Antidiabetic and Immunomodulatory Properties of Peptide Fractions from Sacha Inchi Oil Press-Cake
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SGID and Peptide Fractionation
2.3. In Vitro α-Amylase Inhibition Assay
2.4. Modulatory Effects in RAW 264.7 Cell Model
2.4.1. Culture
2.4.2. Determination of NO Production
2.4.3. Cytokine Production
2.5. Peptide Sequencing and SGID-Resistant Peptides Identification
2.6. In Silico Analysis of SGID-Resistant Peptides
2.6.1. Identification of Inhibitory α-Amylase Peptides
2.6.2. Bioactivity and Bioavailability of Anti-Inflammatory Peptides
2.7. Molecular Docking with Resistant and Bioactive Peptides
2.7.1. Resistant Peptides and α-Amylase
2.7.2. Resistant Peptides and TLR4/MD-2 Complex
2.8. Statistical Analysis
3. Results and Discussion
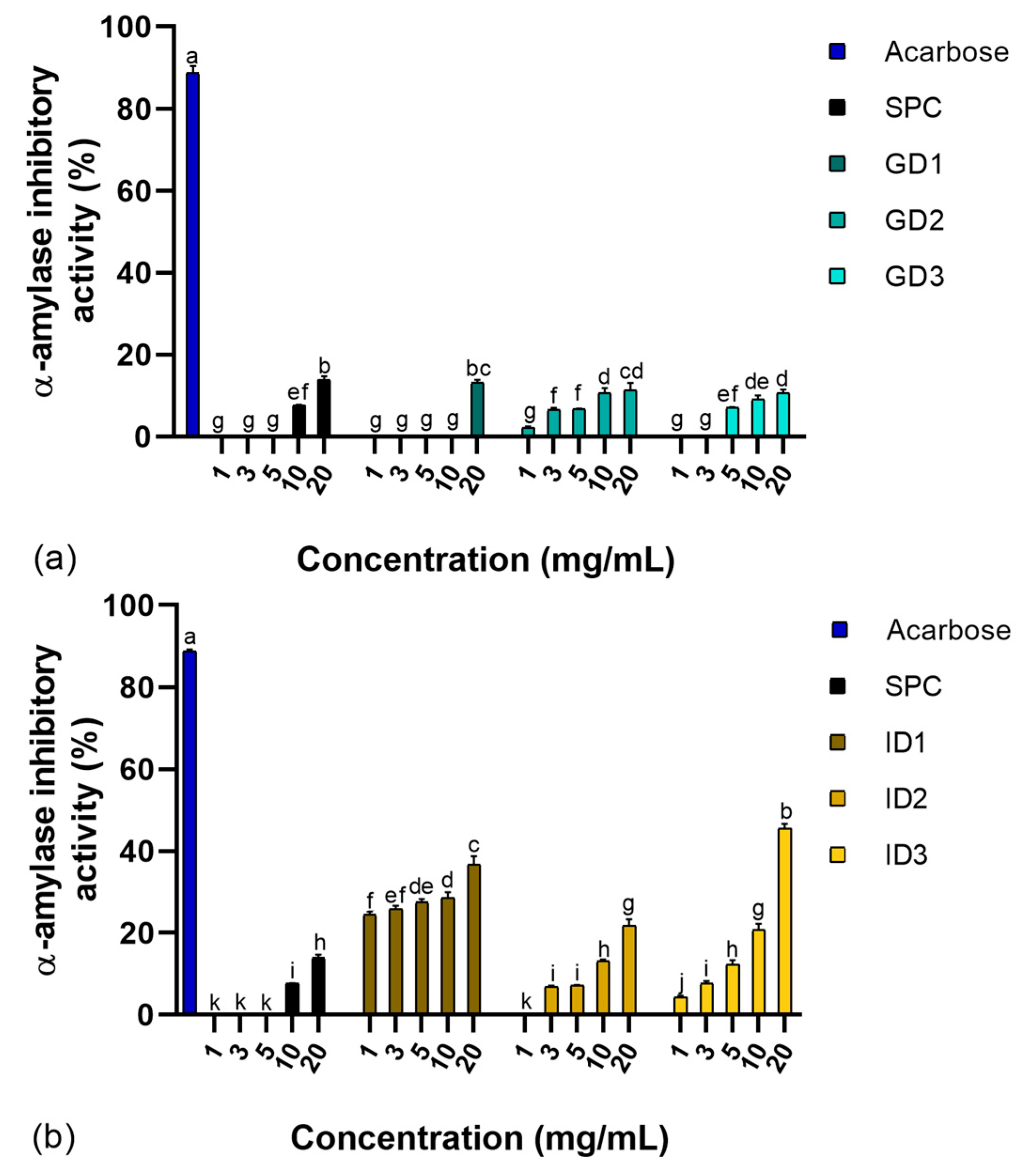
3.1. α-Amylase Inhibition Activity
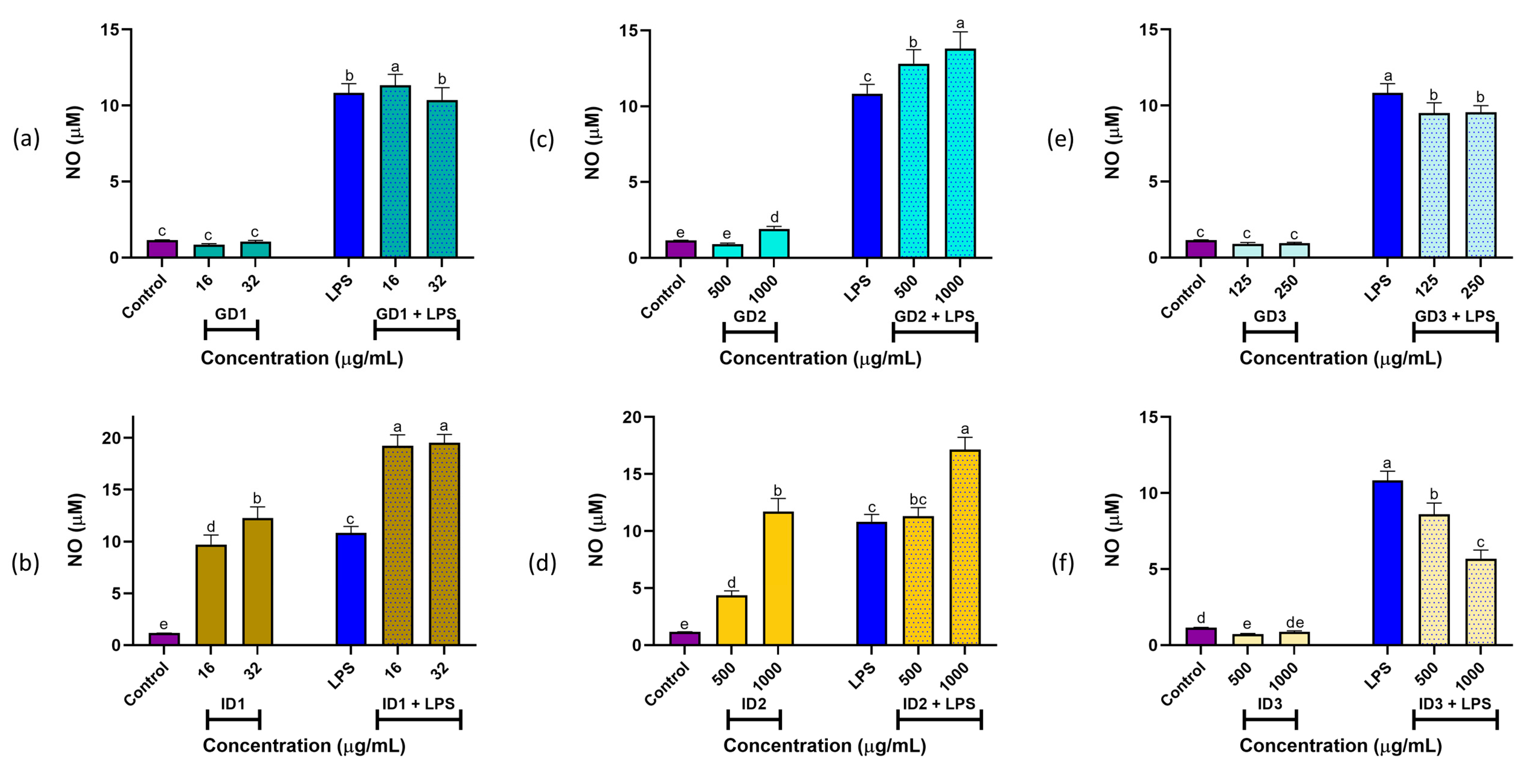
3.2. NO Production
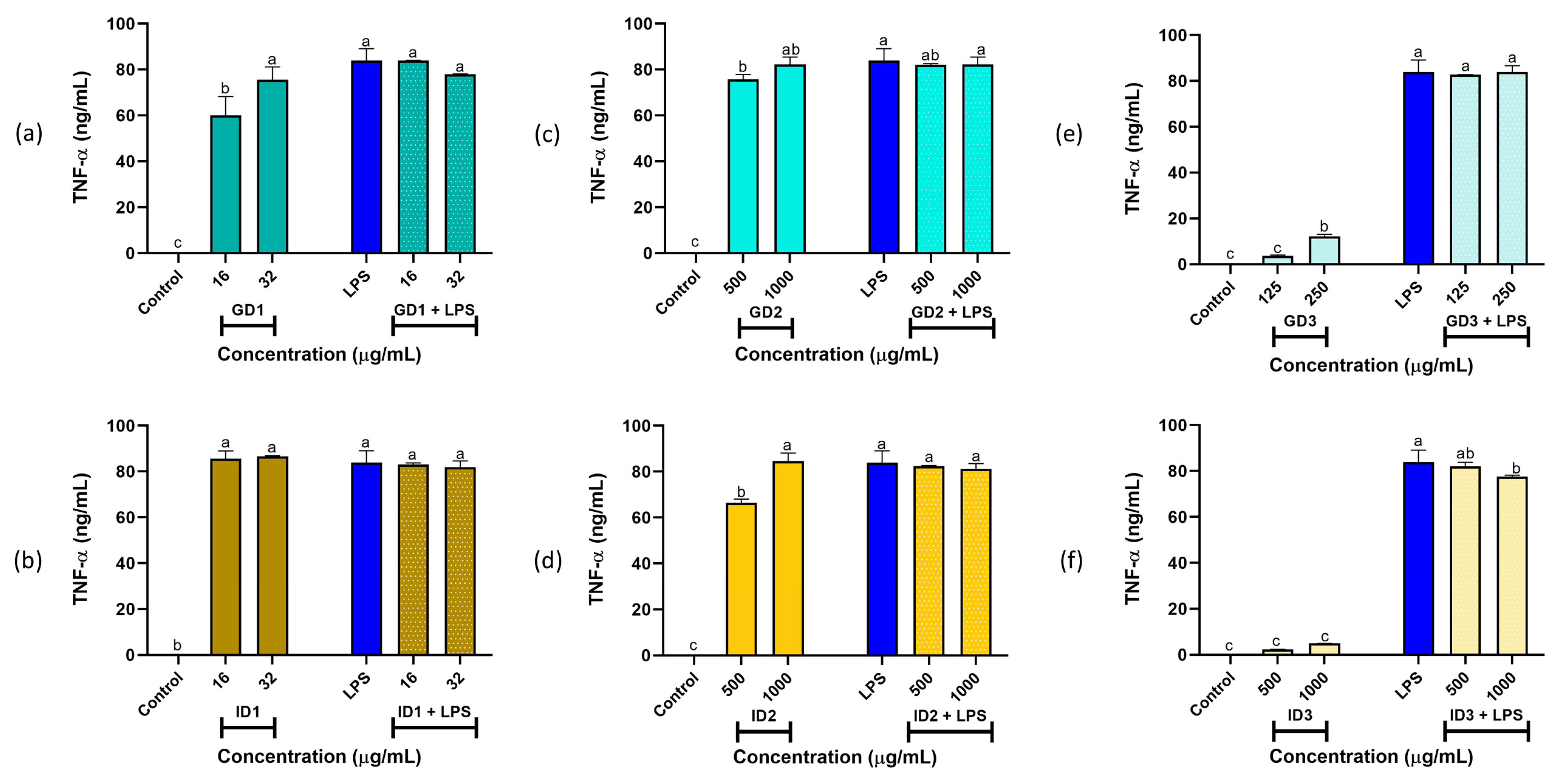
3.3. Cytokines IL-6 and TNF-α Production
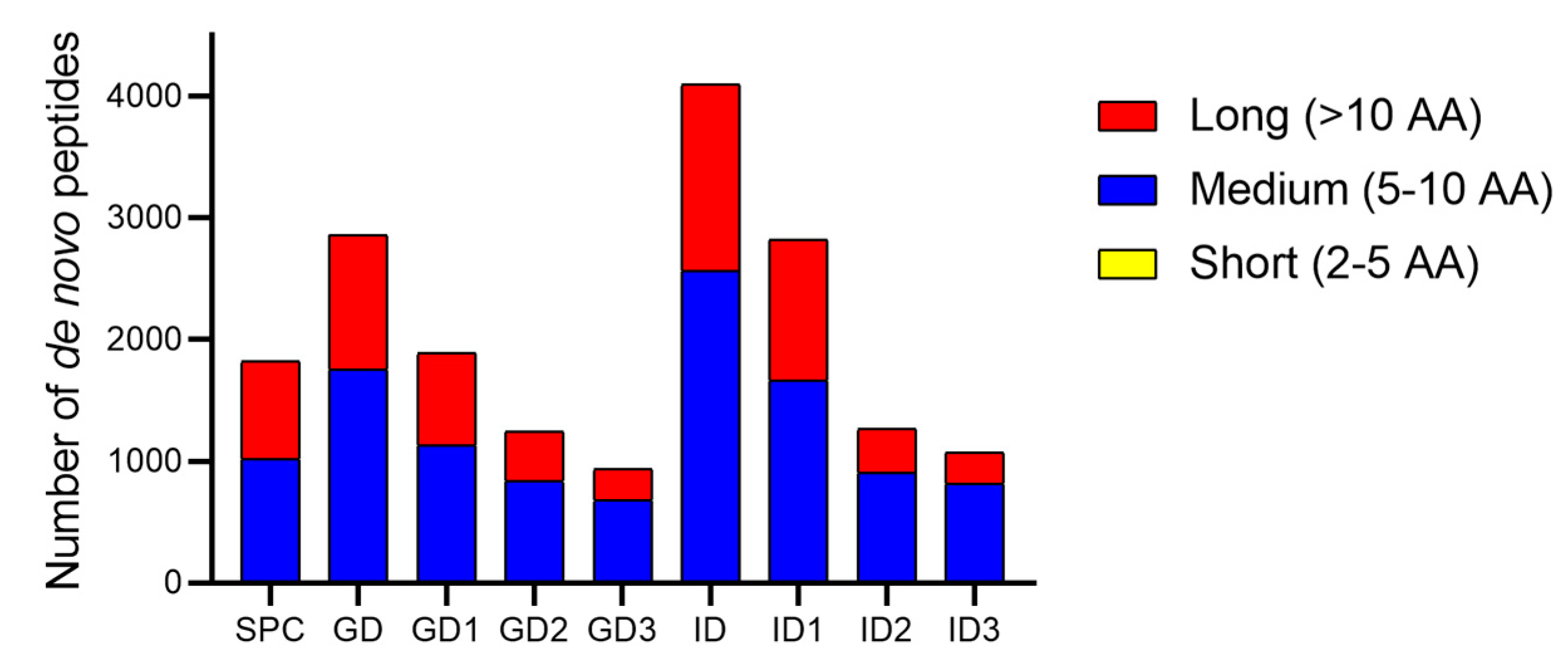
3.4. De Novo Peptides Sequencing and SGID-Resistant Peptides Identification
3.5. In Silico Analysis
3.5.1. Properties of Resistant Peptides with α-Amylase Inhibitory Potential
3.5.2. Bioactivity and Bioavailability of Peptides
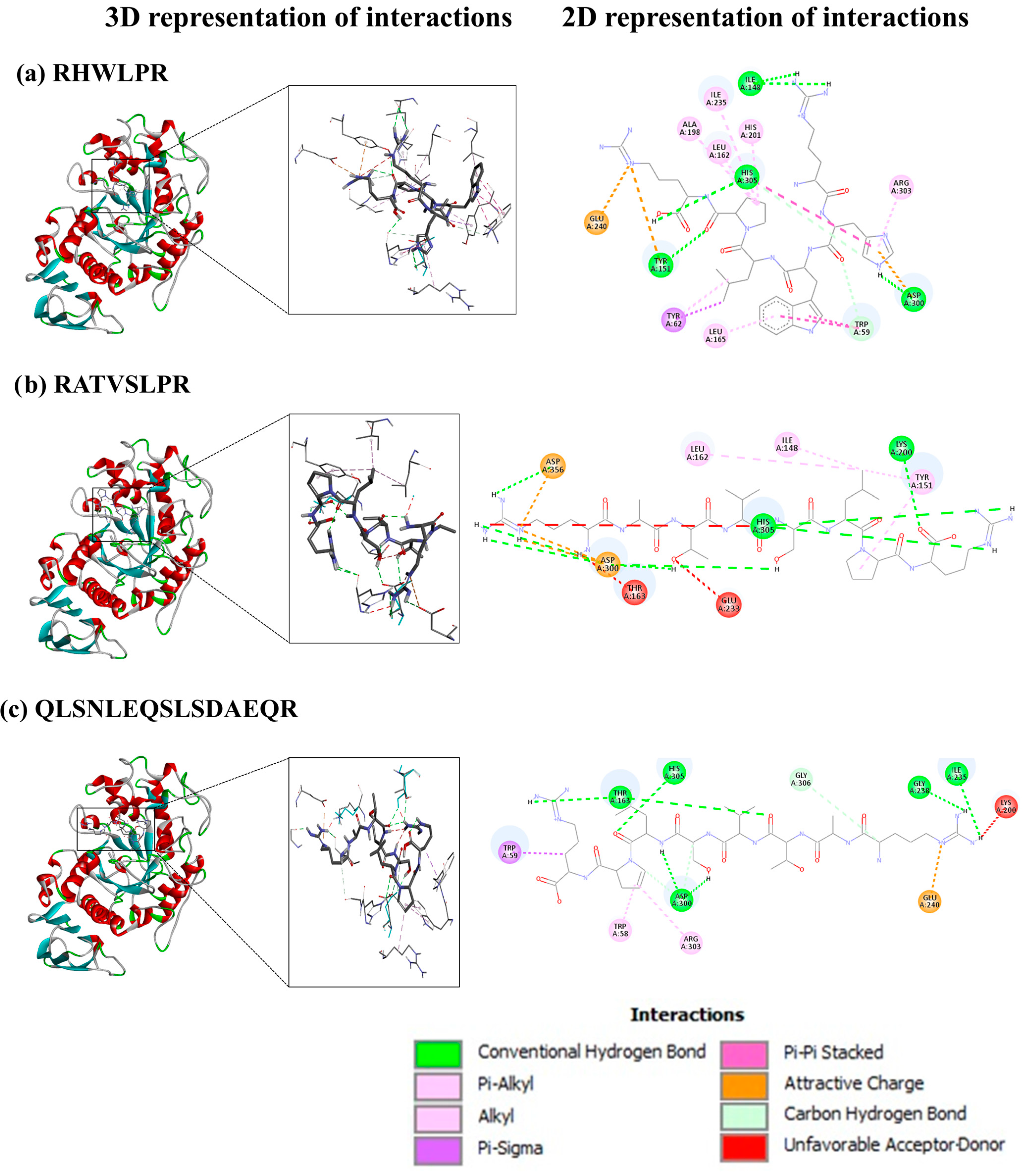
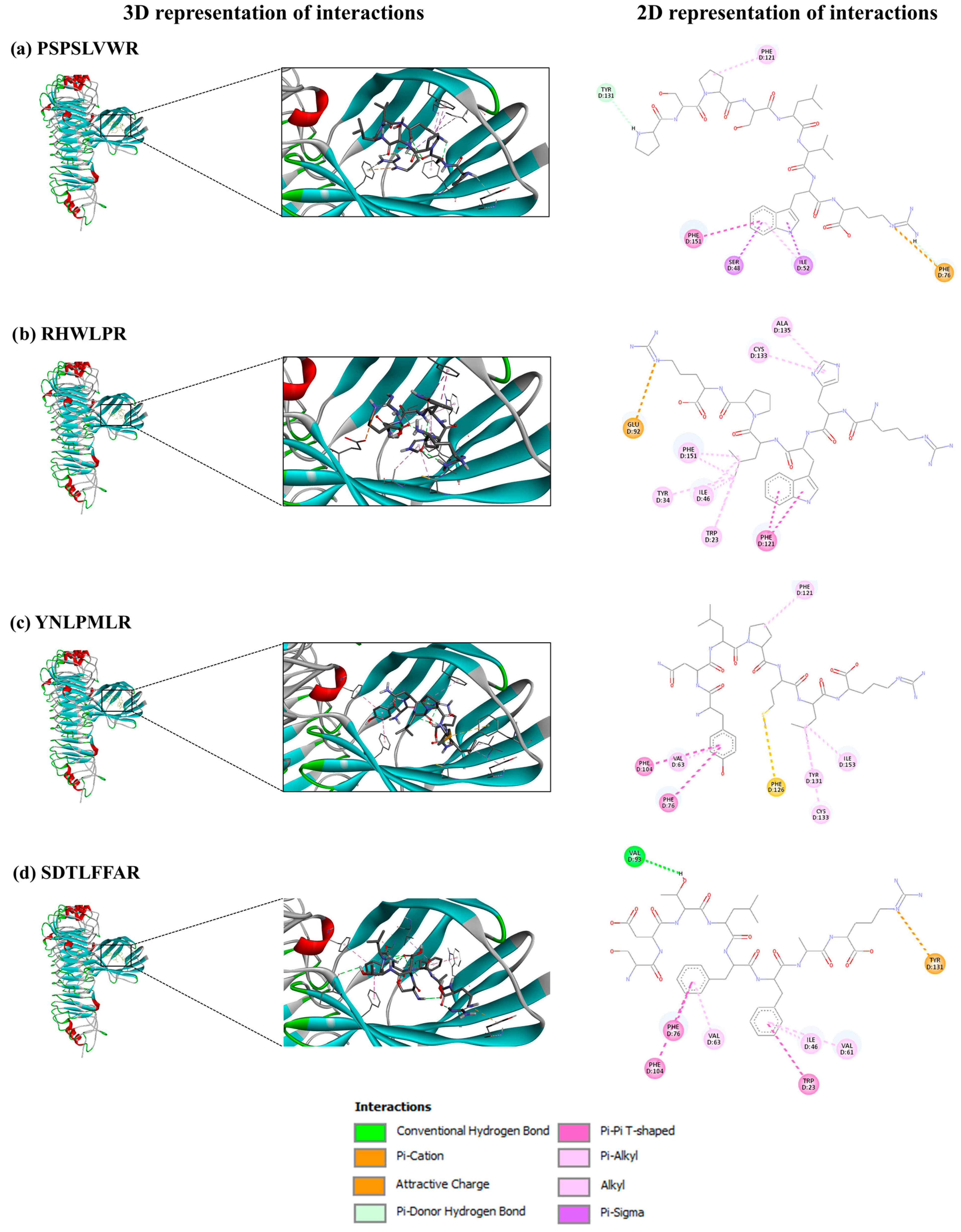
3.6. Molecular Docking
3.6.1. SGID-Resistant Peptides and α-Amylase
3.6.2. SGID-Resistant Peptides and TLR4/MD-2 Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Amino acids |
| ALC | Average local confidence |
| ANOVA | Analysis of variance |
| DM2 | Diabetes mellitus type 2 diabetes |
| DMEM | High-glucose Dulbecco’s modified Eagle’s medium |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DPP-IV | Dipeptidyl peptidase-IV |
| ELISA | Enzyme-linked immunosorbent assay |
| FAO | Food and Agriculture Organization |
| FBS | Fetal bovine serum |
| HPLC-MS/MS | High-performance liquid chromatography coupled with Tandem mass spectrometry |
| IC50 | Half-maximal inhibitory concentration |
| IL-6 | Interleukin-6 |
| LC-MS/MS | Liquid chromatography-Tandem mass spectrometry |
| LPS | Lipopolysaccharide |
| MW | Molecular weight |
| NO | Nitric oxide |
| PBS | Phosphate-buffered saline |
| pI | Isoelectric point |
| RCSB | Research Collaboratory for Structural Bioinformatics |
| SGID | Simulated gastrointestinal digestion |
| TLR4/MD-2 | Toll-like receptor 4/Myeloid differentiation protein-2 |
| TNF-α | Tumor necrosis factor-α |
| UN | United Nations |
