Impact of Fermented Soy Beverages Containing Selected Vaginal Probiotics on the In Vitro Fecal Microbiota of Post-Menopausal Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Production of Microcapsules
2.3. Preparation of Fermented Soy Beverages and Assessment of Viability of Microbial Strains
- C: control, fermented soy beverage containing starter cultures only;
- BC4: fermented soy beverage with starter cultures and L. crispatus BC4;
- BC9: fermented soy beverage with starter cultures and L. gasseri BC9;
- BC4 + BC9: fermented soy beverage containing starter cultures and L. crispatus BC4 + L. gasseri BC9;
- E-BC4: fermented soy beverage with starter cultures and encapsulated L. crispatus BC4;
- E-BC9: fermented soy beverage with starter cultures and encapsulated L. gasseri BC9;
- E-BC4 + BC9: fermented soy beverage with starter cultures and encapsulated L. crispatus BC4 + L. gasseri BC9.
2.4. In Vitro Digestion
2.5. Volunteers and Fecal Sample Collection
2.6. Fecal Batch Cultures
2.7. pH and Gas Monitoring in Fecal Cultures
2.8. Short-Chain Fatty Acids (SCFAs) Quantification
2.9. Determination of Microbiota Composition
2.10. Statistical Analysis
3. Results and Discussion
3.1. Microbial Strain Viability Assessment in Fermented Product
3.2. pH Variations and Gas Production During Batch Colon Incubation
3.3. Production of Short-Chain Fatty Acids (SCFAS)
3.4. Microbiota Composition
3.4.1. Quantitative PCR Analyses
3.4.2. Effect of Digested Soy Beverages Containing Encapsulated and Non-Encapsulated Probiotics on the Microbial Community Composition in Short-Term Colonic Incubations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colella, M.; Charitos, I.A.; Ballini, A.; Cafiero, C.; Topi, S.; Palmirotta, R.; Santacroce, L. Microbiota Revolution: How Gut Microbes Regulate Our Lives. World J. Gastroenterol. 2023, 29, 4368–4383. [Google Scholar] [CrossRef] [PubMed]
- Aumeistere, L.; Ķibilds, J.; Siksna, I.; Neimane, L.V.; Kampara, M.; Ļubina, O.; Ciproviča, I. The Gut Microbiome among Postmenopausal Latvian Women in Relation to Dietary Habits. Nutrients 2022, 14, 3568. [Google Scholar] [CrossRef]
- Peters, B.A.; Santoro, N.; Kaplan, R.C.; Qi, Q. Spotlight on the Gut Microbiome in Menopause: Current Insights. Int. J. Women’s Health 2022, 14, 1059–1072. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Li, X.; Sun, Q.; Qin, P.; Wang, Q. Compositional and Functional Features of the Female Premenopausal and Postmenopausal Gut Microbiota. FEBS Lett. 2019, 593, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Muhleisen, A.L.; Herbst-Kralovetz, M.M. Menopause and the Vaginal Microbiome. Maturitas 2016, 91, 42–50. [Google Scholar] [CrossRef]
- Kim, J.-M.; Park, Y.J. Probiotics in the Prevention and Treatment of Postmenopausal Vaginal Infections: Review Article. J. Menopausal Med. 2017, 23, 139–145. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, H.; Wang, G.; Zhao, J.; Chen, H.; Lu, X.; Chen, W. Lactic Acid Bacteria: A Promising Tool for Menopausal Health Management in Women. Nutrients 2022, 14, 4466. [Google Scholar] [CrossRef] [PubMed]
- Dover, S.E.; Aroutcheva, A.A.; Faro, S.; Chikindas, M.L. Natural Antimicrobials and Their Role in Vaginal Health: A Short Review. Int. J. Probiotics Prebiotics 2008, 3, 219–230. [Google Scholar]
- Foschi, C.; Salvo, M.; Cevenini, R.; Parolin, C.; Vitali, B.; Marangoni, A. Vaginal Lactobacilli Reduce Neisseria Gonorrhoeae Viability through Multiple Strategies: An in Vitro Study. Front. Cell Infect. Microbiol. 2017, 7, 502. [Google Scholar] [CrossRef]
- Gupta, S.; Kakkar, V.; Bhushan, I. Crosstalk between Vaginal Microbiome and Female Health: A Review. Microb. Pathog. 2019, 136, 103696. [Google Scholar] [CrossRef]
- Nardini, P.; Nãhui Palomino, R.A.; Parolin, C.; Laghi, L.; Foschi, C.; Cevenini, R.; Vitali, B.; Marangoni, A. Lactobacillus Crispatus Inhibits the Infectivity of Chlamydia Trachomatis Elementary Bodies, in Vitro Study. Sci. Rep. 2016, 6, 29024. [Google Scholar] [CrossRef] [PubMed]
