Comparative Evaluation of Qualitative and Nutraceutical Parameters in Fresh Fruit and Processed Products of ‘Lady Cot’ and Vesuvian ‘Pellecchiella’ Apricot Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Reagents
2.3. Physico-Chemical Characterization of Fruits
2.4. Methanolic Extraction and UHPLC-Q-Orbitrap HRMS Analysis of Fruits
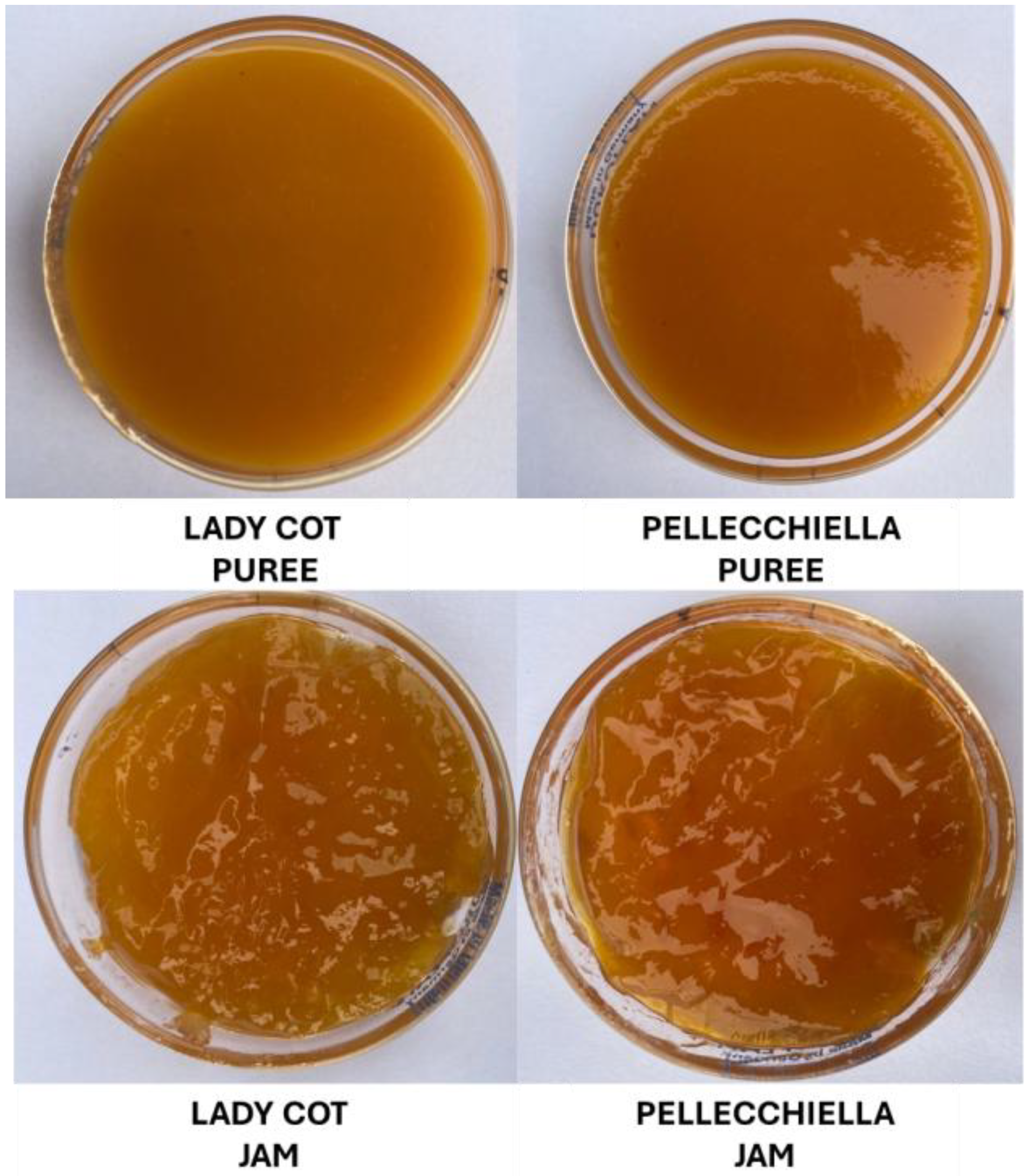
2.5. Puree and Jam Preparation
2.6. Color Analysis from Fruit to Jam
2.7. Preparation of Methanolic Extracts for Spectrophotometer Analysis
2.8. Total Phenol Content
2.9. Antioxidant Activity by ABTS, DPPH and FRAP Assays
2.10. Determination of Carotenoids
2.11. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Characterization of the Apricot Fruits
3.2. Determination of Polyphenol Content in the Apricot Fruits
3.3. Evolution of Color, Carotenoids, Total Polyphenols, and Antioxidant Activity: From Fresh Fruit to Puree and Jam
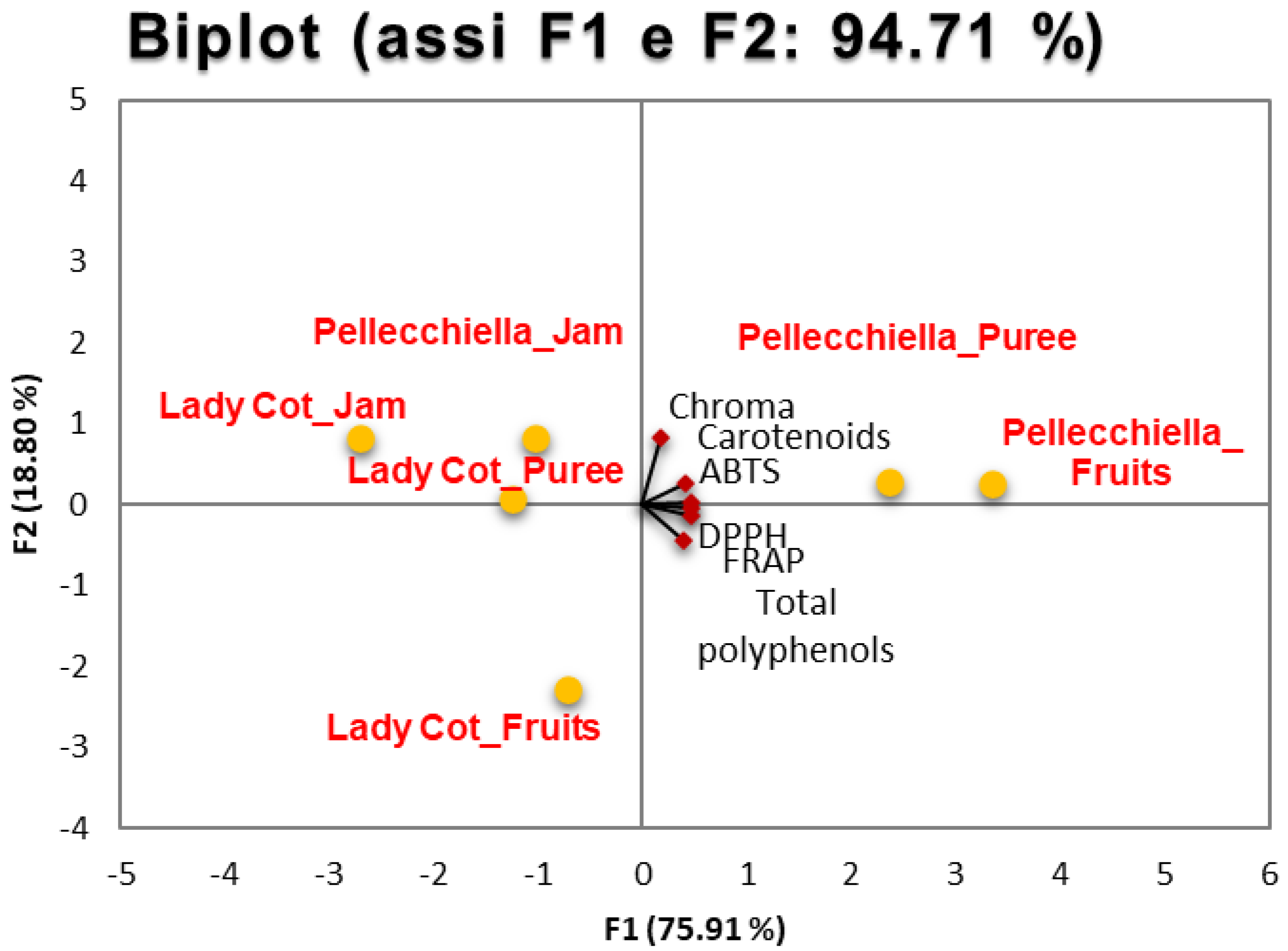
3.4. Principal Component Analysis of Bioactive Parameters and Color from Whole Fruit to Puree and Jam
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Corato, U. Improving the shelf-life and quality of fresh and minimally-processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. Nutr. 2020, 60, 940–975. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, B.E.; Ruiz, D.; Salazar, J.A.; Rubio, M.; Martínez-García, P.J.; Martínez-Gómez, P. Analysis of Metabolites and Gene Expression Changes Relative to Apricot (Prunus armeniaca L.) Fruit Quality During Development and Ripening. Front. Plant Sci. 2020, 11, 1269. [Google Scholar] [CrossRef] [PubMed]
- Taiti, C.; Vivaldo, G.; Masi, E.; Giordani, E.; Nencetti, V. Postharvest Monitoring and Consumer Choice on Traditional and Modern Apricot Cultivars. Eur. Food Res. Technol. 2023, 249, 2719–2739. [Google Scholar] [CrossRef]
- Piagnani, M.C.; Castellari, L.; Sgarbi, P.; Bassi, D. Fruit quality evaluation of diverse apricot cultivars. Asp. Appl. Biol. 2013, 119, 139–144. [Google Scholar]
- Di Vaio, C.; Cirillo, C.; Pannico, A.; Graziani, G.; Ritieni, A.; Famiani, F. Bioactive Compounds and Fruit Quality Traits of Vesuvian Apricot Cultivars (Prunus armeniaca L.) and Use of Skin Cover Colour as a Harvesting Index. Aust. J. Crop Sci. 2019, 13, 2022–2029. [Google Scholar] [CrossRef]
