Preparation, Characterization and Bioactivities of Strawberry Polysaccharides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ultrasonic Degradation of the Native Polysaccharide
2.3. Determination of Physicochemical Properties of DSFP-500 and DSFP-700
2.3.1. Chemical Composition
2.3.2. Particle Sizes and Mws
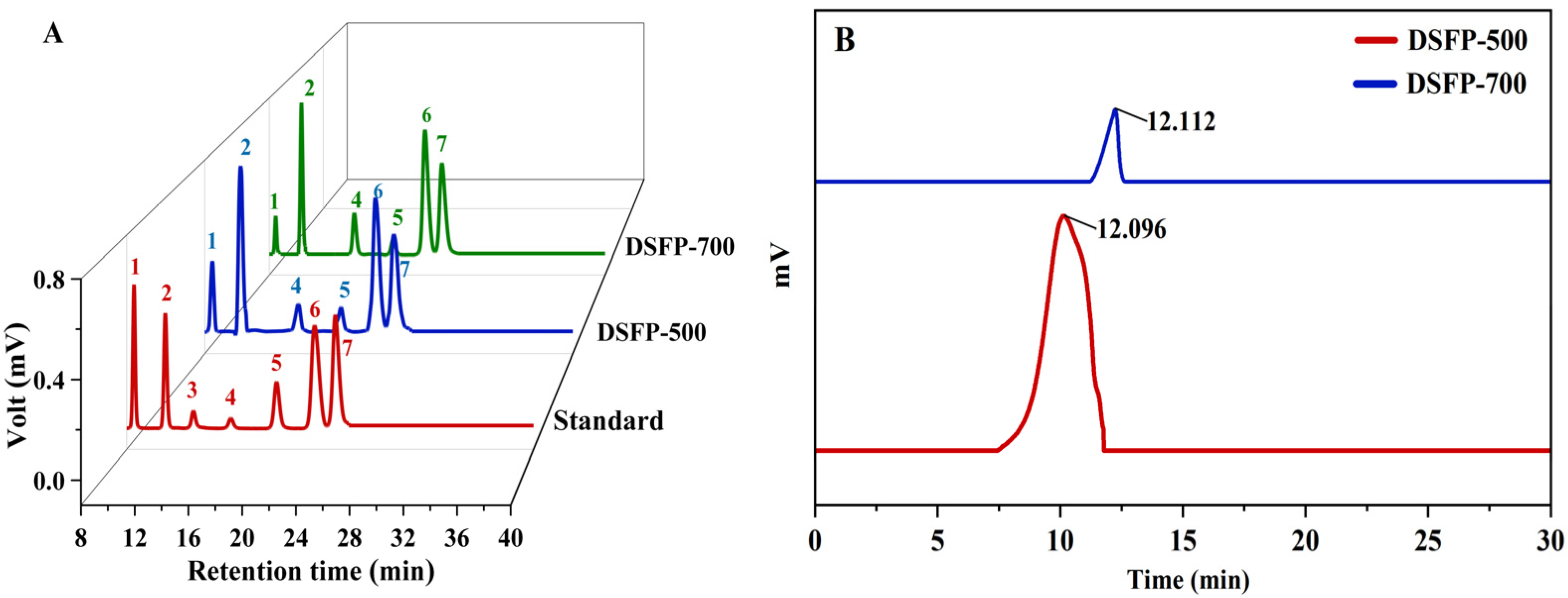
2.3.3. Monosaccharide Composition
2.4. Structural Characterization of DSFP-500 and DSFP-700
2.4.1. Ultraviolet-Visible Spectrophotometer (UV-Vis) and Fourier Transform Infrared Spectroscopy (FT-IR)
2.4.2. Nuclear Magnetic Resonance (NMR)
2.4.3. Congo Red Test
2.4.4. X-Ray Diffraction (XRD) and Scanning Electron Microscopy (SEM)
2.5. Determination of Functional Properties of DSFP-500 and DSFP-700
2.5.1. Water Holding Capacity (WHC) and Oil Holding Capacity (OHC)
2.5.2. Emulsion Properties
2.5.3. Thermal Stability Analysis
2.5.4. Rheological Analysis
2.6. Measurement of Free Radical Scavenging Activities of Two Polysaccharides
2.6.1. The ABTS+• Scavenging Activity
2.6.2. The DPPH• Scavenging Activity
2.7. Measurement of Anti-Complement Activities of Two Degraded Polysaccharides
2.7.1. Anti-Complement Activities Through the Classical Pathway
2.7.2. Anti-Complement Activities Through the Alternative Pathway
2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparation of DSFP-500 and DSFP-700
3.2. Characterization of DSFP-500 and DSFP-700
3.2.1. UV-Vis and FT-IR Spectra
3.2.2. NMR Analysis
3.2.3. Congo Red Test Analysis
3.2.4. XRD and SEM Analysis
3.3. Functional Properties of DSFP-500 and DSFP-700
3.3.1. WHC and OHC
3.3.2. Emulsification
3.3.3. Thermal Analysis
3.3.4. Rheological Behaviors
3.4. Free Radical Scavenging Activities of DSFP-500 and DSFP-700
3.4.1. The Scavenging Activity on ABTS+•
3.4.2. The Scavenging Activities on DPPH•
3.5. Anti-Complement Activities of DSFP-500 and DSFP-700
3.5.1. Anti-Complement Activity Through the Classical Pathway
3.5.2. Anti-Complement Activity Through the Alternative Pathway
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tudu, M.; Samanta, A. Natural polysaccharides: Chemical properties and application in pharmaceutical formulations. Eur. Polym. J. 2023, 184, 111801. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Sotiropoulos, K. Current advances of polysaccharide-based nanogels and microgels in food and biomedical sciences. Polymers 2022, 14, 813. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ren, Q.; Wang, S.; Gao, J.; Shen, C.; Zhang, S.; Wang, Y.; Guan, F. Chemical modification of polysaccharides: A review of synthetic approaches, biological activity and the structure-activity relationship. Molecules 2023, 28, 6073. [Google Scholar] [CrossRef]
- Yarley, O.P.N.; Kojo, A.B.; Zhou, C.; Yu, X.; Gideon, A.; Kwadwo, H.H.; Richard, O. Reviews on mechanisms of in vitro antioxidant, antibacterial and anticancer activities of water-soluble plant polysaccharides. Int. J. Biol. Macromol. 2021, 183, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Hajjati, S.; Mahmudi, Z.; Tyagi, I.; Agarwal, S.; Maity, A.; Gupta, V.K. Modeling of competitive ultrasonic assisted removal of the dyes—Methylene blue and Safranin-O using Fe3O4 nanoparticles. Chem. Eng. J. 2015, 268, 28–37. [Google Scholar] [CrossRef]
