Characterization of Cellulose from Gagome Kelp and Its Effect on Dough, Gluten, and Starch as Novel Bread Improvers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Determination on Water Holding Capacity of the GC
2.2.2. Determination of Oil Holding Capacity of the GC
2.2.3. Determination on Unsaturated Fat and Saturated Fat-Adsorptive Capacity of GC
2.2.4. Determination on Cholesterol Adsorptive Capacity of the GC
2.2.5. Determination on α-Amylase Inhibition Activity of the GC
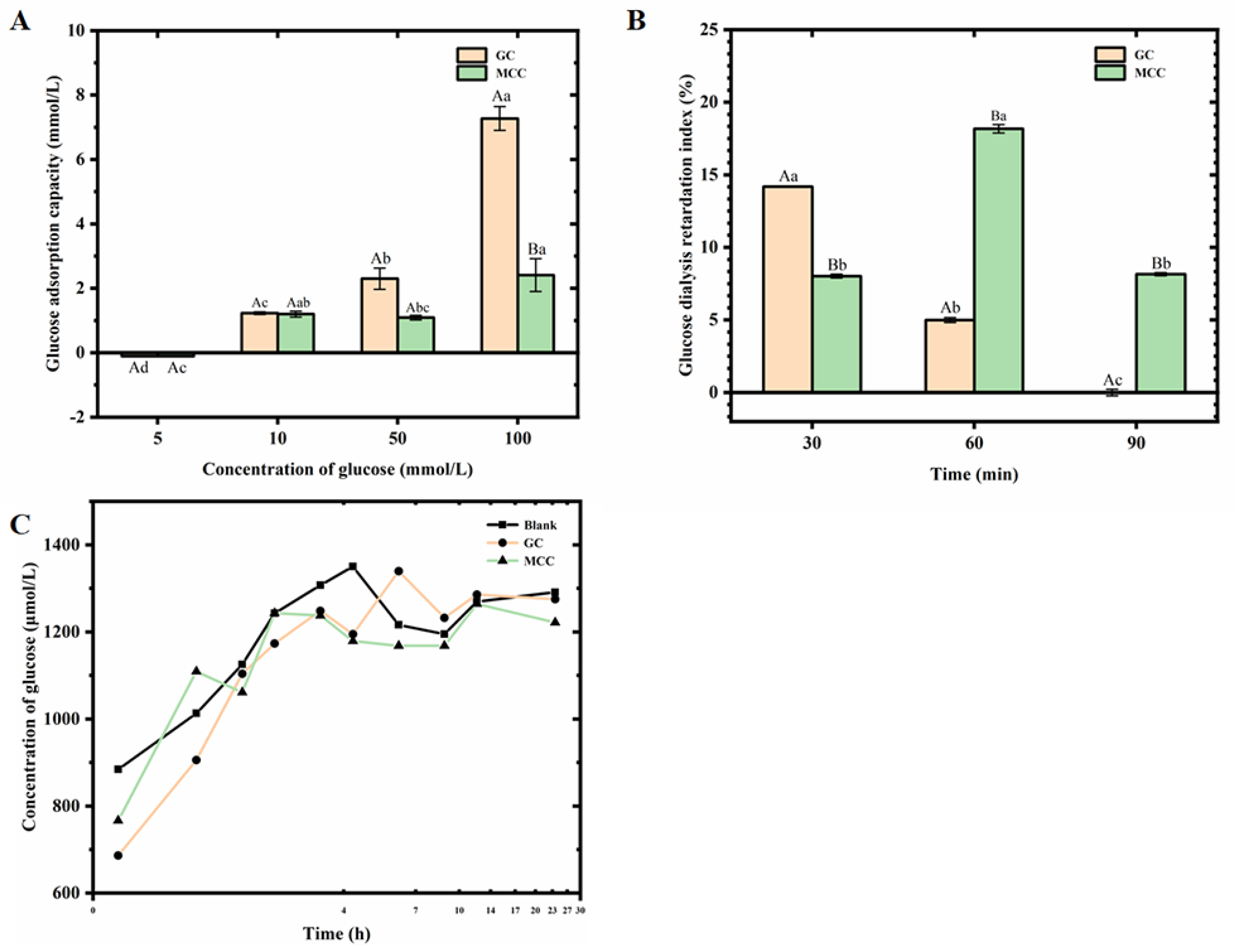
2.2.6. Determination on Glucose Adsorptive Capacity of the GC
2.2.7. Determination on Glucose Diffusion Capacity and the Glucose Dialysis Retardation Index (GDRI) of the GC
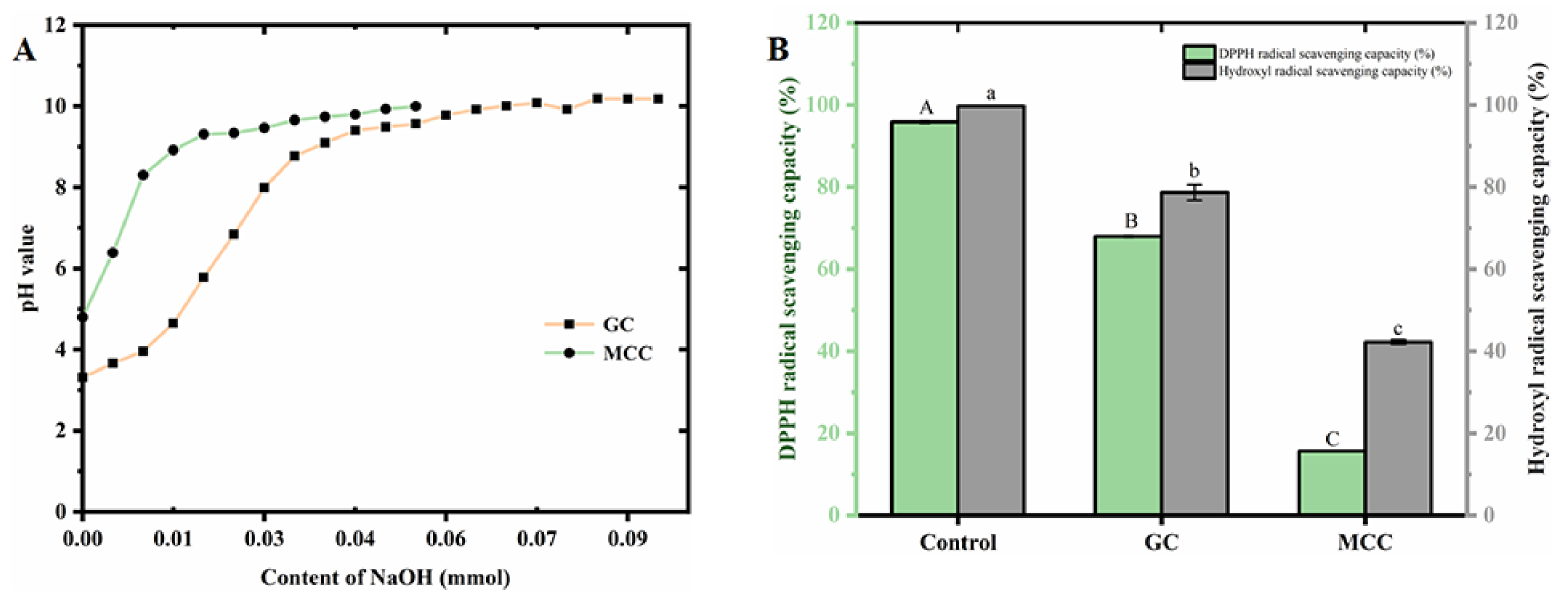
2.2.8. Determination on Cation Exchange Capacity of the GC
2.2.9. Determination on DPPH Free-Radical Scavenging Activity of the GC
2.2.10. Determination on Hydroxyl Radical Scavenging Activity of the GC
2.2.11. Determination on the Volume, pH and Color of the GC-Dough
2.2.12. Separation of the GC-Gluten and GC-Starch
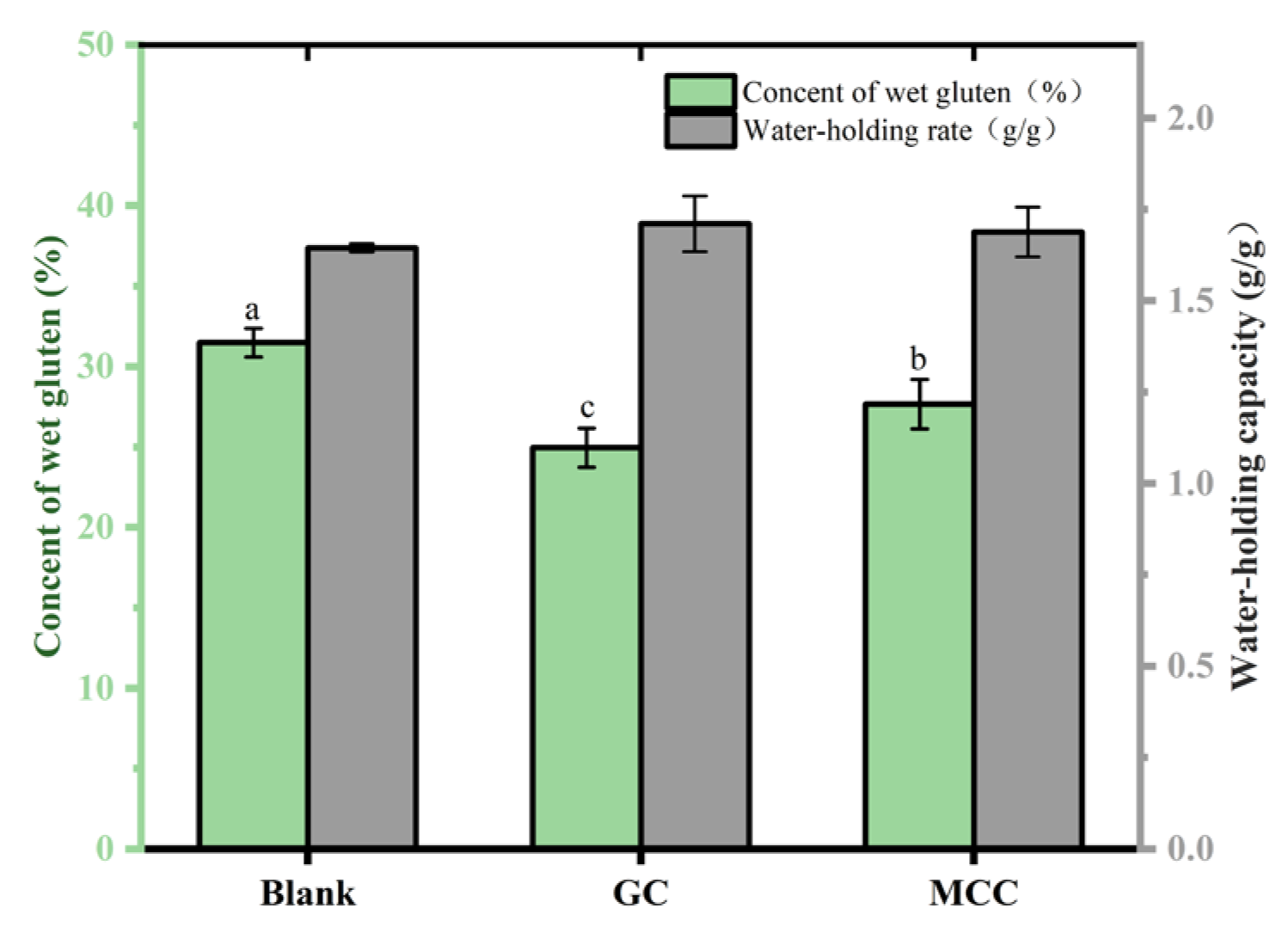
2.2.13. Determination of the Content and Water Holding Capacity of GC-Gluten
2.2.14. The Secondary Structure of GC-Gluten Protein
2.2.15. Determination on the Content of Glutenin and Gliadin
2.2.16. Determination on the Solubility and Swelling of GC-Starch
2.2.17. Preparation of the GC-Bread
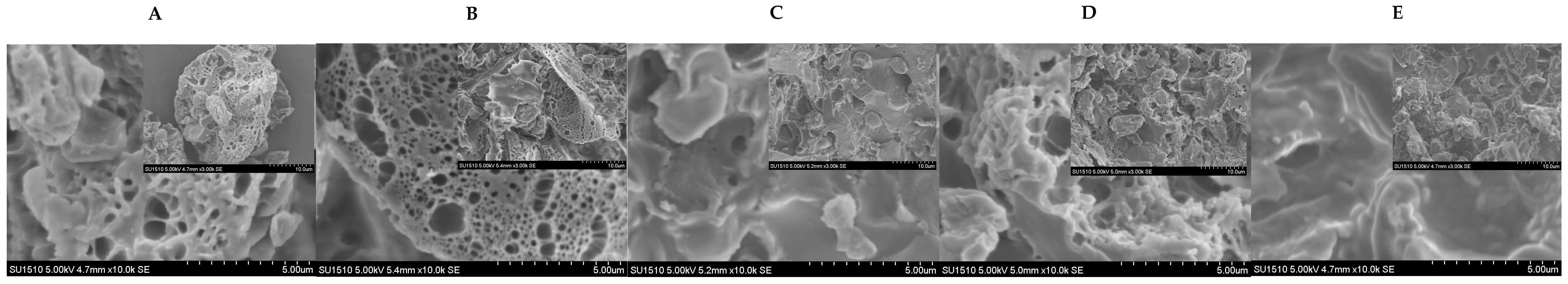
2.2.18. The Scanning Electron Microscopy (SEM) Observation of the GC-Bread
2.2.19. Determination on Texture Profile of the GC-Bread
2.2.20. Sensory Test Description of the GC-Bread
2.2.21. Determination on the Moisture Content and Hardness of the Cold-Stored GC-Bread
2.2.22. Statistical Analysis
3. Results and Discussion
3.1. Water and Oil Holding Capacity of the GC
3.2. Interaction Between the GC and Fat