- Parolin, C.; Marangoni, A.; Laghi, L.; Foschi, C.; Palomino, R.A.Ñ.; Calonghi, N.; Cevenini, R.; Vitali, B. Isolation of Vaginal Lactobacilli and Characterization of Anti-Candida Activity. PLoS ONE 2015, 10, e0131220. [Google Scholar] [CrossRef]
- Ñahui Palomino, R.A.; Zicari, S.; Vanpouille, C.; Vitali, B.; Margolis, L. Vaginal Lactobacillus Inhibits HIV-1 Replication in Human Tissues Ex Vivo. Front. Microbiol. 2017, 8, 906. [Google Scholar] [CrossRef]
- De Alberti, D.; Russo, R.; Terruzzi, F.; Nobile, V.; Ouwehand, A.C. Lactobacilli Vaginal Colonisation after Oral Consumption of Respecta® Complex: A Randomised Controlled Pilot Study. Arch. Gynecol. Obstet. 2015, 292, 861–867. [Google Scholar] [CrossRef]
- Bertuccini, L.; Russo, R.; Iosi, F.; Superti, F. Effects of Lactobacillus Rhamnosus and Lactobacillus Acidophilus on Bacterial Vaginal Pathogens. Int. J. Immunopathol. Pharmacol. 2017, 30, 163–167. [Google Scholar] [CrossRef]
- Mezzasalma, V.; Manfrini, E.; Ferri, E.; Boccarusso, M.; Di Gennaro, P.; Schiano, I.; Michelotti, A.; Labra, M. Orally Administered Multispecies Probiotic Formulations to Prevent Uro-Genital Infections: A Randomized Placebo-Controlled Pilot Study. Arch. Gynecol. Obstet. 2017, 295, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal Microbiota and the Potential of Lactobacillus Derivatives in Maintaining Vaginal Health. Microb. Cell Fact. 2020, 19, 203. [Google Scholar] [CrossRef]
- Marziali, G.; Foschi, C.; Parolin, C.; Vitali, B.; Marangoni, A. In-Vitro Effect of Vaginal Lactobacilli against Group B Streptococcus. Microb. Pathog. 2019, 136, 103692. [Google Scholar] [CrossRef]
- Ñahui Palomino, R.A.; Vanpouille, C.; Laghi, L.; Parolin, C.; Melikov, K.; Backlund, P.; Vitali, B.; Margolis, L. Extracellular Vesicles from Symbiotic Vaginal Lactobacilli Inhibit HIV-1 Infection of Human Tissues. Nat. Commun. 2019, 10, 5656. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Parolin, C.; Ñahui Palomino, R.A.; Vitali, B.; Lanciotti, R. Determination of Antibacterial and Technological Properties of Vaginal Lactobacilli for Their Potential Application in Dairy Products. Front. Microbiol. 2017, 8, 166. [Google Scholar] [CrossRef]
- Patrignani, F.; Parolin, C.; D’Alessandro, M.; Siroli, L.; Vitali, B.; Lanciotti, R. Evaluation of the Fate of Lactobacillus Crispatus BC4, Carried in Squacquerone Cheese, throughout the Simulator of the Human Intestinal Microbial Ecosystem (SHIME). Food Res. Int. 2020, 137, 109580. [Google Scholar] [CrossRef] [PubMed]
- Vujic, G.; Jajac Knez, A.; Despot Stefanovic, V.; Kuzmic Vrbanovic, V. Efficacy of Orally Applied Probiotic Capsules for Bacterial Vaginosis and Other Vaginal Infections: A Double-Blind, Randomized, Placebo-Controlled Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Heczko, P.B.; Tomusiak, A.; Adamski, P.; Jakimiuk, A.J.; Stefanski, G.; Mikolajczyk-Cichonska, A.; Suda-Szczurek, M.; Strus, M. Supplementation of Standard Antibiotic Therapy with Oral Probiotics for Bacterial Vaginosis and Aerobic Vaginitis: A Randomised, Double-Blind, Placebocontrolled Trial. BMC Women’s Health 2015, 15, 115. [Google Scholar] [CrossRef]
- Rasika, D.; Vidanarachchi, J.K.; Rocha, R.S.; Balthazar, C.F.; Cruz, A.G.; Ana, A.S.S.; Ranadheera, C.S. ScienceDirect Plant-Based Milk Substitutes as Emerging Probiotic Carriers. Curr. Opin. Food Sci. 2021, 38, 8–20. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Gottardi, D.; Parolin, C.; Glicerina, V.T.; Vitali, B.; Lanciotti, R.; Patrignani, F. Development and Characterization of Fermented Soy Beverages Containing Encapsulated or Non-Encapsulated Vaginal Probiotics. LWT 2023, 180, 114713. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D. A Standardised Static in Vitro Digestion Method Suitable for Food–an International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Nogacka, A.M.; Salazar, N.; Arboleya, S.; Ruas-Madiedo, P.; Mancabelli, L.; Suarez, A.; Martinez-Faedo, C.; Ventura, M.; Tochio, T.; Hirano, K.; et al. In Vitro Evaluation of Different Prebiotics on the Modulation of Gut Microbiota Composition and Function in Morbid Obese and Normal-Weight Subjects. Int. J. Mol. Sci. 2020, 21, 906. [Google Scholar] [CrossRef]