| WHO | World Health Organization |
References
- Rawdkuen, S.; Ketnawa, S. Extraction, Characterization, and Application of Agricultural and Food Processing By-Products. In Food Preservation and Waste Exploitation; Socaci, S.A., Fărcaş, A.C., Aussenac, T., Laguerre, J.-C., Eds.; IntechOpen: London, UK, 2019; pp. 1–32. [Google Scholar]
- Hamaker, B.R.; Valles, C.; Gilman, R.; Hardmeier, R.M.; Clark, D.; Garcia, H.H.; Gonzales, A.E.; Kohlstad, I.; Castro, M.; Valdivia, R.; et al. Amino Acid and Fatty Acid Profiles of the Inca Peanut (Plukenetia volubilis). Cereal Chem. 1992, 69, 461–463. [Google Scholar]
- Chirinos, R.; Aquino, M.; Pedreschi, R.; Campos, D. Optimized Methodology for Alkaline and Enzyme-Assisted Extraction of Protein from Sacha Inchi (Plukenetia volubilis) Kernel Cake. J. Food Process Eng. 2016, 3, e12412. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Rodzi, N.; Pinijsuwan, S. Characterization of Sacha Inchi Protein Hydrolysates Produced by Crude Papain and Calotropis Proteases. LWT-Food Sci. Technol. 2018, 98, 18–24. [Google Scholar] [CrossRef]
- Gravel, A.; Doyen, A. The Use of Edible Insect Proteins in Food: Challenges and Issues Related to Their Functional Properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Gençdağ, E.; Görgüç, A.; Yılmaz, F.M. Recovery of Bioactive Peptides from Food Wastes and Their Bioavailability Properties. Turkish J. Agric.-Food Sci. Technol. 2020, 8, 855–863. [Google Scholar] [CrossRef]
- Amigo, L.; Hernández-Ledesma, B. Current Evidence on the Bioavailability of Food Bioactive Peptides. Molecules 2020, 25, 4479. [Google Scholar] [CrossRef]
- Rahmi, A.; Arcot, J. In Vitro Assessment Methods for Antidiabetic Peptides from Legumes: A Review. Foods 2023, 12, 631. [Google Scholar] [CrossRef] [PubMed]
- Oishi, S.; Yoshioka, Y.; Dohra, H.; Miyoshi, N. Chickpea Proteins Are Potential Sources of Bioactive Peptides That Induce Glucose Uptake via AMP-Activated Protein Kinase. Food Biosci. 2024, 59, 104029. [Google Scholar] [CrossRef]
- Berraquero-García, C.; Rivero-Pino, F.; Ospina, J.L.; Pérez-Gálvez, R.; Espejo-Carpio, F.J.; Guadix, A.; García-Moreno, P.J.; Guadix, E.M. Activity, Structural Features and in Silico Digestion of Antidiabetic Peptides. Food Biosci. 2023, 55, 102954. [Google Scholar] [CrossRef]
- Cao, R.; Li, W.; Zhang, J.; Bao, X.; Feng, H.; Sun, J.; Liu, X.; Sun, L. Milk Casein Hydrolysate Peptides Regulate Starch Digestion through Inhibition of α-Glucosidase: An Insight into the Active Oligopeptide Screening, Enzyme Inhibition Behaviors, and Oligopeptide-Enzyme Binding Interactions. Food Hydrocoll. 2024, 152, 109926. [Google Scholar] [CrossRef]
