Development of Vegetable Creams Enriched with Different Microalgae Species: A Study on the Physicochemical and Sensory Stability over Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Vegetable Cream Preparation
2.2. Vegetable Creams Characterization
2.2.1. Physicochemical Parameters
2.2.2. Microbiological Analyses
2.2.3. Sensory Analysis
2.3. Analysis of the Results
3. Results and Discussion
3.1. Nutritional Composition
3.2. Evaluation of Changes Related to Formulation
3.2.1. Physicochemical Properties
3.2.2. Sensory Properties
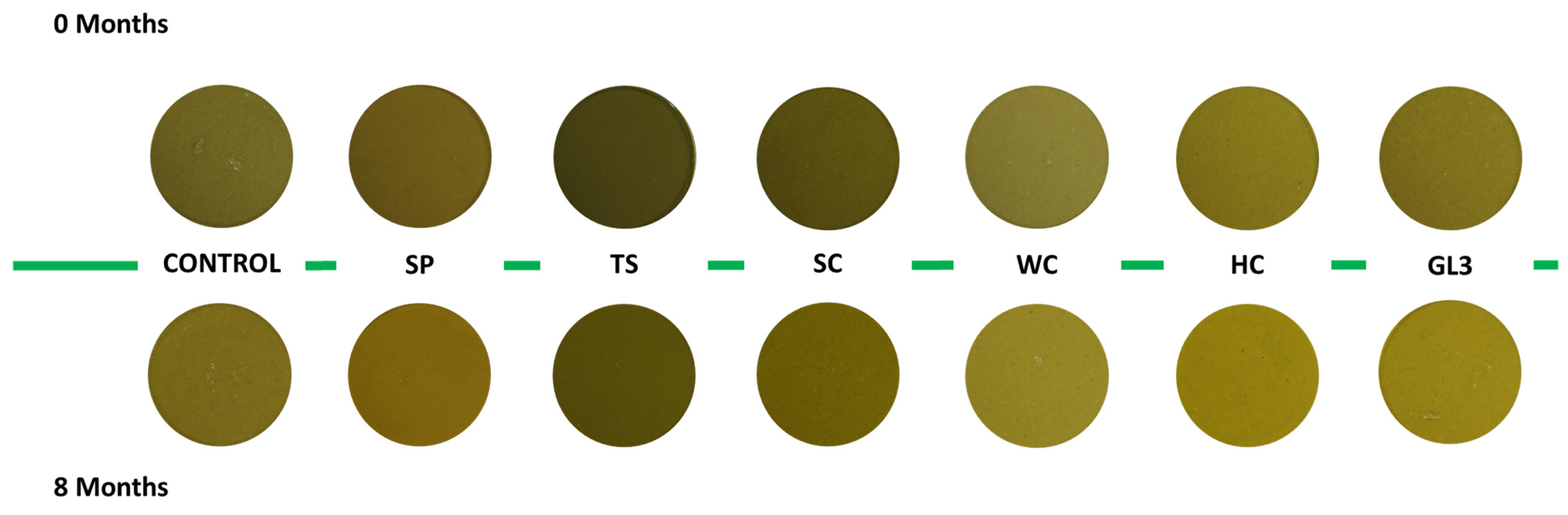
3.3. Quality over Time Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Ingredients | Energy, (kcal/100 g) | Fat (g/100 g) | Saturated Fatty Acids (g/100 g) | Carbohydrates (g/100 g) | Sugars (g/100 g) | Dietary Fibers (g/100 g) | Protein (g/100 g) | Salt (NaCl) (g/100 g) |
|---|---|---|---|---|---|---|---|---|
| Spinach | 30.0 | 0.50 | 0.10 | 1.10 | 0.90 | 3.40 | 3.60 | 0.20 |
| Zucchini | 25.0 | 0.30 | 0.10 | 2.60 | 2.50 | 1.30 | 2.40 | 0.00 |
| Chickpea | 13.1 | 2.00 | 0.30 | 17.7 | 1.00 | 6.20 | 7.40 | 0.00 |
| Leek | 33.0 | 0.30 | 0.00 | 4.20 | 3.90 | 3.10 | 1.80 | 0.00 |
| Broccoli | 30.0 | 0.40 | 0.10 | 2.30 | 2.20 | 2.90 | 2.90 | 0.00 |
| Chard | 30.0 | 0.30 | 0.00 | 3.60 | 0.70 | 1.30 | 2.80 | 0.50 |
| Water | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sunflower oil | 81.0 | 90.0 | 9.80 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Salt | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 98.0 |
| Spirulina | 38.8 | 7.70 | 2.70 | 20.3 | 3.10 | 3.20 | 57.5 | 0.50 |
| Tetraselmis chui | 33.6 | 7.90 | 7.63 | 15.5 | 4.30 | 11.2 | 45.3 | 1.40 |
| Smooth Chlorella | 30.0 | 6.00 | 1.80 | 36.0 | 0.00 | 20.0 | 36.0 | 0.35 |
| White Chlorella | 29.6 | 5.92 | 1.48 | 57.8 | 4.00 | 20.0 | 26.3 | 0.30 |
| Honey Chlorella | 35.0 | 6.00 | 1.80 | 56.0 | 2.50 | 9.00 | 30.0 | 0.25 |
| GL3 strain Chlorella | 40.8 | 10.5 | 0.00 | 38.2 | 1.00 | 13.1 | 33.5 | 0.20 |

| Phase | Time (min) | Temperature (°C) | Pressure (mbar) |
|---|---|---|---|
| 1 | 15 | 116 | 2213 |
| 2 | 74 | 116 | 2213 |
| 3 | 10 | 30 | 2213 |
| 4 | 10 | 30 | 1013 |

| Sample | Batch | Replicate | pH | A-MB | A-MS | A-TS | An-MS | An-TS |
|---|---|---|---|---|---|---|---|---|
| Control | 1 | 1 | 5.68 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 |
| 1 | 2 | 5.66 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 2 | 1 | 5.71 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 2 | 2 | 5.67 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 3 | 1 | 5.67 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 3 | 2 | 5.66 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| SP | 1 | 1 | 6.16 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1 | 2 | 6.16 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 2 | 1 | 6.17 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 2 | 2 | 6.17 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 3 | 1 | 6.16 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 3 | 2 | 6.14 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| TS | 1 | 1 | 5.93 | 0.0 | 0.0 | 0.0 | 2.0 | 0.0 |
