Dynamics of the Dissipation of Acetamiprid, Azoxystrobin, and β-Cyfluthrin in Jalapeño Pepper (Capsicum annuum L.) Produced Under Greenhouse and Open-Field Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Pesticide Standards and Reagents
2.2. Crop Development and Agronomic Management
2.3. Pesticide Application and Sampling
2.4. Measurement of Climatic Conditions During Pesticide Application
2.5. Sample Preparation and Application of the QuEChERS Method
2.6. Identification and Quantification of Acetamiprid, Azoxystrobin Using UHPLC-MS/MS, and β-Cyfluthrin Using GC-uECD
2.6.1. UHPLC-MS/MS
2.6.2. GC-µECD
2.7. Analytical Method Validation
2.8. Degradation, Half-Life, and Permanence of Acetamiprid, Azoxystrobin, and β-Cyfluthrin in Jalapeño Pepper
2.9. Experimental Design
3. Results and Discussion
3.1. Method Validation
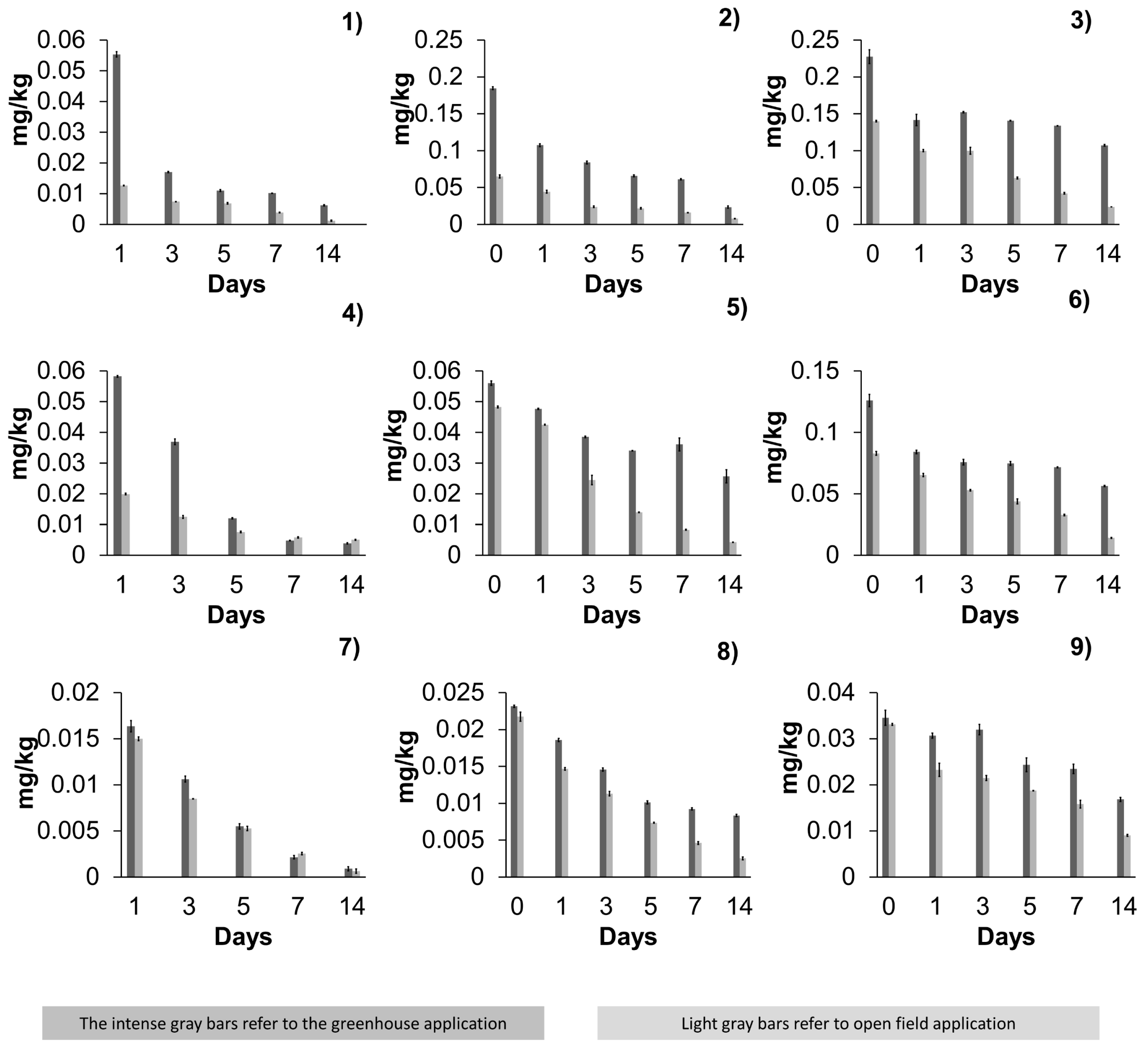
3.2. Residuality of Acetamiprid, Azoxystrobin, and β-Cyfluthrin in Jalapeño Peppers in a Greenhouse and Open Field
3.2.1. Residuality of Acetamiprid
3.2.2. Residuality of Azoxystrobin
3.2.3. Residuality of β-Cyfluthrin
3.3. Climatic Conditions and Their Relationship with the Dissipation of Acetamiprid, Azoxystrobin, and β-Cyfluthrin in Jalapeño Pepper
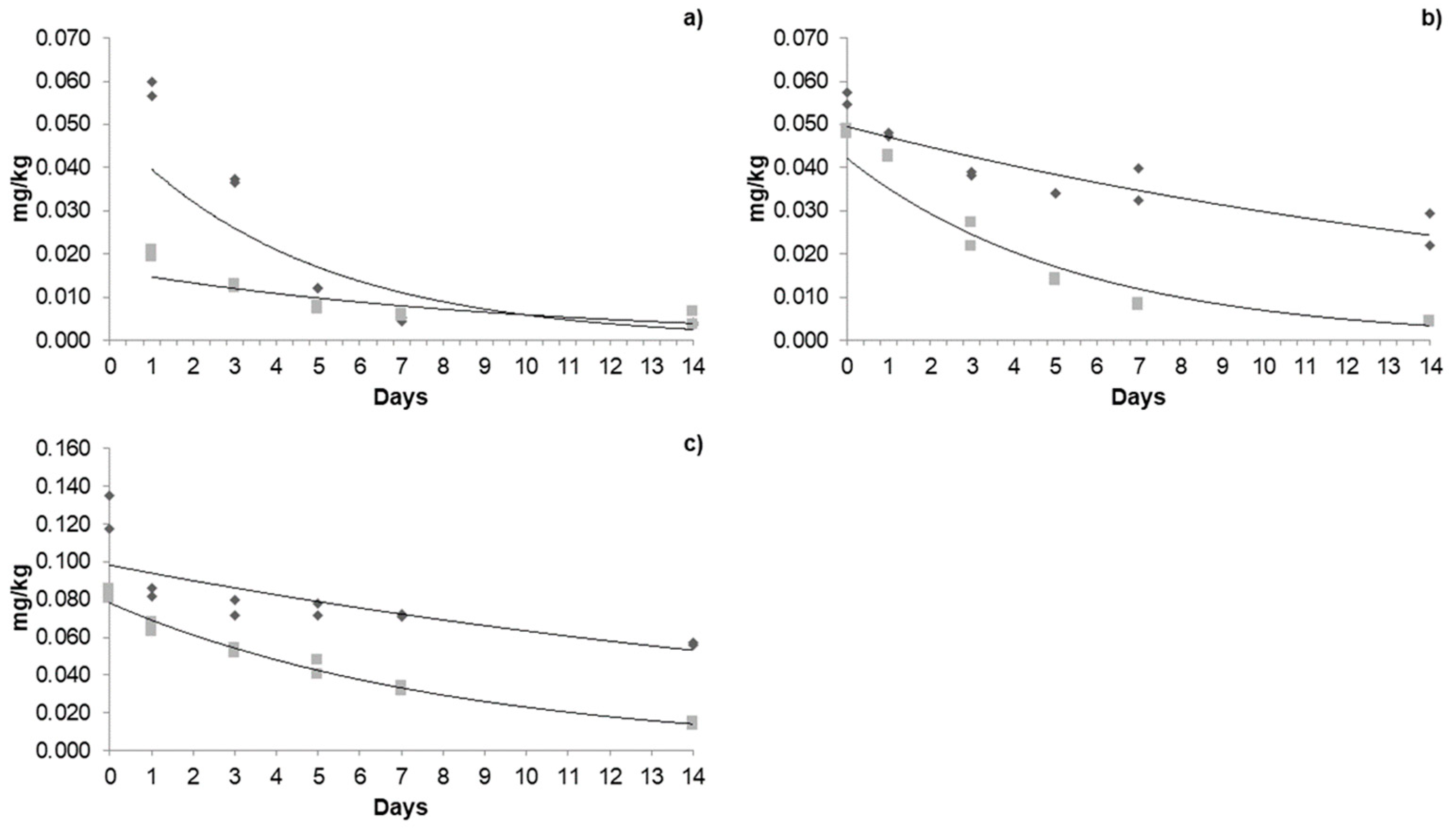
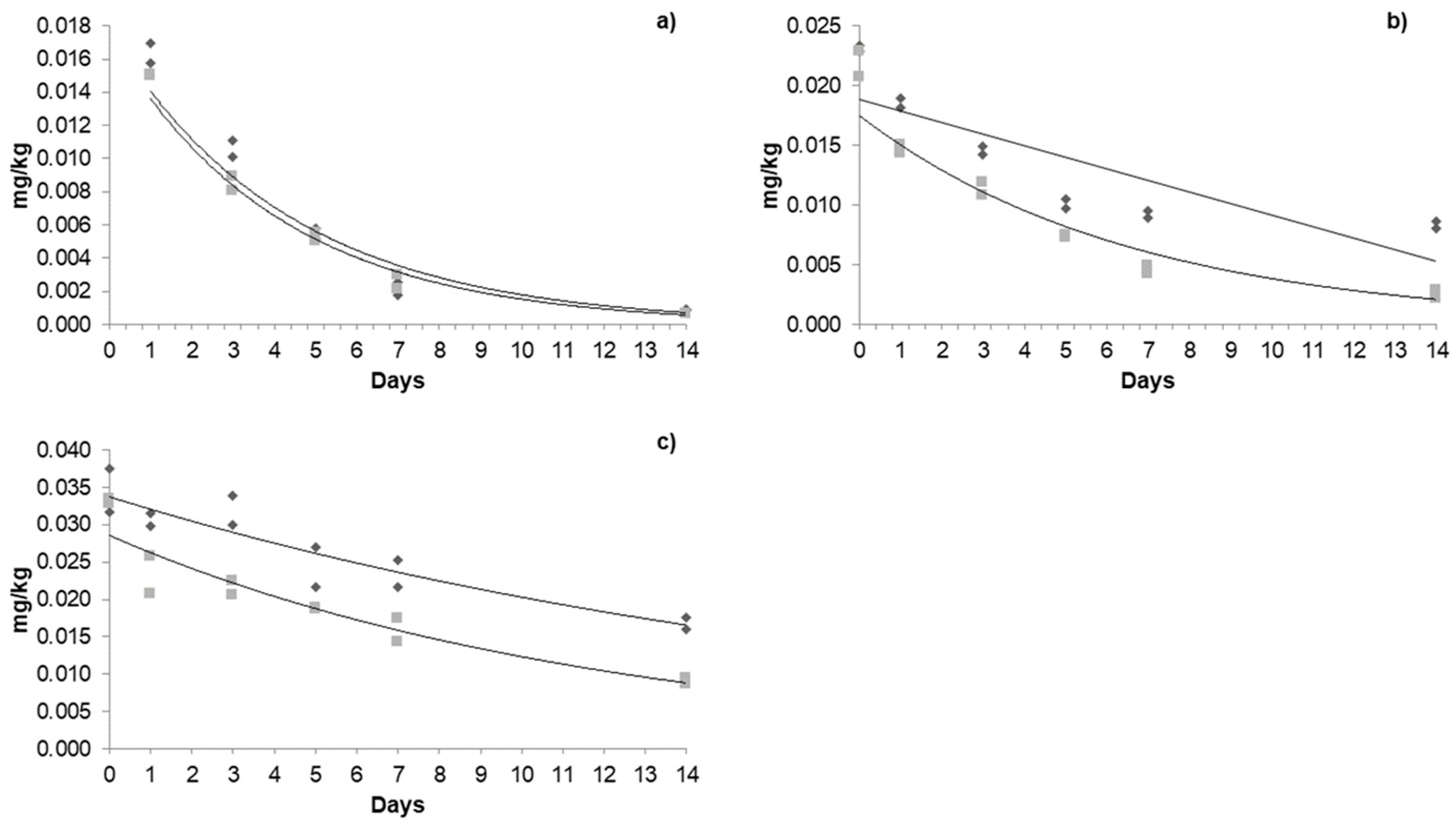
3.4. Dissipation Kinetics of Acetamiprid, Azoxystrobin, and β-Cyfluthrin in Jalapeño Pepper in a Greenhouse and Open Field
3.4.1. Dissipation Kinetics of Acetamiprid in a Greenhouse and Open Field
3.4.2. Dissipation Kinetics of Azoxystrobin in a Greenhouse and Open Field
3.4.3. Dissipation Kinetics of β-Cyfluthrin in a Greenhouse and Open Field
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cooper, J.; Dobson, H. The benefits of pesticides to mankind and the environment. Crop Prot. 2007, 26, 1337–1348. [Google Scholar] [CrossRef]
- Souza, M.C.O.; Cruz, J.C.; Cesila, C.A.; Gonzalez, N.; Rocha, B.A.; Adeyemi, J.A.; Nadal, M.; Domingo, J.L.; Barbosa, F. Recent trends in pesticides in crops: A critical review of the duality of risks-benefits and the Brazilian legislation issue. Environ. Res. 2023, 228, 115811. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization/World Health Organization. Maximum Residue Limits (MRL). 2023. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/maximum-residue-limits/es/#:~:text=Un%20l%C3%ADmite%20m%C3%A1ximo%20de%20residuos,a%20las%20buenas%20pr%C3%A1cticas%20agr%C3%ADcolas (accessed on 16 November 2023).