- González-Montoya, M.; Hernández-Ledesma, B.; Mora-Escobedo, R.; Martínez-Villaluenga, C. Bioactive Peptides from Germinated Soybean with Anti-Diabetic Potential by Inhibition of Dipeptidyl Peptidase-IV, α-Amylase, and α-Glucosidase Enzymes. Int. J. Mol. Sci. 2018, 19, 2883. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejía, E.G. Optimization of Enzymatic Production of Anti-Diabetic Peptides from Black Bean (Phaseolus vulgaris L.) Proteins, Their Characterization and Biological Potential. Food Funct. 2016, 7, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Suwanangul, S.; Alashi, M.A.; Aluko, R.E.; Tochampa, W.; Ruttarattanamongkol, K. Inhibition of α-Amylase, α-Glucosidase and Pancreatic Lipase Activities in Vitro by Sacha Inchi (Plukenetia volubilis L.) Protein Hydrolysates and Their Fractionated Peptides. Maejo Int. J. Sci. Technol. 2021, 15, 13–26. [Google Scholar]
- Zhang, A.; Wang, K.; Liu, X.; Zhang, X. Isolation and Identification of Dipeptidyl Peptidase-IV Inhibitory Peptides from Sacha Inchi Meal. J. Sci. Food Agric. 2023, 103, 2926–2938. [Google Scholar] [CrossRef] [PubMed]
- Suwanangul, S.; Sangsawad, P.; Alashi, M.A.; Aluko, R.E.; Tochampa, W.; Chittrakorn, S.; Ruttarattanamongkol, K. Antioxidant Activities of Sacha Inchi (Plukenetia volubilis L.) Protein Isolate and Its Hydrolysates Produced with Different Proteases. Maejo Int. J. Sci. Technol. 2021, 15, 48–60. [Google Scholar]
- Zhan, Q.; Wang, Q.; Liu, Q.; Guo, Y.; Gong, F.; Hao, L.; Wu, H. The Antioxidant Activity of Protein Fractions from Sacha Inchi Seeds after a Simulated Gastrointestinal Digestion. LWT 2021, 145, 111356. [Google Scholar] [CrossRef]
- Shu, T.; Wang, K.; Zhang, X. Antioxidant and Hypoglycemic Activity of Sacha Inchi Meal Protein Hydrolysate. Appl. Sci. 2023, 13, 6528. [Google Scholar] [CrossRef]
- Torres-Sánchez, E.; Lorca-Alonso, I.; González-de la Fuente, S.; Hernández-Ledesma, B.; Gutiérrez, L.-F. Antioxidant Peptides from Sacha Inchi Meal: An In Vitro, Ex Vivo, and In Silico Approach. Foods 2024, 13, 3924. [Google Scholar] [CrossRef]
- Li, P.; Wen, J.; Ma, X.; Lin, F.; Jiang, Z.; Du, B. Structural, Functional Properties and Immunomodulatory Activity of Isolated Inca Peanut (Plukenetia volubilis L.) Seed Albumin Fraction. Int. J. Biol. Macromol. 2018, 118, 1931–1941. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-lacanal, C.; Boutrou, R.; Carrière, F. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar]
