Mechanism of Textural Reorganization in Silkworm Chrysalis and Pea Protein Extrusion: Structural Evolution and Quality Characteristic
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Formulation of Raw Materials
2.3. High-Moisture Extrusion Process
2.4. Water-Holding Capacity (WHC)
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Color Testing
2.7. Texture Properties
2.8. Scanning Electron Microscope (SEM)
2.9. Rheological Properties
2.9.1. Construction of the BP Neural Network
2.9.2. Small Amplitude Oscillation Shear Stress Measurement (SAOS)
2.10. Thermal Characteristics Test
2.11. Amino Acid Test
2.12. Data Analysis
3. Results and Discussion
3.1. Apparent Characteristics and SEM Analysis
3.2. Color Analysis
3.3. Texture Analysis
3.4. FTIR Analysis
3.5. Rheological Analysis
3.5.1. Steady Shear Test Analysis
3.5.2. Small Amplitude Oscillation Shear Stress Measurement Analysis
3.6. Water-Holding-Capacity Analysis
3.7. Amino Acid Analysis
3.8. Thermal Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCP | Silkworm Chrysalis Protein |
| PPI | Pea Protein Isolate |
| GHG | Global Greenhouse Gas |
| HME | High-Moisture Extrusion |
| WHC | Water Holding Capacity |
| FTIR | Fourier Transform Infrared |
| SEM | Scanning electron microscope |
References
- Laura, G.; Gabriele, A.; Paolo, B.; Dalle Zotte, A.; Danieli, P.P.; De Angelis, A.; Fortina, R.; Marino, R.; Parisi, G.; Piccolo, G.; et al. Insect and fish by-products as sustainable alternatives to conventional animal proteins in animal nutrition. Ital. J. Anim. Sci. 2020, 19, 360–372. [Google Scholar] [CrossRef]
- Pam, B.I.; Lasika, L.S.; Alicia, S.; Brackenridge, A. Protein demand: Review of plant and animal proteins used in alternative protein product development and production. Anim. Front. 2020, 10, 53–63. [Google Scholar] [CrossRef]
- Havlík, P.; Valin, H.; Herrero, M.; Obersteiner, M.; Schmid, E.; Rufino, M.C.; Mosnier, A.; Thornton, P.K.; Böttcher, H.; Conant, R.T.; et al. Climate change mitigation through livestock system transitions. Proc. Natl. Acad. Sci. USA 2014, 111, 3709–3714. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Liu, Z.; Zhi, L.; Jiao, B.; Hu, H.; Ma, X.; Agyei, D.; Shi, A. Plant protein-based emulsifiers: Mechanisms, techniques for emulsification enhancement and applications. Food Hydrocoll. 2023, 144, 109008. [Google Scholar] [CrossRef]
- Lee, H.J.; Yong, H.I.; Kim, M.; Choi, Y.-S.; Jo, C. Status of meat alternatives and their potential role in the future meat market—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1533. [Google Scholar] [CrossRef]