| 1 | 2 | 5.92 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 2 | 1 | 5.93 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 2 | 2 | 5.92 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 3 | 1 | 5.92 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 3 | 2 | 5.93 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | |
| SC | 1 | 1 | 5.63 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1 | 2 | 5.62 | 0.0 | 0.0 | 0.0 | 1.3 | 0.0 | |
| 2 | 1 | 5.62 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 2 | 2 | 5.62 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 3 | 1 | 5.63 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 3 | 2 | 5.63 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| WC | 1 | 1 | 5.64 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1 | 2 | 5.65 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 2 | 1 | 5.66 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 2 | 2 | 5.65 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 3 | 1 | 5.68 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 3 | 2 | 5.64 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| HC | 1 | 1 | 5.64 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 |
| 1 | 2 | 5.64 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 2 | 1 | 5.63 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 2 | 2 | 5.67 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 3 | 1 | 5.66 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | |
| 3 | 2 | 5.65 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| GL3 | 1 | 1 | 5.58 | 1.3 | 0.0 | 1.0 | 0.0 | 0.0 |
| 1 | 2 | 5.59 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 2 | 1 | 5.60 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 2 | 2 | 5.59 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 3 | 1 | 5.60 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 3 | 2 | 5.59 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
References
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical Review: Vegetables and Fruit in the Prevention of Chronic Diseases. Eur. J. Nut. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- European Commission-Directorate-General for Agriculture and Rural Development. Fruit and Vegetables, Market Data on National and European Agriculture. Browse Visualisations about Prices, SIV, Imports, Exports and Short-Term Outlook. 2022. Available online: https://agridata.ec.europa.eu/extensions/DataPortal/fruit-and-vegetables.html (accessed on 25 June 2024).
- Fortune Business Insights. Soup Market Size, Share & COVID-19 Impact Analysis, by Type (Instant, Dehydrated, Canned, Chilled, and Others), Distribution Channel (Hypermarket/Supermarket, Convenience Store, Online Retail, and Others), and Regional Forecast, 2020–2027. 2023. Available online: https://www.fortunebusinessinsights.com/press-release/soup-market-9964.html (accessed on 25 June 2024).
- Innova Market Insights. Top Ten Trends for 2023—Explore the Top Ten Latest Trends in the Food and Beverage Industry for 2023. 2020. Available online: https://www.innovamarketinsights.com/trends/top-ten-trends-for-2023-redefining-value-in-a-volatile-world/ (accessed on 25 June 2024).