- Chaipoot, S.; Punfa, W.; Ounjaijean, S.; Phongphisutthinant, R.; Kulprachakarn, K.; Parklak, W.; Phaworn, L.; Rotphet, P.; Boonyapranai, K. Antioxidant, Anti-Diabetic, Anti-Obesity, and Antihypertensive Properties of Protein Hydrolysate and Peptide Fractions from Black Sesame Cake. Molecules 2023, 28, 211. [Google Scholar] [CrossRef] [PubMed]
- Paterson, S.; Fernández-Tomé, S.; Galvez, A.; Hernández-Ledesma, B. Evaluation of the Multifunctionality of Soybean Proteins and Peptides in Immune Cell Models. Nutrients 2023, 15, 1220. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sánchez, E.; Morato, E.; Hernández-Ledesma, B.; Gutiérrez, L.-F. Proteomic Analysis of the Major Alkali-Soluble Inca Peanut (Plukenetia volubilis) Proteins. Foods 2024, 13, 3275. [Google Scholar] [CrossRef]
- Mohd Rodhi, A.; Yap, P.G.; Olalere, O.A.; Gan, C.Y. Unveiling α-Amylase Inhibition: A Bioinformatics Perspective on Peptide Properties and Amino Acid Contributions. J. Mol. Struct. 2024, 1305, 137768. [Google Scholar] [CrossRef]
- Lear, S.; Cobb, S.L. Pep-Calc.Com: A Set of Web Utilities for the Calculation of Peptide and Peptoid Properties and Automatic Mass Spectral Peak Assignment. J. Comput. Aided. Mol. Des. 2016, 30, 271–277. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Krutz, N.L.; Limviphuvadh, V.; Lopata, A.L.; Gerberick, G.F.; Maurer-Stroh, S. AllerCatPro 2.0: A Web Server for Predicting Protein Allergenicity Potential. Nucleic Acids Res. 2022, 50, W36–W43. [Google Scholar] [CrossRef]
- Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the Improved Discovery and Design of Functional Peptides: Common Features of Diverse Classes Permit Generalized Prediction of Bioactivity. PLoS ONE 2012, 7, e45012. [Google Scholar] [CrossRef]
- Khatun, M.S.; Hasan, M.M.; Kurata, H. PreAIP: Computational Prediction of Anti-Inflammatory Peptides by Integrating Multiple Complementary Features. Front. Genet. 2019, 10, 129. [Google Scholar] [CrossRef]
- Manavalan, B.; Patra, M.C. MLCPP 2.0: An Updated Cell-Penetrating Peptides and Their Uptake Efficiency Predictor. J. Mol. Biol. 2022, 434, 167604. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Liu, Y.; Bi, H.; Zhou, X.; Wen, L.; Yang, B. Characterisation of Functional Pea Protein Hydrolysates and Their Immunomodulatory Activity. Int. J. Food Sci. Technol. 2024, 59, 3317–3330. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Millan-Linares, M.C.; Rivero-Pino, F.; Gonzalez-de la Rosa, T.; Villanueva, A.; Montserrat-de la Paz, S. Identification, Characterization, and Molecular Docking of Immunomodulatory Oligopeptides from Bioavailable Hempseed Protein Hydrolysates. Food Res. Int. 2024, 176, 113712. [Google Scholar] [CrossRef] [PubMed]