- Fratianni, F.; d’Acierno, A.; Albanese, D.; Matteo, M.D.; Coppola, R.; Nazzaro, F. Biochemical Characterization of Traditional Varieties of Apricots (Prunus armeniaca L.) of the Campania Region, Southern Italy. Foods 2021, 11, 100. [Google Scholar] [CrossRef]
- Iovane, M.; Izzo, L.G.; Cirillo, A.; Romano, L.E.; Di Vaio, C.; Aronne, G. Flowering and pollen resilience to high temperature of apricot cultivars. Sci. Hortic. 2022, 304, 111261. [Google Scholar] [CrossRef]
- Cirillo, A.; De Luca, L.; Izzo, L.; Cepparulo, M.; Graziani, G.; Ritieni, A.; Romano, R.; Di Vaio, C. Biochemical and Nutraceutical Characterization of Different Accessions of the Apricot (Prunus armeniaca L.). Horticulturae 2023, 9, 546. [Google Scholar] [CrossRef]
- Lo Bianco, R.; Farina, V.; Indelicato, S.G.; Filizzola, F.; Agozzino, P. Fruit Physical, Chemical and Aromatic Attributes of Early, Intermediate and Late Apricot Cultivars. J. Sci. Food Agric. 2010, 90, 1008–1019. [Google Scholar] [CrossRef]
- Fratianni, A.; Cinquanta, L.; Panfili, G. Degradation of Carotenoids in Orange Juice during Microwave Heating. LWT–Food Sci. Technol. 2010, 43, 867–871. [Google Scholar] [CrossRef]
- Syamaladevi, R.M.; Andrews, P.K.; Davies, N.M.; Walters, T.; Sablani, S.S. Storage Effects on Anthocyanins, Phenolics and Antioxidant Activity of Thermally Processed Conventional and Organic Blueberries. J. Sci. Food Agric. 2012, 92, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef] [PubMed]
- Rebeaud, S.G. Cultivar, Maturity at Harvest and Postharvest Treatments Influence Softening of Apricots. Postharvest Biol. Technol. 2023, 195, 112134. [Google Scholar]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Bladé, C.; Arola-Arnal, A.; Muguerza, B. Optimization of Extraction Methods for Characterization of Phenolic Compounds in Apricot Fruit (Prunus armeniaca). Food Funct. 2019, 10, 6492–6502. [Google Scholar] [CrossRef]
- Izzo, L.; Rodríguez-Carrasco, Y.; Pacifico, S.; Castaldo, L.; Narváez, A.; Ritieni, A. Colon Bioaccessibility under In Vitro Gastrointestinal Digestion of a Red Cabbage Extract Chemically Profiled through UHPLC-Q-Orbitrap HRMS. Antioxidants 2020, 9, 955. [Google Scholar] [CrossRef]
- Cirillo, A.; Izzo, L.; Ciervo, A.; Ledenko, I.; Cepparulo, M.; Piscitelli, A.; Di Vaio, C. Optimizing apricot yield and quality with biostimulant interventions: A comprehensive analysis. Horticulturae 2024, 10, 447. [Google Scholar] [CrossRef]
- Falciano, A.; Moresi, M.; Masi, P. Phenomenology of Neapolitan pizza baking in a traditional wood-fired oven. Foods 2023, 12, 890. [Google Scholar] [CrossRef]