- Ferreira, A.; Guerra, I.; Costa, M.; Silva, J.; Gouveia, L. Future Perspectives of Microalgae in the Food Industry. In Cultured Microalgae for the Food Industry: Current and Potential Applications, 1st ed.; Lafarga, T., Acien, G., Eds.; Academic Press: Cambridge, MA, USA, 2021; Chapter 15; pp. 387–433. [Google Scholar]
- Ahmad, A.; Ashraf, S.S. Sustainable Food and Feed Sources from Microalgae: Food Security and the Circular Bioeconomy. Algal Res. 2023, 74, 103185. [Google Scholar] [CrossRef]
- Olabi, A.G.; Shehata, N.; Sayed, E.T.; Rodriguez, C.; Anyanwu, R.C.; Russell, C.; Abdelkareem, M.A. Role of Microalgae in Achieving Sustainable Development Goals and Circular Economy. Sci. Total Environ. 2023, 854, 158689. [Google Scholar] [CrossRef]
- Spínola, M.P.; Alfaia, C.M.; Costa, M.M.; Pinto, R.M.A.; Lopes, P.A.; Pestana, J.M.; Tavares, J.C.; Mendes, A.R.; Mourato, M.P.; Tavares, B.; et al. Impact of High Spirulina Diet, Extruded or Supplemented with Enzymes, on Blood Cells, Systemic Metabolites, and Hepatic Lipid and Mineral Profiles of Broiler Chickens. Front. Vet. Sci. 2024, 11, 1342310. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Hu, Q. Microalgae as Feed Sources and Feed Additives for Sustainable Aquaculture: Prospects and Challenges. Rev. Aquac. 2024, 16, 818–835. [Google Scholar] [CrossRef]
- Sun, T.; Kalia, S.; Wyman, B.M.; Ou, K.J.; Lei, X.G. Impacts of Feeding Three Strains of Microalgae Alone or in Combination on Growth Performance, Protein Metabolism, and Meat Quality of Broiler Chickens. Algal Res. 2024, 83, 103691. [Google Scholar] [CrossRef]
- Camacho, F.; Macedo, A.; Malcata, F. Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef]
- Hernández-López, I.; Alamprese, C.; Cappa, C.; Prieto-Santiago, V.; Abadias, M.; Aguiló-Aguayo, I. Effect of Spirulina in Bread Formulated with Wheat Flours of Different Alveograph Strength. Foods 2023, 12, 3724. [Google Scholar] [CrossRef]
- Baune, M.C.; Fanari, F.; Lickert, T.; Schilling, F.; Claret, A.; Guerrero, L.; Bindrich, U.; Heinz, V.; Terjung, N. Physical and Sensory Properties of Vegan Organic Microalgae Pasta with High Protein and/or Fiber Content. Appl. Sci. 2025, 15, 1639. [Google Scholar] [CrossRef]
- Marzec, A.; Kramarczuk, P.; Kowalska, H.; Kowalska, J. Effect of Type of Flour and Microalgae (Chlorella vulgaris) on the Rheological, Microstructural, Textural, and Sensory Properties of Vegan Muffins. Appl. Sci. 2023, 13, 7632. [Google Scholar] [CrossRef]
- Fanari, F.; Comaposada, J.; Boukid, F.; Climent, E.; Claret Coma, A.; Guerrero, L.; Castellari, M. Enhancing Energy Bars with Microalgae: A Study on Nutritional, Physicochemical and Sensory Properties. J. Funct. Foods 2023, 109, 105768. [Google Scholar] [CrossRef]
- Oliveira, S.; Torres Pérez, M.D.; Sousa, I.; Raymundo, A. 3D-Printed Chlorella vulgaris Snacks: A Contribution to an Healthy Diet. Front. Food Sci. Technol. 2023, 3, 1265828. [Google Scholar]
- Caporgno, M.P.; Böcker, L.; Müssner, C.; Stirnemann, E.; Haberkorn, I.; Adelmann, H.; Handschin, S.; Windhab, E.J.; Mathys, A. Extruded Meat Analogues Based on Yellow, Heterotrophically Cultivated Auxenochlorella protothecoides Microalgae. Innov. Food Sci. Emerg. Technol. 2020, 59, 102275. [Google Scholar] [CrossRef]
- Palanisamy, M.; Töpfl, S.; Berger, R.G.; Hertel, C. Physico-Chemical and Nutritional Properties of Meat Analogues Based on Spirulina/Lupin Protein Mixtures. Eur. Food Res. Technol. 2019, 245, 1889–1898. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [PubMed]