- BIOVIA Discovery Studio. Available online: https://www.3ds.com/support/ (accessed on 17 September 2024).
- Vilcacundo, R.; Martínez-Villaluenga, C.; Hernández-Ledesma, B. Release of Dipeptidyl Peptidase IV, α-Amylase and α-Glucosidase Inhibitory Peptides from Quinoa (Chenopodium quinoa Willd.) during in Vitro Simulated Gastrointestinal Digestion. J. Funct. Foods 2017, 35, 531–539. [Google Scholar] [CrossRef]
- Montoya-Rodríguez, A.; de Mejía, E.G.; Dia, V.P.; Reyes-Moreno, C.; Milán-Carrillo, J. Extrusion Improved the Anti-Inflammatory Effect of Amaranth (Maranthus hypochondriacus) Hydrolysates in LPS-Induced Human THP-1 Macrophage-like and Mouse RAW 264.7 Macrophages by Preventing Activation of NF-ΚB Signaling. Mol. Nutr. Food Res. 2014, 58, 1028–1041. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, M.; Sun, C.; Brennan, M.; Li, H.; Wang, G.; Lai, F.; Wu, H. Enzymatic Preparation of Immunomodulatory Hydrolysates from Defatted Wheat Germ (Triticum vulgare) Globulin. Int. J. Food Sci. Technol. 2016, 51, 2556–2566. [Google Scholar] [CrossRef]
- Ren, G.; Zhu, Y.; Shi, Z.; Li, J. Detection of Lunasin in Quinoa (Chenopodium quinoa Willd.) and the in Vitro Evaluation of Its Antioxidant and Anti-Inflammatory Activities. J. Sci. Food Agric. 2017, 97, 4110–4116. [Google Scholar] [CrossRef]
- de Mejia, E.G.; Dia, V.P. Lunasin and Lunasin-like Peptides Inhibit Inflammation through Suppression of NF-ΚB Pathway in the Macrophage. Peptides 2009, 30, 2388–2398. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, K.; Hendrie, C.; Liang, C.; Li, M.; Doherty-Kirby, A.; Lajoie, G. PEAKS: Powerful Software for Peptide de Novo Sequencing by Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2337–2342. [Google Scholar] [CrossRef]
- Su, N.; Yi, L.; He, J.; Ming, L.; Jambal, T.; Mijiddorj, B.; Maizul, B.; Enkhtuul, T.; Ji, R. Identification and Molecular Docking of a Novel Antidiabetic Peptide from Protamex-Camel Milk Protein Hydrolysates against α-Amylase and DPP-IV. Int. Dairy J. 2024, 152, 105884. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Kannan, A.; Hettiarachchy, N.; Marshall, M. Food Proteins and Peptides as Bioactive Agents. In Bioactive Food Progeins and Peptides-Applications in Human Health; Hettiarachchy, N.S., Sato, K., Marshall, M.R., Kannan, A., Eds.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2012; p. 348. ISBN 9781420093179. [Google Scholar]
