Characterization of Changes in Key Odorants in Blueberries During Simulated Commercial Storage and Marketing by Sensory-Directed Flavor Analysis and Determination of Differences in Overall Perceived Aroma
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit
2.2. Determination of Weight Loss, Firmness, Total Soluble Solids, and Total Acidity
2.3. Sensory Evaluation Based on R-Index Analysis
2.4. Determination of Volatile Compounds
2.4.1. Chemicals
2.4.2. Unlabeled Reference Standards
2.4.3. Isotopically Labeled Standards
2.4.4. Aroma Extraction by Solvent-Assisted Flavor Evaporation (SAFE)
2.4.5. Gas Chromatography–Mass Spectrometry–Olfactometry (GC-MS-O)
2.4.6. Aroma Extract Dilution Analysis (AEDA)
2.4.7. Compound Identification
2.4.8. Quantitation of Selected Volatile Compounds
Determination of Selected Volatile Compounds by SAFE-GC-MS
Determination of Selected Volatile Compounds by Headspace Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry (HS-SPME-GC-MS)
2.4.9. Calculation of Amounts of Selected Volatile Compounds
2.4.10. Determination of Odor Activity Values (OAVs)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of Storage Conditions on Total Acidity, Firmness, and Soluble Solids in Blueberries
3.2. Effect of Storage Conditions on Character Impact Odorants of Blueberries
3.2.1. AEDA Analysis by GC-MS-O
Esters
Aldehydes and Corresponding Alcohols: Lipid Oxidation Products from the Lipoxygenase Pathway
Terpenes: Products of Terpene Synthase
Benzenoids and Phenolic Compounds: Derived Products of Phenylalanine
Miscellaneous Compounds
3.2.2. Quantitation of Selected Key Odorants and OAVs
| Target Odorants | RI | Odor Threshold (µg/L) 2 | Concentrations (µg/kg) 1 | OAVs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Storage Treatments | Storage Treatments | |||||||||
| SC | S1 | S2 | S3 | SC | S1 | S2 | S3 | |||
| Methyl-3-methylbutyrate | 1022 | 4.4 | 9.21 ± 0.96 C | 22.33 ± 1.53 A | 6.79 ± 0.94 C | 15.5 ± 0.89 B | 2 | 5 | 2 | 4 |
| Ethyl-3-methylbutyrate | 1072 | 0.11 | 1.09 ± 0.04 A | 0.98 ± 0.05 B | 1.04 ± 0.03 AB | 0.99 ± 0.02 AB | 10 | 9 | 9 | 9 |
| Hexanal | 1083 | 10 | 84.09 ± 1.76 A | 9.72 ±1.31 B | 10.82 ± 1.59 BC | 16.74 ± 1.92 CD | 8 | 1 | 1 | 2 |
| (Z)-3-Hexenal | 1145 | 0.21 | 28.38 ± 1.36 C | 38.39 ± 1.75 B | 42.43 ± 3.27 B | 83.14 ± 0.78 A | 135 | 183 | 202 | 396 |