- Dao, M.C.; Clément, K. Gut Microbiota and Obesity: Concepts Relevant to Clinical Care. Eur. J. Intern. Med. 2018, 48, 18–24. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Holck, J.; Lorentzen, A.; Vigsnæs, L.K.; Licht, T.R.; Mikkelsen, J.D.; Meyer, A.S. Feruloylated and Nonferuloylated Arabino-Oligosaccharides from Sugar Beet Pectin Selectively Stimulate the Growth of Bifidobacterium spp. in Human Fecal in Vitro Fermentations. J. Agric. Food Chem. 2011, 59, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Shin, S.Y.; Choi, H.S.; Joo, W.; Cho, S.K.; Li, L.; Kang, J.-H.; Kim, T.-J.; Han, N.S. In Vitro Digestion and Fermentation Properties of Linear Sugar-Beet Arabinan and Its Oligosaccharides. Carbohydr. Polym. 2015, 131, 50–56. [Google Scholar] [CrossRef]
- Sulek, K.; Vigsnaes, L.K.; Schmidt, L.R.; Holck, J.; Frandsen, H.L.; Smedsgaard, J.; Skov, T.H.; Meyer, A.S.; Licht, T.R. A Combined Metabolomic and Phylogenetic Study Reveals Putatively Prebiotic Effects of High Molecular Weight Arabino-Oligosaccharides When Assessed by in Vitro Fermentation in Bacterial Communities Derived from Humans. Anaerobe 2014, 28, 68–77. [Google Scholar] [CrossRef]
- Astó, E.; Méndez, I.; Rodríguez-Prado, M.; Cuñé, J.; Espadaler, J.; Farran-Codina, A. Effect of the Degree of Polymerization of Fructans on Ex Vivo Fermented Human Gut Microbiome. Nutrients 2019, 11, 1293. [Google Scholar] [CrossRef]
- Koutsos, A.; Lima, M.; Conterno, L.; Gasperotti, M.; Bianchi, M.; Fava, F.; Vrhovsek, U.; Lovegrove, J.A.; Tuohy, K.M. Effects of Commercial Apple Varieties on Human Gut Microbiota Composition and Metabolic Output Using an in Vitro Colonic Model. Nutrients 2017, 9, 533. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Chioua, M.; Garrido, I.; Cueva, C.; Samadi, A.; Marco-Contelles, J.; Moreno-Arribas, M.V.; Bartolomé, B.; Monagas, M. Synthesis, Analytical Features, and Biological Relevance of 5-(3′,4′-Dihydroxyphenyl)-γ-Valerolactone, a Microbial Metabolite Derived from the Catabolism of Dietary Flavan-3-Ols. J. Agric. Food Chem. 2011, 59, 7083–7091. [Google Scholar] [CrossRef]
- Tuncil, Y.E.; Thakkar, R.D.; Marcia, A.D.R.; Hamaker, B.R.; Lindemann, S.R. Divergent Short-Chain Fatty Acid Production and Succession of Colonic Microbiota Arise in Fermentation of Variously-Sized Wheat Bran Fractions. Sci. Rep. 2018, 8, 16655. [Google Scholar] [CrossRef]
- Vollmer, M.; Esders, S.; Farquharson, F.M.; Neugart, S.; Duncan, S.H.; Schreiner, M.; Louis, P.; Maul, R.; Rohn, S. Mutual Interaction of Phenolic Compounds and Microbiota: Metabolism of Complex Phenolic Apigenin-C- and Kaempferol-O-Derivatives by Human Fecal Samples. J. Agric. Food Chem. 2018, 66, 485–497. [Google Scholar] [CrossRef]
- Nogacka, A.M.; de los Reyes-Gavilán, C.G.; Arboleya, S.; Ruas-Madiedo, P.; Martínez-Faedo, C.; Suarez, A.; He, F.; Harata, G.; Endo, A.; Salazar, N.; et al. In Vitro Selection of Probiotics for Microbiota Modulation in Normal-Weight and Severely Obese Individuals: Focus on Gas Production and Interaction With Intestinal Epithelial Cells. Front. Microbiol. 2021, 12, 630572. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Ghyselinck, J.; Marzorati, M.; Koch, A.-M.; Lambert, W.; Michiels, J.; Chalvon-Demersay, T. The Effect of Amino Acids on Production of SCFA and BCFA by Members of the Porcine Colonic Microbiota. Microorganisms 2022, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, P.B.; Clausen, M.R. Short-Chain Fatty Acids in the Human Colon: Relation to Gastrointestinal Health and Disease. Scand. J. Gastroenterol. 