- Larder, C.E.; Iskandar, M.M.; Kubow, S. Assessment of Bioavailability after in Vitro Digestion and First Pass Metabolism of Bioactive Peptides from Collagen Hydrolysates. Curr. Issues Mol. Biol. 2021, 43, 1592–1605. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.D.; Blagg, J.; Price, D.A.; Bailey, S.; Decrescenzo, G.A.; Devraj, R.V.; Ellsworth, E.; Fobian, Y.M.; Gibbs, M.E.; Gilles, R.W.; et al. Physiochemical Drug Properties Associated with in Vivo Toxicological Outcomes. Bioorg. Med. Chem. Lett. 2008, 18, 4872–4875. [Google Scholar] [CrossRef]
- Hameed, S.; Saleem, F.; Özil, M.; Baltaş, N.; Salar, U.; Ashraf, S.; Ul-Haq, Z.; Taha, M.; Khan, K.M. Indenoquinoxaline-Phenylacrylohydrazide Hybrids as Promising Drug Candidates for the Treatment of Type 2 Diabetes: In Vitro and in Silico Evaluation of Enzyme Inhibition and Antioxidant Activity. Int. J. Biol. Macromol. 2024, 263, 129517. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.; Bao, X.; Xiao, W.; Chen, G. Toll-like Receptor 4 (TLR4) Inhibitors: Current Research and Prospective. Eur. J. Med. Chem. 2022, 235, 114291. [Google Scholar] [CrossRef]
- Wang, Y.; Su, L.; Morin, M.D.; Jones, B.T.; Whitby, L.R.; Surakattula, M.M.R.P.; Huang, H.; Shi, H.; Choi, J.H.; Wang, K.W.; et al. TLR4/MD-2 Activation by a Synthetic Agonist with No Similarity to LPS. Proc. Natl. Acad. Sci. USA 2016, 113, E884–E893. [Google Scholar] [CrossRef]
- Manzoor, M.; Singh, J.; Gani, A. Exploration of Bioactive Peptides from Various Origin as Promising Nutraceutical Treasures: In Vitro, in Silico and in Vivo Studies. Food Chem. 2022, 373, 131395. [Google Scholar] [CrossRef]
- Achek, A.; Shah, M.; Seo, J.Y.; Kwon, H.-K.; Gui, X.; Shin, H.-J.; Cho, E.-Y.; Lee, B.S.; Kim, D.-J.; Lee, S.H.; et al. Linear and Rationally Designed Stapled Peptides Abrogate TLR4 Pathway and Relieve Inflammatory Symptoms in Rheumatoid Arthritis Rat Model. J. Med. Chem. 2019, 62, 6495–6511. [Google Scholar] [CrossRef]
- Cochet, F.; Facchini, F.A.; Zaffaroni, L.; Billod, J.-M.; Coelho, H.; Holgado, A.; Braun, H.; Beyaert, R.; Jerala, R.; Jimenez-Barbero, J.; et al. Novel Carboxylate-Based Glycolipids: TLR4 Antagonism, MD-2 Binding and Self-Assembly Properties. Sci. Rep. 2019, 9, 919. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, F.; Chen, H.; Zhang, T.; Yang, K.; Wang, Y.; Jiang, Z.; Rice, K.C.; Watkins, L.R.; Hutchinson, M.R.; et al. Dissecting the Innate Immune Recognition of Opioid Inactive Isomer (+)-Naltrexone Derived Toll-like Receptor 4 (TLR4) Antagonists. J. Chem. Inf. Model. 