- Muela, T.; Abellán, A.; Bande-De León, C.; Gómez, P.; Gil, M.D. Effect of Macro and Microalgae Addition on Nutritional, Physicochemical, Sensorial, and Functional Properties of a Vegetable Cream. Foods 2024, 13, 1651. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Comaposada, J.; Ribas-Agustí, A.; Castellari, M. Development of High-Protein Vegetable Creams by Using Single-Cell Ingredients from Some Microalgae Species. Foods 2021, 10, 2550. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Acién-Fernández, F.G.; Castellari, M.; Villaró, S.; Bobo, G.; Aguiló-Aguayo, I. Effect of Microalgae Incorporation on the Physicochemical, Nutritional, and Sensorial Properties of an Innovative Broccoli Soup. LWT 2019, 111, 167–174. [Google Scholar] [CrossRef]
- Fernández-López, J.; Botella-Martínez, C.; Navarro-Rodríguez de Vera, C.; Sayas-Barberá, M.E.; Viuda-Martos, M.; Sánchez-Zapata, E.; Pérez-Álvarez, J.A. Vegetable Soups and Creams: Raw Materials, Processing, Health Benefits, and Innovation Trends. Plants 2020, 9, 1769. [Google Scholar] [CrossRef]
- Amiri Samani, S.; Naji, M.H. Effect of Homogenizer Pressure and Temperature on Physicochemical, Oxidative Stability, Viscosity, Droplet Size, and Sensory Properties of Sesame Vegetable Cream. Food Sci. Nutr. 2019, 7, 899–906. [Google Scholar] [CrossRef]
- Gheysen, L.; Demets, R.; Devaere, J.; Bernaerts, T.; Goos, P.; Van Loey, A.; De Cooman, L.; Foubert, I. Impact of Microalgal Species on the Oxidative Stability of N-3 LC-PUFA Enriched Tomato Puree. Algal Res. 2019, 40, 101502. [Google Scholar] [CrossRef]
- European Parliament. EU Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods. Off. J. Eur. Union 2015, L327, 1–22. [Google Scholar]
- European Parliament. EC Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Off. J. Eur. Union 2006, L404, 1–17. [Google Scholar]
- Verdú, S.; Pérez, A.J.; Barat, J.M.; Grau, R. Laser Backscattering Imaging as a Control Technique for Fluid Foods: Application to Vegetable-Based Creams Processing. J. Food Eng. 2019, 241, 58–66. [Google Scholar] [CrossRef]
- Luo, M.R. CIELAB. In Encyclopedia of Color Science and Technology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–7. [Google Scholar]
- Diantom, A.; Curti, E.; Carini, E.; Vittadini, E. Effect of Added Ingredients on Water Status and Physico-Chemical Properties of Tomato Sauce. Food Chem. 2017, 236, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, R.R.; Mccarthy, K.L. Relationship Between the Bostwick Measurement and Fluid Properties. J. Texture Stud. 2006, 37, 640–654. [Google Scholar] [CrossRef]
- 4833-1:2013; ISO Microbiology of the Food Chain. Horizontal Method for the Enumeration of Microorganisms. Part 1: Colony Count at 30 °C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013.
- Turnbull, P.C.B.; Frawley, D.A.; Bull, R.L. Heat Activation/Shock Temperatures for Bacillus Anthracis Spores and the Issue of Spore Plate Counts versus True Numbers of Spores. J. Microbiol. Methods 2007, 68, 353–357. [Google Scholar] [CrossRef]
- Macfie, H.J.; Bratchell, N.; Greenhof, K.; Vallis, L.V. Designs to Balance the Effect of Order of Presentation and First-Order Carry-Over Effects in Hall Tests. J. Sens. Stud. 1989, 4, 129–148. [Google Scholar] [CrossRef]
- 8589:2007; ISO Sensory Analysis. General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2007.
- 11132:2021; ISO Sensory Analysis. Methodology. Guidelines for the Measurement of the Performance of a Quantitative Descriptive Sensory Panel. ISO: Geneva, Switzerland, 2021.
- European Parliament. European Parliament and Council of the European Union Regulation (EU) No 1169/2011 of 25 October 2011 on the Provision of Food Information to Consumers. Off. J. Eur. Union 2011, L304, 1–60. [Google Scholar]
- Kumar, A.; Mohanty, V.; Yashaswini, P. Development of High Protein Nutrition Bar Enriched with Spirulina Plantensis for Undernourished Children. Curr. Nutr. Food Sci. 2018, 6, 835–844. [Google Scholar] [CrossRef]