- Fantke, P.; Juraske, R. Variability of Pesticide Dissipation Half-Lives in Plants. Environ. Sci. Technol. 2013, 47, 3548–3562. [Google Scholar] [CrossRef]
- Shim, J.-H.; Eun, J.-B.; Zaky, A.A.; Hussein, A.S.; Hacimüftüoğlu, A.; Abd El-Aty, A.M. A Comprehensive Review of Pesticide Residues in Peppers. Foods 2023, 12, 970. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Food and Agriculture Data. 2023. Available online: https://www.fao.org/faostat/en/#home (accessed on 16 November 2023).
- SAGARPA. Planeación Agrícola Nacional. Chiles y Pimientos Mexicanos. 2017. Available online: https://www.gob.mx/cms/uploads/attachment/file/257072/Potencial-Chiles_y_Pimientos-parte_uno.pdf (accessed on 16 November 2023).
- SIAP. Anuario Estadístico de la Producción Agrícola. 2024. Available online: https://www.gob.mx/siap/acciones-y-programas/produccion-agricola-33119 (accessed on 14 March 2025).
- Faraji, M.; Noorbakhsh, R.; Shafieyan, H.; Ramezani, M. Determination of acetamiprid, imidacloprid, and spirotetramat and their relevant metabolites in pistachio using modified QuEChERS combined with liquid chromatography-tandem mass spectrometry. Food Chem. 2018, 240, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Mandal, S.; Majumder, B.; Paul, A.; Paul, T.; Sahana, N.; Mondal, P. A liquid chromatographic method for determination of acetamiprid and buprofezin residues and their dissipation kinetics in paddy matrices and soil. Environ. Sci. Pollut. Res. 2022, 29, 1401–1412. [Google Scholar] [CrossRef]
- Abdelraheem, E.M.H.; Hassan, S.M.; Arief, M.M.H.; Mohammad, S.G. Validation of quantitative method for azoxystrobin residues in green beans and peas. Food Chem. 2015, 182, 246–250. [Google Scholar] [CrossRef]
- Gautam, M.; Fomsgaard, I.S. Liquid chromatography-tandem mass spectrometry method for simultaneous quantification of azoxystrobin and its metabolites, azoxystrobin free acid and 2-hydroxybenzonitrile, in greenhouse-grown lettuce. Food Addit. Contam. Part A 2017, 34, 2173–2180. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukherjee, S.; Das, G.K.; Bhattacharyya, A. Analytical method validation and comparison of two extraction techniques for screening of azoxystrobin from widely used crops using LC–MS/MS. J. Food Meas. Charact. 2015, 9, 517–524. [Google Scholar] [CrossRef]
- Laskowski, D.A. Physical and Chemical Properties of Pyrethroids. In Reviews of Environmental Contamination and Toxicology: Continuation of Residue Reviews; Ware, G.W., Ed.; Springer: New York, NY, USA, 2002; pp. 49–170. [Google Scholar]
- Chawla, S.; Shah, P.G.; Patel, A.R.; Patel, H.K.; Vaghela, K.M.; Solanki, P.P. Residue determination of β-cyfluthrin and imidacloprid as mix formulation in/on chickpea (Cicer arietinum) pods and soil and its risk assessment. Food Qual. Saf. 2018, 2, 75–81. [Google Scholar] [CrossRef]
- Liu, W.; Gan, J.J. Separation and Analysis of Diastereomers and Enantiomers of Cypermethrin and Cyfluthrin by Gas Chromatography. J. Agric. Food Chem. 2004, 52, 755–761. [Google Scholar] [CrossRef]
- You, J.; Wang, D.; Lydy, M.J. Determination of pyrethroid insecticides in sediment by gas chromatography—Ion trap tandem mass spectrometry. Talanta 2010, 81, 136–141. [Google Scholar] [CrossRef]
- European Food Safety, A.; Arena, M.; Auteri, D.; Brancato, A.; Bura, L.; Carrasco Cabrera, L.; Chiusolo, A.; Court Marques, D.; Crivellente, F.; De Lentdecker, C.; et al. Peer review of the pesticide risk assessment of the active substance beta-cyfluthrin. EFSA J. 2020, 18, e06058. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.G.d.; Kurz, M.H.S.; Guimarães, M.C.M.; Martins, M.L.; Prestes, O.D.; Zanella, R.; Ribeiro, J.N.d.S.; Gonçalves, F.F. Development and validation of a method for the analysis of pyrethroid residues in fish using GC–MS. Food Chem. 2019, 297, 124944. [Google Scholar] [CrossRef]
- Tian, F.; Qiao, C.; Luo, J.; Guo, L.; Pang, T.; Pang, R.; Li, J.; Wang, C.; Wang, R.; Xie, H. Method development and validation of ten pyrethroid insecticides in edible mushrooms by Modified QuEChERS and gas chromatography-tandem mass spectrometry. Sci. Rep. 2020, 10, 7042. [Google Scholar] [CrossRef] [PubMed]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- Lehotay, S.J. Determination of Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate: Collaborative Study. J. Aoac Int. 2007, 90, 485–520. [Google Scholar] [CrossRef]
- SENASICA. GÚIA DE VALIDACIÓN DE MÉTODOS PARA EL ANÁLISIS DE PLAGUICIDAS. 2022. Available online: https://www.gob.mx/cms/uploads/attachment/file/702365/SMEC-PR-GVP_G_IA_DE_VALIDACI_N_DE_M_TODOS_PARA_EL_AN_LISIS_DE_PLAGUICIDAS__1_.pdf (accessed on 15 February 2025).