2018, 58, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Sestito, S.E.; Facchini, F.A.; Morbioli, I.; Billod, J.-M.; Martin-Santamaria, S.; Casnati, A.; Sansone, F.; Peri, F. Amphiphilic Guanidinocalixarenes Inhibit Lipopolysaccharide (LPS)- and Lectin-Stimulated Toll-like Receptor 4 (TLR4) Signaling. J. Med. Chem. 2017, 60, 4882–4892. [Google Scholar] [CrossRef] [PubMed]

| CPP e | Aller. d | Toxicity c | Distribution c | Absorption c | Drug-likeness c | PreAIP b | Ranks a | Peptide | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prob. | Class. | Skin | ROA | PPB | HIA | Caco-2 | Pfizer Rule | ||||
| 0.16 | Non-CPP | No ev. | 0.21 | 0.04 | 0.16 | 0.92 | −7.10 | Accepted | 0.61 | 0.92 | QCCDFMK |
| 0.40 | Non-CPP | No ev. | 0.48 | 0.19 | 0.31 | 1.00 | −6.90 | Accepted | 0.38 | 0.87 | HGGGGGGFGGGGFSR |
| 0.42 | Non-CPP | No ev. | 0.25 | 0.01 | 0.17 | 0.97 | −6.77 | Accepted | 0.66 | 0.86 | KCCDMMK |
| 0.22 | Non-CPP | No ev. | 0.12 | 0.45 | 0.23 | 0.99 | −6.97 | Accepted | 0.42 | 0.85 | PSPSLVWR |
| 0.12 | Non-CPP | No ev. | 0.59 | 0.01 | 0.20 | 0.97 | −7.52 | Accepted | 0.56 | 0.85 | EWGGGGCGGGGGVSSLR |
| 0.33 | Non-CPP | No ev. | 0.32 | 0.14 | 0.23 | 0.83 | −7.52 | Accepted | 0.42 | 0.84 | SGGFGGNFGNR |
| 0.72 | CPP | No ev. | 0.15 | 0.63 | 0.16 | 0.80 | −6.24 | Accepted | 0.38 | 0.83 | RHWLPR |
| 0.07 | Non-CPP | No ev. | 0.24 | 0.08 | 0.16 | 0.92 | −7.60 | Accepted | 0.45 | 0.81 | SDWPELLGR |
| 0.42 | Non-CPP | No ev. | 0.63 | 0.00 | 0.12 | 0.90 | −7.84 | Accepted | 0.40 | 0.79 | SGGGGGGLGSGGSLR |
| 0.02 | Non-CPP | No ev. | 0.14 | 0.36 | 0.15 | 0.86 | −6.87 | Accepted | 0.42 | 0.79 | YNLPMLR |
| 0.39 | Non-CPP | No ev. | 0.5 | 0.07 | 0.31 | 1.00 | −7.24 | Accepted | 0.32 | 0.79 | HGGGGGGFGGGGFDK |
| 0.70 | CPP | No ev. | 0.09 | 0.13 | 0.19 | 0.89 | −7.63 | Accepted | 0.42 | 0.76 | SDTLFFAR |
| 0.11 | Non-CPP | No ev. | 0.19 | 0.47 | 0.20 | 0.94 | −7.00 | Accepted | 0.43 | 0.75 | LLFPMSR |
| Bound Sites | Affinity | Peptide Sequence |
|---|---|---|
| Trp58, Trp59 **, Thr163, Asp197 *, Lys200, His201, Ile235, Asp300 *, Ala307, Asp356 | −7.4 | LHALEDPNR |
| Trp59 **, Tyr62 **, Tyr151 **, Leu162 Leu165, Ile168, Ala198, His201, Ile235, Glu240, Asp300 *, Arg303, His305 | −9.2 | RHWLPR |
| Thr163, Leu165, Ile 235, Leu237, Glu240, Lys257, Asp300 *, Asp356 | −7.0 | LPTQSWKVPR |
| Ile148, Tyr151**, Leu162, Thr163, Lys200, Glu233 *, Asp300 *, His305, Asp356 | −8.1 | RATVSLPR |