- Pereira, T.; Costa, S.; Barroso, S.; Teixeira, P.; Mendes, S.; Gil, M.M. Development and Optimization of High-Protein and Low-Saturated Fat Bread Formulations Enriched with Lupin and Microalgae. LWT 2024, 191, 115612. [Google Scholar] [CrossRef]
- Ahda, M.; Suhendra; Permadi, A. Spirulina Platensis Microalgae as High Protein-Based Products for Diabetes Treatment. Food Rev. Int. 2024, 40, 1796–1804. [Google Scholar] [CrossRef]
- Banovic, M.; Lähteenmäki, L.; Arvola, A.; Pennanen, K.; Duta, D.E.; Brückner-Gühmann, M.; Grunert, K.G. Foods with Increased Protein Content: A Qualitative Study on European Consumer Preferences and Perceptions. Appetite 2018, 125, 233–243. [Google Scholar] [CrossRef]
- Fei, X.; Jones, O.G.; Reuhs, B.L.; Campanella, O.H. Soluble Pectin Acts as a Particle Stabilizer of Tomato Suspensions: The Impact on Tomato Products Rheological Characterization. LWT 2021, 139, 110508. [Google Scholar] [CrossRef]
- Grossmann, L.; Ebert, S.; Hinrichs, J.; Weiss, J. Formation and Stability of Emulsions Prepared with a Water-Soluble Extract from the Microalga Chlorella protothecoides. J. Agric. Food Chem. 2019, 67, 6551–6558. [Google Scholar] [CrossRef]
- Uribe-Wandurraga, Z.N.; Martínez-Sánchez, I.; Savall, C.; García-Segovia, P.; Martínez-Monzó, J. Microalgae Fortification of Low-Fat Oil-in-Water Food Emulsions: An Evaluation of the Physicochemical and Rheological Properties. J. Food Sci. Technol. 2021, 58, 3701–3711. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Boye, J.I. Advances in the Design and Production of Reduced-Fat and Reduced-Cholesterol Salad Dressing and Mayonnaise: A Review. Food Bioprocess Technol. 2013, 6, 648–670. [Google Scholar] [CrossRef]
- Vermeir, I.; Roose, G. Visual Design Cues Impacting Food Choice: A Review and Future Research Agenda. Foods 2020, 9, 1495. [Google Scholar] [CrossRef]
- Hoppu, U.; Puputti, S.; Sandell, M. Factors Related to Sensory Properties and Consumer Acceptance of Vegetables. Crit. Rev. Food Sci. Nutr. 2021, 61, 1751–1761. [Google Scholar] [CrossRef]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina Platensis Fortification on Physicochemical, Textural, Antioxidant and Sensory Properties of Yogurt during Fermentation and Storage. LWT 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Du, Q.; Wang, S.; Lyu, F.; Liu, J.; Ding, Y. The Interfacial Covalent Bonding of Whey Protein Hydrolysate and Pectin under High Temperature Sterilization: Effect on Emulsion Stability. Colloids Surf. B Biointerfaces 2021, 206, 111936. [Google Scholar] [CrossRef]
- Olia, M.S.J.; Azin, M.; Moazami, N. Comparison of Different Pretreatment Methods to Facilitate the Carbohydrate Release from Two Microalgae Isolates: A Critical Step in Bioethanol Production. Biomass Convers. Biorefin. 2023, 13, 17119–17132. [Google Scholar] [CrossRef]
- Villaró, S.; Viñas, I.; Lafarga, T. Consumer Acceptance and Attitudes toward Microalgae and Microalgal-Derived Products as Food. In Cultured Microalgae for the Food Industry: Current and Potential Applications, 1st ed.; Lafarga, T., Acien, G., Eds.; Academic Press: Cambridge, MA, USA, 2023; Chapter 14; pp. 367–385. [Google Scholar] [CrossRef]
- Matos, Â.P.; Novelli, E.; Tribuzi, G. Use of Algae as Food Ingredient: Sensory Acceptance and Commercial Products. Front. Food Sci. Technol. 2022, 2, 989801. [Google Scholar] [CrossRef]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Encapsulation of Microalgae-Based Products for Food and Feed Applications. In Handbook of Food and Feed from Microalgae: Production, Application, Regulation, and Sustainability, 1st ed.; Jacob-Lopes, E., Maroneze, M.M., Queiroz, M.I., Zepka, L.Q., Eds.; Academic Press: Cambridge, MA, USA, 2023; Chapter 30; pp. 371–393. [Google Scholar] [CrossRef]
