Protective Effects of a Polyherbal Mixture on Intestinal Injury via the NF-κB Signaling Pathway and Gut Microbiota Modulation in Hyperuricemic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of PHT
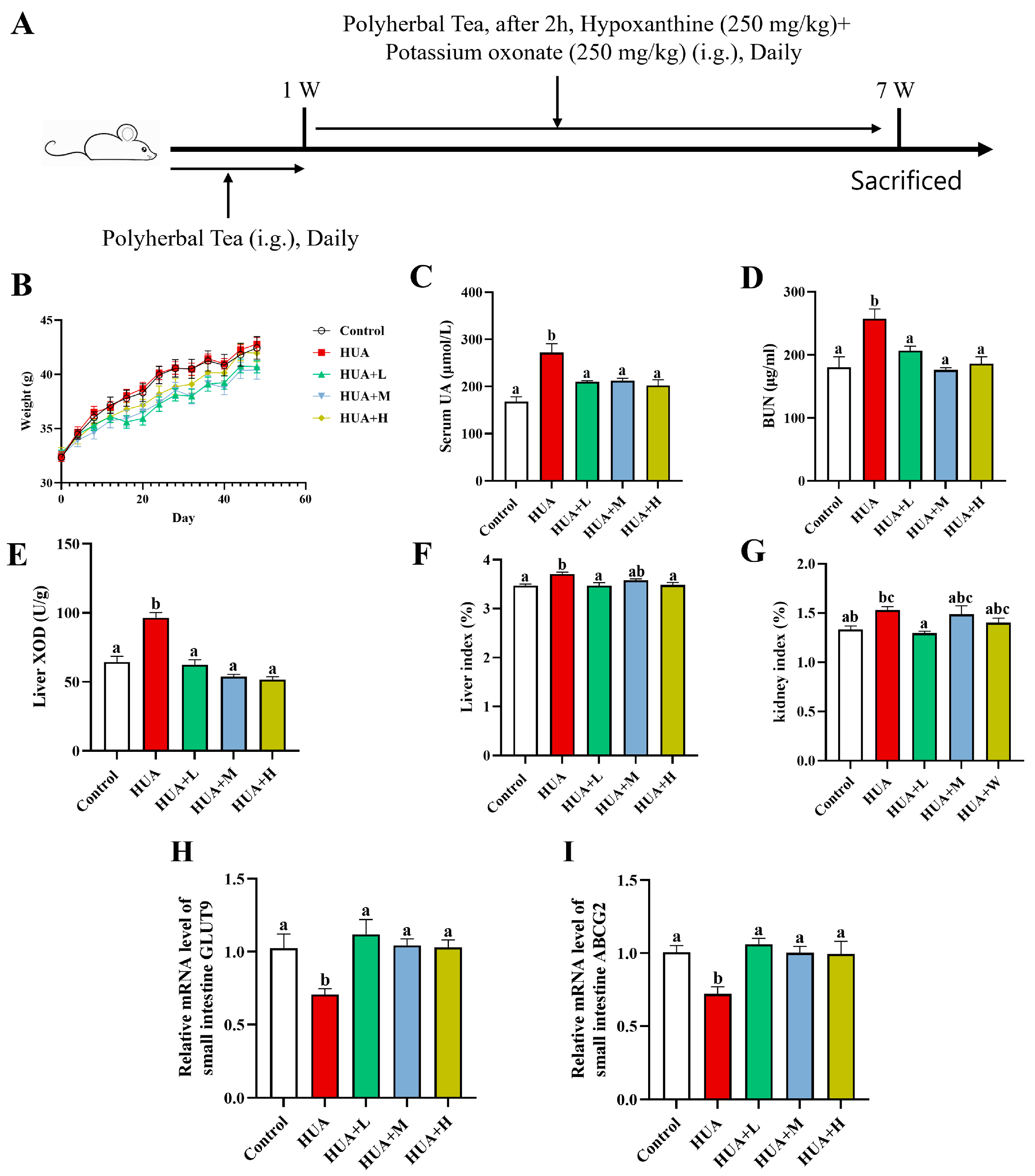
2.3. Animals and Experimental Design
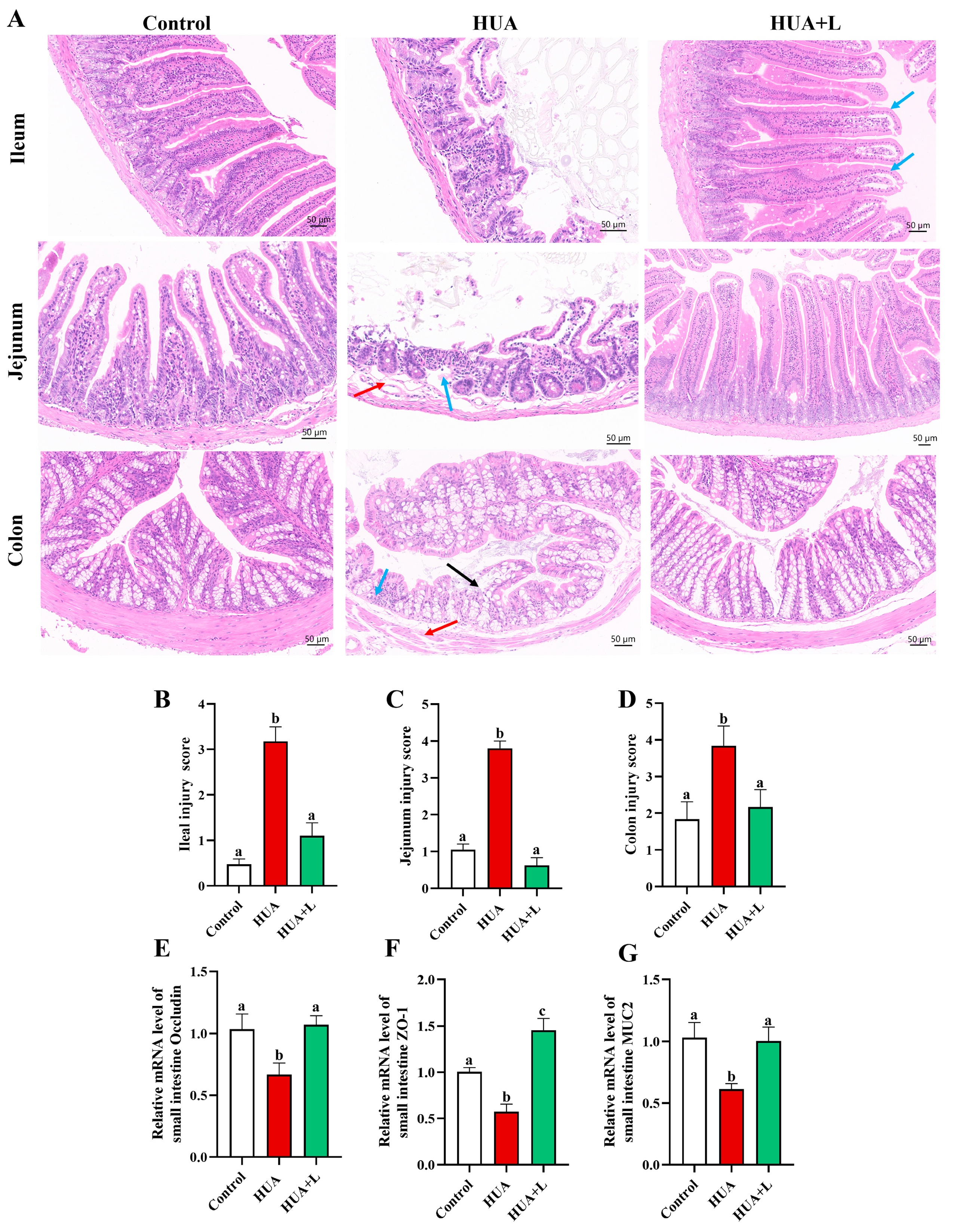
2.4. Histological Analysis
2.5. Real-Time Quantitative PCR (RT–qPCR) Analysis
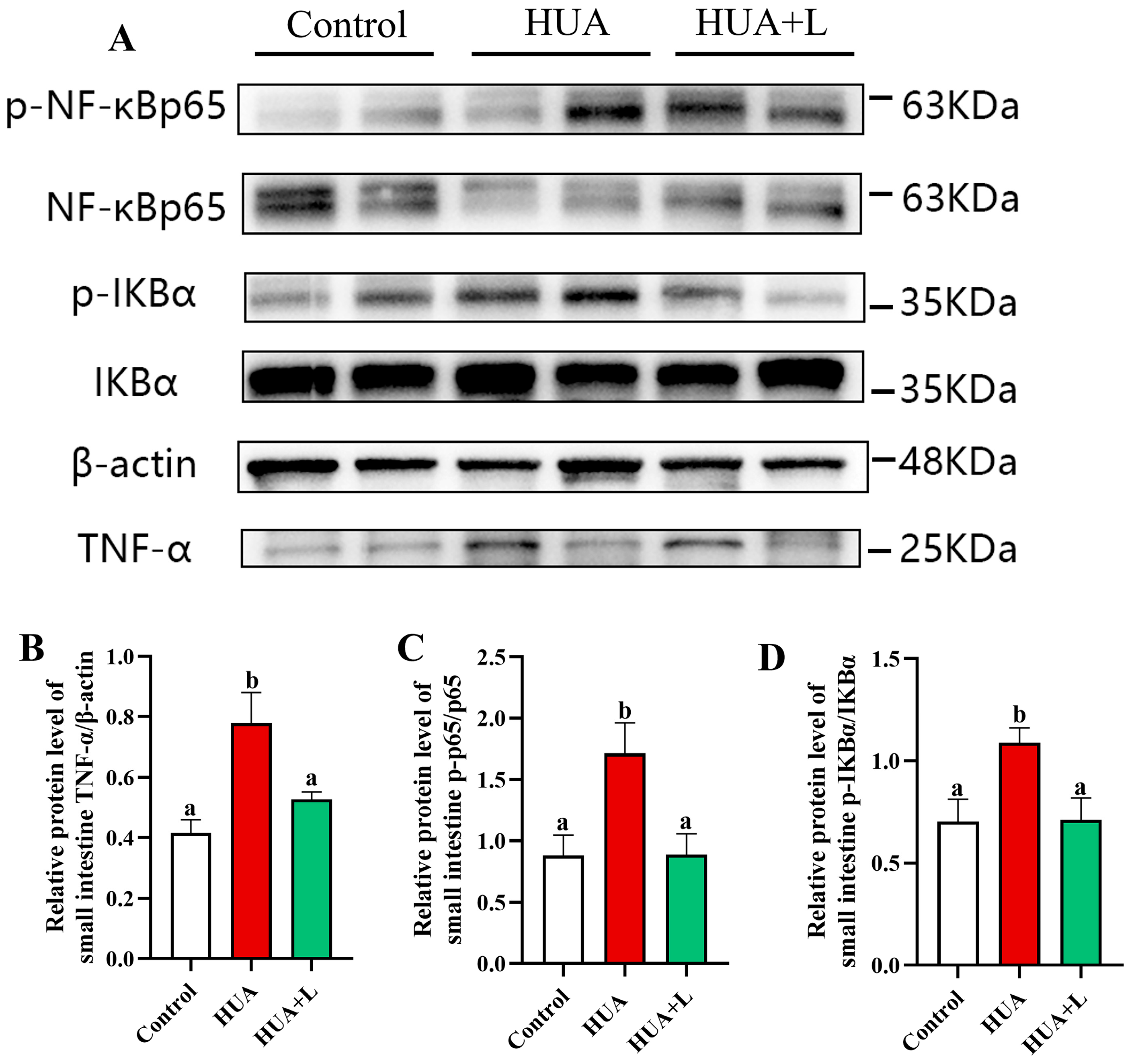
2.6. Western Blotting
2.7. Gut Microbial Analysis
2.8. UPLC–MS Analysis of PHT and Cecum Contents
2.9. Statistical Analysis
3. Results
3.1. PHT Alleviated HUA Induced by Potassium Oxonate and Hypoxanthine
3.2. PHT Improved Intestinal Injury and Maintained Barrier Integrity
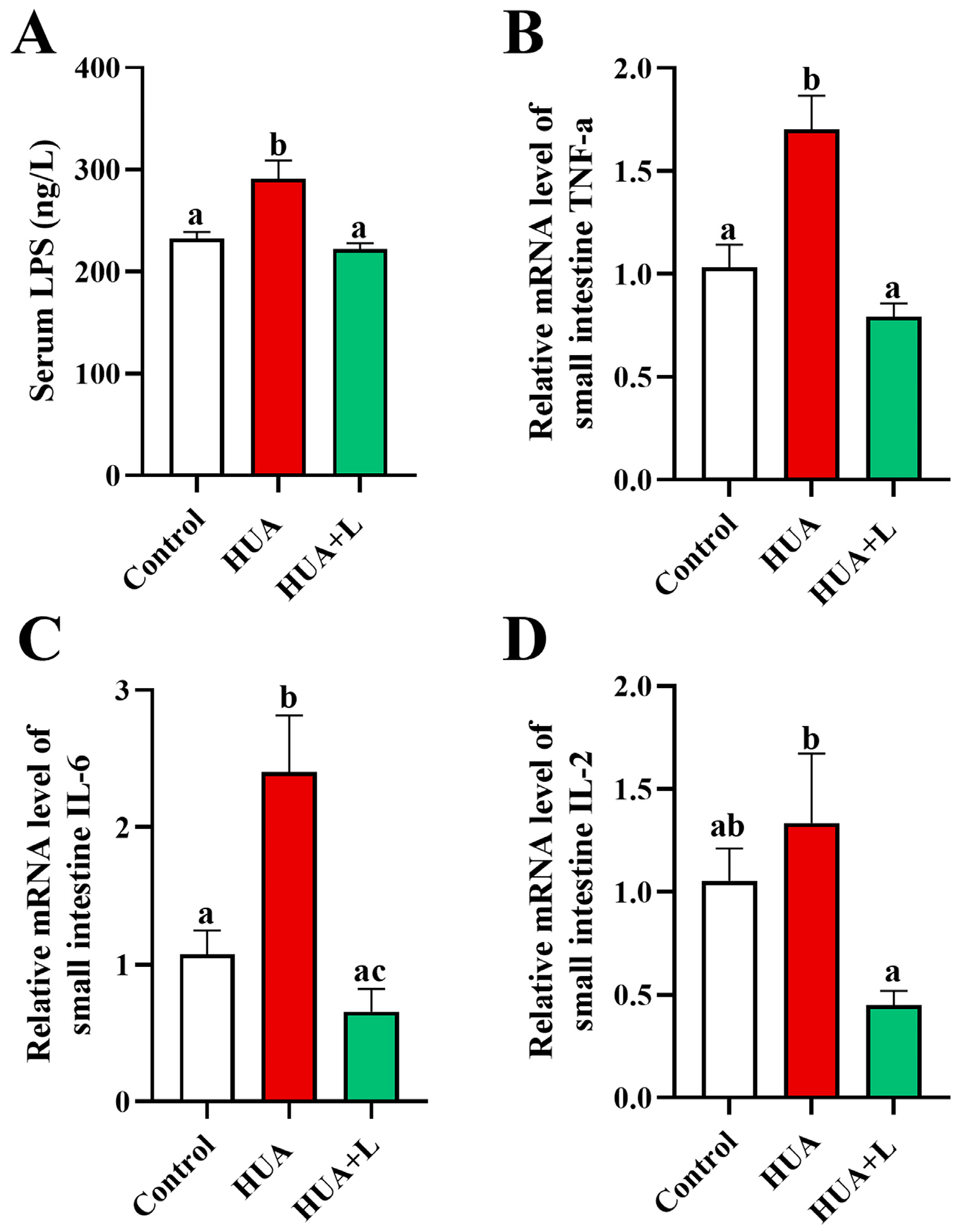
3.3. PHT Relieved Intestinal Inflammation in Hyperuricemic Mice
3.4. PHT Inhibited the NF-κB Signaling Pathway in the Gut
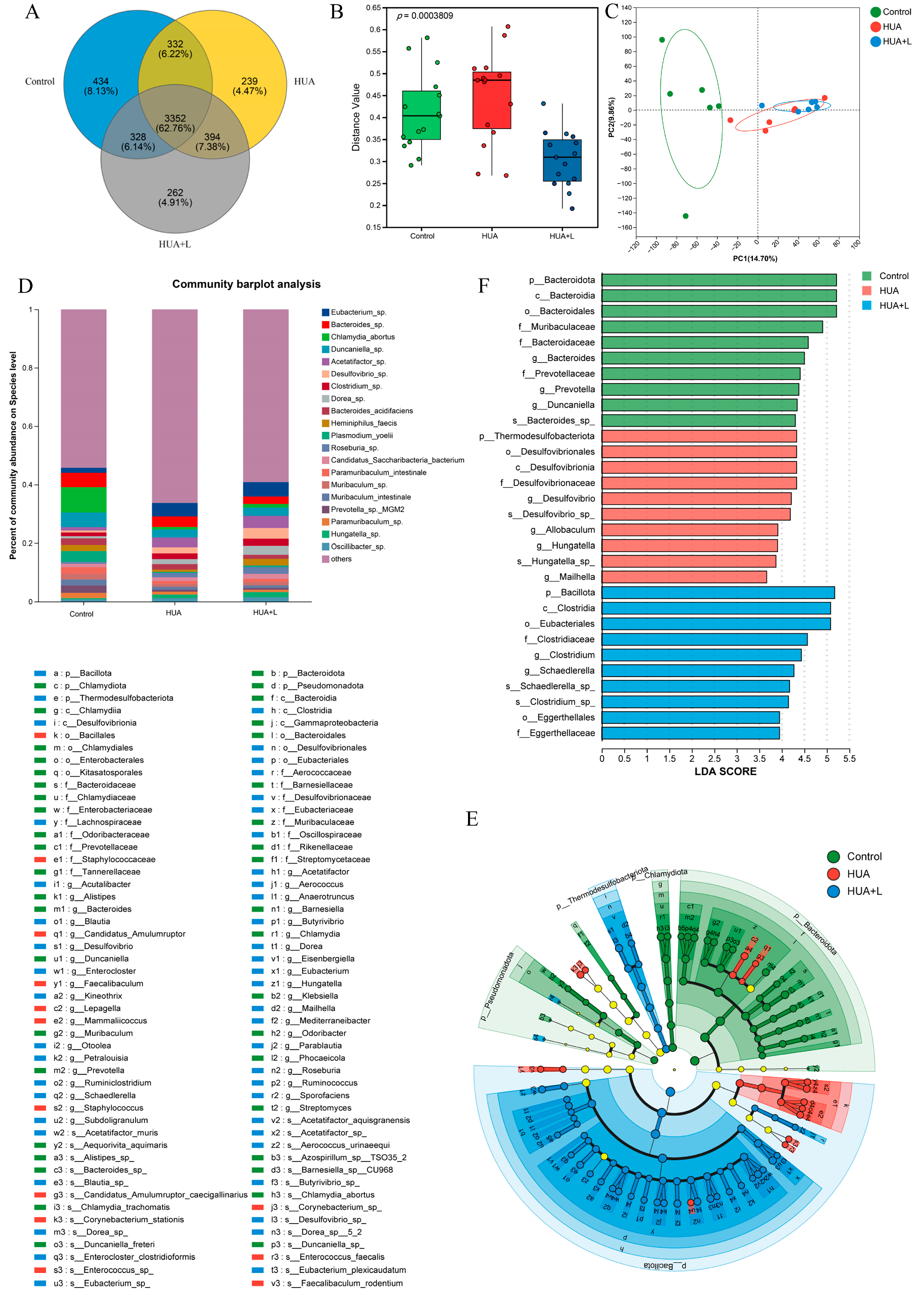
3.5. PHT Modulated the Gut Microbiota in Hyperuricemic Mice
3.6. PHT Functional Composition Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehmood, A.; Iftikhar, A.; Chen, X. Food-derived bioactive peptides with anti-hyperuricemic activity: A comprehensive review. Food Chem. 2024, 451, 139444. [Google Scholar]
- Du, L.; Zong, Y.; Li, H.; Wang, Q.; Xie, L.; Yang, B.; Pang, Y.; Zhang, C.; Zhong, Z.; Gao, J. Hyperuricemia and its related diseases: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 212. [Google Scholar]
- Pathmanathan, K.; Robinson, P.C.; Hill, C.L.; Keen, H.I. The prevalence of gout and hyperuricaemia in Australia: An updated systematic review. Semin. Arthritis Rheum. 2021, 51, 121–128. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, X.; Wu, J.; Huang, Z.; Zhao, Z.; Zhang, X.; Xue, Y.; Wan, W.; Li, C.; Zhang, W.; et al. Prevalence of Hyperuricemia Among Chinese Adults: Findings From Two Nationally Representative Cross-Sectional Surveys in 2015–16 and 2018–19. Front. Immunol. 2022, 12, 791983. [Google Scholar]
- Kim, Y.J.; Kim, S.; Seo, J.H.; Cho, S.K. Prevalence and Associations Between Metabolic Syndrome Components and Hyperuricemia by Race: Findings From US Population, 2011–2020. Arthritis Care Res. 2024, 76, 1195–1202. [Google Scholar]
- Basnet, T.B.; Du, S.; Feng, R.; Gao, J.; Gong, J.; Ye, W. Fatty liver mediates the association of hyperuricemia with prediabetes and diabetes: A weighting-based mediation analysis. Front. Endocrinol. 2023, 14, 1133515. [Google Scholar]
- Gu, T.; Cao, G.; Luo, M.; Zhang, N.; Xue, T.; Hou, R.; Leng, M. A systematic review and meta-analysis of the hyperuricemia risk from certain metals. Clin. Rheumatol. 2022, 41, 3641–3660. [Google Scholar] [CrossRef]
- Yu, Y.; Quan, X.; Wang, H.; Zhang, B.; Hou, Y.; Su, C. Assessing the health risk of hyperuricemia in participants with persistent organic pollutants exposure—A systematic review and meta-analysis. Ecotox. Environ. Safe 2023, 251, 114525. [Google Scholar]
- Lv, Q.; Zhou, J.; Wang, C.; Yang, X.; Han, Y.; Zhou, Q.; Yao, R.; Sui, A. A dynamics association study of gut barrier and microbiota in hyperuricemia. Front. Microbiol. 2023, 14, 1287468. [Google Scholar] [CrossRef]
- Liu, X.; Lv, Q.; Ren, H.; Gao, L.; Zhao, P.; Yang, X.; Yang, G.; Xu, D.; Wang, G.; Yang, W.; et al. The altered gut microbiota of high-purine-induced hyperuricemia rats and its correlation with hyperuricemia. Peerj 2020, 8, e8664. [Google Scholar]
- Ge, H.; Jiang, Z.; Li, B.; Xu, P.; Wu, H.; He, X.; Xu, W.; Huang, Z.; Xiong, T.; Wang, P.; et al. Dendrobium officinalis Six Nostrum Promotes Intestinal Urate Underexcretion via Regulations of Urate Transporter Proteins in Hyperuricemic Rats. Comb. Chem. High Throughput Screen. 2023, 26, 848–861. [Google Scholar] [CrossRef]