1996, 31, 132–148. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Hosseini, E.; Grootaert, C.; Verstraete, W.; Van de Wiele, T. Propionate as a Health-Promoting Microbial Metabolite in the Human Gut. Nutr. Rev. 2011, 69, 245–258. [Google Scholar] [CrossRef]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.S.; Comelli, E.M. Adiposity, Gut Microbiota and Faecal Short Chain Fatty Acids Are Linked in Adult Humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Gao, X.; Yi, Y.; Hou, Y.; Meng, X.; Jia, C.; Chao, B.; Fan, W.; Li, X.; et al. Effects of Inulin Propionate Ester on Obesity-Related Metabolic Syndrome and Intestinal Microbial Homeostasis in Diet-Induced Obese Mice. ACS Omega 2020, 5, 12865–12876. [Google Scholar] [CrossRef]
- Jung, T.H.; Park, J.H.; Jeon, W.M.; Han, K.S. Butyrate Modulates Bacterial Adherence on LS174T Human Colorectal Cells by Stimulating Mucin Secretion and MAPK Signaling Pathway. Nutr. Res. Pract. 2015, 9, 343–349. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Aguirre, M.; Eck, A.; Koenen, M.E.; Savelkoul, P.H.M.; Budding, A.E.; Venema, K. Diet Drives Quick Changes in the Metabolic Activity and Composition of Human Gut Microbiota in a Validated in Vitro Gut Model. Res. Microbiol. 2016, 167, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Pieper, R.; Kröger, S.; Richter, J.F.; Wang, J.; Martin, L.; Bindelle, J.; Htoo, J.K.; von Smolinski, D.; Vahjen, W.; Zentek, J. Fermentable Fiber Ameliorates Fermentable Protein-Induced Changes in Microbial Ecology, but Not the Mucosal Response, in the Colon of Piglets. J. Nutr. 2012, 142, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Hald, S.; Schioldan, A.G.; Moore, M.E.; Dige, A.; Lærke, H.N.; Agnholt, J.; Bach Knudsen, K.E.; Hermansen, K.; Marco, M.L.; Gregersen, S. Effects of Arabinoxylan and Resistant Starch on Intestinal Microbiota and Short-Chain Fatty Acids in Subjects with Metabolic Syndrome: A Randomised Crossover Study. PLoS ONE 2016, 11, e0159223. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Sada, A.; Orlando, P. Fermentative Ability of Alginate-Prebiotic Encapsulated Lactobacillus Acidophilus and Survival under Simulated Gastrointestinal Conditions. J. Funct. Foods 2009, 1, 319–323. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, M.; Xin, Y.; Qin, X.; Cheng, Z.; Shi, L.; Tang, Z. Alginate-based and Protein-based Materials for Probiotics Encapsulation: A Review. Int. J. Food Sci. Technol. 2013, 48, 1339–1351. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, T.; Chen, X.; Tian, D. Effect of Vanillin-Conjugated Chitosan-Stabilized Emulsions on Dough and Bread Characteristics. Curr. Res. Food Sci. 2024, 8, 100691. [Google Scholar] [CrossRef]
- Gbassi, G.K.; Vandamme, T. Probiotic Encapsulation Technology: From Microencapsulation to Release into the Gut. Pharmaceutics 2012, 4, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; van Sinderen, D.; Ventura, M. Bifidobacteria: Insights into the Biology of a Key Microbial Group of Early Life Gut Microbiota. Microbiome Res. Rep. 2021, 1, 2. [Google Scholar] [CrossRef]
- Derrien, M.; Turroni, F.; Ventura, M.; van Sinderen, D. Insights into Endogenous Bifidobacterium Species in the Human Gut Microbiota during Adulthood. Trends Microbiol. 2022, 30, 940–947. [Google Scholar] [CrossRef]
- Turroni, F.; Duranti, S.; Milani, C.; Lugli, G.A.; van Sinderen, D.; Ventura, M. Bifidobacterium Bifidum: A Key Member of the Early Human Gut Microbiota. Microorganisms 2019, 7, 544. [Google Scholar] [CrossRef]