- Nunes, M.C.; Ferreira, J.; Raymundo, A. Volatile Fingerprint Impact on the Sensory Properties of Microalgae and Development of Mitigation Strategies. Curr. Opin. Food Sci. 2023, 51, 101040. [Google Scholar] [CrossRef]
- Van Durme, J.; Goiris, K.; De Winne, A.; De Cooman, L.; Muylaert, K. Evaluation of the Volatile Composition and Sensory Properties of Five Species of Microalgae. J. Agric. Food Chem. 2013, 61, 10881–10890. [Google Scholar] [CrossRef]
- Grácio, M.; Ferreira, J.; Steinrücken, P.; Kleinegris, D.M.M.; Sousa, I.; Nunes, M.C.; Raymundo, A. The Volatile Composition and the Potential Health Benefits of Different Microalgae Strains. Foods 2024, 13, 2174. [Google Scholar] [CrossRef]
- Kurihara, K. Umami the Fifth Basic Taste: History of Studies on Receptor Mechanisms and Role as a Food Flavor. BioMed Res. Int. 2015, 2015, 189402. [Google Scholar] [CrossRef]
- Prandi, B.; Boukid, F.; Van De Walle, S.; Cutroneo, S.; Comaposada, J.; Van Royen, G.; Sforza, S.; Tedeschi, T.; Castellari, M. Protein Quality and Protein Digestibility of Vegetable Creams Reformulated with Microalgae Inclusion. Foods 2023, 12, 2395. [Google Scholar] [CrossRef]
- Zhao, Y.; Bai, M.; Qiu, D.; Zhang, J.; Zhao, N.; Feng, G.; Wu, H.; Zeng, M.; Obadina, A.O. Comparative Evaluation of Sensory and Instrumental Flavor Profiles of Four Edible Microalgae: Spirulina Platensis, Chlorella Pyrenoidosa, Chlamydomonas Reinhardtii, and Haematococcus Pluvialis. Algal Res. 2024, 82, 103628. [Google Scholar] [CrossRef]
- Guo, X.; Liu, B.; Zhang, Y.; Zhou, Y.; Gong, Z.; Wu, Y.; Wang, Q.; Liu, X. Interfacial Structure Modification and Enhanced Emulsification Stability of Microalgae Protein through Interaction with Anionic Polysaccharides. Int. J. Biol. Macromol. 2024, 283, 137404. [Google Scholar] [CrossRef]
- Lima, V.S.; de Oliveira, D.R.B.; da Silva, C.A.S.; Santana, R.d.C.; Soares, N.d.F.F.; de Oliveira, E.B.; Martins, M.A.; Coimbra, J.S.d.R. Stabilization of Oil–Water Emulsions with Protein Concentrates from the Microalga Tetradesmus Obliquus. J. Food Sci. Technol. 2023, 60, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Weemaes, C.A.; Ooms, V.; Van Loey, A.M.; Hendrickx, M.E. Kinetics of Chlorophyll Degradation and Color Loss in Heated Broccoli Juice. J. Agric. Food Chem. 1999, 47, 2404–2409. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2012, 6, 36–60. [Google Scholar] [CrossRef]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, Flavor, Texture, and Nutritional Quality of Fresh-Cut Fruits and Vegetables: Desirable Levels, Instrumental and Sensory Measurement, and the Effects of Processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.V.; Denoya, G.I.; Agüero, M.V.; Jagus, R.J.; Vaudagna, S.R. Optimization of High Pressure Processing Parameters to Preserve Quality Attributes of a Mixed Fruit and Vegetable Smoothie. Innov. Food Sci. Emerg. Technol. 2018, 47, 170–179. [Google Scholar] [CrossRef]
- Sonar, C.R.; Rasco, B.; Tang, J.; Sablani, S.S. Natural Color Pigments: Oxidative Stability and Degradation Kinetics during Storage in Thermally Pasteurized Vegetable Purees. J. Sci. Food Agric. 2019, 99, 5934–5945. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, V.; Gurak, P.D.; Ferreira Marczak, L.D.; Tessaro, I.C. Tracking Bioactive Compounds with Colour Changes in Foods—A Review. Dye Pigments 2013, 98, 601–608. [Google Scholar] [CrossRef]
- Ferioli, F.; Castagnetti, G.B.; Caboni, M.F. Effect of Different Storage Conditions on the Lipid Fraction of a Vegetable Cream. J. Food Qual. 2008, 31, 446–464. [Google Scholar] [CrossRef]