- EPA. ASSIGNING VALUES TO NONDETECTED/NON-QUANTIFIED PESTICIDE RESIDUES IN HUMAN HEALTH FOOD EXPOSURE ASSESSMENTS. 2000. Available online: https://archive.epa.gov/pesticides/trac/web/pdf/trac3b012.pdf (accessed on 15 February 2025).
- Zhang, J.J.; Yang, H. Metabolism and detoxification of pesticides in plants. Sci. Total Environ. 2021, 790, 148034. [Google Scholar] [CrossRef]
- Sanyal, D.; Chakma, D.; Alam, S. Persistence of a Neonicotinoid Insecticide, Acetamiprid on Chili (Capsicum annum L.). Bull. Environ. Contam. Toxicol. 2008, 81, 365–368. [Google Scholar] [CrossRef]
- Varghese, T.S.; Mathew, T.B.; George, T.; Beevi, S.N.; Xavier, G. Persistence and dissipation of neonicotinoid insecticides on chilli fruits. Qual. Assur. Saf. Crops Foods 2015, 7, 487–491. [Google Scholar] [CrossRef]
- Fenoll, J.; Ruiz, E.; Hellín, P.; Lacasa, A.; Flores, P. Strobilurin residue levels in greenhouse-grown pepper and under cold-storage conditions. J. Sci. Food Agric. 2009, 89, 299–303. [Google Scholar] [CrossRef]
- Bian, Y.; Guo, G.; Liu, F.; Chen, X.; Wang, Z.; Hou, T. Meptyldinocap and azoxystrobin residue behaviors in different ecosystems under open field conditions and distribution on processed cucumber. J. Sci. Food Agric. 2020, 100, 648–655. [Google Scholar] [CrossRef]
- Jankowska, M.; Kaczynski, P.; Hrynko, I.; Lozowicka, B. Dissipation of six fungicides in greenhouse-grown tomatoes with processing and health risk. Environ. Sci. Pollut. Res. 2016, 23, 11885–11900. [Google Scholar] [CrossRef]
- Ahlawat, S.; Chauhan, R.; Malik, K.; Yadav, S.S.; Kumari, N. Persistence and processing effects in reduction of residues of β-cyfluthrin + imidacloprid and its metabolite in hot pepper. Int. J. Environ. Anal. Chem. 2021, 101, 411–421. [Google Scholar] [CrossRef]
- Mandal, K.; Chahil, G.S.; Sahoo, S.K.; Battu, R.S.; Singh, B. Dissipation Kinetics of β-Cyfluthrin and Imidacloprid in Brinjal and Soil Under Subtropical Conditions of Punjab, India. Bull. Environ. Contam. Toxicol. 2010, 84, 225–229. [Google Scholar] [CrossRef]
- Jiang, S.; Xinguang, W.; Pei, D.; Zheng, S.; Fu, S.; Wang, T. Effective Method of Estimating the Daily Evapotranspiration of Greenhouse Grapes in the Cold Area of Northeast China. ACS Omega 2022, 7, 15666–15680. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Gao, M.; Wang, J.; Li, B.; Mao, L.; Zhao, M.; Xu, Z.; Niu, H.; Wang, T.; Sun, L.; et al. Estimating Evapotranspiration of Greenhouse Tomato under Different Irrigation Levels Using a Modified Dual Crop Coefficient Model in Northeast China. Agriculture 2023, 13, 1741. [Google Scholar] [CrossRef]
- Shukla, V.R.; Parmar, K.; Vaghela, K.M.; Patel, J.; Chawla, S.; Patel, A.R.; Upadhyay, P.; Shah, P.; Pathan, F.K. Persistence of Pesticides in Capsicum (Capsicum annuum L.) under Greenhouse and Open Field. Pestic. Res. J. 2016, 28, 159–167. [Google Scholar]
- Pathipati, V.; Singh, T.V.K.; Vemuri, S.B.; Reddy, R.V.S.K.; Bharathi, N.B. Dissipation Dynamics of flubendiamideon Capsicum in Open Field and Poly House Conditions. Int. J. For. Hortic. 2017, 3, 30–35. [Google Scholar] [CrossRef]