- Sun, D.; Wu, M.; Zhou, C.; Wang, B. Transformation of high moisture extrusion on pea protein isolate in melting zone during: From the aspects of the rheological property, physicochemical attributes and modification mechanism. Food Hydrocoll. 2022, 133, 108016. [Google Scholar] [CrossRef]
- Wu, M.; Sun, D.; Zhang, T.; Zhou, C.; Zhang, B. Study on the Function of Conveying, Kneading Block and Reversing Elements on the Mixing Efficiency and Dispersion Effect Inside the Barrel of an Extruder with Numerical Simulation. Foods 2023, 12, 3503. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, J.; Sun, C.; Zhao, Y.; Cao, Y.; Lu, W.; Zhang, Y.; Fang, Y. Multihole nozzle-mediated high-moisture extrusion of soy proteins into fiber-rich structures. Food Hydrocoll. 2024, 151, 109819. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, X.; Cheng, Y.; Cui, B.; El-Aty, A.M.A. Impact of high moisture contents on the structure and functional properties of pea protein isolate during extrusion. Food Hydrocoll. 2022, 127, 107508. [Google Scholar] [CrossRef]
- Diaz, J.M.R.; Kantanen, K.; Edelmann, J.M.; Suhonen, H.; Sontag-Strohm, T.; Jouppila, K.; Piironen, V. Fibrous meat analogues containing oat fiber concentrate and pea protein isolate: Mechanical and physicochemical characterization. Innov. Food Sci. Emerg. Technol. 2022, 77, 102954. [Google Scholar] [CrossRef]
- Xia, X.; Li, Y.; Qian, H.; Zhang, H.; Wang, L. Effects of modification treatments on structural characteristics and functional properties of pea protein. J. Food Sci. Technol. 2021, 39, 32–38+48. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, B.; Zhou, C.; Wang, B.; Wu, M. Study on high moisture extruded pea protein isolate based on acid-induced process: Physicochemical properties, conformational changes and fibrous structure mechanism. Food Hydrocoll. 2023, 141, 108746. [Google Scholar] [CrossRef]
- Sun, D.; Wu, M.; Zhang, T.; Wei, D.; Zhou, C.; Shang, N. Conformational changes and physicochemical attributes of texturized pea protein isolate-konjac gum: With a new perspective of residence duration time during extrusion. Food Res. Int. 2023, 165, 112500. [Google Scholar] [CrossRef] [PubMed]
- Day, L. Protein: Food Sources. Encycl. Food Health 2016, 530–537. [Google Scholar] [CrossRef]
- Grasso, S.; Jaworska, S. Part Meat and Part Plant: Are Hybrid Meat Products Fad or Future? Foods 2020, 9, 1888. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Xiong, Y.; Jiang, J. The interplay of muscle and pea proteins in low-salt gels: An insight into in situ structure formation in hybrid meat alternatives. Food Chem. 2024, 455, 139870. [Google Scholar] [CrossRef]
- Goes, E.S.d.R.; de Souza, M.L.R.; Campelo, D.A.V.; Yoshida, G.M.; Xavier, T.O.; de Moura, L.B.; Monteiro, A.R.G. Extruded snacks with the addition of different fish meals. Food Sci. Technol. 2015, 35, 683–689. [Google Scholar] [CrossRef]