| Eucalyptol | 1210 | 1.1 | 35.77 ± 2.05 B | 55.5 ± 1.13 A | 32.53 ± 1.29 B | 50.73 ± 1.33 A | 33 | 50 | 30 | 46 |
| (E)-2-Hexenal | 1221 | 190 | 109.72 ± 10.65 A | 21.64 ± 4.38 B | 27.02 ± 1.91 B | 41.77 ± 4.31 B | 1 | <1 | <1 | <1 |
| 1-Octen-3-one | 1278 | 0.036 | 13.73 ± 1.51 A | 0.68 ± 0.14 C | 5.34 ± 1.34 BC | 10.45 ± 1.88 AB | 381 | 19 | 148 | 290 |
| (E)-3-Hexen-1-ol | 1374 | 110 | 10.27 ± 0.53 A | 5.05 ± 0.08 D | 8.55 ± 0.21 B | 7.09 ± 0.15 C | <1 | <1 | <1 | <1 |
| (Z)-3-Hexen-1-ol | 1387 | 13 | 8.18 ± 0.04 C | 15.08 ± 0.41 B | 26.56 ± 0.82 A | 11.26 ± 0.48 BC | 1 | 1 | 2 | 1 |
| 1-Octen-3-ol | 1453 | 1 | 53.81 ± 4.51 B | 2.15 ± 0.10 B | 4.76 ± 0.31 B | 266.97 ± 30.70 A | 54 | 2 | 5 | 267 |
| Linalool | 1550 | 6 | 678.16 ± 17.95 A | 221.02 ± 7.71 C | 364.21 ± 14.26 B | 267.47 ± 14.88 C | 113 | 37 | 61 | 45 |
| (E,Z)-2,6-Nonadienal | 1587 | 0.01 | 19.62 ± 0.24 B | 0.10 ± 0.01 D | 1.45 ± 0.35 C | 89.23 ± 0.58 A | 1962 | 10 | 145 | 8923 |
| 3-Methylbutyric acid | 1678 | 132 | 63.06 ± 4.05 A | 25.52 ± 0.17 B | 29.13 ± 0.29 B | 21.99 ± 2.12 B | <1 | <1 | <1 | <1 |
| α-Terpineol | 1701 | 330 | 82.17 ± 12.37 A | 82.57 ± 6.89 A | 59.2 ± 12.03 A | 92.43 ± 21.75 A | <1 | <1 | <1 | <1 |
| Citronellol | 1784 | 40 | 0.74 ± 0.01 C | 0.002 ± 0.00 C | 2.26 ± 0.34 B | 287.41 ± 11.76 A | <1 | <1 | <1 | <1 |
| Nerol | 1802 | 300 | 6.93 ± 0.03 B | 0.02 ± 0.00 B | 4.44 ± 0.04 B | 236.94 ± 15.93 A | <1 | <1 | <1 | <1 |
| Guaiacol | 1861 | 2.5 | 2.11 ± 0.08 B | 0.005 ± 0.00 B | 0.60 ± 0.15 B | 15.92 ± 1.67 A | 1 | <1 | <1 | 6 |
| Geraniol | 1867 | 5 | 3.75 ± 0.56 B | 0.01 ± 0.00 B | 2.60 ± 0.03 B | 48.38 ± 8.94 A | 1 | <1 | 1 | 10 |
| (E)-Cinnamaldehyde | 2038 | 790 | 0.15 ± 0.03 B | 0.01 ± 0.00 B | 0.28 ± 0.02 B | 4.35 ± 1.09 A | <1 | <1 | <1 | <1 |
| γ-Decalactone | 2165 | 11 | 0.24 ± 0.00 C | 0.01 ± 0.00 D | 0.81 ± 0.01 B | 2.21 ± 0.08 A | <1 | <1 | <1 | <1 |
| Eugenol | 2171 | 1.3 | 5.15 ± 0.00 C | 5.28 ± 0.13 C | 14.88 ± 0.29 A | 11.88 ± 0.27 B | 4 | 4 | 11 | 9 |
| 4-Vinylguaiacol | 2187 | 20 | 0.06 ± 0.01 C | 0.01 ± 0.00 C | 1.50 ± 0.20 B | 3.19 ± 0.17 A | <1 | <1 | <1 | <1 |
| Vanillin | 2569 | 25 | 57.27 ± 0.46 B | 29.36 ± 2.26 C | 82.39 ± 2.23 A | 57.89 ± 2.66 B | 2 | 1 | 3 | 2 |
| Storage Treatments | R-Index JB a |
|---|---|
| S1 | 81 * |
| S2 | 57 |
| S3 | 62 |
3.3. Effects of Storage Conditions on the Overall Perceived Aroma of Blueberries