- Morimoto, C.; Tamura, Y.; Asakawa, S.; Kuribayashi-Okuma, E.; Nemoto, Y.; Li, J.; Murase, T.; Nakamura, T.; Hosoyamada, M.; Uchida, S.; et al. ABCG2 expression and uric acid metabolism of the intestine in hyperuricemia model rat. Nucleosides Nucleotides Nucleic Acids 2020, 39, 744–759. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, Y.; Zhang, W.; Wen, Y.; Yang, R.; Dong, J.; Zhang, X.; Hua, Y.; Ji, P.; Wei, Y. Pathological mechanism of intestinal mucosal barrier injury of large intestine dampness-heat syndrome rats and the protective effect of Yujin powder. Res. Vet. Sci. 2022, 152, 485–496. [Google Scholar] [CrossRef]
- Dong, L.; Luo, P.; Zhang, A. Intestinal microbiota dysbiosis contributes to the liver damage in subchronic arsenic-exposed mice. Acta Biochim. Biophys. Sin. 2024, 56, 1774–1788. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, C.; Li, B.; Wang, S.; Sun, J.; Mao, X. Multi-omics analysis reveals that agaro-oligosaccharides with different degrees of polymerization alleviate colitis in mice by regulating intestinal flora and arginine synthesis. Food Funct. 2024, 15, 10628–10643. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J.; Sun, R.; Wang, M.; Wang, K.; Li, Y.; Shang, H.; Hou, J.; Jiang, Z. Lactobacillus plantarum 23-1 improves intestinal inflammation and barrier function through the TLR4/NF-κB signaling pathway in obese mice. Food Funct. 2022, 13, 5971–5986. [Google Scholar] [CrossRef]
- Zhong, J.; Qiu, X.; Yu, Q.; Chen, H.; Yan, C. A novel polysaccharide from Acorus tatarinowii protects against LPS-induced neuroinflammation and neurotoxicity by inhibiting TLR4-mediated MyD88/NF-κB and PI3K/Akt signaling pathways. Int. J. Biol. Macromol. 2020, 163, 464–475. [Google Scholar] [CrossRef]
- Chen, N.; Wang, R.; Li, H.; Wang, W.; Wang, L.; Yin, X.; Yao, R.; Yang, B. Flavonoid extract of saffron by-product alleviates hyperuricemia via inhibiting xanthine oxidase and modulating gut microbiota. Phytother. Res. 2022, 36, 4604–4619. [Google Scholar] [CrossRef]
- Chu, Y.; Sun, S.; Huang, Y.; Gao, Q.; Xie, X.; Wang, P.; Li, J.; Liang, L.; He, X.; Jiang, Y.; et al. Metagenomic analysis revealed the potential role of gut microbiome in gout. Npj Biofilms Microbomes 2021, 7, 66. [Google Scholar] [CrossRef]
- Shi, R.; Ye, J.; Fan, H.; Xiao, C.; Wang, D.; Xia, B.; Zhao, Z.; Zhao, B.; Dai, X.; Liu, X. Lactobacillus plantarum LLY-606 supplementation ameliorates hyperuricemia via modulating intestinal homeostasis and relieving inflammation. Food Funct. 2023, 14, 5663–5677. [Google Scholar] [CrossRef]
- Zou, Y.; Ro, K.; Jiang, C.; Yin, D.; Zhao, L.; Zhang, D.; Du, L.; Xie, J. The anti-hyperuricemic and gut microbiota regulatory effects of a novel purine assimilatory strain, Lactiplantibacillus plantarum X7022. Eur. J. Nutr. 2024, 63, 697–711. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, N.; Werlinger, P.; Suh, D.; Lee, H.; Cho, J.; Cheng, J. Probiotic Characterization of Lactobacillus brevis MJM60390 and In Vivo Assessment of Its Antihyperuricemic Activity. J. Med. Food 2022, 25, 367–380. [Google Scholar]
- Li, Y.; Zhu, J.; Lin, G.; Gao, K.; Yu, Y.; Chen, S.; Chen, L.; Chen, Z.; Li, L. Probiotic effects of Lacticaseibacillus rhamnosus 1155 and Limosilactobacillus fermentum 2644 on hyperuricemic rats. Front. Nutr. 2022, 9, 993951. [Google Scholar]
- Wang, J.; Chen, Y.; Zhong, H.; Chen, F.; Regenstein, J.; Hu, X.; Cai, L.; Feng, F. The gut microbiota as a target to control hyperuricemia pathogenesis: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. Nutr. 2022, 62, 3979–3989. [Google Scholar]
- Hutchinson, A.N.; Tingö, L.; Brummer, R.J. The Potential Effects of Probiotics and ω-3 Fatty Acids on Chronic Low-Grade Inflammation. Nutrients 2020, 12, 2402. [Google Scholar] [CrossRef]
- Xu, L.; Lu, L.L.; Gao, J.D. Traditional Chinese Herbal Medicine Plays a Role in the Liver, Kidney, and Intestine to Ameliorate Hyperuricemia according to Experimental Studies. Evid.-Based Complement Altern. Med. 2021, 13, 4618352. [Google Scholar]
- Huang, C.; Chen, T.; Tsai, G. Hypouricemic Effect of Submerged Culture of Ganoderma lucidum in Potassium Oxonate-Induced Hyperuricemic Rats. Metabolites 2022, 12, 553. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Ou, J.; Wan, Q.; Shi, L.; Li, Y.; He, F.; Wang, H.; He, L.; Gao, J. Effect and Mechanism of ShiZhiFang on Uric Acid Metabolism in Hyperuricemic Rats. Evid.-Based Complement Altern. Med. 2018, 11, 6821387. [Google Scholar]
