, and Tocopherol Contents. J. Food Sci. Technol. 2020, 57, 2017–2025. [Google Scholar] [CrossRef]
- Hacıbekiroğlu, I.; Yılmaz, P.K.; Haşimi, N.; Kılınç, E.; Tolan, V.; Kolak, U. In Vitro Biological Activities and Fatty Acid Profiles of Pistacia terebinthus Fruits and Pistacia khinjuk Seeds. Nat. Prod. Res. 2015, 29, 444–446. [Google Scholar] [CrossRef]
- Pulaj, B.; Mustafa, B.; Nelson, K.; Quave, C.L.; Hajdari, A. Chemical Composition and In Vitro Antibacterial Activity of Pistacia terebinthus Essential Oils Derived from Wild Populations in Kosovo. BMC Complement. Altern. Med. 2016, 16, 147. [Google Scholar] [CrossRef]
- İnan, M. Seasonal Variation of Fatty and Essential Oil in Terebinth (Pistacia terebinthus L.) Fruit. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12171. [Google Scholar] [CrossRef]
- Akyuz, M.; Yabo-Dambagi, L.; Kilic, T.; Cakir, A. Antidiabetic, Neuroprotective, and Antioxidant Potentials of Different Parts of Pistacia terebinthus Fruits. S. Afr. J. Bot. 2022, 147, 443–456. [Google Scholar] [CrossRef]
- Kaya, F.; Özer, A. Characterization of Extracted Oil from Seeds of Terebinth (Pistacia terebinthus L.) Growing Wild in Turkey. Turk. J. Sci. Technol. 2015, 10, 49–57. [Google Scholar]
- Kaya, F.; Özer, A. A Study on the Investigation of Diffusion Coefficient for Oil Extraction from Terebinth (Pistacia terebinthus L.) Seeds. Curr. Phys. Chem. 2020, 10, 144–153. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Papageorgiou, V.P. GC-MS Analysis of Penta- and Tetra-Cyclic Triterpenes from Resins of Pistacia Species. Part II. Pistacia terebinthus var. Chia. Biomed. Chromatogr. 2005, 19, 586–605. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Marzouki, H.; Maxia, A.; Marengo, A.; Porcedda, S.; Falconieri, D.; Salgueiro, L. Chemical Characterization and Biological Activity of Leaf Essential Oils Obtained from Pistacia terebinthus Growing Wild in Tunisia and Sardinia Island. Nat. Prod. Res. 2017, 31, 2684–2689. [Google Scholar] [CrossRef]
- Hamlat, N.; Alqaraleh, M.; Kasabri, V.; Mizher, H.; Hassani, A.; Afifi, F.; Al Alawi, S.; Ouafi, S.; Khwaldeh, A. Dual Amylase/Glucosidase Inhibition, Antilipolytic and Antiproliferative Potential of the Aerial Parts of Pistacia atlantica, Pistacia lentiscus, and Pistacia terebinthus on an Obesity-Related Colorectal Cancer Cell Line Panel. Curr. Issues Pharm. Med. Sci. 2024, 37, 131–137. [Google Scholar] [CrossRef]
- Uysal, S.; Sinan, K.I.; Jekő, J.; Cziáky, Z.; Zengin, G. Chemical Characterization, Comprehensive Antioxidant Capacity, and Enzyme Inhibitory Potential of Leaves from Pistacia terebinthus L. (Anacardiaceae). Food Biosci. 2022, 48, 101820. [Google Scholar] [CrossRef]
- Aşan Özüsağlam, M. Investigation of New Potential Uses of Menengiç (Pistacia terebinthus) for Various Areas. Kahramanmaraş Sütçü İmam Üniv. Tarım Doğa Derg. 2024, 27, 793–801. [Google Scholar] [CrossRef]
- Barbouchi, M.; Elamrani, K.; El Idrissi, M.; Choukrad, M. The Effect of Solvent Extracts on the Measurement of Total Polyphenol, Flavonoid, and Tannin Contents and Its Relation to Antioxidant Capacities from Various Parts of Terebinth (Pistacia terebinthus L.). Mor. J. Chem. 2019, 7, 290–300. [Google Scholar] [CrossRef]