- Dóka, O.; Ficzek, G.; Luterotti, S.; Bicanic, D.; Spruijt, R.; Buijnsters, J.G.; Szalay, L.; Végvári, G. Simple and Rapid Quantification of Total Carotenoids in Lyophilized Apricots (Prunus armeniaca L.) by Means of Reflectance Colorimetry and Photoacoustic Spectroscopy. Food Technol. Biotechnol. 2013, 51, 453–459. [Google Scholar]
- Falciano, A.; Sorrentino, A.; Masi, P.; Di Pierro, P. Development of functional pizza base enriched with jujube (Ziziphus jujuba) powder. Foods 2022, 11, 1458. [Google Scholar] [CrossRef]
- Izzo, L.; Castaldo, L.; Lombardi, S.; Gaspari, A.; Grosso, M.; Ritieni, A. Bioaccessibility and Antioxidant Capacity of Bioactive Compounds From Various Typologies of Canned Tomatoes. Front. Nutr. 2022, 9, 849163. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Rodrigues, S.; García-Pérez, J.V.; Cárcel, J.A. Effects of Ultrasound-Assisted Air-Drying on Vitamins and Carotenoids of Cherry Tomatoes. Dry. Technol. 2016, 34, 986–996. [Google Scholar] [CrossRef]
- Rodrigues, M.; Baptistella, J.L.C.; Horz, D.C.; Bortolato, L.M.; Mazzafera, P. Organic Plant Biostimulants and Fruit Quality—A Review. Agronomy 2020, 10, 988. [Google Scholar] [CrossRef]
- Valero, C.; Crisosto, C.H.; Slaughter, D. Relationship between Nondestructive Firmness Measurements and Commercially Important Ripening Fruit Stages for Peaches, Nectarines and Plums. Postharvest Biol. Technol. 2007, 44, 248–253. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Garner, D.; Crisosto, G.M.; Bowerman, E. Increasing ‘Blackamber’ Plum (Prunus salicina Lindell) Consumer Acceptance. Postharvest Biol. Technol. 2004, 34, 237–244. [Google Scholar] [CrossRef]
- Ayour, J. Evolution of Pigments and Their Relationship with Skin Color Based on Ripening in Fruits of Different Moroccan Genotypes of Apricots (Prunus armeniaca L.). Sci. Hortic. 2016, 207, 168–175. [Google Scholar] [CrossRef]
- Carbone, K.; Ciccoritti, R.; Paliotta, M.; Rosato, T.; Terlizzi, M.; Cipriani, G. Chemometric Classification of Early-Ripening Apricot (Prunus armeniaca, L.) Germplasm Based on Quality Traits, Biochemical Profiling and in Vitro Biological Activity. Sci. Hortic. 2018, 227, 187–195. [Google Scholar] [CrossRef]
- Alajil, O.; Sagar, V.R.; Kaur, C.; Rudra, S.G.; Sharma, R.R.; Kaushik, R.; Verma, M.K.; Tomar, M.; Kumar, M.; Mekhemar, M. Nutritional and Phytochemical Traits of Apricots (Prunus armeniaca L.) for Application in Nutraceutical and Health Industry. Foods 2021, 10, 1344. [Google Scholar] [CrossRef]