3.3. Cholesterol Adsorptive Capacity of the GC
3.4. The α-Amylase Inhibition Activity of the GC
3.5. Interaction Between the GC and Glucose
3.6. Cation Exchange Capacity of the GC
3.7. Antioxidant Activity of the GC
3.8. Effect of the GC on Dough of Bread
3.9. Effect of the GC on Gluten of Bread
3.10. Structure of the Protein in GC-Gluten
3.11. Effect of the GC on Starch of Bread
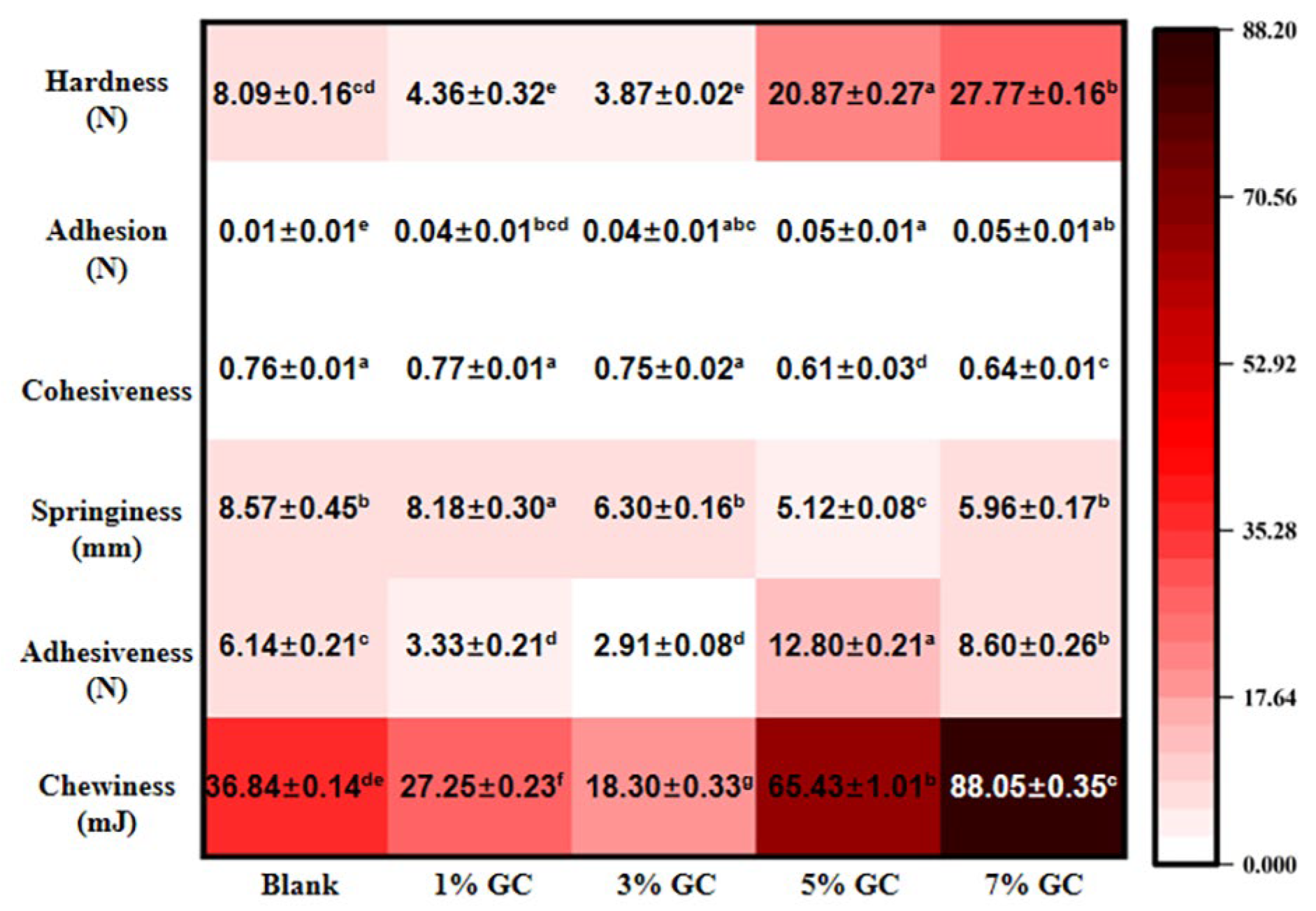
3.12. Characterization of Bread with Added GC
3.13. Cold-Stored Stability of the GC-Bread
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verbeke, C.; Debonne, E.; Versele, S.; Van Bockstaele, F.; Eeckhout, M. Technological Evaluation of Fiber Effects in Wheat-Based Dough and Bread. Foods 2024, 13, 2582. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Bustamante, A.; Echeverría, F.; Encina, C.; Palma, M.; Sanhueza, L.; Sambra, V.; Pando, M.E.; Jiménez, P. A Feasible Approach to Developing Fiber-Enriched Bread Using Pomegranate Peel Powder: Assessing Its Nutritional Composition and Glycemic Index. Foods 2023, 12, 2798. [Google Scholar] [CrossRef] [PubMed]
- Collar, C.; Villanueva, M.; Ronda, F. Structuring Diluted Wheat Matrices: Impact of Heat-Moisture Treatment on Protein Aggregation and Viscoelasticity of Hydrated Composite Flours. Food Bioprocess Technol. 2020, 13, 475–487. [Google Scholar]
- Culetu, A.; Duta, D.E.; Papageorgiou, M.; Varzakas, T. The Role of Hydrocolloids in Gluten-Free Bread and Pasta; Rheology, Characteristics, Staling and Glycemic Index. Foods 2021, 10, 3121. [Google Scholar] [CrossRef]
- Jaksics, E.; Paszerbovics, B.; Egri, B.; Rakszegi, M.; Tremmel-Bede, K.; Vida, G.; Gergely, S.; Németh, R.; Tömösközi, S. Complex Rheological Characterization of Normal, Waxy and High-amylose Wheat Lines. J. Cereal Sci. 2020, 93, 102982. [Google Scholar]