- Pöri, P.; Aisala, H.; Liu, J.; Lille, M.; Sozer, N. Structure, texture, and sensory properties of plant-meat hybrids produced by high-moisture extrusion. LWT 2023, 173, 114345. [Google Scholar] [CrossRef]
- Smetana, S.; Larki, N.A.; Pernutz, C.; Franke, K.; Bindrich, U.; Toepfl, S.; Heinz, V. Structure design of insect-based meat analogs with high-moisture extrusion. J. Food Eng. 2018, 229, 83–85. [Google Scholar] [CrossRef]
- García-Segovia, P.; Igual, M.; Noguerol, A.T.; Martínez-Monzó, J. Use of insects and pea powder as alternative protein and mineral sources in extruded snacks. Eur. Food Res. Technol. 2020, 246, 703–712. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Q.; Kaplan, D.L.; Wang, Q. High-moisture extruded protein fiber formation toward plant-based meat substitutes applications: Science, technology, and prospect. Trends Food Sci. Technol. 2022, 128, 202–216. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Rothman, J.M.; Pontzer, H.; Simpson, S.J. Macronutrient contributions of insects to the diets of hunter–gatherers: A geometric analysis. J. Hum. Evol. 2014, 71, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Halloran, A.; Roos, N.; Eilenberg, J.; Cerutti, A.; Bruun, S. Life cycle assessment of edible insects for food protein: A review. Agron. Sustain. Dev. 2016, 36, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.Y.; Lim, H.Y.; Sofian-Seng, N.-S.; Mustapha, W.A.W.; Razali, N.S.M. Physicochemical Characteristics and Microbiological Quality of Silkworm (Bombyx mori) Larval and Pupae Powder: Comparative Study. Sains Malays. 2022, 51, 547–558. [Google Scholar] [CrossRef]
- Wu, X.; He, K.; Velickovic, T.C.; Liu, Z. Nutritional, functional, and allergenic properties of silkworm pupae. Food Sci. Nutr. 2021, 9, 4655–4665. [Google Scholar] [CrossRef]
- Herman, R.A.; Yan, C.H.; Wang, J.Z.; Xun, X.M.; Wu, C.K.; Li, Z.N.; Ayepa, E.; You, S.; Gong, L.C.; Wang, J. Insight into the silkworm pupae: Modification technologies and functionality of the protein and lipids. Trends Food Sci. Technol. 2022, 129, 408–420. [Google Scholar] [CrossRef]
- Park, Y.S.; Choi, Y.S.; Hwang, K.E.; Kim, T.-K.; Lee, C.-W.; Shin, D.-M.; Han, S.G. Physicochemical properties of meat batter added with edible silkworm pupae (Bombyx mori) and transglutaminas. Korean J. Food Sci. Anim. Resour. 2017, 37, 351. [Google Scholar] [CrossRef]