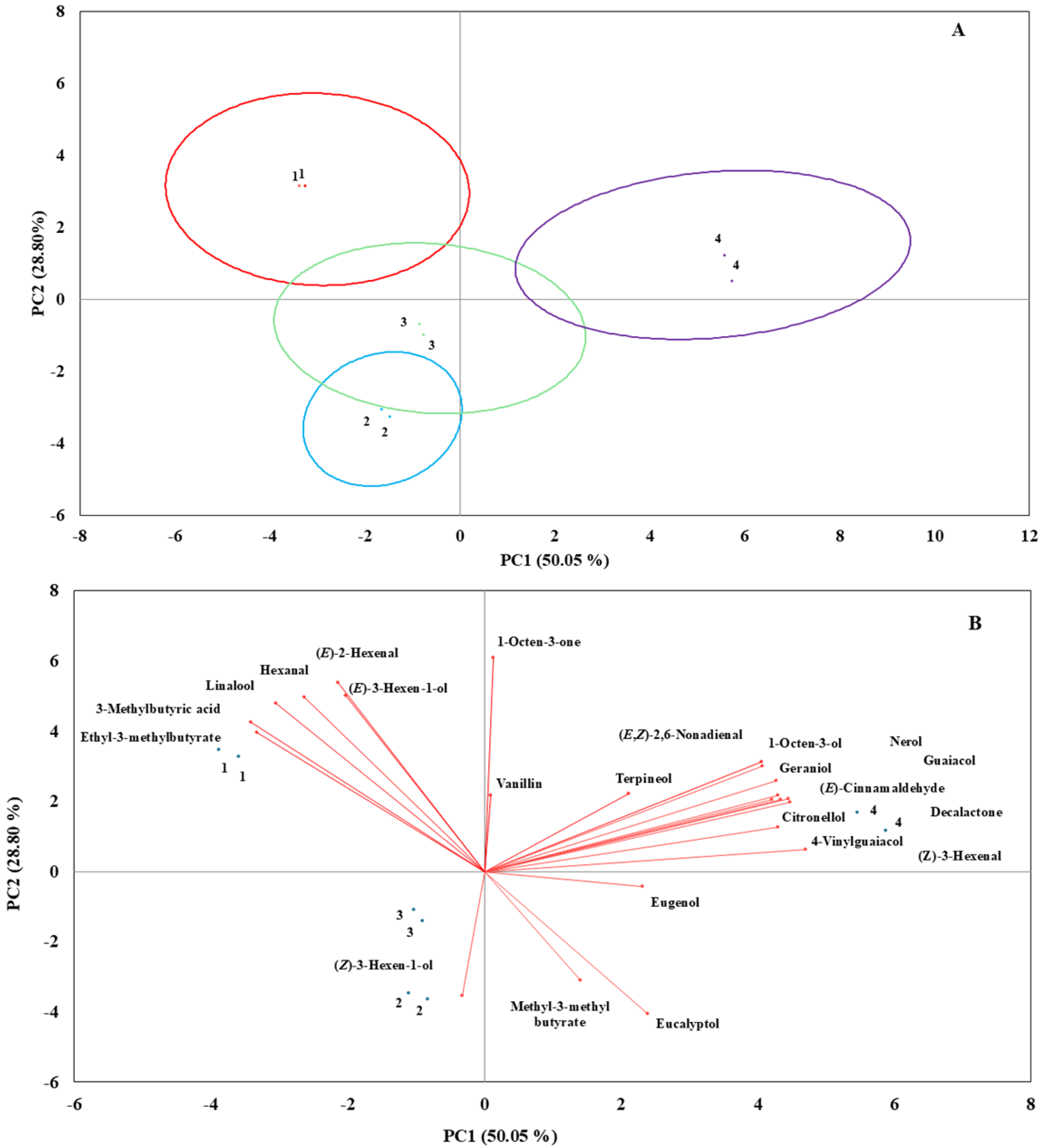
3.4. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC-MS | gas chromatography–mass spectrometry |
| GC-O | gas chromatography–olfactometry |
| OAVs | odor activity values |
| ODTs | odor detection thresholds |
| AEDA | aroma-extracted dilution analysis |
| SC | control; blueberries soon after harvest |
| S2 | 1 °C ± 0.5 °C for 3 weeks |
| S3 | 1 °C ± 0.5 °C for 3 weeks and 10 °C ± 0.5 °C for 1 week |
| S4 | 10 °C ± 0.5 °C for 1 week and removed to 20 °C ± 0.5 °C for 2 days |
| RH | relative humidity |
| SSC | soluble solids concentration |
| TA | titratable acidity |
| IRB | Institutional Review Board |
| DCM | dichloromethane |
| HCl | hydrochloric acid |
| NaOH | sodium hydroxide |
| Na2SO4 | sodium sulfate |
| UHP | ultrahigh purity |
| GC-FID | GC–flame ionization detection |
| SAFE | solvent-assisted flavor evaporation |
| GC-MS-O | gas chromatography–mass spectrometry–olfactometry |
| AEDA | aroma extract dilution analysis |
| FD | flavor dilution |
| MSD | mass-selective detector |
| RI | retention index |
| MS | mass spectral |
| SAFE-GC-MS | solvent-assisted flavor evaporation–gas chromatography–mass spectrometry |
| SIM | selective ion monitoring |
| Rf | response factors |
| HS-SPME-GC-MS | headspace solid-phase microextraction–gas chromatography–mass spectrometry |
| NaF | sodium fluoride |
| NaCl | sodium chlorine |
| PTFE | polytetrafluoroethylene |
| TOF-MS | time-of-flight mass spectrometer |
| HS | headspace |
| IS | internal standard |
| t | target analyte |
| PCA | principal component analysis |
| ANOVA | analysis of variance |
| SPME-GC-O | solid-phase microextraction–gas chromatography–olfactometry |
| CoA | coenzyme A |
| PTV | programmable temperature vaporizer |
References
- Jimenez, M.J.; Carpenter, F.; Molinar, R.H.; Wright, K.; Day, K.R. Blueberry research launches exciting new California specialty crop. Calif. Agric. 2005, 59, 65–69. [Google Scholar]
- Lobos, G.A.; Callow, P.; Hancock, J.F. The effect of delaying harvest date on fruit quality and storage of late highbush blueberry cultivars (Vaccinium corymbosum L.). Postharvest Biol. Technol. 2014, 87, 133–139. [Google Scholar]
- USDA-NASS. 2023 Blueberry Statistics. Available online: https://www.nass.usda.gov/Statistics_by_State/New_Jersey/Publications/Blueberry_Statistics/NJ-2023-Blueberry.pdf (accessed on 26 March 2025).