- Aqil, M.; Ahad, A.; Sultana, Y.; Ali, A. Status of Terpenes as Skin Penetration Enhancers. Drug Discov. Today 2007, 12, 1061–1067. [Google Scholar] [CrossRef]
- Gómez-Favela, M.A.; Santos-Ballardo, D.U.; Bergés-Tiznado, M.E.; Ambriz-Pérez, D.L. Nanoformulations Applied to the Delivery of Terpenes. In Nanotechnology in Biomedicine, Phytochemical Nanodelivery Systems as Potential Biopharmaceuticals; Heredia, J.B., Gutiérrez-Grijalva, E.P., Licea-Claverie, A., Gutierrez-Uribe, J.A., Patra, J.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 221–256. [Google Scholar] [CrossRef]
- Karlberg, A.-T.; Lepoittevin, J.-P. One Hundred Years of Allergic Contact Dermatitis Due to Oxidized Terpenes: What We Can Learn from Old Research on Turpentine Allergy. Contact Dermatitis 2021, 85, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Sukakul, T.; Bruze, M.; Mowitz, M.; Antelmi, A.; Bergendorff, O.; Björk, J.; Dahlin, J.; Hamnerius, N.; Hauksson, I.; Isaksson, M.; et al. Contact Allergy to Oxidized Linalool and Oxidized Limonene: Patch Testing in Consecutive Patients with Dermatitis. Contact Dermat. 2022, 86, 15–24. [Google Scholar] [CrossRef]
- Hu, W.; Meng, X.; Wu, Y.; Li, X.; Chen, H. Terpenoids, a Rising Star in Bioactive Constituents for Alleviating Food Allergy: A Review about the Potential Mechanism, Preparation, and Application. J. Agric. Food Chem. 2024, 72, 26599–26616. [Google Scholar] [CrossRef]
- Nazım, B.; Houssem, F.; Ismaıl, B.; Yassıne, M. Antimicrobial Activity of Leaf, Fruit, and Gall Extract of Pistacia terebinthus Growing in Tessala. Curr. Perspect. Med. Aromat. Plants 2021, 4, 87–92. [Google Scholar] [CrossRef]
- Dodange, S.; Shekarchizadeh, H.; Kadivar, M. Development and Characterization of Antioxidant Bilayer Film Based on Poly Lactic Acid–Bitter Vetch (Vicia ervilia) Seed Protein Incorporated with Pistacia terebinthus Extract for Active Food Packaging. Curr. Res. Food Sci. 2023, 7, 100613. [Google Scholar] [CrossRef] [PubMed]
- Schoina, V.; Terpou, A.; Bosnea, L.; Kanellaki, M.; Nigam, P.S. Entrapment of Lactobacillus casei ATCC393 in the Viscus Matrix of Pistacia terebinthus Resin for Functional Myzithra Cheese Manufacture. LWT 2018, 89, 441–448. [Google Scholar] [CrossRef]
- Naghmachi, M.; Raissi, A.; Baziyar, P.; Homayoonfar, F.; Amirmahani, F.; Danaei, M. Green Synthesis of Silver Nanoparticles (AgNPs) by Pistacia terebinthus Extract: Comprehensive Evaluation of Antimicrobial, Antioxidant and Anticancer Effects. Biochem. Biophys. Res. Commun. 2022, 608, 163–169. [Google Scholar] [CrossRef]
- Köten, M.; Ünsal, A.S. Nutritional, Chemical, and Cooking Properties of Noodles Enriched with Terebinth (Pistacia terebinthus) Fruits Roasted at Different Temperatures. Food Sci. Technol. 2021, 42, e47120. [Google Scholar] [CrossRef]
- Ergenekon, M.; Akbulut Çakır, Ç. Impact of Pistacia terebinthus on the Antioxidant Activity and Total Phenolics of Ice Cream Depending on Roasting Conditions and Incorporation Time. Eur. J. Food Sci. Technol. 2024, 8, 81–91. [Google Scholar]
- Ali, G.M.A.; Bilen, S.; Güney, K. Growth Promoter, Immunostimulant and Antioxidant for Rainbow Trout (Oncorhynchus mykiss): Terebinth (Pistacia terebinthus) Extract. Mar. Sci. Technol. Bull. 2022, 11, 98–112. [Google Scholar] [CrossRef]