- Torres, K.S.; Sampaio, R.F.; Ferreira, T.H.B.; Argondoña, E.J.S. Development of cookie enriched with silkworm pupae (Bombyx mori). J. Food Meas. Charact. 2022, 16, 1540–1548. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Kaplan, D.L.; Wang, Q. Protein-amylose/amylopectin molecular interactions during high-moisture extruded texturization toward plant-based meat substitutes applications. Food Hydrocoll. 2022, 127, 107559. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Meng, S.; Wang, Q. Rheological properties of pea protein isolate-amylose/amylopectin mixtures and the application in the high-moisture extruded meat substitutes. Food Hydrocoll. 2021, 117, 106732. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, M.; Sun, D.; Wei, W.; Yu, H.; Zhang, T. Twin-screw extrusion of oat: Evolutions of rheological behavior, thermal properties and structures of extruded oat in different extrusion zones. Foods 2022, 11, 2206. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, M.; Xie, J.; Su, E.; Wan, Z.; Sagis, L.M.; Yang, X. Large amplitude oscillatory shear (laos) for nonlinear rheological behavior of heterogeneous emulsion gels made from natural supramolecular gelators. Food Res. Int. 2021, 140, 110076. [Google Scholar] [CrossRef] [PubMed]
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Saari, N. Texturized mung bean protein as a sustainable food source: Effects of extrusion on its physical, textural and protein quality. Innov. Food Sci. Emerg. Technol. 2021, 67, 102591. [Google Scholar] [CrossRef]
- Zha, F.; Yang, Z.; Rao, J.; Chen, B. Conjugation of pea protein isolate via maillard-driven chemistry with saccharide of diverse molecular mass: Molecular interactions leading to aggregation or glycation. J. Agric. Food Chem. 2020, 68, 10157–10166. [Google Scholar] [CrossRef]
- Ma, X.; Gao, J.; Tong, P.; Li, X.; Chen, H. Tracking the behavior of Maillard browning in lysine/arginine–sugar model systems under high hydrostatic pressure. J. Sci. Food Agric. 2017, 97, 5168–5175. [Google Scholar] [CrossRef]
- Wu, X.; Yang, J.; Mumby, W.; Zhang, Y.; Zhang, Y.; Wang, C.; Chen, X.; Suo, H.; Song, J. Silkworm pupa protein and its peptides: Preparation, biological activity, applications in foods, and advantages. Trends Food Sci. Technol. 2023, 139, 104129. [Google Scholar] [CrossRef]
- Bartkuvienė, I.; Keršienė, M.; Petrikaitė, V.; Leskauskaitė, D. Modulation of pea protein isolate hydrogels by adding kappa-carrageenan: Gelling properties and formation mechanism. Int. J. Food Sci. Technol. 2024, 59, 6598–6610. [Google Scholar] [CrossRef]
- Wang, W.; Wang, N.; Liu, C.; Jin, J. Effect of silkworm pupae peptide on the fermentation and quality of yogurt. J. Food Process. Preserv. 2017, 41, e12893. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.; Jeong, Y.J.; Choi, I.; Han, J. Impact of interactions between soy and pea proteins on quality characteristics of high-moisture meat analogues prepared via extrusion cooking process. Food Hydrocoll. 2023, 139, 108567. [Google Scholar] [CrossRef]