- Pathipati, V.L.; Singh, T.V.K.; Vemuri, S.B.; Reddy, R.V.S.K.; Bharathi, N.B.; Reddy, N.R.; Aruna. Dissipation Studies of Thiamethoxam on Capsicum under Field and Poly House Conditions. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1688–1693. [Google Scholar] [CrossRef]
- Sharma, D.; Hebbar, S.S.; Divakara, J.V.; Mohapatra, S. Residues of pesticides acephate and methamidophos in capsicum grown in greenhouse and open field. Qual. Assur. Saf. Crops Foods 2012, 4, e33–e37. [Google Scholar] [CrossRef]
- Mandal, S.; Poi, R.; Hazra, D.K.; Bhattacharyya, S.; Banerjee, H.; Karmakar, R. Assessment of variable agroclimatic impact on dissipation kinetics of ready-mix fungicide formulation in green chili for harmonization of food safety. J. Food Compos. Anal. 2022, 110, 104541. [Google Scholar] [CrossRef]
- Garau, V.L.; Angioni, A.; Del Real, A.A.; Russo, M.; Cabras, P. Disappearance of Azoxystrobin, Pyrimethanil, Cyprodinil, and Fludioxonil on Tomatoes in a Greenhouse. J. Agric. Food Chem. 2002, 50, 1929–1932. [Google Scholar] [CrossRef]
- Galietta, G.; Egaña, E.; Gemelli, F.; Maeso, D.; Casco, N.; Conde, P.; Nuñez, S. Pesticide dissipation curves in peach, pear and tomato crops in Uruguay*. J. Environ. Sci. Health Part B 2010, 46, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Malhat, F.; Loutfy, N.M.; Ahmed, M.T. Dissipation pattern and risk assessment of the synthetic pyrethroid Lambda-cyhalothrin applied on tomatoes under dryland conditions, a case study. Int. J. Food Contam. 2016, 3, 8. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Kohli, S.K.; Kaur, R.; Kaur, T.; Arora, S.; Thukral, A.K.; Bhardwaj, R. Pesticide Metabolism in Plants, Insects, Soil Microbes and Fishes. In Pesticides in Crop Production; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 35–53. [Google Scholar]
- Gupta, S.; Gajbhiye, V.T.; Gupta, R.K. Effect of Light on the Degradation of Two Neonicotinoids viz Acetamiprid and Thiacloprid in Soil. Bull. Environ. Contam. Toxicol. 2008, 81, 185–189. [Google Scholar] [CrossRef]
- Nicol, E.; Varga, Z.; Vujovic, S.; Bouchonnet, S. Laboratory scale UV–visible degradation of acetamiprid in aqueous marketed mixtures—Structural elucidation of photoproducts and toxicological consequences. Chemosphere 2020, 248, 126040. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, S.; Wang, M.; Liang, X.; Xie, Y.; Zhang, Y.; Zhang, C. Dissipation behavior, residue distribution and dietary risk assessment of cyromazine, acetamiprid and their mixture in cowpea and cowpea field soil. J. Sci. Food Agric. 2020, 100, 4540–4548. [Google Scholar] [CrossRef]
- Abdallah, O.; Abdel Ghani, S.; Hrouzková, S. Development of validated LC-MS/MS method for imidacloprid and acetamiprid in parsley and rocket and evaluation of their dissipation dynamics. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 392–399. [Google Scholar] [CrossRef]