- Meng, J.; Tian, J.; Zhao, Y.; Li, C.; Yi, Y.; Zhang, Y.; Han, J.; Wang, L.; Pan, C.; Liu, S.; et al. Ameliorative effect of cheqianzi decoction on hyperuricemia and kidney injury and underlying mechanism in rats. Heliyon 2023, 9, e15333. [Google Scholar]
- Wang, Z.; Liu, J.; Mou, Y.; Liao, W.; Li, Y.; Liu, J.; Tang, J. Anti-inflammatory and uric acid lowering effects of Euodiae fructus on hyperuricemia and gout mice. Front. Pharmacol. 2024, 15, 1296075. [Google Scholar]
- Chen, G.; Tan, M.; Li, K.; Leung, P.; Ko, C. Green tea polyphenols decrease uric acid level through xanthine oxidase and renal urate transporters in hyperuricemic mice. J. Ethnopharmacol. 2015, 175, 14–20. [Google Scholar]
- Zhu, C.; Xu, Y.; Liu, Z.; Wan, X.; Li, D.; Tai, L. The anti-hyperuricemic effect of epigallocatechin-3-gallate (EGCG) on hyperuricemic mice. Biomed. Pharmacother. 2018, 97, 168–173. [Google Scholar]
- Huang, L.; Deng, J.; Chen, G.; Zhou, M.; Liang, J.; Yan, B.; Shu, J.; Liang, Y.; Huang, H. The anti-hyperuricemic effect of four astilbin stereoisomers in Smilax glabra on hyperuricemic mice. J. Ethnopharmacol. 2019, 238, 111777. [Google Scholar]
- Liang, D.; Yong, T.; Diao, X.; Chen, S.; Chen, D.; Xiao, C.; Zuo, D.; Xie, Y.; Zhou, X.; Hu, H. Hypouricaemic and nephroprotective effects of Poria cocos in hyperuricemic mice by up-regulating ATP-binding cassette super-family G member 2. Pharm. Biol. 2021, 59, 273–284. [Google Scholar]
- Zhu, L.; Dong, Y.; Na, S.; Han, R.; Wei, C.; Chen, G. Saponins extracted from Dioscorea collettii rhizomes regulate the expression of urate transporters in chronic hyperuricemia rats. Biomed. Pharmacother. 2017, 93, 88–94. [Google Scholar]
- Hou, S.; Chen, S.; Shen, J.; Chen, H.; Wang, S.; Wang, C.; Man, K.; Liu, P.; Tsai, M.; Chen, Y.; et al. Emodin, a Natural Anthraquinone, Increases Uric Acid Excretion in Rats with Potassium Oxonate-Induced Hyperuricemia. Pharmaceuticals 2023, 16, 789. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, M.; Lu, G.; Yang, Z.; Ji, H.; Hu, Q. Emodinol ameliorates urate nephropathy by regulating renal organic ion transporters and inhibiting immune inflammatory responses in rats. Biomed. Pharmacother. 2017, 96, 727–735. [Google Scholar]
- Wang, H.; Wang, C.; Guo, S.; Chen, Z.; Peng, Z.; Duan, R.; Dong, T.T.X.; Tsim, K.W.K. Polysaccharide deriving from Ophiopogonis Radix promotes metabolism of ginsenosides in the present of human gut microbiota based on UPLC-MS/MS assay. J. Pharm. Biomed. Anal. 2019, 175, 112779. [Google Scholar]
- Wei, X.; Liang, J.; Liu, J.; Dai, Y.; Leng, X.; Cheng, Y.; Chi, L. Anchang Yuyang Decoction inhibits experimental colitis-related carcinogenesis by regulating PPAR signaling pathway and affecting metabolic homeostasis of host and microbiota. J. Ethnopharmacol. 2024, 326, 117995. [Google Scholar]
- Xu, S.Y.; Bian, R.L.; Chen, X. Pharmacological Experimental Methodology; People’s Medical Publishing House: Beijing, China, 1982. [Google Scholar]
- Li, X.; Gao, X.; Zhang, H.; Liu, Y.; Sarker, M.M.R.; Wu, Y.; Chen, X.; Zhao, C. The anti-hyperuricemic effects of green alga Enteromorpha prolifera polysaccharide via regulation of the uric acid transporters in vivo. Food Chem. Toxicol. 2021, 158, 112630. [Google Scholar]
- Xie, D.; Shen, Y.; Su, E.; Du, L.; Xie, J.; Wei, D. Anti-Hyperuricemic, Nephroprotective, and Gut Microbiota Regulative Effects of Separated Hydrolysate of α-Lactalbumin on Potassium Oxonate- and Hypoxanthine-Induced Hyperuricemic Mice. Mol. Nutr. Food Res. 2023, 67, 2200162. [Google Scholar]
- Shen, L.; Yang, Y.; Zhang, J.; Feng, L.; Zhou, Q. Diacylated anthocyanins from purple sweet potato (Ipomoea batatas L.) attenuate hyperglycemia and hyperuricemia in mice induced by a high-fructose/high-fat diet. J. Zhejiang Univ.-Sci. B 2023, 24, 587–601. [Google Scholar]