- Harker, F.R.; Johnston, J.W. Importance of Texture in Fruit and Its Interaction with Flavour. In Fruit and Vegetable Flavour; Elsevier: Amsterdam, The Netherlands, 2008; pp. 132–149. ISBN 978-1-84569-183-7. [Google Scholar]
- Ali, S. Physico-Chemical Characteristics of Apricot (Prunus armeniaca L.) Grown in Northern Areas of Pakistan. Sci. Hortic. 2011, 130, 386–392. [Google Scholar] [CrossRef]
- Melgarejo, P.; Calín-Sánchez, Á.; Carbonell-Barrachina, Á.A.; Martínez-Nicolás, J.J.; Legua, P.; Martínez, R.; Hernández, F. Antioxidant Activity, Volatile Composition and Sensory Profile of Four New Very-Early Apricots (Prunus armeniaca L.): Fruit Quality of Very-Early-Season Apricots. J. Sci. Food Agric. 2014, 94, 85–94. [Google Scholar] [CrossRef]
- Ribeiro, H.; Cunha, M.; Abreu, I. Quantitative Forecasting of Olive Yield in Northern Portugal Using a Bioclimatic Model. Aerobiologia 2008, 24, 141–150. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals: Prooxidant Activity of Polyphenols and Carotenoids. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- MacRae, K.; Connolly, K.; Vella, R.; Fenning, A. Epicatechin’s Cardiovascular Protective Effects Are Mediated via Opioid Receptors and Nitric Oxide. Eur. J. Nutr. 2019, 58, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.; Burgos, V.; Marín, V.; Camins, A.; Olloquequi, J.; Gonzalez-Chavarría, I.; Ulrich, H.; Wyneken, U.; Ortiz, L.; Paz, C. Therapeutic Potential of Caffeic Acid Phenethyl Ester (Cape) and Derivatives With Anti-Inflammatory, Antioxidant, and Neuroprotective Activities. Exp. Ther. Med. 2023, 9, 1582–1588. [Google Scholar]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Poiana, M.A.; Alexa, E.; Mateescu, C. Tracking antioxidant properties and color changes in low-sugar bilberry jam as effect of processing, storage and pectin concentration. Chem. Cent. J. 2012, 6, 4. [Google Scholar] [CrossRef]
- Chironi, S.; Ingrassia, M. Study of the importance of emotional factors connected to the colors of fresh-cut cactus pear fruits in consumer purchase choices for a marketing positioning strategy. In ISHS Acta Horticulturae 1067, Proceedings of the VIII International Congress on Cactus Pear and Cochineal, Palermo, Italy, 28 October 2013; 2013; pp. 209–215. [Google Scholar]