- Lee, J.; Kim, B.J.; Kim, E.; Kim, J.-H. Dissipation Kinetics and the Pre-Harvest Residue Limits of Acetamiprid and Chlorantraniliprole in Kimchi Cabbage Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2019, 24, 2616. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Ismail, A.M.E.; Ibrahim, A.I.H. Quantitative analysis of acetamiprid and imidacloprid residues in tomato fruits under greenhouse conditions. J. Environ. Sci. Health Part B 2019, 54, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, Y.; Zhan, H.; Bhatt, P.; Chen, S. An Overview of Strobilurin Fungicide Degradation:Current Status and Future Perspective. Front. Microbiol. 2020, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Noegrohati, S.; Sulasmi, S.; Hernadi, E.; Asviastuti, S. Dissipation pattern of azoxystrobin and difenoconazole in red dragon fruit (Hylocereus polyrhizus) cultivated in Indonesian highland (West Java) and coastal area (D.I. Jogyakarta) and its implication for dietary risk assessment. Food Qual. Saf. 2019, 3, 99–106. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Chahil, G.S.; Mandal, K.; Battu, R.S.; Singh, B. Estimation of β-cyfluthrin and imidacloprid in okra fruits and soil by chromatography techniques. J. Environ. Sci. Health Part B 2012, 47, 42–50. [Google Scholar] [CrossRef] [PubMed]


| Commercial Name | Active Ingredient | Dosage | a.i/ha (g) | Safety Interval (Days) | Vapor Pressure (mPa) | ADI * (mg/kg) | MRL * (mg/L) |
|---|---|---|---|---|---|---|---|
| Bulldock 125® (Bayer®) | β-cyfluthrin (5%) | 100 cm3/ha | 5 | 5 | 3 × 10−4 | 0.02 | 0.5 |
| Rescate 20 PS® (SummitAgro®) | Acetamiprid (20%) | 250 g/ha | 40 | 7 | 1.73 × 10−4 | 0.07 | 1 |
| Amistar® (Syngenta®) | Azoxystrobin (50%) | 115 g/ha | 57.5 | 3 | 1.1 × 10−7 | 0.2 | 3 |
| Pesticide | Recovery (%R) | Accuracy Under Repeatability Conditions (%CV) | Limit of Detection (mg/kg) | Limit of Quantitation (mg/kg) | Linearity (R2) |
|---|---|---|---|---|---|
| Acetamiprid | 106.7 ± 1.6 | 17.99 | 0.022 | 0.068 | 0.998 |
| Azoxystrobin | 104.3 ± 6.8 | 6.88 | 0.011 | 0.034 | 0.997 |
| β-cyfluthrin | 109.9 ± 7.7 | 8.30 | 0.013 | 0.039 | 0.996 |
| Criteria | 70–120% | CV ≤ 20% | R2 ≥ 0.99 |
| Pesticide | Days | First Application | Second Application | Third Application | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Greenhouse | Open Field | Greenhouse | Open Field | Greenhouse | Open Field | ||||||||
| mg/kg | D (%) | mg/kg | D (%) | mg/kg | D (%) | mg/kg | D (%) | mg/kg | D (%) | mg/kg | D (%) | ||
| Acetamiprid | 0 | 0.185 | 0 | 0.065 | 0 | 0.227 | 0 | 0.140 | 0 | ||||
| 1 | 0.055 | 0 | 0.013 | 0 | 0.107 | 39 | 0.044 | 41 | 0.141 | 48 | 0.100 | 30 | |
| 3 | 0.017 | 70 | 0.007 | 45 | 0.084 | 52 | 0.024 | 68 | 0.152 | 37 | 0.100 | 22 | |
| 5 | 0.011 | 80 | 0.007 | 44 | 0.066 | 65 | 0.022 | 66 | 0.141 | 43 | 0.063 | 56 | |
| 7 | 0.010 | 82 | 0.004 | 67 | 0.061 | 67 | 0.016 | 77 | 0.134 | 45 | 0.042 | 68 | |
| 14 | 0.006 | 88 | 0.001 | 89 | 0.023 | 89 | 0.008 | 88 | 0.107 | 55 | 0.024 | 83 | |
| Azoxystrobin | 0 | 0.056 | 0 | 0.048 | 0 | 0.126 | 0 | 0.083 | 0 | ||||
| 1 | 0.058 | 0 | 0.020 | 0 | 0.048 | 16 | 0.042 | 12 | 0.084 | 39 | 0.065 | 16 | |
| 3 | 0.037 | 35 | 0.013 | 42 | 0.039 | 32 | 0.024 | 55 | 0.076 | 47 | 0.053 | 36 | |
| 5 | 0.012 | 79 | 0.008 | 61 | 0.034 | 41 | 0.014 | 71 | 0.075 | 47 | 0.044 | 50 | |
| 7 | 0.005 | 91 | 0.006 | 71 | 0.036 | 31 | 0.008 | 82 | 0.072 | 47 | 0.033 | 58 | |
| 14 | 0.004 | 94 | 0.005 | 69 | 0.026 | 62 | 0.004 | 91 | 0.056 | 59 | 0.014 | 81 | |