- Chiu, C.; McArdle, A.H.; Brown, R.; Scott, H.J.; Gurd, F.N. Intestinal Mucosal Lesion in Low-Flow States: I. A Morphological, Hemodynamic, and Metabolic Reappraisal. Arch. Surg. 1970, 101, 478–483. [Google Scholar]
- Ala, M.; Fallahpour Khoshdel, M.R.; Mohammad Jafari, R.; Sadrkhanloo, M.; Goudarzi, S.; Asl Soleimani, M.; Dehpour, A.R. Low-dose sumatriptan improves the outcome of acute mesenteric ischemia in rats via downregulating kynurenine. Pharmacol. Rep. 2023, 75, 623–633. [Google Scholar]
- Jang, Y.J.; Kim, W.; Han, D.H.; Lee, K.; Ko, G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes 2019, 10, 696–711. [Google Scholar]
- Okumura, R.; Takeda, K. The role of the mucosal barrier system in maintaining gut symbiosis to prevent intestinal inflammation. Semin. Immunopathol. 2024, 47, 2. [Google Scholar]
- Li, J.; Zhang, R.; Ma, J.; Tang, S.; Li, Y.; Li, Y.; Wan, J. Mucosa-Associated Microbial Profile Is Altered in Small Intestinal Bacterial Overgrowth. Front. Microbiol. 2021, 12, 710940. [Google Scholar]
- Sun, Y.; Wu, D.; Zeng, W.; Chen, Y.; Guo, M.; Lu, B.; Li, H.; Sun, C.; Yang, L.; Jiang, X.; et al. The Role of Intestinal Dysbacteriosis Induced Arachidonic Acid Metabolism Disorder in Inflammaging in Atherosclerosis. Front. Cell. Infect. Microbiol. 2021, 11, 618265. [Google Scholar]
- Kim, H.W.; Wang, M.; Leber, C.A.; Nothias, L.; Reher, R.; Kang, K.B.; van der Hooft, J.J.J.; Dorrestein, P.C.; Gerwick, W.H.; Cottrell, G.W. NPClassifier: A Deep Neural Network-Based Structural Classification Tool for Natural Products. J. Nat. Prod. 2021, 84, 2795–2807. [Google Scholar]
- Adachi, S.; Oyama, M.; Kondo, S.; Yagasaki, K. Comparative effects of quercetin, luteolin, apigenin and their related polyphenols on uric acid production in cultured hepatocytes and suppression of purine bodies-induced hyperuricemia by rutin in mice. Cytotechnology 2021, 73, 343–351. [Google Scholar]
- Wang, F.; Zhao, X.; Su, X.; Song, D.; Zou, F.; Fang, L. Isorhamnetin, the xanthine oxidase inhibitor from Sophora japonica, ameliorates uric acid levels and renal function in hyperuricemic mice. Food Funct. 2021, 12, 12503–12512. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Fang, Z.; Lu, S. Hesperetin acts as a potent xanthine oxidase inhibitor: New evidence from its reactive oxygen suppression and enzyme binding. Int. J. Biol. Macromol. 2025, 306, 141429. [Google Scholar] [CrossRef]
- Xiang, L.; Huang, Y.; Li, R.; Tao, Y.; Wu, T.; Pan, S.; Xu, X. Artemisia selengensis Turcz. leaves extract ameliorates hyperuricemia in mice by inhibiting hepatic xanthine oxidase activity, modulating renal uric acid transporters, and improving metabolic disorders. Food Biosci. 2023, 56, 102639. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Wang, R.; Yu, Y.; Liu, X.; Tian, Z. Protective effect of sodium butyrate on intestinal barrier damage and uric acid reduction in hyperuricemia mice. Biomed. Pharmacother. 2023, 161, 114568. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, G.; Niu, T.; Guo, Y.; Wang, C.; Wang, X.; Yu, J. Polysaccharide isolated from Dioscorea septemloba improves hyperuricemia and alleviates renal fibrosis through gut-kidney axis in mice. Int. J. Biol. Macromol. 2024, 282, 137112. [Google Scholar] [CrossRef]
- Lv, Q.; Xu, D.; Zhang, X.; Yang, X.; Zhao, P.; Cui, X.; Liu, X.; Yang, W.; Yang, G.; Xing, S. Association of Hyperuricemia With Immune Disorders and Intestinal Barrier Dysfunction. Front. Physiol. 2020, 11, 524236. [Google Scholar] [CrossRef]
- Fei, W.; Zhang, J.; Wang, L.; Yang, Y.; Chen, Y.; Chen, Y.; Tao, R.; Zhu, Y. A clinical study to observe the efficacy and safety of Besunyen Detox Tea for constipation. Medicine 2022, 101, e30729. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, C.; Sawadogo, W.R.; Bian, Z.; Yuan, C. Herbal Medicines for Constipation and Phytochemical Comparison of Active Components. Am. J. Chin. Med. 2022, 50, 723–732. [Google Scholar]