- Mosibo, O.K.; Ferrentino, G.; Udenigwe, C.C. Microalgae Proteins as Sustainable Ingredients in Novel Foods: Recent Developments and Challenges. Foods 2024, 13, 733. [Google Scholar] [CrossRef]
- Long, Z.; Zhao, M.; Zhao, Q.; Yang, B.; Liu, L. Effect of Homogenisation and Storage Time on Surface and Rheology Properties of Whipping Cream. Food Chem. 2012, 131, 748–753. [Google Scholar] [CrossRef]
- Alemaskin, K.; Manas-Zloczower, I.; Kaufman, M. Entropic Analysis of Color Homogeneity. Polym. Eng. Sci. 2005, 45, 1031–1038. [Google Scholar] [CrossRef]
- Chen, G.-W.; Yang, H.; Ciesielski, W.; Khachatryan, K. Production and Purification of Novel Hypocholesterolemic Peptides from Lactic Fermented Spirulina Platensis through High Hydrostatic Pressure-Assisted Protease Hydrolysis. Catalysts 2021, 11, 873. [Google Scholar] [CrossRef]
- Keenan, D.F.; Brunton, N.P.; Mitchell, M.; Gormley, R.; Butler, F. Flavour Profiling of Fresh and Processed Fruit Smoothies by Instrumental and Sensory Analysis. Food Res. Int. 2012, 45, 17–25. [Google Scholar] [CrossRef]
- Van der Stricht, H.; Hung, Y.; Fischer, A.R.H.; Verbeke, W. Consumer Segments Less or More Willing to Adopt Foods with Microalgae Proteins. Food Qual. Prefer. 2024, 113, 105047. [Google Scholar] [CrossRef]
- Lamanna, M.; Muca, E.; Buonaiuto, G.; Formigoni, A.; Cavallini, D. From Posts to Practice: Instagram’s Role in Veterinary Dairy Cow Nutrition Education—How Does the Audience Interact and Apply Knowledge? A Survey Study. J. Dairy Sci. 2025, 108, 1659–1671. [Google Scholar] [CrossRef]
| Ingredient | Control | SP | TS | SC | WC | HC | GL3 |
|---|---|---|---|---|---|---|---|
| Spirulina | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Tetraselmis chui | 0.0 | 2.0 | |||||
| Smooth Chlorella | 0.0 | 4.0 | |||||
| White Chlorella | 0.0 | 2.0 | |||||
| Honey Chlorella | 0.0 | 4.0 | |||||
| Chlorella GL3 | 0.0 | 4.0 | |||||
| Spinach | 15.4 | 15.2 | 14.5 | 14.5 | 14.5 | 14.5 | 14.5 |
| Zucchini | 13.5 | 13.3 | 13.1 | 12.7 | 13.1 | 12.7 | 12.7 |
| Chickpea | |||||||
| Leek | 9.7 | 9.5 | 9.4 | 9.10 | 9.40 | 9.10 | 9.10 |
| Broccoli | |||||||
| Chard | 5.8 | 5.7 | 5.6 | 5.4 | 5.6 | 5.4 | 5.4 |
| Water | 29.0 | ||||||
| Oil | 2.9 | ||||||
| Salt | 0.5 | ||||||
| Sample | Energy (kcal/100 g) | Carbohydrates (g/100 g) | Proteins (g/100 g) | Fats (g/100 g) | Fiber (g/100 g) | %Kcal Protein |
|---|---|---|---|---|---|---|
| Control | 57.02 | 3.75 | 2.50 | 3.08 | 2.19 | 17.51% |
| SP | 60.44 | 3.91 | 3.04 | 3.17 | 2.19 | 20.12% |
| TS | 62.83 | 3.95 | 3.34 | 3.23 | 2.35 | 21.26% |
| SC | 67.18 | 4.98 | 4.98 | 3.30 | 2.87 | 20.01% |
| WC | 62.02 | 4.81 | 2.95 | 3.19 | 2.53 | 20.13% |
| HC | 68.21 | 5.80 | 3.56 | 3.30 | 2.06 | 20.59% |
| GL3 | 71.56 | 5.07 | 3.70 | 3.48 | 2.59 | 20.71% |
| Bostwick (cm) | pH | L* | a* | b* | ΔE | MC% | Sy% | °Brix | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 5.87 d | 5.75 b | 43.78 b | −1.64 b | 21.66 e | 0.54 f | 87.52 a | 68.02 a | 4.05 c |
| SP | 6.39 cd | 5.73 c | 41.91 c | 0.71 a | 22.99 d | 3.36 e | 86.70 ab | 57.50 cd | 5.00 b |
| TS | 6.98 bc | 5.99 a | 37.40 e | −1.94 cd | 15.78 f | 8.69 b | 85.87 bc | 63.30 ab | 5.30 ab |
| SC | 8.12 a | 5.70 e | 39.14 d | −1.83 bc | 21.72 e | 4.73 d | 84.21 d | 57.00 d | 5.70 a |
| WC | 7.08 bc | 5.72 de | 47.46 a | −2.15 d | 24.54 c | 4.74 d | 85.76 bc | 60.11 bcd | 5.02 b |
| HC | 7.58 ab | 5.72 d | 47.62 a | −2.06 cd | 31.81 a | 10.9 a | 84.15 d | 56.83 d | 5.20 b |
| GL3 | 6.97 bc | 5.68 f | 46.63 a | −1.91 c | 27.86 b | 6.86 c | 85.57 c | 62.60 bc | 4.87 b |
| Global Odor Intensity | Green Leafy Vegetables | Leek | Fishy | Legumes/Mealy | Shrimp/Shellfish | |
|---|---|---|---|---|---|---|
| Control | 6.34 c | 5.71 a | 5.82 a | 0.00 d | 3.27 a | 0.00 d |
| SP | 6.57 bc | 4.53 b | 3.74 b | 0.72 bcd | 2.99 a | 0.79 bc |