- Komeroski, M.R.; Oliveira, V.R. Influence of the Amount and Type of Whey Protein on the Chemical, Technological, and Sensory Quality of Pasta and Bakery Products. Foods 2023, 12, 2801. [Google Scholar] [CrossRef]
- Naseer, B.; Naik, H.R.; Hussain, S.Z.; Zargar, I.; Beenish; Bhat, T.A.; Nazir, N. Effect of carboxymethyl cellulose and baking conditions on in-vitro starch digestibility and physico-textural characteristics of low glycemic index gluten-free rice cookies. LWT 2021, 141, 110885. [Google Scholar]
- Alba, K.; Rizou, T.; Paraskevopoulou, A.; Campbell, G.M.; Kontogiorgos, V. Effects of Blackcurrant Fibre on Dough Physical Properties and Bread Quality Characteristics. Food Biophys. 2020, 15, 313–322. [Google Scholar] [CrossRef]
- Zhang, X.; Yelle, D.J.; Kitin, P.; Tong, G.; Zhu, J.Y. Producing Cellulose Microfibrils at a High Solid Content with and without Mechanical or Enzymatic Pretreatment. Biomacromolecules 2024, 25, 2509–2519. [Google Scholar] [CrossRef]
- Yuan, H.; Wei, X.; Chen, C.; Yu, H.; Huang, J.; Tian, H. Recent Developments in Cellulose-Based Emulsion Systems for Food Applications: A Review. Food Hydrocoll. 2025, 163, 111089. [Google Scholar]
- Dai, Y.; Tyl, C. A Review on Mechanistic Aspects of Individual Versus Combined uses of Enzymes as Clean Label-friendly Dough Conditioners in Breads. J. Food Sci. 2021, 86, 1583–1598. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kerr, W.L.; Kong, F.; Dee, D.R.; Lin, M. Influence of Nano-fibrillated Cellulose (NFC) on Starch Digestion and Glucose Absorption. Carbohydr. Polym. 2018, 196, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Linter, B.R.; Foster, T.J. Starch Replacement in Gluten Free Bread by Cellulose and Fibrillated Cellulose. Food Hydrocoll. 2020, 107, 105957. [Google Scholar] [CrossRef]
- Stanford, E.C.C. Stanford’s New Method of Treating Seaweed. J. Soc. Chem. Ind. 1885, 4, 518–520. [Google Scholar]