- Turroni, F.; Milani, C.; Ventura, M.; van Sinderen, D. The Human Gut Microbiota during the Initial Stages of Life: Insights from Bifidobacteria. Curr. Opin. Biotechnol. 2022, 73, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Averina, O.V.; Poluektova, E.U.; Marsova, M.V.; Danilenko, V.N. Biomarkers and Utility of the Antioxidant Potential of Probiotic Lactobacilli and Bifidobacteria as Representatives of the Human Gut Microbiota. Biomedicines 2021, 9, 1340. [Google Scholar] [CrossRef] [PubMed]
- Liévin-Le Moal, V.; Servin, A.L. Anti-Infective Activities of Lactobacillus Strains in the Human Intestinal Microbiota: From Probiotics to Gastrointestinal Anti-Infectious Biotherapeutic Agents. Clin. Microbiol. Rev. 2014, 27, 167–199. [Google Scholar] [CrossRef] [PubMed]
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in Health and Disease, a Driver or Just along for the Ride? Curr. Opin. Biotechnol. 2018, 49, 140–147. [Google Scholar] [CrossRef]
- Walujkar, S.A.; Dhotre, D.P.; Marathe, N.P.; Lawate, P.S.; Bharadwaj, R.S.; Shouche, Y.S. Characterization of Bacterial Community Shift in Human Ulcerative Colitis Patients Revealed by Illumina Based 16S RRNA Gene Amplicon Sequencing. Gut Pathog. 2014, 6, 22. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. Biomed. Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef]
- Hu, Y.; Dun, Y.; Li, S.; Zhao, S.; Peng, N.; Liang, Y. Effects of Bacillus Subtilis KN-42 on Growth Performance, Diarrhea and Faecal Bacterial Flora of Weaned Piglets. Asian-Australas. J. Anim. Sci. 2014, 27, 1131. [Google Scholar] [CrossRef]
- Zhang, H.; Yeh, C.; Jin, Z.; Ding, L.; Liu, B.Y.; Zhang, L.; Dannelly, H.K. Prospective Study of Probiotic Supplementation Results in Immune Stimulation and Improvement of Upper Respiratory Infection Rate. Synth. Syst. Biotechnol. 2018, 3, 113–120. [Google Scholar] [CrossRef]
- Fuhrman, B.J.; Feigelson, H.S.; Flores, R.; Gail, M.H.; Xu, X.; Ravel, J.; Goedert, J.J. Associations of the Fecal Microbiome with Urinary Estrogens and Estrogen Metabolites in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2014, 99, 4632–4640. [Google Scholar] [CrossRef]
- Zhao, D.; Guallar, E.; Ouyang, P.; Subramanya, V.; Vaidya, D.; Ndumele, C.E.; Lima, J.A.; Allison, M.A.; Shah, S.J.; Bertoni, A.G. Endogenous Sex Hormones and Incident Cardiovascular Disease in Post-Menopausal Women. J. Am. Coll. Cardiol. 2018, 71, 2555–2566. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Arnoriaga-Rodríguez, M.; Luque-Córdoba, D.; Priego-Capote, F.; Pérez-Brocal, V.; Moya, A.; Burokas, A.; Maldonado, R.; Fernández-Real, J.M. Gut Microbiota Steroid Sexual Dimorphism and Its Impact on Gonadal Steroids: Influences of Obesity and Menopausal Status. Microbiome 2020, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Park, Y.-H.; Sim, M.; Kim, S.-A.; Joung, H.; Shin, D.-M. Serum Level of Sex Steroid Hormone Is Associated with Diversity and Profiles of Human Gut Microbiome. Res. Microbiol. 2019, 170, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhuo, Y.; Liu, Y.; Chen, Y.; Ning, Y.; Yao, J. Association between Premature Ovarian Insufficiency and Gut Microbiota. BMC Pregnancy Childbirth 2021, 21, 418. [Google Scholar] [CrossRef] [PubMed]
- La Reau, A.J.; Suen, G. The Ruminococci: Key Symbionts of the Gut Ecosystem. J. Microbiol. 2018, 56, 199–208. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia Muciniphila: Paradigm for next-Generation Beneficial Microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of Ethanol-Induced Akkermansia Muciniphila Depletion Ameliorates Alcoholic Liver Disease. Gut 2018, 67, 892–902. [Google Scholar] [CrossRef]
- Reider, S.; Watschinger, C.; Längle, J.; Pachmann, U.; Przysiecki, N.; Pfister, A.; Zollner, A.; Tilg, H.; Plattner, S.; Moschen, A.R. Short- and Long-Term Effects of a Prebiotic Intervention with Polyphenols Extracted from European Black Elderberry—Sustained Expansion of Akkermansia spp. J. Pers. Med. 2022, 12, 1479. [Google Scholar] [CrossRef]
- Park, M.G.; Cho, S.; Oh, M.M. Menopausal Changes in the Microbiome—A Review Focused on the Genitourinary Microbiome. Diagnostics 2023, 13, 1193. [Google Scholar] [CrossRef]