- Dragovicuzelac, V.; Levaj, B.; Mrkic, V.; Bursac, D.; Boras, M. The Content of Polyphenols and Carotenoids in Three Apricot Cultivars Depending on Stage of Maturity and Geographical Region. Food Chem. 2007, 102, 966–975. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J. Phenotypic Diversity and Relationships of Fruit Quality Traits in Apricot (Prunus armeniaca L.) Germplasm. Euphytica 2008, 163, 143–158. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive compounds of citrus fruits: A review of composition and health benefits of carotenoids, flavonoids, limonoids, and terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Saini, R.K. Carotenoid Extraction Methods: A Review of Recent Developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.; Egea, J.; Tomás-Barberán, F.A.; Gil, M.I. Carotenoids from New Apricot (Prunus armeniaca L.) Varieties and Their Relationship with Flesh and Skin Color. J. Agric. Food Chem. 2005, 53, 6368–6374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhao, S.; Xu, M.; Niu, Y.; Nasier, M.; Fan, G.; Quan, S.; Zhang, S.; Wang, Y.; Liao, K. Identification of Key Genes Controlling Carotenoid Metabolism during Apricot Fruit Development by Integrating Metabolic Phenotypes and Gene Expression Profiles. J. Agric. Food Chem. 2021, 69, 9472–9483. [Google Scholar] [CrossRef]
- Gecer, M.K.; Kan, T.; Gundogdu, M.; Ercisli, S.; Ilhan, G.; Sagbas, H.I. Physicochemical Characteristics of Wild and Cultivated Apricots (Prunus armeniaca L.) from Aras Valley in Turkey. Genet. Resour. Crop Evol. 2020, 67, 935–945. [Google Scholar] [CrossRef]
- Fratianni, F.; Cozzolino, R.; d’Acierno, A.; Ombra, M.N.; Spigno, P.; Riccardi, R.; Malorni, L.; Stocchero, M.; Nazzaro, F. Biochemical Characterization of Some Varieties of Apricot Present in the Vesuvius Area, Southern Italy. Front. Nutr. 2022, 9, 854868. [Google Scholar] [CrossRef]
- Kuşçu, A.; Bulantekin, Ö. Determination of Phenolics, Organic Acids, Minerals and Volatile Compounds of Jujube (Ziziphus jujuba Miller) Jam Produced by under Vacuum Evaporation Compared with Open Pan Method. J. Food Meas. Charact. 2021, 15, 1127–1138. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Cilla, A.; Bosch, L.; Barberá, R.; Alegría, A. Effect of Processing on the Bioaccessibility of Bioactive Compounds—A Review Focusing on Carotenoids, Minerals, Ascorbic Acid, Tocopherols and Polyphenols. J. Food Compos. Anal. 2018, 68, 3–15. [Google Scholar] [CrossRef]