- California Blueberry Commission. Annual Report 2017–2018. Available online: www.calblueberry.org/uploads/1/2/5/0/125053236/2017-2018_ar_final.pdf (accessed on 22 August 2024).
- Ehlenfeldt, M.K. Postharvest research and technology in Vaccinium. Acta Hort. 2000, 574, 31–38. [Google Scholar]
- Yan, X.; Yan, J.; Pan, S.; Yuan, F. Changes of the aroma composition and other quality traits of blueberry ‘Garden Blue’ during the cold storage and subsequent shelf life. Foods 2020, 9, 1223. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Wang, F.; Obenland, D.; Xiao, C.L. Effects of peroxyacetic acid on postharvest diseases and quality of blueberries. Plant Dis. 2021, 105, 3231–3237. [Google Scholar]
- Qian, Y.L.; Zhang, D.; An, Y.; Zhou, Q.; Qian, M.C. Characterization of aroma-active compounds in northern highbush blueberries “Bluecrop” (Vaccinium corymbosum “Bluecrop”) and “Elliott” (Vaccinium corymbosum “Elliott”) by gas chromatography–olfactometry dilution analysis and odor activity value. J. Agric. Food Chem. 2021, 69, 5691–5701. [Google Scholar]
- Paniagua, A.C.; East, A.R.; Hindmarsh, J.P.; Heyes, J.A. Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Biol. Technol. 2013, 79, 13–19. [Google Scholar]
- Sater, H.M.; Bizzio, L.N.; Tieman, D.M.; Munoz, P.D. A Review of the fruit volatiles found in blueberry and other Vaccinium species. J. Agric. Food Chem. 2020, 68, 5777–5786. [Google Scholar]
- Ktenioudaki, A.; O’Donnell, C.P.; Emond, J.P.; do Nascimento Nunes, M.C. Blueberry supply chain: Critical steps impacting fruit quality and application of a boosted regression tree model to predict weight loss. Postharvest Biol. Technol. 2021, 179, 111590. [Google Scholar]
- Sater, H.; Ferrão, L.F.V.; Olmstead, J.; Munoz, P.R.; Bai, J.; Hopf, A.; Plotto, A. Exploring environmental and storage factors affecting sensory, physical and chemical attributes of six southern highbush blueberry cultivars. Sci. Hortic. 2021, 289, 110468. [Google Scholar]
- Brattoli, M.; De Gennaro, G.; De Pinto, V.; Loiotile, A.D.; Lovascio, S.; Penza, M.J.S. Odour detection methods: Olfactometry and chemical sensors. Sensors 2011, 11, 5290–5322. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Chang, S.; Li, S.; Tan, Y.; Zhou, Y.; Pang, X. Characterization of the key aroma compounds in different varieties of hops by application of the sensomics approach. Food Chem. 2024, 460, 140448. [Google Scholar] [PubMed]
- Callejón, R.M.; Morales, M.L.; Troncoso, A.M.; Silva Ferreira, A.C. Targeting key aromatic substances on the typical aroma of sherry vinegar. J. Agric. Food Chem. 2008, 56, 6631–6639. [Google Scholar] [PubMed]
- Kulapichitr, F.; Borompichaichartkul, C.; Suppavorasatit, I.; Cadwallader, K.R. Impact of drying process on chemical composition and key aroma components of Arabica coffee. Food Chem. 2019, 291, 49–58. [Google Scholar]
- O’Mahony, M. Understanding discrimination tests: A user-friendly treatment of response bias, rating and ranking R-index tests and their relationship to signal detection. J. Sens. Stud. 1992, 7, 1–47. [Google Scholar]
- Hausch, B.J.; Lorjaroenphon, Y.; Cadwallader, K.R. Flavor chemistry of lemon-lime carbonated beverages. J. Agric. Food Chem. 2015, 63, 112–119. [Google Scholar]
- Zhu, W.; Cadwallader, K.R.J.F.C.X. Streamlined approach for careful and exhaustive aroma characterization of aged distilled liquors. Food Chem. 2019, 3, 100038. [Google Scholar]