| Trp59 **, His101, Tyr151 **, Leu162, Thr163, Lys200, His201, Ile235, Glu240, His299, Asp300 *, Hys305, Gly306 | −7.6 | LSASGHVVLR |
| - | n.d | QNSNLQKSLSDAEQR |
| Trp58, Trp59, Thr163, Lys200, Ile235, Gly238, Glu240, Asp300 *, Arg303, His305, Gly306 | −7.8 | QLSNLEQSLSDAEQR |
| - | n.d | LQSNLQKSLSDAEQR |
| - | n.d | SPSNLQKSLSDAEQR |
| - | n.d | KNSNLQQSLSDAEQR |
| Neutral e (%) | Basic d (%) | Acidic c (%) | Hydrophobic b (%) | MW a | Length a | Affinity | Peptide |
|---|---|---|---|---|---|---|---|
| 42.86 | 14.29 | 14.29 | 28.57 | 874.07 | 7 | −5.50 | QCCDFMK |
| 73.33 | 13.33 | 0.00 | 13.33 | 1263.28 | 15 | −8.50 | HGGGGGGFGGGGFSR |
| 28.57 | 28.57 | 14.29 | 28.57 | 858.14 | 7 | −4.80 | KCCDMMK |
| 25.00 | 12.50 | 0.00 | 62.50 | 941.08 | 8 | −7.60 | PSPSLVWR |
| 70.59 | 5.88 | 5.88 | 17.65 | 1492.58 | 17 | −6.80 | EWGGGGCGGGGGVSSLR |
| 72.73 | 9.09 | 0.00 | 18.18 | 1069.09 | 11 | −6.90 | SGGFGGNFGNR |
| 0.00 | 50.00 | 0.00 | 50.00 | 864.01 | 6 | −7.80 | RHWLPR |
| 22.22 | 11.11 | 22.22 | 44.44 | 1072.17 | 9 | −7.30 | SDWPELLGR |
| 81.25 | 6.25 | 0.00 | 12.50 | 1175.21 | 15 | −6.50 | SGGGGGGLGSGGSLR |
| 28.57 | 14.29 | 0.00 | 57.14 | 906.11 | 7 | −7.80 | YNLPMLR |
| 66.67 | 13.33 | 6.67 | 13.33 | 1263.28 | 15 | −6.40 | HGGGGGGFGGGGFDK |
| 25.00 | 12.50 | 12.50 | 50.00 | 956.05 | 8 | −7.80 | SDTLFFAR |
| 14.29 | 14.29 | 0.00 | 71.43 | 863.08 | 7 | −7.60 | LLFPMSR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Sánchez, E.; Martínez-Villaluenga, C.; Paterson, S.; Hernández-Ledesma, B.; Gutiérrez, L.-F. Antidiabetic and Immunomodulatory Properties of Peptide Fractions from Sacha Inchi Oil Press-Cake. Foods 2025, 14, 1231. https://doi.org/10.3390/foods14071231
Torres-Sánchez E, Martínez-Villaluenga C, Paterson S, Hernández-Ledesma B, Gutiérrez L-F. Antidiabetic and Immunomodulatory Properties of Peptide Fractions from Sacha Inchi Oil Press-Cake. Foods. 2025; 14(7):1231. https://doi.org/10.3390/foods14071231
Chicago/Turabian StyleTorres-Sánchez, Erwin, Cristina Martínez-Villaluenga, Samuel Paterson, Blanca Hernández-Ledesma, and Luis-Felipe Gutiérrez. 2025. "Antidiabetic and Immunomodulatory Properties of Peptide Fractions from Sacha Inchi Oil Press-Cake" Foods 14, no. 7: 1231. https://doi.org/10.3390/foods14071231
APA StyleTorres-Sánchez, E., Martínez-Villaluenga, C., Paterson, S., Hernández-Ledesma, B., & Gutiérrez, L.-F. (2025). Antidiabetic and Immunomodulatory Properties of Peptide Fractions from Sacha Inchi Oil Press-Cake. Foods, 14(7), 1231. https://doi.org/10.3390/foods14071231