- Xu, X.; Li, C.; Zhou, P.; Jiang, T. Uric acid transporters hiding in the intestine. Pharm. Biol. 2016, 54, 3151–3155. [Google Scholar] [CrossRef]
- Qi, X.; Ma, Y.; Guan, K.; Zhao, L.; Ma, Y.; Wang, R. Whey Protein Peptide Pro-Glu-Trp Ameliorates Hyperuricemia by Enhancing Intestinal Uric Acid Excretion, Modulating the Gut Microbiota, and Protecting the Intestinal Barrier in Rats. J. Agric. Food Chem. 2024, 72, 2573–2584. [Google Scholar] [CrossRef]
- DeBosch, B.J.; Kluth, O.; Fujiwara, H.; Schürmann, A.; Moley, K. Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9. Nat. Commun. 2014, 5, 4642. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kamada, N.; Moon, J.J. Oral nanomedicine for modulating immunity, intestinal barrier functions, and gut microbiome. Adv. Drug Deliv. Rev. 2021, 179, 114021. [Google Scholar] [PubMed]
- Meng, W.; Chen, L.; Ouyang, K.; Lin, S.; Zhang, Y.; He, J.; Wang, W. Chimonanthus nitens Oliv. leaves flavonoids alleviate hyperuricemia by regulating uric acid metabolism and intestinal homeostasis in mice. Food Sci. Hum. Wellness 2023, 12, 2440–2450. [Google Scholar] [CrossRef]
- Liu, C.; Ruan, F.; Chen, Z.; Han, J.; Ding, X.; Han, C.; Ye, L.; Yang, C.; Yu, Y.; Zuo, Z.; et al. Phenanthrene-induced hyperuricemia with intestinal barrier damage and the protective role of theabrownin: Modulation by gut microbiota-mediated bile acid metabolism. Sci. Total Environ. 2024, 949, 174923. [Google Scholar]
- Wang, Q.; Liang, J.; Zou, Q.; Wang, W.; Yan, G.; Guo, R.; Yuan, T.; Wang, Y.; Liu, X.; Liu, Z. Tryptophan Metabolism-Regulating Probiotics Alleviate Hyperuricemia by Protecting the Gut Barrier Integrity and Enhancing Colonic Uric Acid Excretion. J. Agric. Food Chem. 2024, 72, 26746–26761. [Google Scholar] [CrossRef]
- Liu, X.; Lin, C.; Dreffs, A.A.; Su, E.J.; Lawrence, D.A.; Antonetti, D.A. Occludin carboxy terminus is a dynein adaptor required for mouse embryonic development. Investig. Ophthalmol. Vis. Sci. 2024, 65, 315. [Google Scholar]
- Kuo, W.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef]
- Yao, D.; Dai, W.; Dong, M.; Dai, C.; Wu, S. MUC2 and related bacterial factors: Therapeutic targets for ulcerative colitis. Ebiomedicine 2021, 74, 103751. [Google Scholar] [CrossRef]
- Hansson, G.C. Mucins and the Microbiome. Annu. Rev. Biochem. 2020, 89, 769–793. [Google Scholar]
- Liu, Y.; Yu, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression. Int. J. Biol. Macromol. 2020, 164, 884–891. [Google Scholar]
- Zhou, Y.; Zhao, M.; Pu, Z.; Xu, G.; Li, X. Relationship between oxidative stress and inflammation in hyperuricemia: Analysis based on asymptomatic young patients with primary hyperuricemia. Medicine 2018, 97, e13108. [Google Scholar]
- He, J.; Han, S.; Li, X.; Wang, Q.; Cui, Y.; Chen, Y.; Gao, H.; Huang, L.; Yang, S. Diethyl Blechnic Exhibits Anti-Inflammatory and Antioxidative Activity via the TLR4/MyD88 Signaling Pathway in LPS-Stimulated RAW264.7 Cells. Molecules 2019, 24, 4502. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Niu, H.; Bian, M.; Zhang, X.; Zhang, X.; Zhou, Z. Study on the mechanism of Orthosiphon aristatus (Blume) Miq. in the treatment of hyperuricemia by microbiome combined with metabonomics. J. Ethnopharmacol. 2023, 317, 116805. [Google Scholar] [PubMed]
- Lakhal, R.; Pradel, N.; Postec, A.; Hamdi, M.; Ollivier, B.; Godfroy, A.; Fardeau, M. Vallitaleaguaymasensis gen. nov., sp. nov., isolated from marine sediment. Int. J. Syst. Evol. Microbiol. 2013, 63, 3019–3023. [Google Scholar]
- Morotomi, M.; Nagai, F.; Watanabe, Y. Description of Christensenella minuta gen. nov., sp. nov., isolated from human faeces, which forms a distinct branch in the order Clostridiales, and proposal of Christensenellaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2012, 62, 144–149. [Google Scholar]
- Yoon, J.; Kang, S.; Park, S.; Lee, S.; Oh, T. Reclassification of Aquaspirillum itersonii and Aquaspirillum peregrinum as Novispirillum itersonii gen. nov., comb. nov. and Insolitispirillum peregrinum gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 2830–2835. [Google Scholar]