- Marques, M.C.; Hacke, A.; Neto, C.A.C.; Mariutti, L.R. Impact of Phenolic Compounds in the Digestion and Absorption of Carotenoids. Curr. Opin. Food Sci. 2021, 39, 190–196. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Rababah, T.M.; Al-Mahasneh, M.A.; Kilani, I.; Yang, W.; Alhamad, M.N.; Ereifej, K.; Al-u’datt, M. Effect of Jam Processing and Storage on Total Phenolics, Antioxidant Activity, and Anthocyanins of Different Fruits: Effect of Processing on Jam Constituents. J. Sci. Food Agric. 2011, 91, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Delalibera, P.; Branco Tiago Queiroz, B.; Fernandes Marques De Souza, R.L.; De Araújo, R.L.; Queiroz Assis, R.; Oliveira Guimarães Abud, C.; De Oliveira Rios, A.; Becker Pertuzatti, P. Unlocking the Potential of Pequi (Caryocar Brasiliense Camb.): Stability of Phenolic Compounds, Carotenoid Profile and Vitamin A after Drying. Food Res. Int. 2024, 197, 115196. [Google Scholar] [CrossRef] [PubMed]
- Drogoudi, P.D.; Vemmos, S.; Pantelidis, G.; Petri, E.; Tzoutzoukou, C.; Karayiannis, I. Physical Characters and Antioxidant, Sugar, and Mineral Nutrient Contents in Fruit from 29 Apricot (Prunus armeniaca L.) Cultivars and Hybrids. J. Agric. Food Chem. 2008, 56, 10754–10760. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elnoor, A. Effect of home-processing on the antioxidant properties of apricot products. Alex. Sci. Exch. J. 2019, 40, 629–639. [Google Scholar] [CrossRef]
- Giuffrida, D.; Torre, G.; Dugo, P.; Dugo, G. Determination of the Carotenoid Profile in Peach Fruits, Juice and Jam. Fruits 2013, 68, 39–44. [Google Scholar] [CrossRef][Green Version]
- Hwang, E.; Stacewicz-Sapuntzakis, M.; Bowen, P.E. Effects of Heat Treatment on the Carotenoid and Tocopherol Composition of Tomato. J. Food Sci. 2012, 77, C1109–C1114. [Google Scholar] [CrossRef]
- Azizah, A.H.; Wee, K.C.; Azizah, O.; Azizah, M. Effect of boiling and stir frying on total phenolics, carotenoids and radical scavenging activity of pumpkin (Cucurbita moschato). Int. Food Res. J. 2009, 16, 45–51. [Google Scholar]
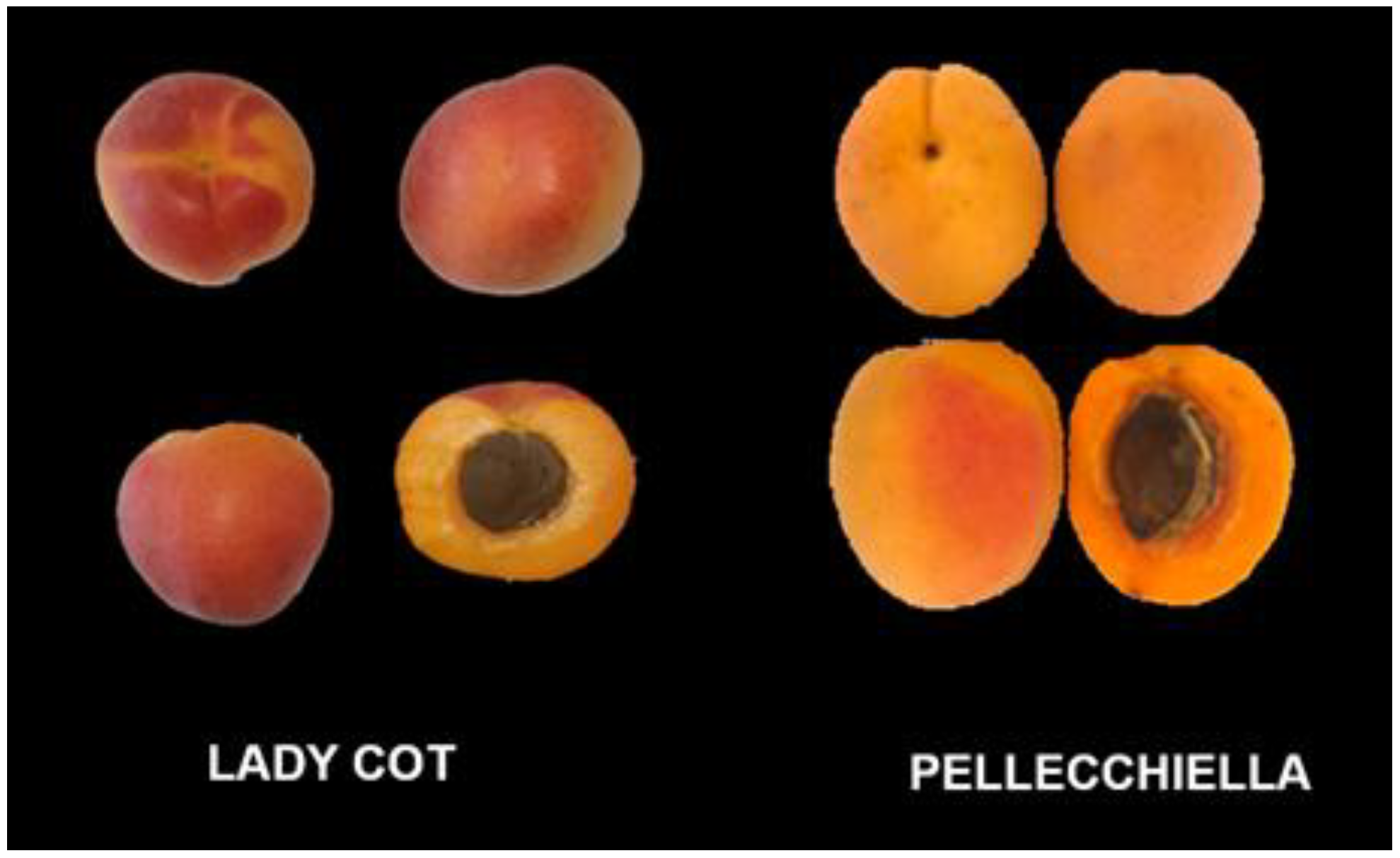
| Characteristic | Lady Cot | Pellecchiella |
|---|---|---|
| Origin | Selected in France | Native to the Vesuvian area |
| Fruit shape | Round, symmetrical | Oval, slightly elongated |
| Skin color | Bright orange with red streaks | Intense yellow |
| Flesh color | Light orange, firm | Yellow, juicy |