- Ferawati, F.; Zahari, I.; Barman, M.; Hefni, M.; Ahlström, C.; Witthöft, C.; Östbring, K. High-moisture meat analogues produced from yellow pea and faba bean protein isolates/concentrate: Effect of raw material composition and extrusion parameters on texture properties. Foods 2021, 10, 843. [Google Scholar] [CrossRef]
- Sun, X.; Sun, Z.; Guo, Y.; Zhao, J.; Zhao, J.; Ge, X.; Shen, H.; Zhang, Q.; Yan, W. Effect of twin-screw extrusion combined with cold plasma on multi-scale structure, physicochemical properties, and digestibility of potato starches. Innov. Food Sci. Emerg. Technol. 2021, 74, 102855. [Google Scholar] [CrossRef]
- Gao, K.; Zha, F.; Yang, Z.; Rao, J.; Chen, B. Structure characteristics and functionality of water-soluble fraction from high-intensity ultrasound treated pea protein isolate. Food Hydrocoll. 2022, 125, 107409. [Google Scholar] [CrossRef]
- Shrestha, S.; van’t Hag, L.; Haritos, V.; Dhital, S. Comparative study on molecular and higher-order structures of legume seed protein isolates: Lentil, mungbean and yellow pea. Food Chem. 2023, 411, 135464. [Google Scholar] [CrossRef]
- Gong, J.; Wang, L.; Wu, J.; Yuan, Y.; Mu, R.-J.; Du, Y.; Wu, C.; Pang, J. The rheological and physicochemical properties of a novel thermosensitive hydrogel based on konjac glucomannan/gum tragacanth. LWT 2019, 100, 271–277. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, F.; Xue, W.; Lee, L. FTIR spectra studies on the secondary structures of 7S and 11S globulins from soybean proteins using AOT reverse micellar extraction. Food Hydrocoll. 2008, 22, 568–575. [Google Scholar] [CrossRef]
- Beck, S.M.; Knoerzer, K.; Sellahewa, J.; Emin, M.A.; Arcot, J. Effect of different heat-treatment times and applied shear on secondary structure, molecular weight distribution, solubility and rheo-logical properties of pea protein isolate as investigated by capillary rheometry. J. Food Eng. 2017, 208, 66–76. [Google Scholar] [CrossRef]
- Xia, S.; Xue, Y.; Xue, C.; Jiang, X.; Li, J. Structural and rheological properties of meat analogues from Haematococcus pluvialis residue-pea protein by high moisture extrusion. LWT 2021, 154, 112756. [Google Scholar] [CrossRef]
- Kadival, A.; Mitra, J.; Machavaram, R.; Kaushal, M. 3D printing of rice starch incorporated peanut protein isolate paste: Rheological characterization and simulation of flow properties. Innov. Food Sci. Emerg. Technol. 2024, 94, 103669. [Google Scholar] [CrossRef]
- Li, D.; Zhang, T.; Gao, F.; Zhou, C.; Sun, D.; Gao, Y.; Wu, M. The study of numerical simulation and texture of soybean protein based on high moisture extrusion with different screw elements. Innov. Food Sci. Emerg. Technol. 2024, 92, 103560. [Google Scholar] [CrossRef]