- Wu, T.; Cadwallader, K.R. Identification of Characterizing Aroma Components of Roasted Chicory “Coffee” Brews. J. Agric. Food Chem. 2019, 67, 13848–13859. [Google Scholar]
- Du, X.; Rouseff, R. Aroma active volatiles in four southern highbush blueberry cultivars determined by gas chromatography–olfactometry (GC-O) and gas chromatography–mass spectrometry (GC-MS). J. Agric Food Chem. 2014, 62, 4537–4543. [Google Scholar]
- Semmelroch, P.; Grosch, W. Studies on character impact odorants of coffee brews. J. Agric. Food Chem. 1996, 44, 537–543. [Google Scholar]
- Miller, W.R.; McDonald, R.E.; Cracker, T.E. Quality of two Florida blueberry cultivars after packaging and storage. HortScience 1993, 28, 144–147. [Google Scholar]
- Song, H.; Liu, J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [PubMed]
- Bai, J.; Baldwin, E.A.; Imahori, Y.; Kostenyuk, I.; Burns, J.; Brecht, J.K. Chilling and heating may regulate C6 volatile aroma production by different mechanisms in tomato (Solanum lycopersicum) fruit. Postharvest Biol. Technol. 2011, 60, 111–120. [Google Scholar]
- Du, X.; Plotto, A.; Song, M.; Olmstead, J.; Rouseff, R. Volatile Composition of Four Southern Highbush Blueberry Cultivars and Effect of Growing Location and Harvest Date. J. Agric. Food Chem. 2011, 59, 8347–8357. [Google Scholar]
- Forney, C.F.; Jordan, M.A.; Pennell, K.M.; Fillmore, S. Controlled atmosphere storage impacts fruit quality and flavor chemistry of five cultivars of highbush blueberry (Vaccinium corymbosum). Postharvest Biol. Technol. 2022, 194, 112073. [Google Scholar]
- Belitz, H.D.; Grosh, W.; Schieberle, P. Food Chemistry, New York; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Paré, P.W.; Tumlinson, J.H. Plant Volatiles as a Defense against Insect Herbivores. Plant Physiol. 1999, 121, 325–332. [Google Scholar]
- Farneti, B.; Emanuelli, F.; Khomenko, I.; Ajelli, M.; Biasioli, F.; Giongo, L. Development of a novel phenotypic roadmap to improve blueberry quality and storability. Front. Plant Sci. 2020, 11, 1140. [Google Scholar]
- Dixon, J.; Hewett, E.W. Factors affecting apple aroma/flavour volatile concentration: A review. N. Z. J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar]
- Farcuh, M.; Hopfer, H. Aroma volatiles as predictors of chilling injury development during peach (Prunus persica (L) Batsch) cold storage and subsequent shelf-life. Postharvest Biol. Technol. 2023, 195, 112137. [Google Scholar]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of Plant Volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Gu, I.; Howard, L.; Lee, S.-O. Volatiles in berries: Biosynthesis, composition, bioavailability, and health benefits. Appl. Sci. 2022, 12, 10238. [Google Scholar] [CrossRef]
- Horvat, R.; Senter, S.D. Comparison of the volatile constituents from rabbiteye blueberries (Vaccinium ashei) during ripening. J. Food Sci. 1985, 50, 429–431. [Google Scholar] [CrossRef]
- Kulapichitr, F.; Asensio, C.; Arpaia, M.L.; Walse, S.; Obenland, D. Effect of controlled atmosphere storage on key volatiles and sensory perception of Muscat grapes. ACS Food Sci. Technol. 2024, 4, 766–772. [Google Scholar]
- Matsumoto, H.; Ikoma, Y. Effect of postharvest temperature on the muscat flavor and aroma volatile content in the berries of ‘Shine Muscat’ (Vitis labruscana Baily × V. vinifera L.). Postharvest Biol. Technol. 2016, 112, 256–265. [Google Scholar] [CrossRef]
- Anfora, G.; Tasin, M.; De Cristofaro, A.; Ioriatti, C.; Lucchi, A. synthetic grape volatiles attract mated Lobesia botrana females in laboratory and field Bioassays. J. Chem. Ecol. 2009, 35, 1054–1062. [Google Scholar]