- Dou, Z.; Zhang, Y.; Tang, W.; Deng, Q.; Hu, B.; Chen, X.; Niu, H.; Wang, W.; Li, Z.; Zhou, H.; et al. Ultrasonic effects on the degradation kinetics, structural characteristics and protective effects on hepatocyte lipotoxicity induced by palmitic acid of Pueraria Lobata polysaccharides. Ultras. Sonochem. 2023, 101, 106652. [Google Scholar] [CrossRef]
- Yao, Q.; Pu, L.; Dong, B.; Zhu, D.; Wu, W.; Yang, Q. Effects of ultrasonic degradation on physicochemical and antioxidant properties of Gleditsia sinensis seed polysaccharides. Carb. Res. 2024, 545, 109272. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, X.; Zhan, Q.; Zhong, L.; Hu, Q.; Zhao, L. Effects of ultrasound on the degradation kinetics, physicochemical properties and prebiotic activity of Flammulina velutipes polysaccharide. Ultras. Sonochem. 2022, 82, 105901. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Liu, Y.; Xu, B.; Du, B.; Yang, Y. Effect of ultrasonic irradiation on the physicochemical and structural properties of Laminaria japonica polysaccharides and their performance in biological activities. Molecules 2023, 28, 8. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Yan, X.H.; Zhang, J.L.; Wang, L.Y.; Xue, H.; Jiang, G.C.; Ma, X.T.; Liu, X.J. Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2019, 135, 706–716. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Dun, W.; Han, W.; Xu, C.; Sun, Q.; Wang, Z. Effect of ultrasonic degradation on the physicochemical property and bioactivity of polysaccharide produced by Chaetomium globosum CGMCC 6882. Front. Nutr. 2022, 9, 941524. [Google Scholar] [CrossRef]
- Urün, I.; Attar, S.H.; Sönmez, D.A.; Gündeşli, M.A.; Ercişli, S.; Kafkas, N.E.; Bandić, L.M.; Duralija, B. Comparison of polyphenol, sugar, organic acid, volatile compounds, and antioxidant capacity of commercially grown strawberry cultivars in Turkey. Plants 2021, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, R.C.; Temocico, G.; Fierascu, I.; Ortan, A.; Babeanu, N.E. Fragaria Genus: Chemical composition and biological activities. Molecules 2020, 25, 498. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Lin, J.Y. Protective effects of strawberry and mulberry fruit polysaccharides on inflammation and apoptosis in murine primary splenocytes. J. Food Drug Anal. 2014, 22, 210–219. [Google Scholar] [CrossRef]
- Kumari, N.; Kumar, M.; Radha; Rais, N.; Puri, S.; Sharma, K.; Natta, S.; Dhumal, S.; Damale, R.D.; Kumar, S.; et al. Exploring apple pectic polysaccharides: Extraction, characterization, and biological activities—A comprehensive review. Int. J. Biol. Macromol. 2024, 255, 128011. [Google Scholar] [CrossRef]