- Gao, H.; Duan, B.; Lu, A.; Deng, H.; Du, Y.; Shi, X.; Zhang, L. Fabrication of Cellulose Nanofibers from Waste Brown Algae and their Potential Application as Milk Thickeners. Food Hydrocoll. 2018, 79, 473–481. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Q.; Wang, Q.; He, Y.; Ren, D.; Liu, S.; Wu, L. Structural Characterization and Antitumor Effects of Fucoidans from Brown Algae Kjellmaniella crassifolia Farmed in Northern China. Int. J. Biol. Macromol. 2018, 119, 125–133. [Google Scholar] [CrossRef]
- Tang, S.; Dong, X.; Ma, Y.; Zhou, H.; He, Y.; Ren, D.; Li, X.; Cai, Y.; Wang, Q.; Wu, L. Highly Crystalline Cellulose Microparticles from Dealginated Seaweed Waste Ameliorate High Fat-sugar Diet-induced Hyperlipidemia in Mice by Modulating Gut Microbiota. Int. J. Biol. Macromol. 2024, 263, 130485. [Google Scholar] [CrossRef]
- Angioloni, A.; Collar, C. Physicochemical and Nutritional Properties of Reduced-Caloric Density High-fibre Breads. LWT-Food Sci. Technol. 2011, 44, 747–758. [Google Scholar] [CrossRef]
- Arslan, D.; Özcan, M.M. Evaluation of Drying Methods with Respect to Drying Kinetics, Mineral Content and Colour Characteristics of Rosemary Leaves. Energy Convers. Manag. 2008, 49, 1258–1264. [Google Scholar] [CrossRef]
- Li, X.; Song, L.; Xu, X.; Wen, C.; Ma, Y.; Yu, C.; Du, M.; Zhu, B. One-step Coextraction Method for Flavouring Soybean Oil with the Dried Stipe of Lentinus edodes (Berk.) sing by Supercritical CO2 Fluid Extraction. LWT-Food Sci. Technol. 2020, 120, 108853. [Google Scholar] [CrossRef]
- Day, L.; Augustin, M.A.; Pearce, R.J.; Batey, I.L.; Wrigley, C.W. Enhancement of gluten quality combined with reduced lipid content through a new salt-washing process. J. Food Eng. 2009, 95, 365–372. [Google Scholar]