- Knudsen, J.; Gershenzon, J. The chemical diversity of floral scent. In Biology of Floral Scent; Pichersky, N.D.A.E., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar]
- Ghadiriasli, R.; Lorber, K.; Wagenstaller, M.; Buettner, A. Smoky, Vanilla, or Clove-Like? In Sex, Smoke, and Spirits: The Role of Chemistry; American Chemical Society: Washington, DC, USA, 2019; Volume 1321, pp. 43–54. [Google Scholar]
- Pico, J.; Gerbrandt, E.M.; Castellarin, S.D. Optimization and validation of a SPME-GC/MS method for the determination of volatile compounds, including enantiomeric analysis, in northern highbush blueberries (Vaccinium corymbosum L.). Food Chem. 2022, 368, 130812. [Google Scholar]
- Pavithra, B. Eugenol—A Review. J. Pharm. Sci. Res. 2014, 6, 153–154. [Google Scholar]
- Hirvi, T.; Honkanen, E. The aroma of some hybrids between high-bush blueberry (Vaccinium corymbosum, L.) and bog blueberry (Vaccinium uliginosum, L.). Z. Lebensm. Unters. Forsch. 1983, 176, 346–349. [Google Scholar] [CrossRef]
- Stübner, C.A.; Steinhaus, M. Sotolon and (2E,4E,6Z)-nona-2,4,6-trienal are the key compounds in the aroma of walnuts. J. Agric Food Chem. 2023, 71, 7099–7108. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Pet’ka, J.; Aznar, M. Aroma Extract dilution analysis. Precision and optimal experimental design. J. Agric Food Chem. 2002, 50, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pu, D.; Sun, B.; Ren, F.; Zhang, Y.; Chen, H. Characterization and comparison of key aroma compounds in raw and dry porcini mushroom (Boletus edulis) by aroma extract dilution analysis, quantitation and aroma recombination experiments. Food Chem. 2018, 258, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Maneerat, C.; Hayata, Y.; Kozuka, H.; Sakamoto, K.; Osajima, Y. Application of the Porapak Q column extraction method for tomato flavor volatile analysis. J. Agric Food Chem. 2002, 50, 3401–3404. [Google Scholar] [CrossRef]
- Schomburg, G.; Husmann, H.; Schulz, F.; Teller, G.; Bender, M. Cold sample injection with either the split or splitless mode of temperature-programmed sample transfer: Comparison to cold on-column injection with a commercial device. J. Chromatogr. A. 1983, 279, 259–267. [Google Scholar] [CrossRef]
- Zhu, N.; Zhu, Y.; Yu, N.; Wei, Y.; Zhang, J.; Hou, Y.; Sun, A.-d. Evaluation of microbial, physicochemical parameters and flavor of blueberry juice after microchip-pulsed electric field. Food Chem. 2019, 274, 146–155. [Google Scholar] [CrossRef]
- Erten, E.S.; Cadwallader, K.R. Identification of predominant aroma components of raw, dry roasted and oil roasted almonds. Food Chem. 2017, 217, 244–253. [Google Scholar] [CrossRef]
- Su, X.; Yin, Y. Aroma characterization of regional cascade and chinook hops (Humulus lupulus L.). Food Chem. 2021, 364, 130410. [Google Scholar] [CrossRef]
- Lorrain, B.; Ballester, J.; Thomas-Danguin, T.; Blanquet, J.; Meunier, J.M.; Le Fur, Y. Selection of potential impact odorants and sensory validation of their importance in typical Chardonnay wines. J. Agric. Food Chem. 2006, 54, 3973–3981. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, B.; Zhao, M.; Zheng, F.; Huang, M.; Sun, J.; Sun, X.; Li, H. Characterization of the key odorants in Chinese zhima aroma-type baijiu by gas chromatography–olfactometry, quantitative measurements, aroma recombination, and omission studies. J. Agric. Food Chem. 2016, 64, 5367–5374. [Google Scholar] [CrossRef]