- Chen, S.J.; Li, J.Y.; Zhang, J.M. Extraction of yellow pear residue polysaccharides and effects on immune function and antioxidant activity of immunosuppressed mice. Int. J. Biol. Macromol. 2019, 126, 1273–1281. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Li, G.; Zhang, D.; Qin, D.; Wang, L.; Xu, Y. Functional properties, structural characteristics, and anti-complementary activities of two degraded polysaccharides from strawberry fruits. Int. J. Biol. Macromol. 2024, 269, 132263. [Google Scholar] [CrossRef]
- Ma, C.; Bai, J.; Shao, C.; Liu, J.; Zhang, Y.; Li, X.; Yang, Y.; Xu, Y.; Wang, L. Degradation of blue honeysuckle polysaccharides, structural characteristics and antiglycation and hypoglycemic activities of degraded products. Food Res. Int. 2021, 143, 110281. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar]
- Miller, G. Use of dinitrosalicyclic acid reagent for determination of reducing sugars. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Campos, D.A.; Nunes, C.; Ribeiro, S.; Nunes, J.; Oliveira, A.; Pintado, M. Polyphenol extraction by different techniques for valorisation of non-compliant Portuguese sweet cherries towards a novel antioxidant extract. Sustainability 2022, 12, 5556. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, J.; Li, L.; Huang, J.; Yang, Y.; Xu, Y.; Wang, Y.; Liu, Y. Characterization and biological activities of polysaccharides from dandelion (Taraxacum officinale) leaves. Starch-Stärke 2021, 73, 2000051. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G.; Huang, H. Extraction, derivatization and antioxidant activities of onion polysaccharide. Food Chem. 2022, 388, 133000. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, Y.; Nie, S.; Xu, F.; He, S.; Gong, D.; Wu, G.; Tan, L. Physicochemical properties and in vitro antioxidant activities of polysaccharide from Artocarpus heterophyllus Lam. pulp. Carbohydr. Polym. 2017, 155, 354–361. [Google Scholar] [CrossRef]
- Duan, S.; Zhao, M.; Wu, B.; Wang, S.; Yang, Y.; Xu, Y.; Wang, L. Preparation, characteristics, and antioxidant activities of carboxymethylated polysaccharides from blackcurrant fruits. Int. J. Biol. Macromol. 2020, 155, 1114–1122. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, Z.; Zhao, M.; Wu, C.; Wang, L.; Xu, Y. Microwave-assisted extraction of an acidic polysaccharide from Ribes nigrum L.: Structural characteristics and biological activities. Ind. Crops Prod. 2020, 147, 112249. [Google Scholar] [CrossRef]
- Xia, L.; Deji; Zhu, M.; Chen, D.; Lu, Y. Juniperus pingii var. wilsonii acidic polysaccharide: Extraction, characterization and anticomplement activity. Carbohydr. Polym. 2020, 231, 115728. [Google Scholar] [CrossRef]
- Xiong, F.; Li, X.; Zheng, L.; Hu, N.; Cui, M.; Li, H. Characterization and antioxidant activities of polysaccharides from Passiflora edulis Sims peel under different degradation methods. Carbohydr. Polym. 2019, 218, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.K.; Wang, X.; Guo, Y.Q.; Song, X.X.; Yin, J.Y.; Nie, S.P. Exploring the partial degradation of polysaccharides: Structure, mechanism, bioactivities, and perspectives. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4831–4870. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, H.; Wang, Y.; Yang, D.; Tan, H.; Zhan, Y.; Yang, Y.; Luo, Y.; Chen, G. Optimization extraction, structural features and antitumor activity of polysaccharides from Z. jujuba cv. Ruoqiangzao seeds. Int. J. Biol. Macromol. 2019, 135, 1151–1161. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Song, R.; Cai, J.; Xu, J.; Tang, X.; Li, N. Ginger polysaccharides induced cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Int. J. Biol. Macromol. 2019, 123, 81–90. [Google Scholar] [CrossRef]
- Liu, H.; Sun, X.Y.; Wang, F.X.; Ouyang, J.M. Regulation on calcium oxalate crystallization and protection on HK-2 cells of tea polysaccharides with different molecular weights. Oxidative Med. Cell. Longev. 2020, 2020, 5057123. [Google Scholar] [CrossRef]
- Xiu, W.; Wang, X.; Na, Z.; Yu, S.; Wang, J.; Yang, M.; Ma, Y. Ultrasound-assisted hydrogen peroxide–ascorbic acid method to degrade sweet corncob polysaccharides can help treat type 2 diabetes via multiple pathways in vivo. Ultra. Sonochem. 2023, 101, 106683. [Google Scholar] [CrossRef]
- Dokhaee, Z.; Maghsoudi, A.; Ghiaci, P.; Ghiaci, M. Investigation of the blends of chitosan and tragacanth as potential drug carriers for the delivery of ibuprofen in the intestine. New J. Chem. 2019, 43, 14917–14927. [Google Scholar] [CrossRef]
- Rivas, M.Á.; Casquete, R.; Córdoba, M.d.G.; Ruíz-Moyano, S.; Benito, M.J.; Pérez-Nevado, F.; Martín, A. Chemical composition and functional properties of dietary fibre concentrates from winemaking by-products: Skins, stems and lees. Foods 2021, 10, 1510. [Google Scholar] [CrossRef]
- Xu, H.; Wu, Z.; Zhao, D.; Liang, H.; Yuan, H.; Wang, C. Preparation and characterization of electrospun nanofibers-based facial mask containing hyaluronic acid as a moisturizing component and huangshui polysaccharide as an antioxidant component. Int. J. Biol. Macromol. 2022, 214, 212–219. [Google Scholar] [CrossRef]
- Gutiérrez-Méndez, N.; Chavez-Garay, D.R.; Leal-Ramos, M.Y. Lecithins: A comprehensive review of their properties and their use in formulating microemulsions. J. Food Biochem. 2022, 46, e14157. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Lv, F.; Xie, X.; Zhang, S.; Cai, C.; Jia, R.; Pan, Y.; Liu, F. Anti-complementary activity of a degraded sulfated heterogalactan from red alga Pyropia haitanensis. Int. J. Biol. Macromol. 2020, 147, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Yang, Y.; Zhou, L.; Chen, D.; Lu, Y. Two natural flavonoid substituted polysaccharides from Tamarix chinensis: Structural characterization and anticomplement activities. Molecules 2022, 27, 4532. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y. The antioxidant and anti-complementary activities of crude polysaccharides from Trifoliate Orange (Poncirus trifoliate) seeds. Pre. Nut. Food Sci. 2023, 28, 321–327. [Google Scholar] [CrossRef]
- Du, D.; Lu, Y.; Cheng, Z.; Chen, D. Structure characterization of two novel polysaccharides isolated from the spikes of Prunella vulgaris and their anticomplement activities. J. Ethnopharmacol. 2016, 193, 345–353. [Google Scholar] [CrossRef]