| TS | 8.38 a | 2.66 c | 1.59 c | 5.95 a | 1.13 b | 4.86 a |
| SC | 6.85 b | 4.23 b | 3.19 b | 1.75 b | 2.73 a | 1.33 b |
| WC | 6.47 bc | 4.70 b | 3.97 b | 0.66 cd | 2.78 a | 0.67 bc |
| HC | 6.54 bc | 4.73 b | 3.63 b | 1.17 bc | 2.67 a | 0.95 bc |
| GL3 | 6.17 c | 4.54 b | 3.78 b | 0.25 cd | 2.63 a | 0.16 cd |
| Global Taste Intensity | Salty | Sweet | Umami | Fishy | Shrimp/Shellfish | Bitter | |
|---|---|---|---|---|---|---|---|
| Control | 5.94 d | 4.11 d | 2.68 a | 1.42 c | 0.00 d | 0.00 d | 2.31 d |
| SP | 6.59 c | 4.84 bc | 2.14 ab | 3.31 b | 1.28 bc | 1.67 b | 2.99 bcd |
| TS | 8.72 a | 7.14 a | 0.74 c | 4.66 a | 6.74 a | 5.44 a | 4.44 a |
| SC | 7.17 b | 5.31 b | 1.85 b | 3.56 b | 2.08 b | 1.92 b | 4.01 ab |
| WC | 6.46 c | 4.54 cd | 2.25 ab | 2.70 b | 0.89 c | 0.96 bc | 2.75 cd |
| HC | 6.81 bc | 5.07 bc | 2.09 ab | 3.33 b | 1.44 bc | 1.54 bc | 3.44 bc |
| GL3 | 6.45 c | 5.02 bc | 2.56 ab | 2.63 b | 0.49 cd | 0.57 cd | 2.69 cd |
| Creaminess | Particle Presence | Color Homogeneity | Graininess | Fluidity | |
|---|---|---|---|---|---|
| Control | 5.93 | 3.94 a | 6.92 b | 5.26 abc | 5.25 c |
| SP | 5.92 | 2.78 b | 8.53 a | 2.91 e | 5.84 abc |
| TS | 5.66 | 3.72 a | 8.01 a | 3.78 d | 6.30 a |
| SC | 6.18 | 4.23 a | 7.11 b | 5.34 ab | 5.33 c |
| WC | 5.81 | 3.75 a | 7.04 b | 4.64 bc | 5.66 abc |
| HC | 5.97 | 4.13 a | 6.60 b | 4.57 c | 6.10 ab |
| GL3 | 6.02 | 3.85 a | 6.58 b | 5.46 a | 5.37 bc |
| Bostwick (cm) | pH | L* | a* | b* | Δ | WC% | Sy% | °Brix | |
|---|---|---|---|---|---|---|---|---|---|
| 0 months | 7.17 a | 5.91 a | 43.7 a | −1.67 a | 23.4 b | 0.00 b | 85.6 | 64.1 a | 5.08 |
| 8 months | 6.82 b | 5.75 b | 43.2 b | −1.42 b | 24.2 a | 6.06 a | 85.7 | 57.4 b | 4.96 |
| Olfactory Attributes | |||||||
|---|---|---|---|---|---|---|---|
| Global Intensity | Green Leafy Vegetables | Leek | Fishy | Legumes/Mealy | Shrimp/Shellfish | ||
| 0 months | 6.69 | 4.21 b | 3.76 | 1.70 a | 2.34 b | 1.28 a | |
| 8 months | 6.83 | 4.67 a | 3.58 | 1.28 b | 2.85 a | 1.18 b | |
| Flavor/Taste Attributes | |||||||
| Global intensity | Salty | Sweet | Umami | Fishy | Shrimp/ shellfish | Bitter | |
| 0 months | 6.99 a | 5.15 | 2.13 | 3.11 | 2.25 a | 1.82 | 3.17 |
| 8 months | 6.77 b | 5.14 | 1.96 | 3.06 | 1.38 b | 1.58 | 3.30 |
| Visual/Textural Attributes | |||||||
| Creaminess | Particle Presence | Color Homogeneity | Graininess | Fluidity | |||
| 0 months | 5.57 b | 4.10 a | 7.15 | 4.86 a | 5.36 b | ||
| 8 months | 6.29 a | 3.44 b | 7.37 | 4.27 b | 6.03 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanari, F.; Comaposada, J.; Aymerich, T.; Claret, A.; Guerrero, L.; Castellari, M. Development of Vegetable Creams Enriched with Different Microalgae Species: A Study on the Physicochemical and Sensory Stability over Time. Foods 2025, 14, 1230. https://doi.org/10.3390/foods14071230
Fanari F, Comaposada J, Aymerich T, Claret A, Guerrero L, Castellari M. Development of Vegetable Creams Enriched with Different Microalgae Species: A Study on the Physicochemical and Sensory Stability over Time. Foods. 2025; 14(7):1230. https://doi.org/10.3390/foods14071230
Chicago/Turabian StyleFanari, Fabio, Josep Comaposada, Teresa Aymerich, Anna Claret, Luis Guerrero, and Massimo Castellari. 2025. "Development of Vegetable Creams Enriched with Different Microalgae Species: A Study on the Physicochemical and Sensory Stability over Time" Foods 14, no. 7: 1230. https://doi.org/10.3390/foods14071230
APA StyleFanari, F., Comaposada, J., Aymerich, T., Claret, A., Guerrero, L., & Castellari, M. (2025). Development of Vegetable Creams Enriched with Different Microalgae Species: A Study on the Physicochemical and Sensory Stability over Time. Foods, 14(7), 1230. https://doi.org/10.3390/foods14071230