- Göker, M.; Gronow, S.; Zeytun, A.; Nolan, M.; Lucas, S.; Lapidus, A.; Hammon, N.; Deshpande, S.; Cheng, J.-F.; Pitluck, S. Complete Genome Sequence of Odoribacter Splanchnicus Type Strain (1651/6 T). Stand. Genom. Sci. 2011, 4, 200–209. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M. Increased Systolic and Diastolic Blood Pressure Is Associated with Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertension 2016, 68, 974–981. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wen, S.; Zhang, J.; Peng, J.; Shen, X.; Xu, L. Systematic Review and Meta-analysis: Changes of Gut Microbiota before and after Menopause. Dis. Markers 2022, 2022, 3767373. [Google Scholar] [CrossRef] [PubMed]
- Wexler, A.G.; Goodman, A.L. An Insider’s Perspective: Bacteroides as a Window into the Microbiome. Nat. Microbiol. 2017, 2, 17026. [Google Scholar] [CrossRef] [PubMed]

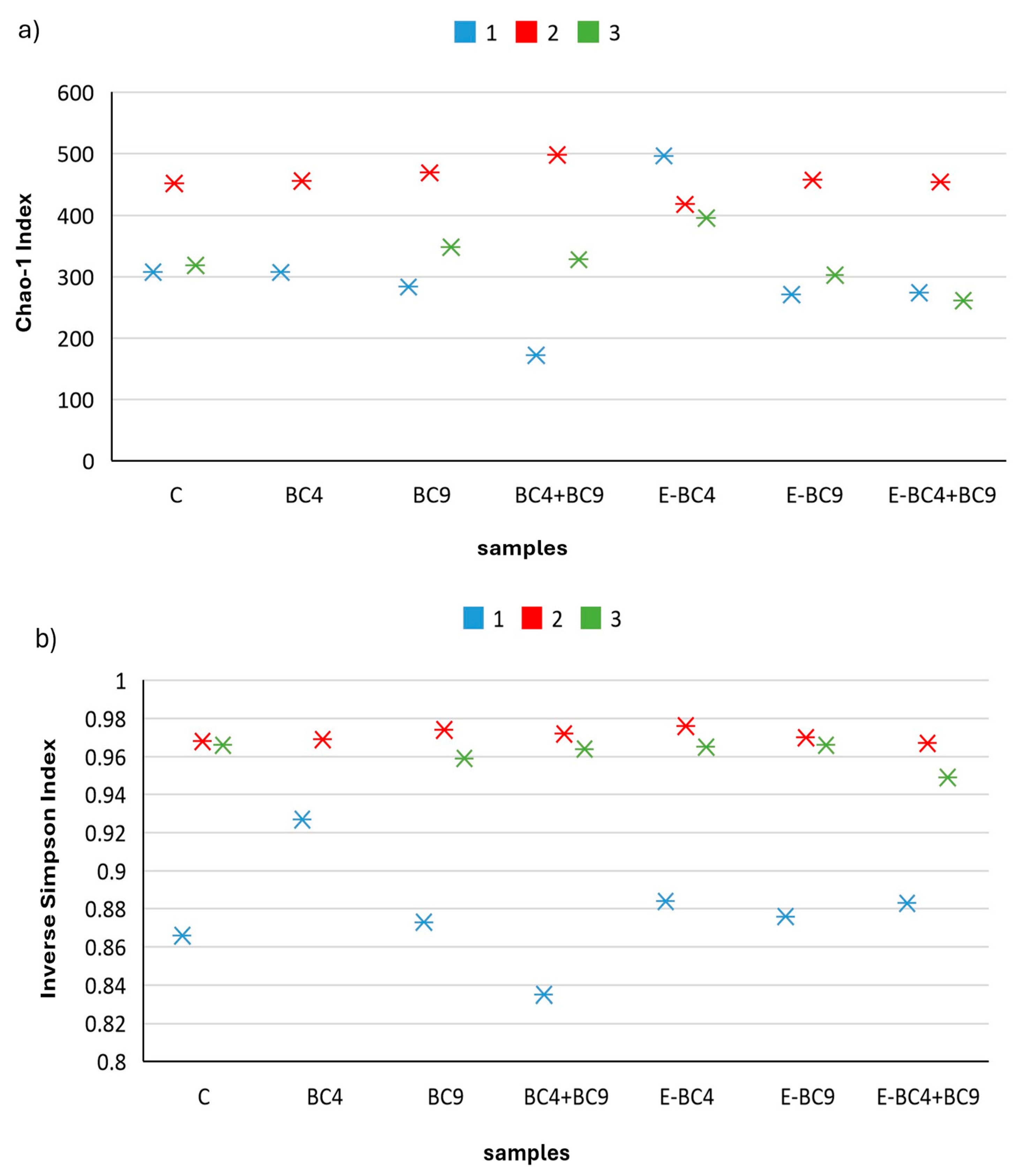


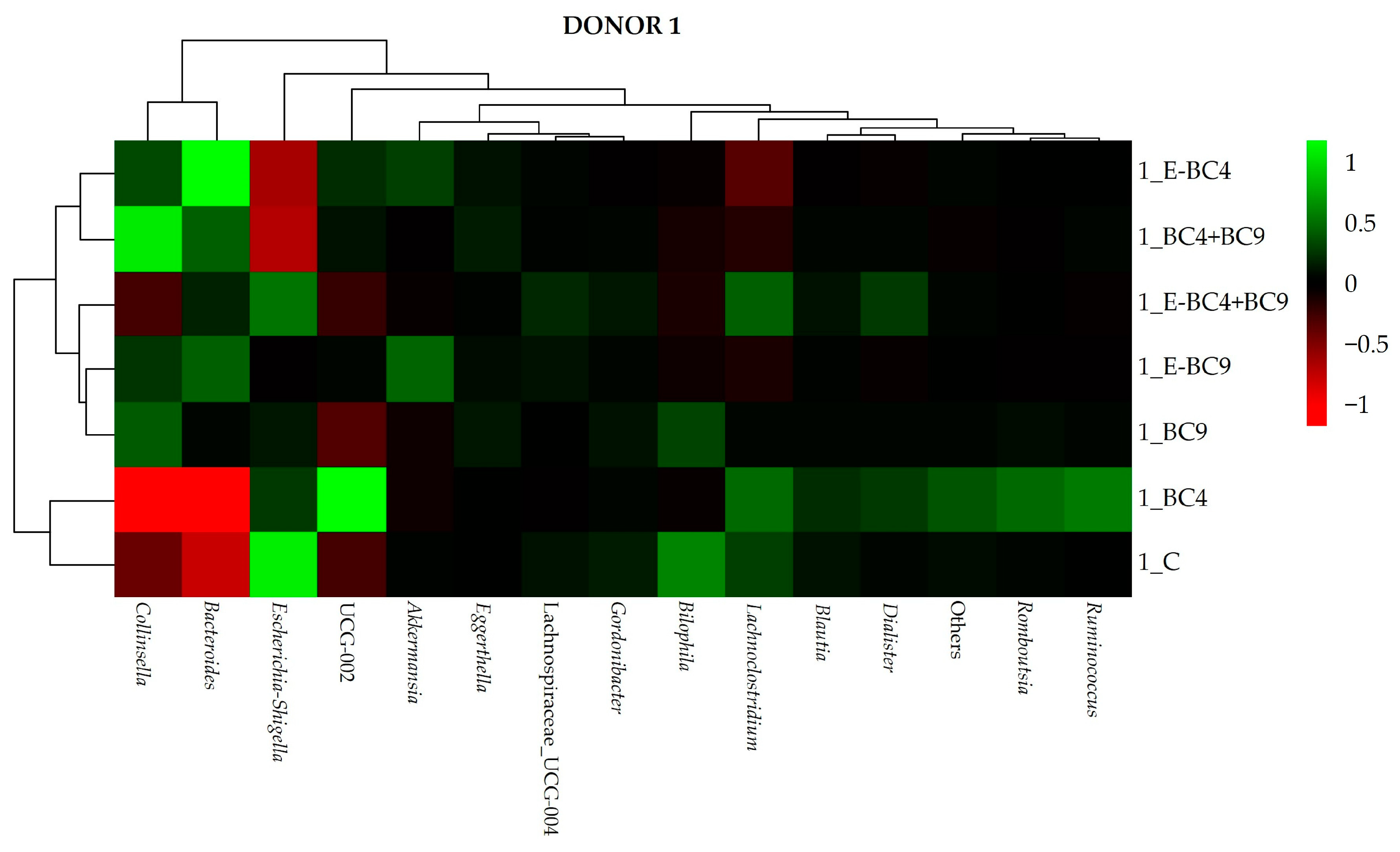
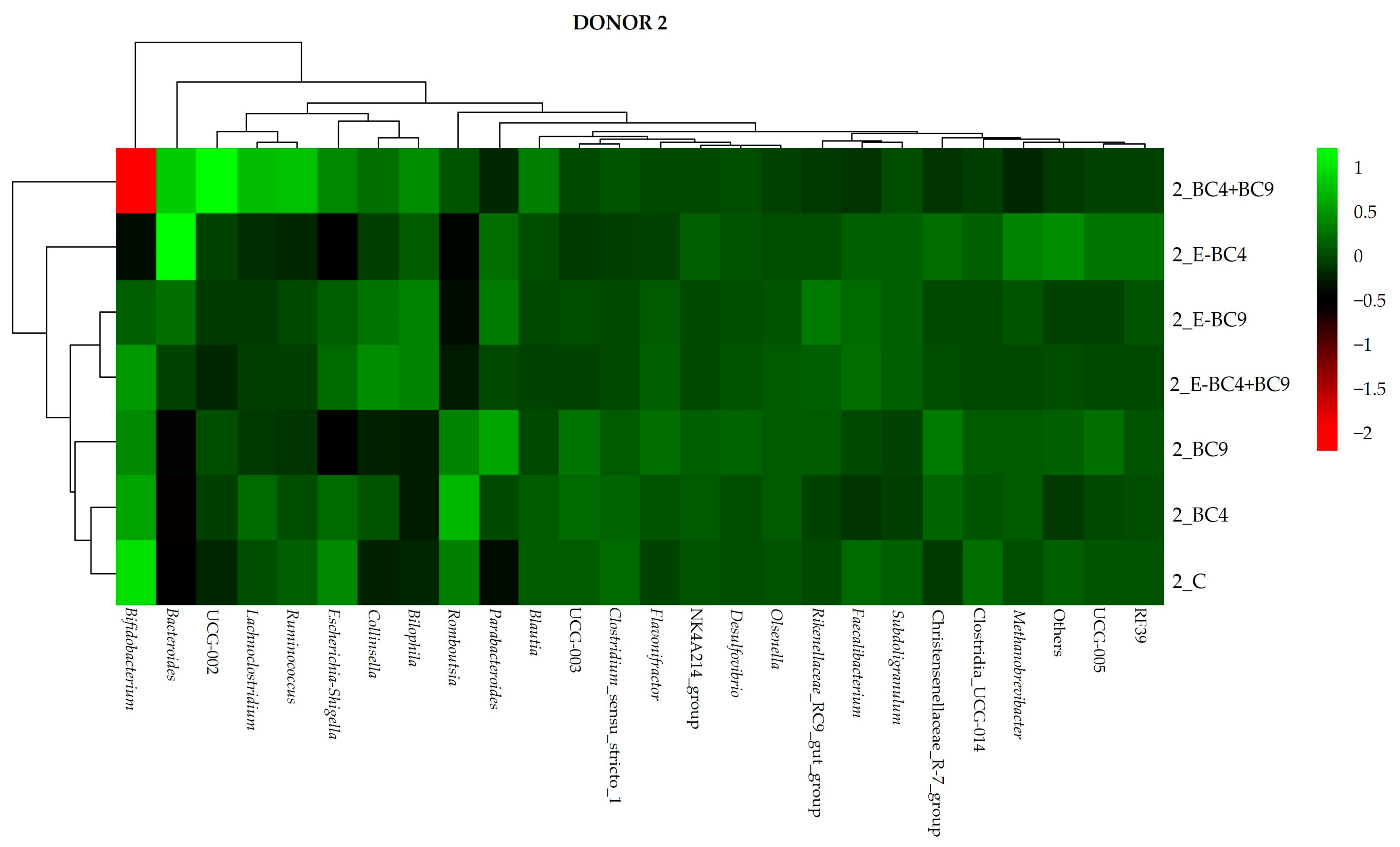

| Samples | ∆ pH Donor 1 | ∆ pH Donor 2 | ∆ pH Donor 3 |
|---|---|---|---|
| C | 0.10 (±0.02) aA | −0.16 (±0.01) aB | −0.05 (±0.03) aC |
| BC4 | 0.11 (±0.02) aA | −0.12 (±0.02) aB | −0.11 (±0.02) abB |
| BC9 | 0.10 (±0.01) aA | −0.11 (±0.03) aB | −0.14 (±0.01) bB |
| BC4 + BC9 | 0.13 (±0.02) aA | −0.12 (±0.02) aB | −0.06 (±0.03) aC |
| E-BC4 | 0.09 (±0.02) aA | −0.18 (±0.04) aB | −0.11 (±0.03) abB |
| E-BC9 | 0.11 (±0.02) aA | −0.16 (±0.02) aB | −0.10 (±0.03) abB |
| E-BC4 + BC9 | 0.08 (±0.03) aA | −0.29 (±0.02) bC | −0.12 (±0.03) abB |
| Donor 1 | Donor 2 | Donor 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | Δ 24-0 | 0 h | 24 h | Δ 24-0 | 0 h | 24 h | Δ24-0 | |
| Acetate | |||||||||
| C | 21.77 (±0.02) fA | 22.37 (±0.04) cA | 0.60 | 10.52 (±0.04) bB | 11.68 (±0.02) aB | 1.16 | 13.89 (±0.07) aC | 19.83 (±0.02) bC | 5.95 |
| BC4 | 17.73 (±0.03) aA | 18.72 (±0.11) bA | 0.99 | 8.65 (±0.06) aB | 20.21 (±0.17) fB | 11.56 | 13.25 (±0.08) aC | 20.46 (±0.20) cB | 7.21 |
| BC9 | 18.88 (±0.01) cA | 22.43 (±0.19) cA | 3.55 | 11.20 (±0.01) cB | 11.48 (±0.04) aB | 0.28 | 13.61 (±0.05) aC | 19.84 (±0.04) bC | 6.23 |
| BC4 + BC9 | 18.46 (±0.06) bA | 14.20 (±0.08) aA | −4.26 | 15.26 (±0.04) eB | 15.86 (±0.16) bB | 0.61 | 14.51 (±0.01) bC | 17.81 (±0.01) aC | 3.30 |
| E-BC4 | 19.68 (±0.03) eA | 23.65 (±0.06) dA | 3.97 | 10.98 (±0.07) bB | 17.54 (±0.04) dB | 6.56 | 15.87 (±0.16) cC | 17.18 (±0.10) aC | 1.30 |
| E-BC9 | 19.26 (±0.01) dA | 22.77 (±0.07) cA | 3.51 | 17.56 (±0.01) fB | 16.24 (±0.02) cB | −1.32 | 16.24 (±0.07) dC | 21.89 (±0.12) dC | 5.65 |
| E-BC4 + BC9 | 19.22 (±0.05) dA | 25.74 (±0.03) eA | 6.51 | 12.77 (±0.08) dB | 19.25 (±0.18) eB | 6.48 | 13.39 (±0.01) aC | 20.81 (±0.25) cC | 7.43 |
| Propionate | |||||||||
| C | 3.75 (±0.01) cA | 4.11 (±0.11) bA | 0.36 | 2.18 (±0.01) aB | 2.43 (±0.04) aB | 0.25 | 3.95 (±0.03) bC | 5.93 (±0.22) bC | 1.98 |
| BC4 | 2.97 (±0.01) aA | 3.43 (±0.05) aA | 0.47 | 1.94 (±0.03) aB | 4.34 (±0.04) dB | 2.41 | 3.76 (±0.03) aC | 6.20 (±0.07) bC | 2.44 |
| BC9 | 3.25 (±0.01) bA | 4.12 (±0.05) bA | 0.88 | 2.45 (±0.21) bB | 2.44 (±0.03) aB | −0.01 | 3.94 (±0.01) bC | 6.11 (±0.01) bC | 2.17 |
| BC4 + BC9 | 3.17 (±0.01) bA | 4.41 (±0.03) cA | 1.24 | 3.35 (±0.11) dA | 3.41 (±0.44) bB | 0.05 | 4.08 (±0.03) cB | 5.06 (±0.13) aC | 0.97 |
| E-BC4 | 3.33 (±0.03) bA | 4.31 (±0.01) cA | 0.97 | 2.35 (±0.10) bB | 3.81 (±0.21) bB | 1.46 | 4.46 (±0.31) dC | 5.17 (±0.01) aC | 0.71 |
| E-BC9 | 3.32 (±0.01) bA | 5.09 (±0.03) dA | 1.77 | 3.91 (±0.01) eB | 3.67 (±0.18) bB | −0.25 | 4.72 (±0.22) dC | 7.08 (±0.08) cC | 2.36 |
| E-BC4 + BC9 | 3.29 (±0.01) bA | 3.90 (±0.11) bA | 0.60 | 2.81 (±0.03) cB | 4.08 (±0.06) cA | 1.27 | 3.91 (±0.02) bC | 6.47 (±0.30) bB | 2.56 |
| Butyrate | |||||||||