- Li, X.; Xu, X.; Song, L.; Bi, A.; Wu, C.; Ma, Y.; Du, M.; Zhu, B. High Internal Phase Emulsion for Food-Grade 3D Printing Materials. ACS Appl. Mater. Interfaces 2020, 12, 45493–45503. [Google Scholar] [PubMed]
- Akyüz, G.; Mazı, B.G. Physicochemical and Sensory Characterization of Bread Produced from Different Dough Formulations by Kluyveromyces lactis. J. Food Process. Preserv. 2020, 44, e14498. [Google Scholar]
- Chinese Standard GB/T 5009.3; Determination of Moisture in Food. Standards Press of China: Beijing, China, 2016. (In Chinese)
- Arp, C.G.; Correa, M.J.; Ferrero, C. Improving quality: Modified Celluloses Applied to Bread Dough with High Level of Resistant Starch. Food Hydrocoll. 2021, 112, 106302. [Google Scholar]
- Tang, S.; Ma, Y.; Dong, X.; Zhou, H.; He, Y.; Ren, D.; Wang, Q.; Yang, H.; Liu, S.; Wu, L. Enzyme-assisted Extraction of Fucoidan from Kjellmaniella crassifolia Based on Kinetic Study of Enzymatic Hydrolysis of Algal Cellulose. Algal Res. 2022, 66, 102795. [Google Scholar]
- Van Dokkum, W.; Pikaar, N.A.; Thissen, J. Physiological Effects of Fibre-rich Types of Bread. Dietary Fibre from Bread: Digestibility by the Intestinal Microflora and Water-holding Capacity in the Colon of Human Subjects. Br. J. Nutr. 1983, 50, 61–74. [Google Scholar]
- Yan, L.; Liu, B.; Liu, C.; Qu, H.; Zhang, Z.; Luo, J.; Zheng, L. Preparation of Cellulose with Controlled Molecular Weight via Ultrasonic Treatment Improves Cholesterol-binding Capacity. J. Food Process. Preserv. 2019, 44, e14340. [Google Scholar]
- Chau, C.F.; Huang, Y.L.; Lee, M.H. In Vitro Hypoglycemic Effects of Different Insoluble Fiber-Rich Fractions Prepared from the Peel of Citrus Sinensis L. cv. J. Agric. Food Chem. 2003, 51, 6623–6626. [Google Scholar]
- Qi, J.; Li, Y.; Masamba, K.G.; Shoemaker, C.F.; Zhong, F.; Majeed, H.; Ma, J. The Effect of Chemical Treatment on the in vitro Hypoglycemic Properties of Rice Bran Insoluble Dietary Fiber. Food Hydrocoll. 2016, 52, 699–706. [Google Scholar]