| Texture | Firm and crunchy | Medium, slightly fibrous |
| Taste | Sweet-tart, well-balanced | Sweet, aromatic |
| Fruit size | Medium | Small to medium |
| Main use | Fresh consumption, processing | Fresh consumption, jam-making |
| Physical Trait | Lady Cot | Pellecchiella | Significance |
|---|---|---|---|
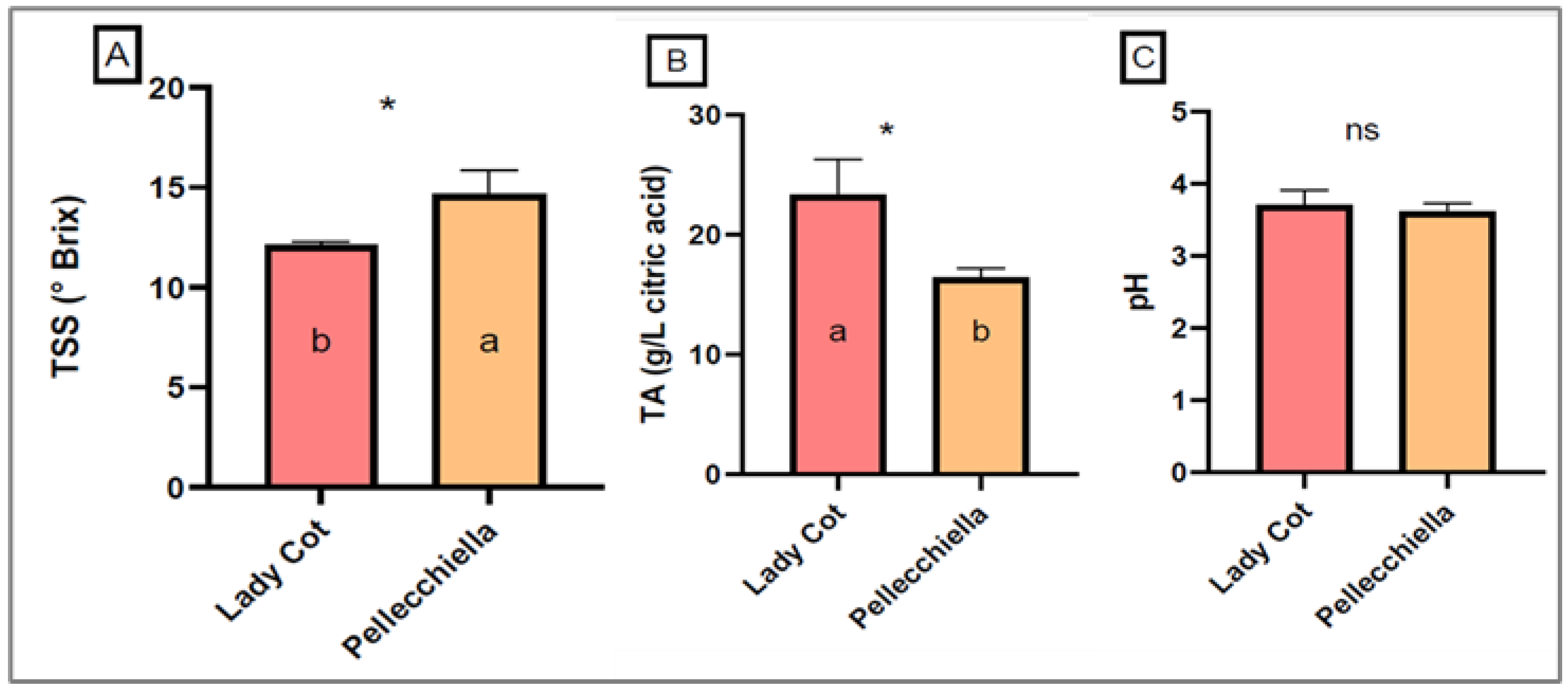
| Fruit Weight (g) | 61.04 ± 8.33 b | 45.68 ± 8.85 a | *** |
| Firmness (kg/0.5 cm2) | 2.33 ± 0.18 a | 1.75 ± 0.22 b | *** |
| Polar Diameter (mm) | 48.92 ± 2.77 a | 47.03 ± 2.95 b | *** |
| Equatorial Diameter (mm) | 46.03 ± 2.76 b | 40.77 ± 3.72 a | *** |
| Transversal Diameter (mm) | 50.50 ± 3.08 a | 47.57 ± 3.29 b | ** |
| L* | 52.88 ± 2.59 b | 62.45 ± 3.59 a | *** |
| a* | 39.37 ± 7.59 b | 41.13 ± 1.59 a | *** |
| b* | 38.93 ± 8.59 b | 61.91 ± 1.59 a | *** |
| C* | 55.38 ± 1.74 b | 74.33 ± 2.11 a | *** |
| Redness Index | 1.01 ± 0.04 a | 0.66 ± 0.07 b | *** |
| Polyphneol (μg/g) | Lady Cot | Pellecchiella | Significance |
|---|---|---|---|
| quinic_acid | 346.42 ± 49.06 a | 241.78 ± 32.41 b | ** |
| protocatechiuc_acid | 1.51 ± 0.22 | 1.27 ± 0.29 | ns |
| caffeic_acid | 16.60 ± 3.93 b | 25.67 ± 4.26 a | * |
| epicatechin | 30.34 ± 7.22 b | 80.94 ± 14 a | *** |
| chlorogenic_acid | 641.14 ± 35.37 | 770.65 ± 147.96 | ns |
| catechin | 76.84 ± 5.85 b | 336.33 ± 62.84 a | *** |
| p-coumaric | 10.10 ± 4.61 | 13.37 ± 0.90 | ns |
| siringic_acid | 24.38 ± 2.94 | 25.9 ± 0.72 | ns |
| ferulic_acid | 58.64 ± 9.62 b | 77.87 ± 11.02 a | * |
| rutin_hydrate | 508.06 ± 135.32 | 471.69 ± 49.55 | ns |
| isorhamnetin-3-rutinoside | 1.89 ± 0.11 | 2.18 ± 0.44 | ns |
| myricitrin | 4.64 ± 1.84 | 3.44 ± 0.39 | ns |
| Color Value | Lady Cot | Pellecchiella | |
|---|---|---|---|
| Fruits: Mesocarp | L* | 63.66 ± 3.16 b | 63.77 ± 3.85 b |
| a* | 23.31 ± 1.13 a | 44.79 ± 2.21 a * | |
| b* | 58.79 ± 2.90 b | 70.02 ± 3.50 b * | |
| C* | 63.24 ± 3.13 b | 83.13 ± 2.19 a * | |
| Redness Index | 0.40 ± 0.03 b | 0.64 ± 0.06 a * | |
| Puree Apricot | L* | 71.39 ± 1.28 a | 73.07 ± 1.55 a |
| a* | 24.88 ± 1.03 a | 28.07 ± 1.43 b * | |
| b* | 74.38 ± 1.76 a | 76.31 ± 2.00 a | |
| C* | 78.42 ± 1.40 a | 81.30 ± 1.85 a | |
| Redness Index | 0.33 ± 0.03 a | 0.37 ± 0.05 b | |
| Jam Apricot | L* | 73.00 ±1.54 a | 67.14 ± 1.61 b * |
| a* | 25.04 ±1.95 a | 30.06 ± 1.75 b * | |
| b* | 75.77 ± 1.98 a | 71.34 ± 1.27 b * | |
| C* | 79.79 ± 1.61 a | 77.17 ± 0.93 b | |