| C | 4.58 (±0.21) bA | 4.98 (±0.01) cA | 0.40 | 2.89 (±0.13) aB | 3.24 (±0.11) aB | 0.35 | 4.40 (±0.12) bA | 5.94 (±0.21) bC | 1.54 |
| BC4 | 3.80 (±0.09) aA | 4.06 (±0.03) bA | 0.26 | 2.62 (±0.02) aB | 6.03 (±0.07) eB | 3.41 | 4.29 (±0.13) bC | 6.35 (±0.05) cC | 2.06 |
| BC9 | 4.26 (±0.22) bA | 5.26 (±0.03) dA | 1.00 | 3.42 (±0.01) bB | 3.36 (±0.02) aB | −0.06 | 3.85 (±0.02) aC | 5.33 (±0.42) bA | 1.49 |
| BC4 + BC9 | 4.23 (±0.22) bA | 3.35 (±0.02) aA | −0.88 | 4.63 (±0.23) cA | 4.54 (±0.03) bB | −0.09 | 4.14 (±0.22) bA | 4.74 (±0.11) aB | 0.60 |
| E-BC4 | 4.31 (±0.31) bA | 5.28 (±0.04) dA | 0.97 | 3.25 (±0.14) bB | 5.10 (±0.03) cB | 1.85 | 4.71 (±0.03) cC | 4.97 (±0.09) aB | 0.26 |
| E-BC9 | 4.40 (±0.11) bA | 5.30 (±0.04) dA | 0.90 | 5.72 (±0.01) dB | 5.05 (±0.01) cB | −0.67 | 5.22 (±0.01) dC | 6.67 (±0.04) cC | 1.45 |
| E-BC4 + BC9 | 4.33 (±0.04) bA | 6.11 (±0.01) eA | 1.78 | 4.00 (±0.21) cB | 5.73 (±0.04) dB | 1.72 | 3.99 (±0.21) abB | 5.73 (±0.25) bB | 1.74 |
| Isovalerate | |||||||||
| C | 0.76 (±0.01) aA | 0.71 (±0.02) bA | −0.05 | 0.53 (±0.04) bB | 0.47 (±0.01) aB | −0.06 | 0.16 (±0.02) aC | 0.29 (±0.01) bC | 0.13 |
| BC4 | 0.66 (±0.01) aA | 0.64 (±0.06) bA | −0.02 | 0.39 (±0.01) aB | 0.77 (±0.01) bA | 0.38 | 0.17 (±0.03) aC | 0.30 (±0.03) bB | 0.13 |
| BC9 | 0.70 (±0.03) aA | 0.74 (±0.02) bA | 0.05 | 0.60 (±0.01) bB | 0.51 (±0.01) aB | −0.10 | 0.14 (±0.01) aC | 0.28 (±0.01) bC | 0.14 |
| BC4 + BC9 | 0.66 (±0.01) aA | 0.48 (±0.02) aA | −0.18 | 0.85 (±0.01) cB | 0.74 (±0.02) bB | −0.10 | 0.14 (±0.01) aC | 0.22 (±0.01) aC | 0.08 |
| E-BC4 | 0.80 (±0.02) abA | 0.88 (±0.01) cA | 0.08 | 0.57 (±0.01) bB | 0.83 (±0.01) cA | 0.26 | 0.19 (±0.02) abC | 0.24 (±0.01) aB | 0.05 |
| E-BC9 | 0.85 (±0.01) bA | 0.93 (±0.01) cA | 0.08 | 1.03 (±0.02) dB | 0.80 (±0.01) bcB | −0.23 | 0.23 (±0.02) bC | 0.42 (±0.01) cC | 0.19 |
| E-BC4 + BC9 | 0.67 (±0.01) aA | 0.81 (±0.08) bcA | 0.14 | 0.73 (±0.09) bcA | 0.90 (±0.02) dB | 0.16 | 0.16 (±0.01) aB | 0.30 (±0.01) bC | 0.14 |
| Isobutyrate | |||||||||
| C | 0.29 (±0.02) abA | 0.27 (±0.01) bA | −0.02 | 0.19 (±0.03) bA | 0.16 (±0.01) aB | −0.02 | 0.04 (±0.01) aB | 0.10 (±0.01) aC | 0.06 |
| BC4 | 0.26 (±0.01) aA | 0.24 (±0.03) bA | −0.02 | 0.13 (±0.01) aB | 0.29 (±0.01) cA | 0.16 | 0.05 (±0.01) aC | 0.12 (±0.02) aB | 0.07 |
| BC9 | 0.26 (±0.01) aA | 0.29 (±0.01) bA | 0.03 | 0.23 (±0.01) bB | 0.20 (±0.01) bB | −0.04 | 0.04 (±0.01) aC | 0.10 (±0.01) aC | 0.06 |
| BC4 + BC9 | 0.27 (±0.01) aA | 0.17 (±0.01) aA | −0.10 | 0.33 (±0.01) cA | 0.30 (±0.01) cB | −0.03 | 0.04 (±0.01) aB | 0.07 (±0.03) aC | 0.03 |
| E-BC4 | 0.31 (±0.01) bA | 0.35 (±0.01) cA | 0.03 | 0.22 (±0.01) bB | 0.33 (±0.02) cA | 0.11 | 0.05 (±0.01) aC | 0.09 (±0.01) aC | 0.03 |
| E-BC9 | 0.34 (±0.01) cA | 0.36 (±0.01) cA | 0.02 | 0.42 (±0.01) dB | 0.33 (±0.03) cA | −0.09 | 0.09 (±0.01) bC | 0.16 (±0.01) bB | 0.08 |
| E-BC4 + BC9 | 0.26 (±0.01) aA | 0.32 (±0.03) bcA | 0.05 | 0.29 (±0.02) cA | 0.37 (±0.01) dA | 0.08 | 0.05 (±0.01) aB | 0.10 (±0.01) aB | 0.06 |
| Total Main SCFAs | |||||||||
| C | 30.10 | 31.45 | 1.36 | 15.59 | 17.34 | 1.76 | 22.24 | 31.71 | 9.47 |
| BC4 | 24.50 | 26.21 | 1.72 | 13.21 | 30.58 | 17.38 | 21.30 | 33.01 | 11.71 |
| BC9 | 26.39 | 31.82 | 5.43 | 17.07 | 17.28 | 0.21 | 21.40 | 31.28 | 9.89 |
| BC4 + BC9 | 25.87 | 21.97 | −3.90 | 23.24 | 23.81 | 0.57 | 22.73 | 27.61 | 4.88 |
| E-BC4 | 27.33 | 33.24 | 5.91 | 16.57 | 26.45 | 9.87 | 25.05 | 27.32 | 2.27 |
| E-BC9 | 26.98 | 33.15 | 6.17 | 27.19 | 24.96 | −2.23 | 26.18 | 35.63 | 9.45 |
| E-BC4 + BC9 | 26.84 | 35.75 | 8.90 | 19.58 | 29.05 | 9.47 | 21.28 | 33.01 | 11.73 |
| Theoretical Ratio (Acetate: Propionate: Butyrate) | |||||||||
| C | 5.81; 1.00; 1.22 | 5.44; 1.00; 1.21 | 4.83; 1.00; 1.33 | 4.81; 1.00; 1.33 | 3.52; 1.00; 1.11 | 3.34; 1.00; 1.00 | |||
| BC4 | 5.97; 1.00; 1.28 | 5.46; 1.00; 1.18 | 4.46; 1.00; 1.35 | 4.66; 1.00; 1.39 | 3.52; 1.00; 1.14 | 3.30; 1.00; 1.02 | |||