- Raffo, A.; Nardo, N.; Tabilio, M.R.; Paoletti, F. Effects of cold storage on aroma compounds of white- and yellow-fleshed peaches. Eur. Food Res. Technol. 2008, 226, 1503–1512. [Google Scholar] [CrossRef]
- Rychlik, M.; Schieberle, P.; Grosch, W. Compilation of Odor Thresholds, Odor Qualities and Retention Indices of Key Food Odorants; Deutsche Forschungsanstalt für Lebensmittelchemie and Institut für Lebensmittelchemie der Technischen Universitat Munchen: Garching, Germany, 1998; pp. 6–63. [Google Scholar]
- Tomaszewski, M.; Dein, M.; Novy, A.; Hartman, T.G.; Steinhaus, M.; Luckett, C.R.; Munafo, J.P., Jr. Quantitation and seasonal variation of key odorants in propolis. J. Agric. Food Chem. 2019, 67, 1495–1503. [Google Scholar]
| Physicochemical Properties | Storage Treatments a | |||
|---|---|---|---|---|
| SC b | S1 | S2 | S3 | |
| Soluble solids (Brix) | 13.43 ± 0.38 A | 13.27 ± 0.06 A | 13.43 ± 0.61 A | 13.90 ± 0.45 A |
| Total acidity (%) | 0.45 ± 0.06 A | 0.41 ± 0.02 AB | 0.35 ± 0.01 B | 0.36 ± 0.01 AB |
| Firmness (g/1 mm) c | 179.61 ± 15.24 A | 164.19 ± 10.69 A | 157.28 ± 9.85 A | 127.89 ± 1.39 B |
| Volatile Compounds | RI | Odor Properties | FD a | Identification b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wax | HP-5 | |||||||||||
| Storage Treatments | Storage Treatments | |||||||||||
| WAX | HP-5 | SC c | S1 | S2 | S3 | SC c | S1 | S2 | S3 | |||
| 2,3-Butanedione | 921 | <800 | Buttery, Dairy, Milky | 4 | 128 | ND d | 16 | 8 | 32 | 8 | 16 | RI, MS, O |
| 3-Methylbutanal | 930 | <800 | Fruity, Green, Malty, Pungent | 64 | 128 | 256 | 512 | 32 | 128 | 128 | 256 | RI, O |
| Methyl-2-methylbutyrate | 1013 | <800 | Blueberry, Fruity, Sweet | 1024 | 128 | 8 | 64 | 1024 | ND | 4 | 16 | RI, O |
| Methyl-3-methylbutyrate | 1022 | <800 | Blueberry, Fruity, Sweet | 1024 | 128 | 64 | 4096 | 1024 | 64 | 32 | 1024 | RI, MS, O |
| Ethyl-2-methylbutyrate | 1054 | 840 | Fruity, Sweet | 32 | 128 | ND | 32 | 128 | ND | ND | 4 | RI, O |
| Ethyl-3-methylbutyrate | 1070 | 848 | Fruity, Sweet | 4096 | 8 | 64 | 64 | 1024 | 4 | 32 | 32 | RI, MS, O |
| Hexanal | 1083 | 811 | Fruity, Grassy, Green | 32 | 16 | 16 | 64 | 16 | 8 | 8 | 32 | RI, MS, O |
| (Z)-3-Hexenal | 1145 | 805 | Apple, Berries, Green | 4096 | 1024 | 16,384 | 16,384 | 2048 | 1024 | 4096 | 4096 | RI, MS, O |
| 2-Heptanone | 1183 | 901 | Green | 64 | 16 | 16 | 8 | 8 | 4 | 4 | 2 | RI, O |
| Eucalyptol | 1210 | 1031 | Minty | 64 | 1024 | 1024 | 256 | 128 | 256 | 512 | 256 | RI, MS, O |
| (E)-2-Hexenal | 1221 | 853 | Cherries, Green, Sweet | 256 | 128 | 64 | 32 | 128 | 64 | 32 | 16 | RI, MS, O |
| 1-Octen-3-one | 1278 | 979 | Green, Meaty, Mushroom | 4096 | 4096 | 16,384 | 16,384 | 2048 | 4096 | 8192 | 8192 | RI, MS, O |
| (E)-3-Hexen-1-ol | 1374 | 867 | Garbage, Grassy, Green, Stinky, Woody | 64 | 32 | 64 | 128 | 64 | 32 | 64 | 128 | RI, MS, O |
| (Z)-3-Hexen-1-ol | 1387 | 850 | Garbage, Grassy, Green, Stinky, Woody | 256 | 128 | 64 | 64 | 128 | 64 | 64 | 64 | RI, MS, O |
| 1-Octen-3-ol | 1453 | 977 | Green, Mushroom | 8192 | 16,384 | 1024 | 8192 | 1024 | 2048 | 1024 | 4096 | RI, MS, O |
| Methional | 1463 | 910 | Fishy, Meaty, Potato | 32 | 64 | 256 | 32 | 64 | 128 | 256 | 32 | RI, O |
| (E)-2-Nonenal | 1548 | 1160 | Cucumber, Floral | 2 | 1 | 1 | 16 | 2 | 1 | 1 | 2 | RI, MS, O |
| Linalool | 1550 | 1096 | Floral | 16,384 | 8192 | 8192 | 16,384 | 1024 | 256 | 256 | 512 | RI, MS, O |