- Fabek, H.; Messerschmidt, S.; Brulport, V.; Goff, H.D. The Effect of in vitro Digestive Processes on the Viscosity of Dietary Fibres and their Influence on Glucose Diffusion. Food Hydrocoll. 2014, 35, 718–726. [Google Scholar]
- Torres-Pérez, R.; Martínez-García, E.; Siguero-Tudela, M.M.; García-Segovia, P.; Martínez-Monzó, J.; Igual, M. Enhancing Gluten-Free Bread Production: Impact of Hydroxypropyl Methylcellulose, Psyllium Husk Fiber, and Xanthan Gum on Dough Characteristics and Bread Quality. Foods 2024, 13, 1691. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Pan, Z.; Li, L.; Zhang, X.; Yuan, Y.; Yang, Y.; Han, S.; Li, B. Improving the Microstructural and Rheological Properties of Frozen Unfermented Wheat Dough with Laccase and Ferulic Acid. Foods 2023, 12, 2772. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, Á.L.; Villanueva, M.; Rico, D.; Harasym, J.; Ronda, F.; Martín-Diana, A.B.; Caballero, P.A. Valorisation of Buckwheat By-Product as a Health-Promoting Ingredient Rich in Fibre for the Formulation of Gluten-Free Bread. Foods 2023, 12, 2781. [Google Scholar] [CrossRef] [PubMed]
- Kontogiorgos, V. Linear Viscoelasticity of Gluten: Decoupling of Relaxation Mechanisms. J. Cereal Sci. 2017, 75, 286–295. [Google Scholar] [CrossRef]
- Correa, M.; Añón, M.; Pérez, G.; Ferrero, C. Effect of Modified Celluloses on Dough Rheology and Microstructure. Food Res. Int. 2010, 43, 780–787. [Google Scholar] [CrossRef]
- Arp, C.G.; Correa, M.J.; Ferrero, C. High-Amylose Resistant Starch as a Functional Ingredient in Breads: A Technological and Microstructural Approach. Food Bioprocess Technol. 2018, 11, 2182–2193. [Google Scholar] [CrossRef]
- Scanlon, M.G.; Zghal, M.C. Bread Properties and Crumb Structure. Food Res. Int. 2001, 34, 841–864. [Google Scholar] [CrossRef]