| BC9 | 5.81; 1.00; 1.31 | 5.44; 1.00; 1.28 | 4.57; 1.00; 1.33 | 4.70; 1.00; 1.38 | 3.45; 1.00; 0.98 | 3.25; 1.00; 0.87 | |||
| E-BC4 | 5.82; 1.00; 1.33 | 3.22; 1.00; 0.76 | 4.56; 1.00; 1.33 | 4.65; 1.00; 1.33 | 3.56; 1.00; 1.01 | 3.52; 1.00; 0.94 | |||
| BC4 + BC9 | 5.91; 1.00; 1.29 | 5.49; 1.00; 1.23 | 4.67; 1.00; 1.38 | 4.60; 1.00; 1.34 | 3.56; 1.00; 1.06 | 3.32; 1.00; 0.96 | |||
| E-BC9 | 5.80; 1.00; 1.33 | 4.47; 1.00; 1.04 | 4.49; 1.00; 1.46 | 4.43; 1.00; 1.38 | 3.44; 1.00; 1.11 | 3.09; 1.00; 0.94 | |||
| E-BC4 + BC9 | 5.84; 1.00; 1.32 | 6.60; 1.00; 1.57 | 4.54; 1.00; 1.42 | 4.72; 1.00; 1.40 | 3.42; 1.00; 1.02 | 3.22; 1.00; 0.89 | |||
| Donor 1 | Donor 2 | Donor 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | Δ24-0 | 0 h | 24 h | Δ24-0 | 0 h | 24 h | Δ24-0 | ||
| Bifidobacterium | C | 7.29 | 7.41 | 0.12 | 5.31 | 7.10 | 1.79 | 8.20 | 8.21 | 0.01 |
| BC4 | 7.41 | 7.59 | 0.17 | 6.59 | 7.78 | 1.19 | 8.20 | 8.25 | 0.04 | |
| BC9 | 7.41 | 7.40 | −0.01 | 4.53 | 7.44 | 2.92 | 8.33 | 8.31 | −0.01 | |
| BC4 + BC9 | 7.49 | 7.48 | −0.01 | 5.62 | 8.44 | 2.82 | 8.34 | 7.58 | −0.76 | |
| E-BC4 | 7.51 | 7.51 | 0.00 | 5.68 | 7.10 | 1.42 | 8.35 | 7.68 | −0.67 | |
| E-BC9 | 7.49 | 7.51 | 0.02 | 4.21 | 7.90 | 3.69 | 8.24 | 7.98 | −0.26 | |
| E-BC4 + BC9 | 7.57 | 7.34 | −0.23 | 6.93 | 7.85 | 0.92 | 8.33 | 7.72 | −0.61 | |
| Lactobacillus | C | 4.27 | 4.36 | 0.09 | b.d.l. 1 | b.d.l. | 4.20 | 4.19 | −0.01 | |
| BC4 | 4.28 | 5.70 | 1.42 | b.d.l. | b.d.l. | 6.04 | 5.84 | −0.20 | ||
| BC9 | 4.80 | 4.59 | −0.21 | b.d.l. | b.d.l. | 5.30 | 4.96 | −0.34 | ||
| BC4 + BC9 | 5.82 | 5.33 | −0.49 | b.d.l. | 4.88 | 6.13 | 5.30 | −0.82 | ||
| E-BC4 | 6.46 | 5.09 | −1.37 | b.d.l. | b.d.l. | 5.92 | 5.22 | −0.70 | ||
| E-BC9 | 6.37 | 5.47 | −0.90 | b.d.l. | 3.61 | 6.35 | 5.33 | −1.01 | ||
| E-BC4 + BC9 | 5.82 | 5.40 | −0.42 | b.d.l. | b.d.l. | 6.31 | 5.81 | −0.50 | ||
| Enterobacteriaceae | C | 8.17 | 8.36 | 0.19 | 6.63 | 6.21 | −0.42 | 8.38 | 7.77 | −0.61 |
| BC4 | 8.25 | 8.45 | 0.20 | 6.87 | 7.41 | 0.54 | 8.29 | 7.40 | −0.89 | |
| BC9 | 8.16 | 8.15 | −0.01 | 6.16 | 7.46 | 1.31 | 8.42 | 6.84 | −1.58 | |
| BC4 + BC9 | 8.27 | 8.24 | −0.03 | 7.06 | 7.55 | 0.49 | 8.25 | 6.38 | −1.86 | |
| E-BC4 | 8.32 | 8.48 | 0.16 | 6.99 | 7.23 | 0.24 | 8.29 | 6.96 | −1.33 | |
| E-BC9 | 8.15 | 8.29 | 0.14 | 5.62 | 7.65 | 2.02 | 7.97 | 6.86 | −1.12 | |
| E-BC4 + BC9 | 8.40 | 8.14 | −0.26 | 7.11 | 7.16 | 0.04 | 8.35 | 6.87 | −1.48 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Alessandro, M.; Gottardi, D.; Arboleya, S.; Alvarado-Jasso, G.M.; Parolin, C.; Vitali, B.; Lanciotti, R.; Gueimonde, M.; Patrignani, F. Impact of Fermented Soy Beverages Containing Selected Vaginal Probiotics on the In Vitro Fecal Microbiota of Post-Menopausal Women. Foods 2025, 14, 1022. https://doi.org/10.3390/foods14061022
D’Alessandro M, Gottardi D, Arboleya S, Alvarado-Jasso GM, Parolin C, Vitali B, Lanciotti R, Gueimonde M, Patrignani F. Impact of Fermented Soy Beverages Containing Selected Vaginal Probiotics on the In Vitro Fecal Microbiota of Post-Menopausal Women. Foods. 2025; 14(6):1022. https://doi.org/10.3390/foods14061022
Chicago/Turabian StyleD’Alessandro, Margherita, Davide Gottardi, Silvia Arboleya, Guadalupe Monserrat Alvarado-Jasso, Carola Parolin, Beatrice Vitali, Rosalba Lanciotti, Miguel Gueimonde, and Francesca Patrignani. 2025. "Impact of Fermented Soy Beverages Containing Selected Vaginal Probiotics on the In Vitro Fecal Microbiota of Post-Menopausal Women" Foods 14, no. 6: 1022. https://doi.org/10.3390/foods14061022
APA StyleD’Alessandro, M., Gottardi, D., Arboleya, S., Alvarado-Jasso, G. M., Parolin, C., Vitali, B., Lanciotti, R., Gueimonde, M., & Patrignani, F. (2025). Impact of Fermented Soy Beverages Containing Selected Vaginal Probiotics on the In Vitro Fecal Microbiota of Post-Menopausal Women. Foods, 14(6), 1022. https://doi.org/10.3390/foods14061022