- Qi, Q.; Zhang, H.; Jin, Z.; Wang, C.; Xia, M.; Chen, B.; Lv, B.; Peres Diaz, L.; Li, X.; Feng, R.; et al. Hydrogen sulfide produced by the gut microbiota impairs host metabolism via reducing GLP-1 levels in male mice. Nat. Metab. 2024, 6, 1601–1615. [Google Scholar]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Gálvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28. [Google Scholar]
- Lagkouvardos, I.; Pukall, R.; Abt, B.; Foesel, B.U.; Meier-Kolthoff, J.P.; Kumar, N.; Bresciani, A.; Martínez, I.; Just, S.; Ziegler, C.; et al. The Mouse Intestinal Bacterial Collection (miBC) provides host-specific insight into cultured diversity and functional potential of the gut microbiota. Nat. Microbiol. 2016, 1, 16131. [Google Scholar]
- Wang, P.; Zhang, X.; Zheng, X.; Gao, J.; Shang, M.; Xu, J.; Liang, H. Folic Acid Protects against Hyperuricemia in C57BL/6J Mice via Ameliorating Gut–Kidney Axis Dysfunction. J. Agric. Food Chem. 2022, 70, 15787–15803. [Google Scholar]
- Liu, X.; Zhang, Y.; Li, W.; Zhang, B.; Yin, J.; Liuqi, S.; Wang, J.; Peng, B.; Wang, S. Fucoidan Ameliorated Dextran Sulfate Sodium-Induced Ulcerative Colitis by Modulating Gut Microbiota and Bile Acid Metabolism. J. Agric. Food Chem. 2022, 70, 14864–14876. [Google Scholar] [PubMed]
- Chen, Y.; Pei, C.; Chen, Y.; Xiao, X.; Zhang, X.; Cai, K.; Deng, S.; Liang, R.; Xie, Z.; Li, P.; et al. Kidney tea ameliorates hyperuricemia in mice via altering gut microbiota and restoring metabolic profile. Chem.-Biol. Interact. 2023, 376, 110449. [Google Scholar]
- Adachi, S.; Kondo, S.; Sato, Y.; Yoshizawa, F.; Yagasaki, K. Anti-hyperuricemic effect of isorhamnetin in cultured hepatocytes and model mice: Structure–activity relationships of methylquercetins as inhibitors of uric acid production. Cytotechnology 2019, 71, 181–192. [Google Scholar] [PubMed]
- Wang, X.; Zhao, J.; Lin, Z.; Li, J.; Li, X.; Xu, X.; Wang, Y.; Lv, G.; Lin, H.; Lin, Z. Analysis of Polyphenol Extract from Hazel Leaf and Ameliorative Efficacy and Mechanism against Hyperuricemia Zebrafish Model via Network Pharmacology and Molecular Docking. Molecules 2024, 29, 317. [Google Scholar] [CrossRef]
- Yao, J.; He, H.; Xue, J.; Wang, J.; Jin, H.; Wu, J.; Hu, J.; Wang, R.; Kuchta, K. Mori Ramulus (Chin.Ph.)—The Dried Twigs of Morus alba L./Part 1: Discovery of Two Novel Coumarin Glycosides from the Anti-Hyperuricemic Ethanol Extract. Molecules 2019, 24, 629. [Google Scholar] [CrossRef]
| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| β-Actin | TATGCTCTCCCTCACGCCATCC | GTCACGCACGATTTCCCTCTCAG |
| occludin | ACGGACCCTGACCACTATGA | TCAGCAGCAGCCATGTACTC |
| ZO-1 | ACCCGAAACTGATGCTGTGGATAG | AAATGGCCGGGCAGAACTTGTGTA |
| MUC2 | TGTGGTCTGTGTGGGAACTT | GCTTACATCTGGGCAAGTGG |
| GLUT9 | TTGCTTTAGCTTCCCTGATGTG | GAGAGGTTGTACCCGTAGAGG |
| ABCG2 | GGCCTGGACAAAGTAGCAGA | GTTGTGGGCTCATCCAGGAA |
| TNF-α | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| IL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| IL-2 | TGAGCAGGATGGAGAATTACAGG | GTCCAAGTTCATCTTCTAGGCAC |
| Class | RT/min | Precursor m/z | Compound Name | Relative Content (%) | Adduct | Formula |
|---|---|---|---|---|---|---|
| Shikimates and phenylpropanoids (47, 30.52%) | 1.94 | 225.07 | (4-Acetyl-2-methoxyphenoxy)acetic acid | 0.036 | [M+H]+ | C11H12O5 |
| 6.93 | 315.08 | Altenuene | 0.421 | [M+Na]+ | C15H16O6 | |
| 3.65 | 317.12 | 4-Fluoro-2′,4′,6′-trimethoxychalcone | 1.474 | [M+H]+ | C18H17FO4 | |
| 18.58 | 391.28 | Dioctyl phthalate | 1.258 | [M+H]+ | C24H38O4 | |
| 4.09 | 593.15 | Vicenin-2 | 0.008 | [M-H]− | C27H30O15 | |
| 5.03 | 639.16 | 2-(3,4-Dihydroxyphenyl)-5-hydroxy-7-methoxy-4-oxo-4H-chromen-3-yl 2-O-.beta.-D-glucopyranosyl-.beta.-D-glucopyranoside | 0.004 | [M-H]− | C28H32O17 | |
| 5.16 | 441.08 | Epicatechin gallate | 0.008 | [M-H]− | C22H18O10 | |
| 8.86 | 285.04 | Kaempferol | 0.005 | [M-H]− | C15H10O6 | |
| 9.16 | 315.05 | Isorhamnetol | 0.083 | [M-H]− | C16H12O7 | |