- Wang, T.; Kaur, L.; Furuhata, Y.; Aoyama, H.; Singh, J. 3D printing of textured soft hybrid meat analogues. Foods 2022, 11, 478. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, X.; Scholten, E. Rheological behavior of emulsion gels stabilized by zein/tannic acid complex particles. Food Hydrocoll. 2018, 77, 363–371. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, J.; Han, X.; Li, Q.; Liao, X.; Zhao, J. Development and characterization of fibrous high moisture extrudates based on pea protein isolate and whey protein. J. Sci. Food Agric. 2024, 105, 760–768. [Google Scholar] [CrossRef]
- Vimbai, M.; Simiso, D.; Mathew, M.N. Chemical, structural and thermal properties of Gonometa postica silk fibroin, a potential biomaterial. Int. J. Biol. Macromol. 2013, 52, 305–311. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, J.; Wang, S.; Qi, M.; Yue, M.; Zhang, S.; Song, J.; Wang, C.; Zhang, D.; Wang, X.; et al. High moisture extrusion of pea protein: Effect of l-cysteine on product properties and the process forming a fibrous structure. Food Hydrocoll. 2022, 129, 107633. [Google Scholar] [CrossRef]
- Dehnad, D.; Emadzadeh, B.; Ghorani, B.; Rajabzadeh, G. High hydrostatic pressure (HHP) as a green technology opens up a new possibility for the fabrication of electrospun nanofibers: Part I-improvement of soy protein isolate properties by HHP. Food Hydrocoll. 2023, 140, 108659. [Google Scholar] [CrossRef]
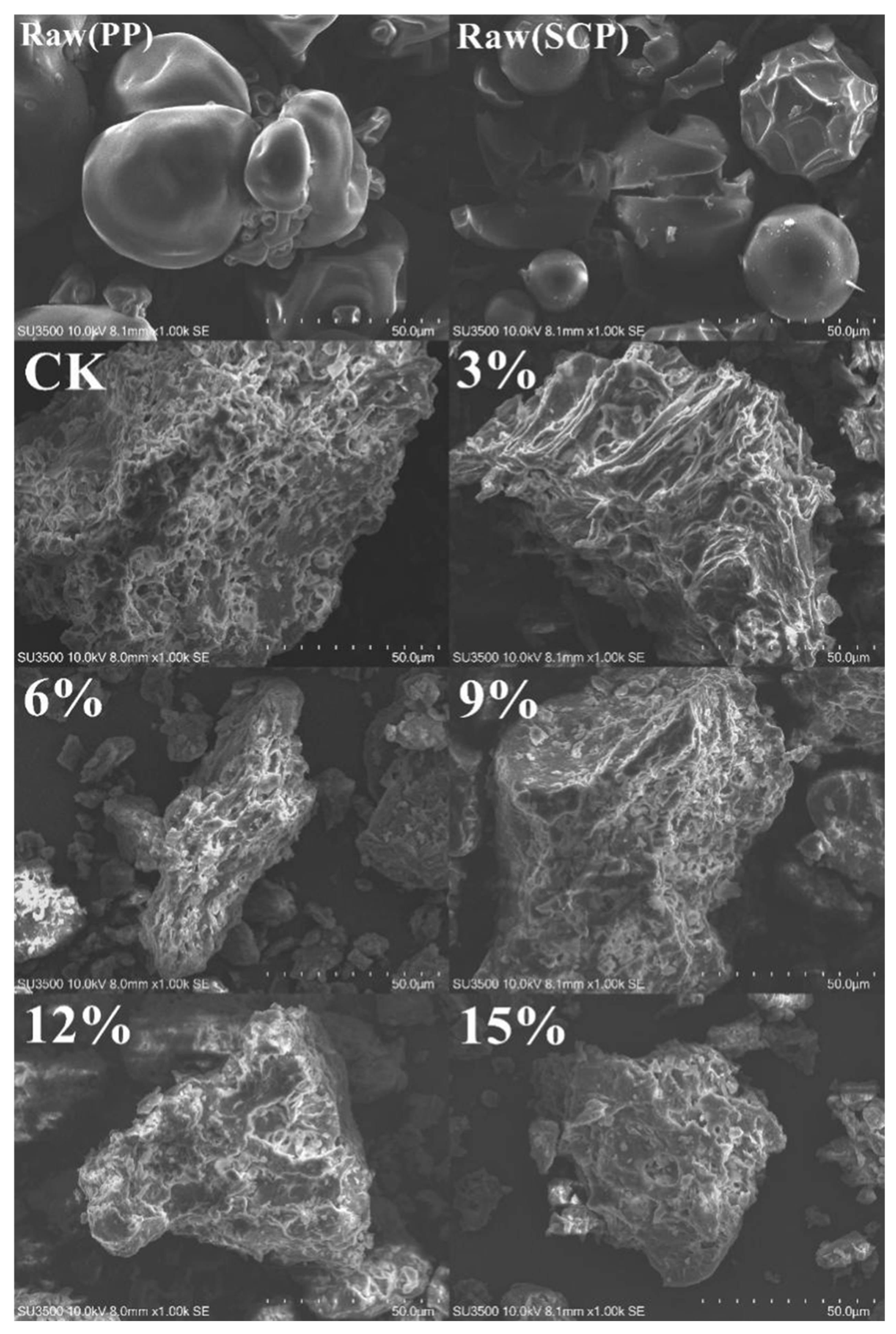

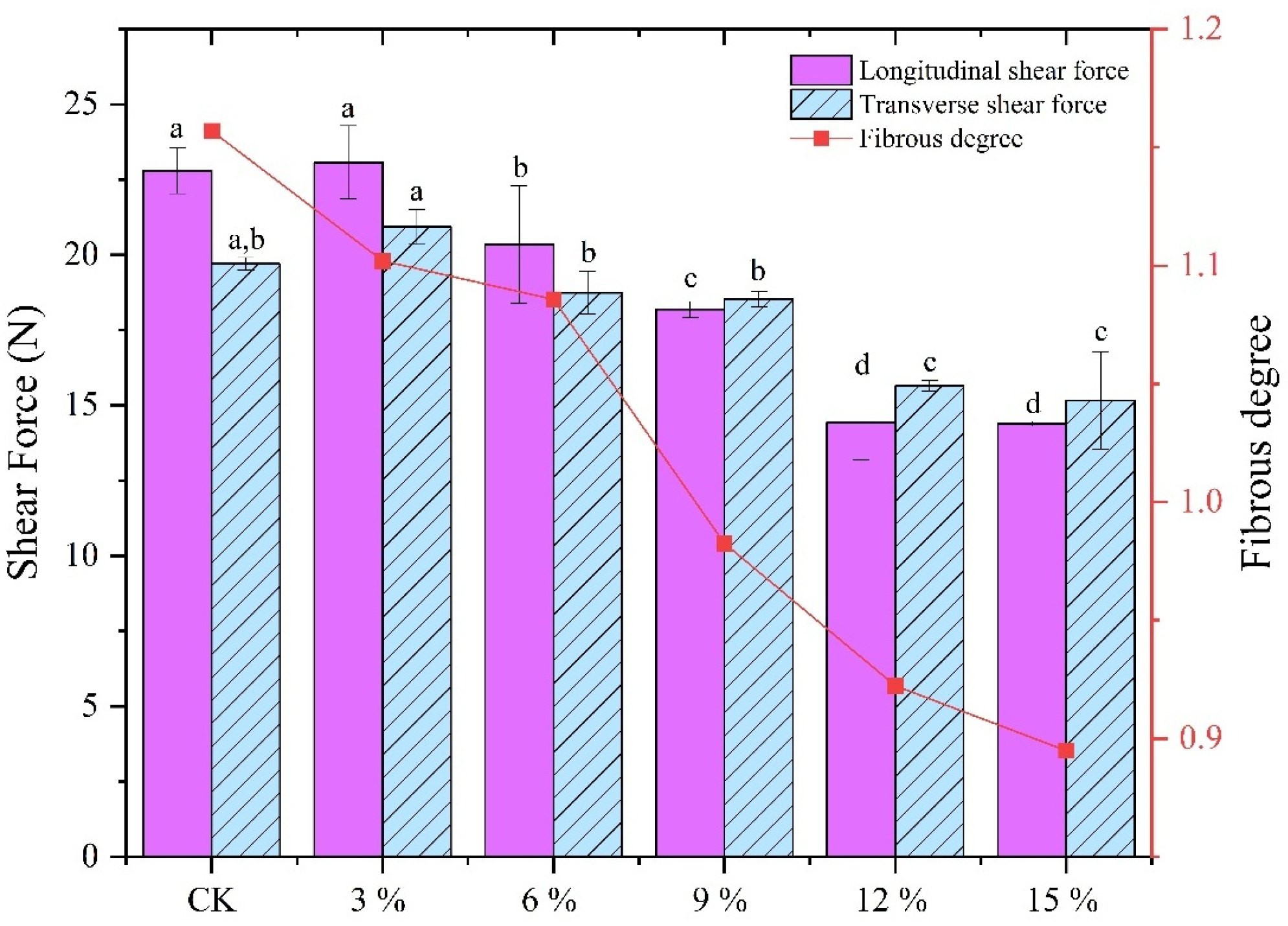
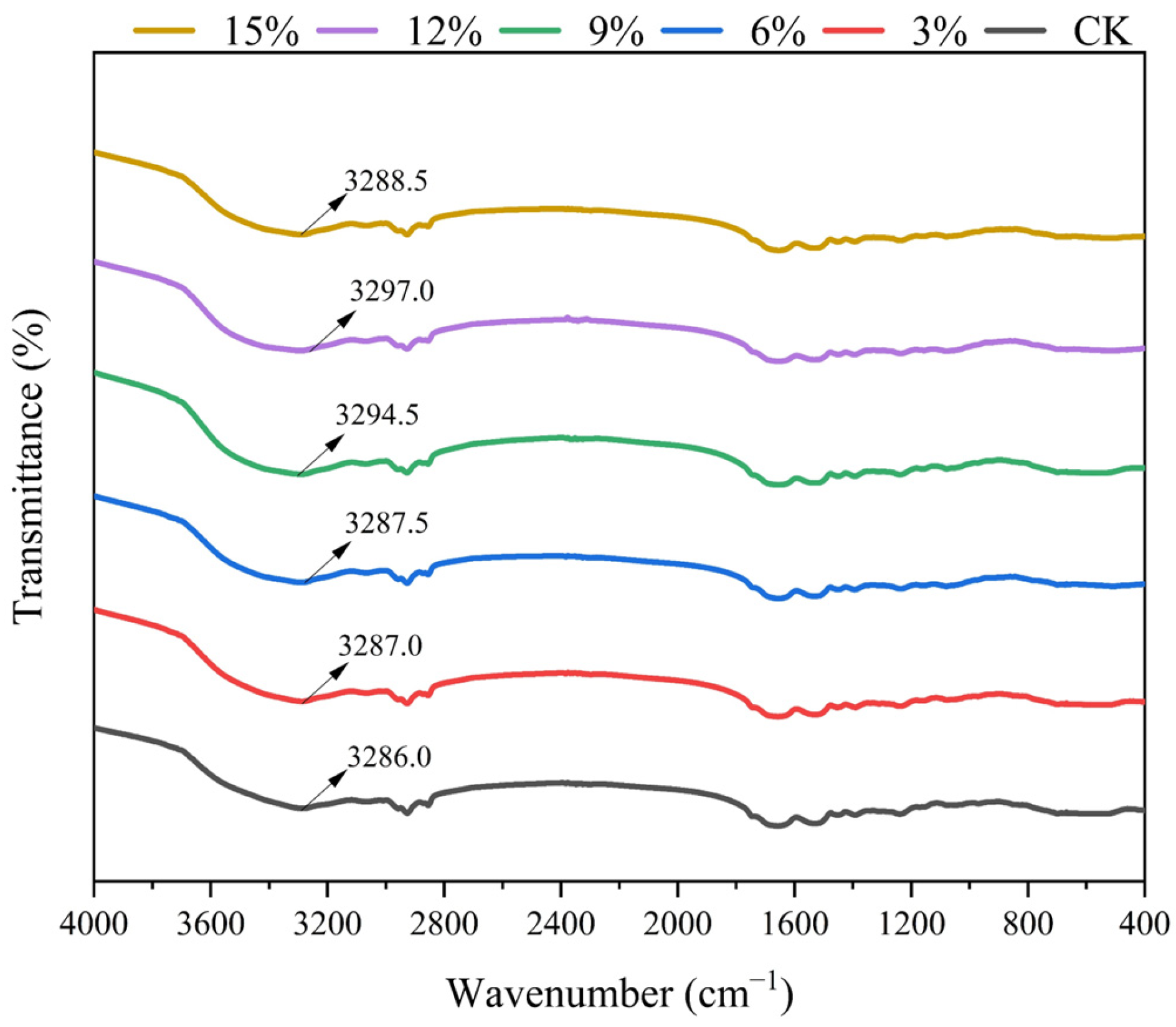


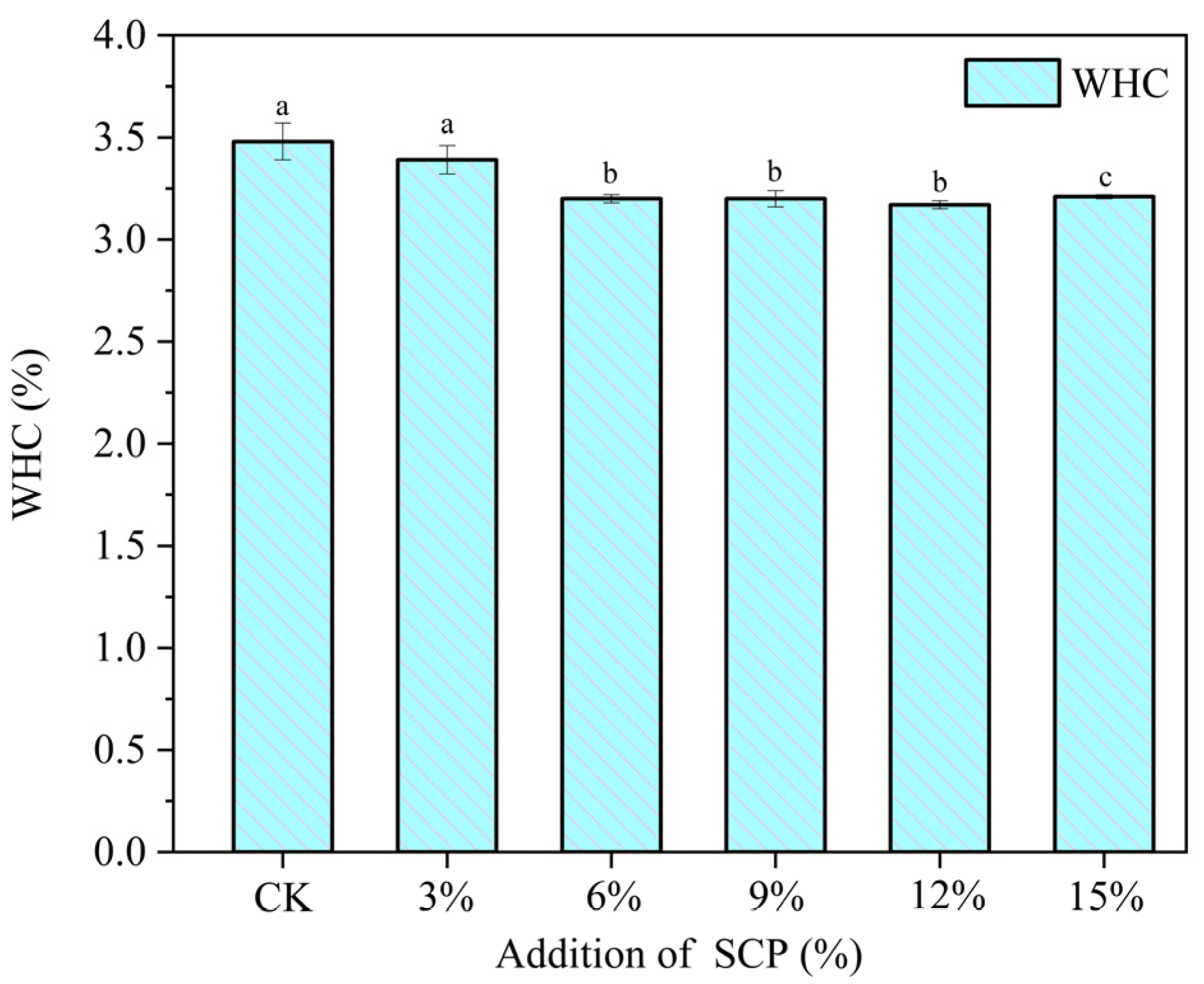
| Addition of SCP | L* | a* | b* |
|---|---|---|---|
| % | |||
| CK | 64.26 ± 1.51 e | 1.44 ± 0.31 b | 17.02 ± 0.72 b |
| 3 | 65.60 ± 1.98 e | 0.01 ± 0.44 c | 17.06 ± 1.12 b,c |
| 6 | 68.30 ± 1.60 d | 0.11 ± 0.38 c | 17.71 ± 0.55 b,c |
| 9 | 71.31 ± 1.86 c | −0.09 ± 0.49 c | 17.25 ± 1.14 b,c |
| 12 | 71.29 ± 1.02 c | −0.07 ± 0.46 c | 17.20 ± 1.74 b,c |
| 15 | 71.61 ± 2.79 c | −1.02 ± 0.87 d | 16.15 ± 0.58 c |
| Raw PPI | 80.24 ± 0.02 b | 2.45 ± 0.03 a | 19.54 ± 0.03 a |
| Raw SCP | 93.39 ± 0.01 a | −2.87 ± 0.01 e | 8.86 ± 0.02 d |
| Addition of SCP | Hardness | Adhesiveness | Resilience | Springiness | Gumminess | Chewiness |
|---|---|---|---|---|---|---|
| % | N | N·s | % | % | ||
| CK | 97.41 ± 1.13 a | 0.92 ± 0.23 a | 36.33 ± 0.44 a | 92.16 ± 1.20 a | 75.36 ± 1.19 a | 69.45 ± 1.91 a |
| 3 | 97.12 ± 3.95 a | 1.10 ± 0.34 a | 35.57 ± 1.06 a,b | 89.64 ± 2.77 a | 74.26 ± 2.10 a | 66.61 ± 3.91 a |
| 6 | 89.11 ± 0.21 b | 1.02 ± 0.12 a | 34.56 ± 0.27 b,c | 91.75 ± 0.60 a | 67.16 ± 0.71 b | 61.61 ± 0.48 b |