- Xin, C.; Nie, L.; Chen, H.; Li, J.; Li, B. Effect of Degree of Substitution of Carboxymethyl Cellulose Sodium on the State of Water, Rheological and Baking Performance of Frozen Bread Dough. Food Hydrocoll. 2018, 80, 8–14. [Google Scholar] [CrossRef]


| Characterizations | MCC | GC | |
|---|---|---|---|
| Water holding capacity (g/g) | 2.98 ± 0.21 | 3.41 ± 0.10 * | |
| Oil holding capacity (g/g) | 3.75 ± 0.21 | 4.11 ± 0.06 * | |
| Unsaturated fat adsorptive capacity (mg/g) | 1.30 ± 0.04 | 1.35 ± 0.04 | |
| Saturated fat adsorptive capacity (mg/g) | 1.62 ± 0.17 | 1.73 ± 0.20 * | |
| Cholesterol-absorbing capacity (mg/g) | pH 2.0 | 5.88 ± 0.42 | 4.48 ± 0.14 * |
| pH 7.0 | 7.60 ± 0.37 | 5.47 ± 0.38 * | |
| α-Amylase inhibitory activities (%) | 24.71 ± 0.20 | 14.31 ± 0.21 * | |
| Sample | 20 min | 40 min | 60 min | |
|---|---|---|---|---|
| volume | Blank | 24.44 ± 0.58 Ac | 31.50 ± 0.50 Bb | 34.70 ± 0.46 Aa |
| GC | 23.33 ± 0.58 Ac | 26.93 ± 1.01 Cb | 32.07 ± 1.01 Ba | |
| MCC | 23.83 ± 0.50 Ab | 33.17 ± 0.29 Aa | 33.34 ± 1.04 ABa | |
| pH value | Blank | 5.18 ± 0.08 Aa | 5.08 ± 0.10 Aa | 5.10 ± 0.06 Aa |
| GC | 5.13 ± 0.10 Aa | 5.01 ± 0.02 Aa | 5.01 ± 0.13 Aa | |
| MCC | 5.16 ± 0.03 Aa | 5.11 ± 0.11 Aab | 5.05 ± 0.05 Ab | |
| color | Blank | 304.41 ± 3.45 Aa | 294.02 ± 0.92 Aa | 311.62 ± 1.74 Aa |
| GC | 299.83 ± 2.73 Aa | 321.39 ± 1.80 Aa | 316.14 ± 1.02 Aa | |
| MCC | 303.56 ± 3.25 Aa | 295.15 ± 4.08 Aa | 306.14 + 1.02 Aa |
| Content (%) | Blank | GC | MCC |
|---|---|---|---|
| Glutenin | 66.54 ± 0.02 c | 71.76 ± 0.05 a | 69.05 ± 0.06 b |
| Gliadin | 36.56 ± 0.01 a | 30.62 ± 0.01 b | 29.33 ± 0.01 c |
| Gliadin/Glutenin | 54.96 ± 0.01 a | 42.81 ± 0.04 b | 42.70 ± 0.05 b |
| α-helix | 15.31 ± 6.23 c | 18.51 ± 4.82 b | 20.35 ± 6.28 a |
| β-sheet | 36.80 ± 6.23 c | 40.92 ± 1.69 b | 47.16 ± 6.88 a |
| β-turn | 32.15 ± 7.50 a | 24.65 ± 2.07 b | 24.65 ± 2.07 b |