| 3.36 | 125.03 | Ganhuangemin | 0.258 | [M-H-C9H6O4]− | C15H12O7 | |
| 3.43 | 137.02 | (-)-Catechin | 0.012 | [M-H-C8H8O3]− | C15H14O6 | |
| 2.61 | 151.04 | 2-Hydroxyphenylacetic acid | 0.002 | [M-H]− | C8H8O3 | |
| 1.87 | 151.04 | 4-Formyl-3-methoxyphenol | 4.358 | [M-H]− | C8H8O3 | |
| 3.13 | 151.04 | 2,5-Dihydroxyacetophenone | 0.003 | [M-H]− | C8H8O3 | |
| 4.36 | 153.02 | Quercetagetinidin cation | 0.061 | [Cat-2H-C8H4O3]− | C15H11O7 | |
| 2.82 | 153.06 | 2-(2-Hydroxyethoxy)phenol | 0.436 | [M-H]− | C8H10O3 | |
| 3.57 | 163.04 | 2,4-Dihydroxy-3,6-dimethylbenzoic acid | 0.032 | [M-H-H2O]− | C9H10O4 | |
| 5.05 | 163.04 | p-Coumaric acid | 0.009 | [M-H]− | C9H8O3 | |
| 3.58 | 165.02 | 3-Formyl-2-hydroxybenzoic acid | 0.003 | [M-H]− | C8H6O4 | |
| 5.15 | 169.02 | 3-Galloylgallocatechin | 0.008 | [M-H-C15H12O6]− | C22H18O11 | |
| 8.07 | 177.06 | Methyl 4-coumarate | 0.237 | [M-H]− | C10H10O3 | |
| 4.97 | 179.04 | 5-Acetylsalicylic acid | 0.005 | [M-H]− | C9H8O4 | |
| 5.86 | 181.05 | 3,4-Dihydroxyhydrocinnamic acid | 0.006 | [M-H]− | C9H10O4 | |
| 7.39 | 181.05 | Homovanillic acid | 0.021 | [M-H]− | C9H10O4 | |
| 1.53 | 189.04 | 3-Dehydroquinic acid | 0.264 | [M-H]− | C7H10O6 | |
| 3.42 | 193.05 | 3-(3,4-Dimethoxyphenyl)-2-hydroxy-3-methoxypropyl (2E)-3-(3,4-dihydroxyphenyl)prop-2-enoate | 0.249 | [M-H-C11H14O4]− | C21H24O8 | |
| 3.81 | 225.05 | 3-Hydroxy-4-methylbenzo[c]chromen-6-one | 9.177 | [M-H]− | C14H10O3 | |
| 7.72 | 227.07 | Resveratrol | 0.770 | [M-H]− | C14H12O3 | |
| 10.71 | 267.07 | 7-Hydroxy-3-(4-hydroxyphenyl)-4-methylcoumarin | 0.327 | [M-H]− | C16H12O4 | |
| 9.89 | 283.06 | 5,7-dihydroxy-2′-methoxyflavone | 0.084 | [M-H]− | C16H12O5 | |
| 7.42 | 285.04 | Luteolin | 0.239 | [M-H]− | C15H10O6 | |
| 3.48 | 299.08 | Salicylic acid beta-D-glucoside | 0.001 | [M-H]− | C13H16O8 | |
| 10.02 | 300.03 | N-(2-Hydroxyphenyl)-2,4,6-trinitrobenzamide | 0.050 | [M-H-HNO2]− | C13H8N4O8 | |
| 3.49 | 313.06 | Salicyl glucuronide | 0.033 | [M-H]− | C13H14O9 | |
| 4.02 | 315.09 | 3-(3,4-Dimethoxyphenyl)-1-(2-hydroxy-4,6-dimethoxyphenyl)propan-1-one | 1.722 | [M-H-C2H6]− | C19H22O6 | |
| 11.10 | 327.09 | 3-Hydroxy-7,8,3′-trimethoxyflavone | 3.306 | [M-H]− | C18H16O6 | |
| 5.06 | 327.09 | 2,4-Bis(4-hydroxyphenyl)cyclobutane-1,3-dicarboxylic acid | 0.008 | [M-H]− | C18H16O6 | |
| 1.89 | 329.09 | Helicid | 0.785 | [M+HCO2]− | C13H16O7 | |
| 11.45 | 337.04 | 6,7′-Dihydroxy-2,2’-dioxo-2H,2’H-[8,8’-bichromen]-7-yl hexopyranoside | 0.008 | [M-H-C6H10O5]− | C24H20O12 | |
| 5.84 | 417.08 | Kaempferol 3-xyloside | 0.341 | [M-H]− | C20H18O10 | |
| 6.43 | 419.14 | Rhapontin | 0.003 | [M-H]− | C21H24O9 | |
| 5.13 | 463.09 | Hyperin | 0.053 | [M-H]− | C21H20O12 | |
| 6.25 | 473.17 | 2-(.beta.-D-Glucopyranosyloxy)-4-(prop-2-en-1-yl)phenyl .beta.-D-glucopyranoside | 1.893 | [M-H]− | C21H30O12 | |
| 4.12 | 609.15 | Cyanin cation | 0.166 | [Cat-2H]− | C27H31O16 | |
| 4.31 | 635.09 | 1,3,6-Trigalloylglucose | 0.037 | [M-H]− | C27H24O18 | |
| 2.62 | 651.20 | 4-O-D-Glucopyranosyl-p-coumaric acid | 0.891 | [2M-H]− | C15H18O8 | |
| 2.44 | 665.06 | Fenoxaprop | 0.029 | [2M-H]− | C16H12ClNO5 | |
| Alkaloids (25, 16.23%) | 1.15 | 110.06 | 3-Cyanocyclopentanone | 1.461 | [M+H]+ | C6H7NO |
| 0.86 | 136.06 | Adenine | 0.266 | [M+H]+ | C5H5N5 | |
| 1.88 | 152.07 | Adrenochrome | 0.346 | [M+H-CO]+ | C9H9NO3 | |
| 1.66 | 166.12 | (-)-Pseudoephedrine | 0.007 | [M+H]+ | C10H15NO | |
| 1.14 | 169.03 | Uric acid | 0.006 | [M+H]+ | C5H4N4O3 | |
| 4.01 | 178.09 | 1-(o-Hydroxyphenyl)-2-pyrrolidinone | 0.304 | [M+H]+ | C10H11NO2 | |
| 1.52 | 203.08 | 2,3-Dimethyl-6-quinoxalinecarboxylic acid | 4.466 | [M+H]+ | C11H10N2O2 | |
| 2.24 | 220.13 | Ritalinic acid | 0.189 | [M+H]+ | C13H17NO2 | |
| 9.92 | 229.08 | Galanthaminone | 0.022 | [M+H-C3H7N]+ | C17H19NO3 | |
| 3.18 | 237.16 | (.+/-.)-Dropropizine | 0.977 | [M+H]+ | C13H20N2O2 | |
| 2.62 | 255.13 | 3-(3,4,5-Trimethoxyphenyl)propanohydrazide | 0.002 | [M+H]+ | C12H18N2O4 | |