| 9 | 84.39 ± 1.39 c | 0.96 ± 0.10 a | 34.52 ± 0.77 b,c | 90.48 ± 1.07 a | 63.21 ± 1.09 c | 57.19 ± 1.48 c |
| 12 | 80.06 ± 0.90 d | 0.49 ± 0.14 b | 33.43 ± 0.58 c | 92.26 ± 1.30 a | 59.27 ± 1.01 d | 54.68 ± 1.06 c |
| 15 | 52.57 ± 0.97 e | 0.11 ± 0.02 c | 30.72 ± 0.82 d | 89.87 ± 0.43 a | 36.43 ± 0.98 e | 32.75 ± 0.86 d |
| Addition of SCP | Random Coil | β-Sheets | α-Helix | β-Turns | α-Helix/β-Sheets |
|---|---|---|---|---|---|
| % | % | % | % | % | |
| CK | 2.41 ± 0.01 e | 58.92 ± 0.04 d | 24.42 ± 0.03 a | 14.26 ± 0.01 a | 0.4146 ± 0.0849 a |
| 3 | 4.35 ± 0.21 a | 63.90 ± 0.34 a | 21.19 ± 0.02 e | 10.57 ± 0.16 e | 0.3315 ± 0.1414 e |
| 6 | 3.33 ± 0.03 c | 60.66 ± 0.06 b | 23.03 ± 0.01 d | 12.99 ± 0.04 c | 0.3796 ± 0.0354 d |
| 9 | 3.52 ± 0.01 c | 60.74 ± 0.06 b | 23.08 ± 0.01 d | 12.61 ± 0.01 d | 0.3799 ± 0.0212 d |
| 12 | 2.95 ± 0.02 d | 59.40 ± 0.18 c | 23.85 ± 0.13 b | 13.81 ± 0.04 b | 0.4015 ± 0.3394 b |
| 15 | 3.57 ± 0.06 b | 60.56 ± 0.06 b | 23.31 ± 0.06 c | 12.56 ± 0.05 d | 0.3849 ± 0.1273 c |
| Addition of SCP % | Power Law Equation: τ = K·γ n Fitting Results | ||
|---|---|---|---|
| K (Pa·sn) | n | R2 | |
| CK | 602.06 ± 14.19 a | 0.25 ± 0.01 c | 0.98 |
| 3 | 307.73 ± 7.31 b | 0.20 ± 0.01 c | 0.96 |
| 6 | 20.45 ± 3.05 c | 0.64 ± 0.04 a,b | 0.95 |
| 9 | 7.13 ± 0.35 e | 0.72 ± 0.01 a | 0.99 |
| 12 | 3.33 ± 0.21 f | 0.67 ± 0.02 a,b | 0.99 |
| 15 | 14.89 ± 1.56 d | 0.57 ± 0.03 b | 0.97 |
| Raw SCP | CK | 3% SCP | 6% SCP | 9% SCP | 12% SCP | 15% SCP | |
|---|---|---|---|---|---|---|---|
| Aspartic acid | 5.26 ± 0.08 e | 8.66 ± 0.07 a | 8.48 ± 0.06 a,b | 8.34 ± 0.01 b,c | 8.24 ± 0.07 c,d | 8.14 ± 0.16 c,d | 8.07 ± 0.03 d |
| Threonine | 1.51 ± 0.02 e | 2.69 ± 0.02 a | 2.63 ± 0.02 a,b | 2.59 ± 0.01 b,c | 2.55 ± 0.02 c,d | 2.51 ± 0.05 d | 2.49 ± 0.02 d |
| Serine | 2.88 ± 0.04 d | 3.73 ± 0.06 a | 3.67 ± 0.02 a,b | 3.62 ± 0.01 b,c | 3.60 ± 0.01 b,c | 3.56 ± 0.07 b,c | 3.55 ± 0.01 c |
| Glutamic acid | 9.22 ± 0.09 d | 13.58 ± 0.16 a | 13.40 ± 0.11 a,b | 13.22 ± 0.01 b,c | 13.11 ± 0.08 b,c | 12.99 ± 0.25 c | 12.93 ± 0.07 c |
| Proline | 12.28 ± 0.05 a | 3.19 ± 0.03 g | 3.51 ± 0.04 f | 3.82 ± 0.02 e | 4.00 ± 0.01 d | 4.30 ± 0.07 c | 4.56 ± 0.04 b |
| Glycine | 24.23 ± 0.05 a | 2.99 ± 0.03 g | 3.58 ± 0.03 f | 4.23 ± 0.02 e | 4.71 ± 0.05 d | 5.28 ± 0.10 c | 5.84 ± 0.02 b |
| Alanine | 8.82 ± 0.02 a | 3.27 ± 0.03 g | 3.44 ± 0.03 f | 3.62 ± 0.01 e | 3.74 ± 0.03 d | 3.92 ± 0.08 c | 4.09 ± 0.02 b |
| Cystine | 0.04 ± 0.02 c | 0.63 ± 0.01 a | 0.63 ± 0.02 a | 0.61 ± 0.02 a | 0.56 ± 0.01 b | 0.57 ± 0.02 b | 0.53 ± 0.01 b |
| Valine | 2.08 ± 0.05 e | 3.87 ± 0.04 a | 3.80 ± 0.03 a,b | 3.73 ± 0.01 b,c | 3.68 ± 0.04 c,d | 3.64 ± 0.07 c,d | 3.58 ± 0.02 d |