| β-cyfluthrin | 0 | 0.023 | 0 | 0.022 | 0 | 0.035 | 0 | 0.033 | 0 | ||||
| 1 | 0.016 | 0 | 0.015 | 0 | 0.019 | 19 | 0.015 | 28 | 0.031 | 34 | 0.023 | 38 | |
| 3 | 0.011 | 34 | 0.009 | 46 | 0.015 | 39 | 0.011 | 43 | 0.032 | 48 | 0.022 | 33 | |
| 5 | 0.005 | 66 | 0.005 | 63 | 0.010 | 58 | 0.007 | 64 | 0.024 | 67 | 0.019 | 44 | |
| 7 | 0.002 | 90 | 0.003 | 80 | 0.009 | 62 | 0.005 | 79 | 0.023 | 81 | 0.016 | 48 | |
| 14 | 0.001 | 95 | 0.001 | 96 | 0.008 | 65 | 0.003 | 89 | 0.017 | 90 | 0.009 | 72 | |
| Climatic Factors | Greenhouse | Open Field |
|---|---|---|
| Maximum temperature (°C) | 38.77 | 32.8 |
| Minimum temperature (°C) | 5.6 | 5.3 |
| Mean temperature (°C) | 28 | 23.3 |
| Mean luminous intensity (Watts/m2) | 640 | 721 |
| Mean relative humidity (%) | 77 | 76 |
| Mean ET0 (mm) | 2.2 | 2.5 |
| Pesticide | Cultivation Type | First Application | Second Application | Third Application | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R0 (mg/kg) | t(1/2) (Days) | Pr (Days) | R0 (mg/kg) | t(1/2) (Days) | Pr (Days) | R0 (mg/kg) | t(1/2) (Days) | Pr (Days) | ||
| Acetamiprid | Greenhouse | 0.055 | 2.15 | 10.2 | 0.185 | 7.02 | 22 | 0.227 | 14.65 | 47 |
| Open field | 0.013 | 4.50 | 11.5 | 0.065 | 6.42 | 14.7 | 0.140 | 4.78 | 28 | |
| Azoxystrobin | Greenhouse | 0.058 | 2.73 | 11.8 | 0.056 | 11.96 | 43.5 | 0.126 | 12.55 | 58.9 |
| Open field | 0.020 | 4.46 | 12 | 0.048 | 3.01 | 11.7 | 0.083 | 5.48 | 23 | |
| β-cyfluthrin | Greenhouse | 0.016 | 3.21 | 6.6 | 0.023 | 6.42 | 15.4 | 0.035 | 12.55 | 39 |
| Open field | 0.015 | 3.18 | 6 | 0.022 | 2.91 | 7.8 | 0.033 | 7.46 | 21 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Ortega, L.A.; Villa-Bojórquez, J.; Bastidas-Bastidas, P.d.J.; Contreras-Martínez, R.; Carrillo-Fasio, J.A.; Báez-Sañudo, M.A. Dynamics of the Dissipation of Acetamiprid, Azoxystrobin, and β-Cyfluthrin in Jalapeño Pepper (Capsicum annuum L.) Produced Under Greenhouse and Open-Field Conditions. Foods 2025, 14, 1023. https://doi.org/10.3390/foods14061023
Jiménez-Ortega LA, Villa-Bojórquez J, Bastidas-Bastidas PdJ, Contreras-Martínez R, Carrillo-Fasio JA, Báez-Sañudo MA. Dynamics of the Dissipation of Acetamiprid, Azoxystrobin, and β-Cyfluthrin in Jalapeño Pepper (Capsicum annuum L.) Produced Under Greenhouse and Open-Field Conditions. Foods. 2025; 14(6):1023. https://doi.org/10.3390/foods14061023
Chicago/Turabian StyleJiménez-Ortega, Luis Alfonso, Jaime Villa-Bojórquez, Pedro de Jesús Bastidas-Bastidas, Rosalba Contreras-Martínez, José Armando Carrillo-Fasio, and Manuel Alonzo Báez-Sañudo. 2025. "Dynamics of the Dissipation of Acetamiprid, Azoxystrobin, and β-Cyfluthrin in Jalapeño Pepper (Capsicum annuum L.) Produced Under Greenhouse and Open-Field Conditions" Foods 14, no. 6: 1023. https://doi.org/10.3390/foods14061023
APA StyleJiménez-Ortega, L. A., Villa-Bojórquez, J., Bastidas-Bastidas, P. d. J., Contreras-Martínez, R., Carrillo-Fasio, J. A., & Báez-Sañudo, M. A. (2025). Dynamics of the Dissipation of Acetamiprid, Azoxystrobin, and β-Cyfluthrin in Jalapeño Pepper (Capsicum annuum L.) Produced Under Greenhouse and Open-Field Conditions. Foods, 14(6), 1023. https://doi.org/10.3390/foods14061023