| 0.83 | 260.11 | 1-Piperazineethanol, 4-(7-nitro-4-benzofurazanyl)- | 0.637 | [M+H-H2O2]+ | C12H15N5O4 | |
| 2.85 | 144.05 | 7-Quinolinol | 0.150 | [M-H]− | C9H7NO | |
| 2.64 | 155.05 | 1,3-Dimethylbarbituric acid | 0.045 | [M-H]− | C6H8N2O3 | |
| 5.02 | 160.04 | 4-[(E)-6,7-Dihydroxy-3,7-dimethyl-oct-2-enoxy]carbostyril | 6.548 | [M-H-C10H18O2]− | C19H25NO4 | |
| 4.72 | 190.05 | 5-Hydroxyindole-3-acetic acid | 0.032 | [M-H]− | C10H9NO3 | |
| 3.53 | 193.10 | 4-(Methylamino)-4-(3-pyridyl)butyric acid | 0.022 | [M-H]− | C10H14N2O2 | |
| 6.67 | 213.11 | Sulfisomidin | 2.936 | [M-H-SO2]− | C12H14N4O2S | |
| 4.01 | 227.06 | 1-Phenyl-1H-pyrazolo[3,4-d]pyrimidine-4,6-diol | 0.536 | [M-H]− | C11H8N4O2 | |
| 14.84 | 250.15 | 2-[(2-Ethylbutanoyl)amino]-4,5-dimethoxybenzoic acid | 0.104 | [M-H-CO2]− | C15H21NO5 | |
| 2.34 | 275.11 | Ofloxacin | 2.422 | [M-H-C4H5O2]− | C18H20FN3O4 | |
| 1.48 | 279.07 | N-(1,3-Dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-phenylacetamide | 0.022 | [M-H]− | C16H12N2O3 | |
| 6.09 | 417.20 | Dauricine | 0.110 | [M-H-C12H16NO2]− | C38H44N2O6 | |
| 7.19 | 449.15 | 3-(3,4-Dihydroxy-1-keto-2,2-dimethyl-3aH-imidaz[1,2-a]indol-4-yl)-2-(4-ketoquinazolin-3-yl)propionic acid | 0.784 | [M-H]− | C23H22N4O6 | |
| 2.66 | 527.25 | Chaetoglobosin A | 1.321 | [M-H]− | C32H36N2O5 | |
| Terpenoids (17, 11.04%) | 9.15 | 180.17 | Rimantadine | 0.433 | [M+H]+ | C12H21N |
| 10.84 | 185.13 | Parthenolide | 1.336 | [M+H-CH4O3]+ | C15H20O3 | |
| 9.43 | 247.13 | Bisbynin | 0.096 | [M+H-2H2O]+ | C15H22O5 | |
| 11.87 | 261.18 | Jaeskeanadiol | 0.001 | [M+Na]+ | C15H26O2 | |
| 9.69 | 367.19 | Uabanin | 0.006 | [M+H-C6H18O8]+ | C29H44O12 | |
| 9.76 | 385.24 | Resinobufagin | 4.430 | [M+H]+ | C24H32O4 | |
| 3.73 | 201.08 | 3-(2-Hydroxybutan-2-yl)-5-oxooxolane-3-carboxylic acid | 0.025 | [M-H]− | C9H14O5 | |
| 6.43 | 203.14 | Viscic acid | 0.002 | [M-H-CH2O2]− | C15H22O3 | |
| 6.43 | 265.15 | Cyperanic acid | 0.749 | [M-H]− | C15H22O4 | |
| 8.39 | 267.16 | 2-((1S,2S,4aR,8aS)-1-hydroxy-4a-methyl-8-methylenedecahydronaphthalen-2-yl)acrylic acid | 0.019 | [M+OH]− | C15H22O3 | |
| 1.76 | 271.13 | 2,6-Dihydroxy-1,1,7-trimethyl-2,9,10,10a-tetrahydrophenanthren-3-one | 0.089 | [M-H]− | C17H20O3 | |
| 11.67 | 287.17 | 4-(4-Hydroxyphenyl)-6,8,9-trimethyl-3-oxabicyclo[3.3.1]non-6-ene-1-methanol | 0.265 | [M-H]− | C18H24O3 | |
| 7.38 | 357.16 | 9-Hydroxyjasmesoside | 0.005 | [M-H-C10H16O6]− | C27H42O14 | |
| 1.88 | 371.10 | 11-Methyloleoside | 0.030 | [M-H-CH4O]− | C17H24O11 | |
| 4.36 | 445.23 | [7′-Formyl-3,4′-dihydroxy-6′-(hydroxymethyl)-4,4,7,8a-tetramethylspiro[2,3,4a,5,6,7-hexahydro-1H-naphthalene-8,2′-3H-1-benzofuran]-2-yl] acetate | 0.012 | [M-H]− | C25H34O7 | |
| 3.66 | 447.19 | 17.beta.-Estradiol 3-.beta.-D-glucuronide | 0.033 | [M-H]− | C24H32O8 | |
| 5.72 | 517.19 | Gossypol | 0.406 | [M-H]− | C30H30O8 | |
| 10.72 | 517.32 | Trachelosperogenin A1 | 0.006 | [M-H-C6H10O5]− | C36H56O12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Xi, Y.; Gu, F.; Peng, L.; Li, J. Protective Effects of a Polyherbal Mixture on Intestinal Injury via the NF-κB Signaling Pathway and Gut Microbiota Modulation in Hyperuricemic Mice. Foods 2025, 14, 1118. https://doi.org/10.3390/foods14071118
Wang H, Xi Y, Gu F, Peng L, Li J. Protective Effects of a Polyherbal Mixture on Intestinal Injury via the NF-κB Signaling Pathway and Gut Microbiota Modulation in Hyperuricemic Mice. Foods. 2025; 14(7):1118. https://doi.org/10.3390/foods14071118
Chicago/Turabian StyleWang, Haoluan, Yu Xi, Fengju Gu, Linlin Peng, and Jian Li. 2025. "Protective Effects of a Polyherbal Mixture on Intestinal Injury via the NF-κB Signaling Pathway and Gut Microbiota Modulation in Hyperuricemic Mice" Foods 14, no. 7: 1118. https://doi.org/10.3390/foods14071118
APA StyleWang, H., Xi, Y., Gu, F., Peng, L., & Li, J. (2025). Protective Effects of a Polyherbal Mixture on Intestinal Injury via the NF-κB Signaling Pathway and Gut Microbiota Modulation in Hyperuricemic Mice. Foods, 14(7), 1118. https://doi.org/10.3390/foods14071118