| (E,Z)-2,6-Nonadienal | 1587 | 1147 | Cucumber, Floral, Green | 256 | 16,384 | 8192 | 512 | 256 | 1024 | 64 | 128 | RI, MS, O |
| Phenylacetaldehyde | 1643 | 1040 | Floral, Rosy | 2 | ND | 16 | 2 | 2 | ND | 8 | 2 | RI, MS, O |
| 3-Methylbutyric acid | 1678 | 859 | Acidity, Cheesy, Sour | 32 | 8 | 64 | 4 | 32 | 8 | 32 | 4 | RI, MS, O |
| α-Terpineol | 1701 | 1192 | Floral, Sweet | 16 | 8 | 16 | 8 | 8 | 2 | ND | 4 | RI, MS, O |
| Citronellol | 1778 | 1242 | Citrusy, Floral, Sweet, Orange | 512 | 1024 | 1024 | 512 | 256 | 512 | 512 | 128 | RI, MS, O |
| Nerol | 1810 | 1232 | Floral, Rosy, Sweet | 16,384 | 1024 | 16,384 | 16,384 | 8192 | 1024 | 8192 | 8192 | RI, MS, O |
| Damascenone | 1814 | 1387 | Honey, Sweet | 4 | 16 | 1 | 8 | 4 | 8 | 2 | 4 | RI, MS, O |
| Guaiacol | 1861 | 1090 | Spicy, Phenolic | 16 | 1024 | 256 | 128 | 8 | 1024 | 256 | 128 | RI, MS, O |
| Geraniol | 1862 | 1252 | Citrusy, Floral, Rosy | 16,384 | 16,384 | 256 | 8192 | 8192 | 8192 | 256 | 2048 | RI, MS, O |
| Geranyl acetone | 1877 | 1463 | Floral | 8 | 128 | 64 | 32 | 4 | 64 | 32 | 16 | RI, O |
| (E)-Cinnamaldehyde | 2020 | 1271 | Coconut, Dairy, Sweet, | 1024 | 512 | 128 | 128 | 512 | 256 | 128 | 128 | RI, MS, O |
| m-Cresol | 2027 | 1082 | Phenolic, Rubber | 2 | ND | ND | 128 | 2 | ND | ND | 8 | RI, MS, O |
| p-Cresol | 2035 | 1079 | Phenolic, Rubber | 2 | ND | ND | 32 | 2 | ND | ND | 8 | RI, MS, O |
| γ-Decalactone | 2168 | 1466 | Coconut, Sweet, Peachy | 4096 | 1024 | 64 | 128 | 2048 | 512 | 256 | 512 | RI, MS, O |
| Eugenol | 2171 | 1356 | Clove | 1024 | 128 | 64 | 512 | 512 | 128 | 64 | 128 | RI, MS, O |
| 4-Vinylguaiacol | 2172 | 1330 | Spicy, Phenolic | 32 | 128 | 1024 | 128 | 8 | 16 | 64 | 8 | RI, MS, O |
| Sotolon | 2180 | 1107 | Curry, Spicy | 32 | 128 | 64 | 64 | 16 | 64 | 16 | 16 | RI, O |
| (Z)-Cinnamyl alcohol | 2299 | 1267 | Floral, Rosy | 32 | 128 | 64 | 32 | 32 | 64 | 32 | 8 | RI, O |
| (Z)-Isoeugenol | 2300 | 1414 | Clove | 8 | ND | 64 | 16 | 8 | ND | 4 | 2 | RI, MS, O |
| Vanillin | 2569 | 1399 | Vanilla | 1024 | 128 | 1024 | 128 | 256 | 128 | 1024 | 128 | RI, MS, O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulapichitr, F.; Cadwallader, K.; Obenland, D. Characterization of Changes in Key Odorants in Blueberries During Simulated Commercial Storage and Marketing by Sensory-Directed Flavor Analysis and Determination of Differences in Overall Perceived Aroma. Foods 2025, 14, 1244. https://doi.org/10.3390/foods14071244
Kulapichitr F, Cadwallader K, Obenland D. Characterization of Changes in Key Odorants in Blueberries During Simulated Commercial Storage and Marketing by Sensory-Directed Flavor Analysis and Determination of Differences in Overall Perceived Aroma. Foods. 2025; 14(7):1244. https://doi.org/10.3390/foods14071244
Chicago/Turabian StyleKulapichitr, Fareeya, Keith Cadwallader, and David Obenland. 2025. "Characterization of Changes in Key Odorants in Blueberries During Simulated Commercial Storage and Marketing by Sensory-Directed Flavor Analysis and Determination of Differences in Overall Perceived Aroma" Foods 14, no. 7: 1244. https://doi.org/10.3390/foods14071244
APA StyleKulapichitr, F., Cadwallader, K., & Obenland, D. (2025). Characterization of Changes in Key Odorants in Blueberries During Simulated Commercial Storage and Marketing by Sensory-Directed Flavor Analysis and Determination of Differences in Overall Perceived Aroma. Foods, 14(7), 1244. https://doi.org/10.3390/foods14071244