| Redness Index | 0.33 ± 0.02 a | 0.42 ± 0.06 b * |
| Lady Cot | Pellecchiella | ||
|---|---|---|---|
| Fruit | Carotenoids (μg/g dw) | 1420.21 ± 60.07 a | 2010.45 ± 116.27 a * |
| Total polyphenols (μg/g dw) | 1715.90 ± 39.43 a | 2047.65 ± 36.79 a * | |
| ABTS (mmol trolox/kg) | 19.15 ± 3.08 a | 42.08 ± 1.61 a * | |
| DPPH (mmol trolox/kg) | 9.47 ± 1.45 a | 15.53 ± 1.72 a * | |
| FRAP (mmol trolox/kg) | 15.73 ± 0.99 a | 30.99 ± 3.17 a * | |
| Puree | Carotenoids (μg/g dw) | 1380.20 ± 20.55 a | 1930.66 ± 30.44 a * |
| Total polyphenols (μg/g dw) | 1300.30 ± 55.87 b | 1766.33 ± 44.78 b * | |
| ABTS (mmol trolox/kg) | 14.52 ± 3.22 b | 40.22 ± 7.31 b * | |
| DPPH (mmol trolox/kg) | 7.24 ± 1.23 b | 13.44 ± 4.32 b * | |
| FRAP (mmol trolox/kg) | 14.04 ± 2.45 b | 25.99 ± 3.22 b * | |
| Jam | Carotenoids (μg/g dw) | 1280.55 ± 17.95 b | 1809.77 ± 33.57 b * |
| Total polyphenols (μg/g dw) | 669.66 ± 22.37 c | 800.99 ± 20.99 c * | |
| ABTS (mmol trolox/kg) | 8.44 ± 1.97 c | 18.66 ± 2.67 c * | |
| DPPH (mmol trolox/kg) | 5.22 ± 0.87 c | 6.25 ± 2.35 c * | |
| FRAP (mmol trolox/kg) | 6.99 ± 1.54 c | 13.67 ± 2.55 c * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falciano, A.; Cirillo, A.; Ramondini, M.; Di Pierro, P.; Di Vaio, C. Comparative Evaluation of Qualitative and Nutraceutical Parameters in Fresh Fruit and Processed Products of ‘Lady Cot’ and Vesuvian ‘Pellecchiella’ Apricot Cultivars. Foods 2025, 14, 945. https://doi.org/10.3390/foods14060945
Falciano A, Cirillo A, Ramondini M, Di Pierro P, Di Vaio C. Comparative Evaluation of Qualitative and Nutraceutical Parameters in Fresh Fruit and Processed Products of ‘Lady Cot’ and Vesuvian ‘Pellecchiella’ Apricot Cultivars. Foods. 2025; 14(6):945. https://doi.org/10.3390/foods14060945
Chicago/Turabian StyleFalciano, Aniello, Aurora Cirillo, Mariachiara Ramondini, Prospero Di Pierro, and Claudio Di Vaio. 2025. "Comparative Evaluation of Qualitative and Nutraceutical Parameters in Fresh Fruit and Processed Products of ‘Lady Cot’ and Vesuvian ‘Pellecchiella’ Apricot Cultivars" Foods 14, no. 6: 945. https://doi.org/10.3390/foods14060945
APA StyleFalciano, A., Cirillo, A., Ramondini, M., Di Pierro, P., & Di Vaio, C. (2025). Comparative Evaluation of Qualitative and Nutraceutical Parameters in Fresh Fruit and Processed Products of ‘Lady Cot’ and Vesuvian ‘Pellecchiella’ Apricot Cultivars. Foods, 14(6), 945. https://doi.org/10.3390/foods14060945