- Gültepe, E.E.; Çetingül, I.S.; Uyarlar, C.; Iqbal, A.; Rahman, A.; Hacisalihoglu, S.; Ozcinar, U.; Bayram, I. Effects of Pistacia terebinthus Seed Meal and Different Storage Times on Egg Quality of Laying Hens. R. Bras. Zootec. 2018, 47, e20170322. [Google Scholar] [CrossRef]
- Çetingül, I.S.; Gültepe, E.E.; Rahman, A.; Iqbal, A.; Uyarlar, C.; Hacisalihoglu, S.; Özcinar, Ü.; Bayram, I. Pistacia terebinthus as a Dietary Supplement for Laying Hens. S. Afr. J. Anim. Sci. 2020, 50, 38–46. [Google Scholar] [CrossRef]
- Poyrazoglu, E.; Ozat, E.; Coksari, G.; Ozat, E.; Konar, N. Effect of Various Process Conditions on Efficiency and Colour Properties of Pistacia terebinthus Oil Encapsulated by Spray Drying. ETP Int. J. Food Eng. 2017, 3, 132–135. [Google Scholar] [CrossRef]
| Plant Part | Ingredient/Method | Main Compounds | Functional Potential | References |
|---|---|---|---|---|
| Fruits | Cold-pressed oil (traditional aqueous-assisted cold pressing) | Crude fat (39% *), crude protein (10%), crude fiber (11%); fatty acids: oleic acid (47%), linoleic acid (23%), palmitic (22%); vitamins: vitamin C (>1%), γ-tocopherol (>1%); minerals: potassium (<1%), calcium (>1%), sodium (>1%), phosphorus (>1%) | Alternative supplement, industrial oil, antioxidant, supports bone health, electrolyte balance, nerve and muscle function, metabolic activity | [4,5,23,24] |
| Fatty acids (solvent extraction with n-hexane) | Oleic acid (53%), palmitic acid (21%), linoleic acid (19%) | Cognitive and cardiovascular health, neuroprotective effects, energy source | [25] | |
| Essential oil (hydrodistillation) | (Z)-β-Ocimene (2–45%), D-limonene (16–24%), α-pinene (13–37%), p-cymen-8-ol (11%), p-anisaldehyde (7%) | Antimicrobial agent food preservative, aroma enhancer | [5,26,27] | |
| Phenolic compounds (sonication with methanol) | Rutin (1%), quercetin (1%), luteolin (<1%), gallic acid (<1%), syringic acid (<1%), catechol (<1%) | Antioxidant, antimicrobial, antidiabetic, flavor enhancer, food preservation | [5,24,28] | |
| Seeds | Cold-pressed oil (Soxhlet method) | Crude oil (47%), crude protein (9%), carbohydrates (21%); fatty acids: oleic acid (45%), palmitic acid (24%), linoleic acid (24%) | Edible oil, functional food applications | [29,30] |
| Protein-rich oil (protein extraction from cold-pressed oil) | Protein (54–66%), carbohydrate (23–34%) | Emulsifying and foaming properties, food industry | [12] | |
| Resin | Triterpenoids (fractionated to acidic and neutral fractions) | Isomasticadienonic acid (26% w/w **), 28-norolean-17-en-3-one (18%), masticadienonic acid (7%) | Anti-inflammatory, antimicrobial, anti-tumor, antioxidant, and gastroprotective activities | [31] |
| Leaves | Essential oil (hydrodistillation) | α-Pinene (33–47%), limonene (17–29%), β-pinene (3–12%) | Fungicidal activity, food preservative | [26,32] |
| Essential oil (hydrodistillation) | α-Pinene (35%), camphene (2%), β-pinene (5%), terpinolene (35%), β-phellandrene (5%) | Fungicidal activity | [32] | |
| Triterpenoids (solvent extraction with ethyl acetate) | Masticadienediol (n.d. ***), isomasticadienonic acid (n.d.), masticadienonic acid (n.d.), masticadienolic acid (n.d.), tirucallone (n.d.), oleanolic acid (n.d.), ursolic acid (n.d.), morolic acid (n.d.) | Anti-inflammatory, nutraceutical applications; targeting food allergy management and immune health | [8] | |