| Random coil | 15.58 ± 0.21 b | 18.98 ± 2.02 a | 15.68 ± 0.01 b |
| Sample | Solubility (%) | Swelling Capacity (%) |
|---|---|---|
| Blank | 33.91 ± 0.63 a | 13.95 ± 0.09 a |
| GC | 23.83 ± 0.36 c | 12.73 ± 0.05 b |
| MCC | 28.45 ± 0.48 b | 14.18 ± 0.44 a |
| Content of the GC | F0 | F7 | Avrami Index (n) | K (d−1) | R2 |
|---|---|---|---|---|---|
| 0 | 4.67 ± 0.74 c | 69.47 ± 2.56 b | 1.37 ± 0.02 d | 8.62 ± 0.56 a | 0.95 ± 0.02 d |
| 1% GC | 3.87 ± 0.02 e | 33.33 ± 2.54 e | 1.08 ± 0.03 c | 3.95 ± 1.45 d | 0.91 ± 0.01 c |
| 3% GC | 4.36 ± 0.31 d | 36.57 ± 1.27 d | 1.06 ± 0.02 e | 3.90 ± 0.55 d | 0.86 ± 0.01 e |
| 5% GC | 13.43 ± 0.20 b | 54.90 ± 3.08 c | 1.16 ± 0.02 b | 5.08 ± 0.48 c | 0.90 ± 0.02 d |
| 7% GC | 23.13 ± 2.46 a | 77.83 ± 0.55 a | 1.31 ± 0.03 a | 7.33 ± 1.88 b | 0.99 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Yang, H.; Yu, X.; Tuo, Y.; Zhou, H.; Cai, Y.; Wu, L. Characterization of Cellulose from Gagome Kelp and Its Effect on Dough, Gluten, and Starch as Novel Bread Improvers. Foods 2025, 14, 1246. https://doi.org/10.3390/foods14071246
Li X, Yang H, Yu X, Tuo Y, Zhou H, Cai Y, Wu L. Characterization of Cellulose from Gagome Kelp and Its Effect on Dough, Gluten, and Starch as Novel Bread Improvers. Foods. 2025; 14(7):1246. https://doi.org/10.3390/foods14071246
Chicago/Turabian StyleLi, Xiang, Heqi Yang, Xiaohui Yu, Ying Tuo, Hui Zhou, Yidi Cai, and Long Wu. 2025. "Characterization of Cellulose from Gagome Kelp and Its Effect on Dough, Gluten, and Starch as Novel Bread Improvers" Foods 14, no. 7: 1246. https://doi.org/10.3390/foods14071246
APA StyleLi, X., Yang, H., Yu, X., Tuo, Y., Zhou, H., Cai, Y., & Wu, L. (2025). Characterization of Cellulose from Gagome Kelp and Its Effect on Dough, Gluten, and Starch as Novel Bread Improvers. Foods, 14(7), 1246. https://doi.org/10.3390/foods14071246