- Zou, M.Y.; Nie, S.P.; Yin, J.Y.; Xie, M.Y. Ascorbic acid induced degradation of polysaccharide from natural products: A review. Int. J. Biol. Macromol. 2020, 151, 483–491. [Google Scholar] [CrossRef]






| Chemical Composition | Samples | |
|---|---|---|
| DSFP-500 | DSFP-700 | |
| Total sugar (%) | 86.04 ± 0.12 | 85.25 ± 0.55 |
| Reducing sugar (%) | 0 | 0 |
| Uronic acid (%) | 25.30 ± 0.17 | 25.59 ± 0.19 |
| Protein (%) | 0.17 ± 0.06 | 0.15 ± 0.08 |
| Polyphenol (%) | 0.13 ± 0.08 | 0.10 ± 0.04 |
| Particle size (nm) | 270.4 ± 0.42 | 257.3 ± 0.33 |
| Mw (kDa) | 809 | 791 |
| Monosaccharide composition (molar ratio) | ||
| Man | 1.00 | 1.00 |
| Rha | 2.82 | 4.37 |
| GalA | 2.79 | 4.61 |
| Glc | 1.06 | 1.06 |
| Gal | 1.51 | 3.30 |
| Ara | 1.56 | 3.36 |
| Sugar Residues | Chemical Shifts of 1H/13C (ppm) | ||||||
|---|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | ||
| A →3,6)-β-D-Galp-(1→ | DSFP-500 | 4.43/103.29 | 3.57/72.71 | 3.86/75.95 | 3.65/72.17 | 3.67/75.23 | 3.46/69.80 |
| DSFP-700 | 4.46/103.55 | 3.54/72.60 | 3.85/76.62 | 3.65/71.06 | 3.68/75.25 | 3.50/69.40 | |
| B →2)-α-L-Rhap-(1→ | DSFP-500 | 5.17/98.37 | 4.00/83.05 | 3.87/73.25 | 3.65/71.05 | 3.45/70.43 | 1.17/16.34 |
| DSFP-700 | 5.16/98.72 | 4.05/82.88 | 3.83/73.29 | 3.65/71.15 | 3.48/70.47 | 1.17/16.51 | |
| C →3)-β-D-Manp-(1→ | DSFP-500 | 4.61/100.62 | 3.75/69.41 | 4.02/80.32 | 3.62/71.34 | 3.94/72.37 | 3.73/61.55 |
| DSFP-700 | 4.66/100.77 | 3.72/69.21 | 4.04/80.51 | 3.64/71.48 | 3.96/72.53 | 3.74/61.26 | |
| D →4)-α-D-GalpA-(1→ | DSFP-500 | 4.92/98.54 | 3.71/72.37 | 4.04/75.71 | 3.64/78.48 | 3.93/69.07 | /173.42 |
| DSFP-700 | 4.92/98.60 | 3.72/72.68 | 4.01/75.67 | 3.68/78.55 | 3.94/69.14 | /173.20 | |
| E α-L-Araf-(1→ | DSFP-500 | 5.16/109.93 | 4.15/81.21 | 3.85/76.53 | 4.01/81.32 | 3.65/61.36 | –/– |
| DSFP-700 | 5.16/109.53 | 4.17/81.35 | 3.86/76.38 | 4.03/81.27 | 3.64/61.52 | –/– | |
| F →4)-β-D-Glcp-(1→ | DSFP-500 | 4.40/102.38 | 4.16/74.12 | 4.09/75.20 | 3.78/79.80 | 3.87/73.70 | 3.74/61.87 |
| DSFP-700 | 4.41/102.58 | 4.14/74.11 | 4.11/75.34 | 3.76/79.92 | 3.89/73.42 | 3.73/61.59 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhao, Y.; Liu, J.; Zhu, L.; Wei, Y.; Cheng, K.; Xu, Y. Preparation, Characterization and Bioactivities of Strawberry Polysaccharides. Foods 2025, 14, 1117. https://doi.org/10.3390/foods14071117
Wang L, Zhao Y, Liu J, Zhu L, Wei Y, Cheng K, Xu Y. Preparation, Characterization and Bioactivities of Strawberry Polysaccharides. Foods. 2025; 14(7):1117. https://doi.org/10.3390/foods14071117
Chicago/Turabian StyleWang, Libo, Yumeng Zhao, Junwen Liu, Ling Zhu, Yanhui Wei, Kun Cheng, and Yaqin Xu. 2025. "Preparation, Characterization and Bioactivities of Strawberry Polysaccharides" Foods 14, no. 7: 1117. https://doi.org/10.3390/foods14071117
APA StyleWang, L., Zhao, Y., Liu, J., Zhu, L., Wei, Y., Cheng, K., & Xu, Y. (2025). Preparation, Characterization and Bioactivities of Strawberry Polysaccharides. Foods, 14(7), 1117. https://doi.org/10.3390/foods14071117