| Flavonoids (aqueous Reflux Extraction) | Kaempferol (4%) and rutin (2%) | Diabetes and obesity management, colorectal cancer prevention | [33] | |
| Phenolics (maceration with methanol) | Gallic, digallic, protocatechuic, and p-coumaric acids (n.d), procyanidin B (n.d.), and various flavonoids (n.d.) such as taxifolin, myricetin, quercitrin, quercetin, cosmosiin, and luteolin | Antioxidant, neuroprotective, glucose metabolism regulation, food preservation | [34] | |
| Galls | Essential oil (hydrodistillation) | α-Pinene (53–66%), limonene (17–23%), β-pinene (0–11%) | Natural preservative | [26] |
| Fatty acids/triterpenoids (Soxhlet extraction with ethanol) | Lauric acid (n.d.), myristic acid (n.d.), lanosterol (n.d.), lupeol (n.d.) | Natural preservative | [16] | |
| Phenolics (Soxhlet extraction with methanol) | Gallic acid (n.d.), rutin (n.d.), caffeoylquinic acid (n.d.) | Potential chemopreventive agent | [17] |
| Technology | Plant Part | Key Findings |
|---|---|---|
| Encapsulation | Fruit oil | Spray-drying with ultrasonic nozzle achieved >90% encapsulation efficiency; α-pinene and linalool retained nearly 100% [6] |
| Food packaging | Fruit extracts | Bilayer film with 15% fruit extract showed strong mechanical properties and high water resistance [43] |
| Emulsion stabilization | Fruits and seeds | Enhanced emulsion capacity and stability in meat emulsions; roasted terebinth provided better results [11] |
| Probiotic immobilization in dairy products | Resin | Improved probiotic viability (>7 log CFU/g in yogurt), antimicrobial effects in cheese, and enhanced aroma [3,13,44] |
| Fermentation carrier for yeast | Resin | Maintained yeast viability for 90 days, enhanced aroma compounds, and improved fermentation efficiency [14,15] |
| Food preservation | Extract (silver nanoparticles) | Strong antimicrobial and antioxidant activity; effective against Gram-positive and Gram-negative bacteria [45] |
| Functional food fortification (noodles, ice cream) | Resin and seeds | Increased antioxidant activity, dietary fiber, and protein content; improved sensory properties [46,47] |
| Animal nutrition (fish feed, poultry feed) | Fruits and seeds | Improved growth, digestion, and immune response in fish; increased egg production and weight in poultry [48,49,50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batovska, D. Advancing Pistacia terebinthus L. (Anacardiaceae) Research: Food Preservation, Functional Foods, and Nutraceutical Potential. Foods 2025, 14, 1245. https://doi.org/10.3390/foods14071245
Batovska D. Advancing Pistacia terebinthus L. (Anacardiaceae) Research: Food Preservation, Functional Foods, and Nutraceutical Potential. Foods. 2025; 14(7):1245. https://doi.org/10.3390/foods14071245
Chicago/Turabian StyleBatovska, Daniela. 2025. "Advancing Pistacia terebinthus L. (Anacardiaceae) Research: Food Preservation, Functional Foods, and Nutraceutical Potential" Foods 14, no. 7: 1245. https://doi.org/10.3390/foods14071245
APA StyleBatovska, D. (2025). Advancing Pistacia terebinthus L. (Anacardiaceae) Research: Food Preservation, Functional Foods, and Nutraceutical Potential. Foods, 14(7), 1245. https://doi.org/10.3390/foods14071245