| Methionine | 0.50 ± 0.03 e | 0.67 ± 0.01 a | 0.67 ± 0.01 a | 0.67 ± 0.01 a,b | 0.65 ± 0.01 c | 0.66 ± 0.01 b,c | 0.64 ± 0.01 d |
| Isoleucine | 1.29 ± 0.03 f | 3.68 ± 0.04 a | 3.59 ± 0.04 a,b | 3.50 ± 0.01 b,c | 3.43 ± 0.04 c,d | 3.38 ± 0.07 d | 3.27 ± 0.02 e |
| Leucine | 2.47 ± 0.05 e | 6.43 ± 0.06 a | 6.26 ± 0.06 b | 6.12 ± 0.03 b,c | 5.99 ± 0.05 c,d | 5.89 ± 0.11 d,e | 5.74 ± 0.04 e |
| Tyrosine | 0.20 ± 0.04 d | 2.74 ± 0.01 a | 2.69 ± 0.05 a | 2.55 ± 0.02 b | 2.49 ± 0.04 b | 2.39 ± 0.06 c | 2.36 ± 0.01 c |
| Phenylalanine | 1.2 ± 0.05 e | 4.02 ± 0.05 a | 3.95 ± 0.05 a | 3.81 ± 0.02 b | 3.72 ± 0.02 b,c | 3.65 ± 0.08 c,d | 3.57 ± 0.04 d |
| Histidine | 0.51 ± 0.04 f | 1.77 ± 0.01 a | 1.72 ± 0.02 a,b | 1.67 ± 0.01 b,c | 1.63 ± 0.01 c,d | 1.60 ± 0.03 d,e | 1.57 ± 0.01 e |
| Lysine | 3.60 ± 0.02 e | 5.56 ± 0.06 a | 5.46 ± 0.04 a,b | 5.38 ± 0.01 b,c | 5.29 ± 0.04 c,d | 5.25 ± 0.10 c,d | 5.18 ± 0.03 d |
| Arginine | 7.07 ± 0.08 a | 6.35 ± 0.06 b | 6.31 ± 0.05 b | 6.31 ± 0.01 b | 6.29 ± 0.01 b | 6.33 ± 0.12 b | 6.36 ± 0.05 b |
| Tyrosine | 0.03 ± 0.01 d | 0.57 ± 0.01 a | 0.55 ± 0.01 b | 0.54 ± 0.01 b | 0.49 ± 0.01 c | 0.50 ± 0.01 c | 0.50 ± 0.01 c |
| ∑ | 83.16 ± 0.38 a | 74.40 ± 0.12 g | 74.32 ± 0.15 f | 74.34 ± 0.36 e | 74.17 ± 0.62 d | 74.56 ± 0.76 c | 74.83 ± 0.86 b |
| Addition of SCP | Td | ΔH |
|---|---|---|
| % | °C | J/g |
| CK | 94.63 ± 0.70 a | 137.30 ± 4.94 c |
| 3 | 93.40 ± 0.01 a | 232.15 ± 0.49 b |
| 6 | 92.01 ± 0.04 a,b | 237.22 ± 0.82 b |
| 9 | 89.63 ± 2.26 b | 232.05 ± 4.87 b |
| 12 | 92.24 ± 0.86 a,b | 252.60 ± 5.66 a |
| 15 | 93.49 ± 2.36 a | 238.35 ± 0.78 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wu, M.; He, T.; Sun, D.; Xu, H.; Zhang, T.; Wei, W. Mechanism of Textural Reorganization in Silkworm Chrysalis and Pea Protein Extrusion: Structural Evolution and Quality Characteristic. Foods 2025, 14, 1134. https://doi.org/10.3390/foods14071134
Zhang X, Wu M, He T, Sun D, Xu H, Zhang T, Wei W. Mechanism of Textural Reorganization in Silkworm Chrysalis and Pea Protein Extrusion: Structural Evolution and Quality Characteristic. Foods. 2025; 14(7):1134. https://doi.org/10.3390/foods14071134
Chicago/Turabian StyleZhang, Xun, Min Wu, Tao He, Dongyu Sun, Huihuang Xu, Tianqi Zhang, and Wenguang Wei. 2025. "Mechanism of Textural Reorganization in Silkworm Chrysalis and Pea Protein Extrusion: Structural Evolution and Quality Characteristic" Foods 14, no. 7: 1134. https://doi.org/10.3390/foods14071134
APA StyleZhang, X., Wu, M., He, T., Sun, D., Xu, H., Zhang, T., & Wei, W. (2025). Mechanism of Textural Reorganization in Silkworm Chrysalis and Pea Protein Extrusion: Structural Evolution and Quality Characteristic. Foods, 14(7), 1134. https://doi.org/10.3390/foods14071134






