Determination of Polar Heterocyclic Aromatic Amines in Meat Thermally Treated in a Roasting Bag with Dried Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
2.3. Meat Preparation
2.4. Heterocyclic Aromatic AMINES Extraction
2.5. HPLC-DAD-FLD Analysis
2.6. Establishment of Basic Validation Parameters for the Determination of HAAs
2.7. Statistical Analysis
3. Results
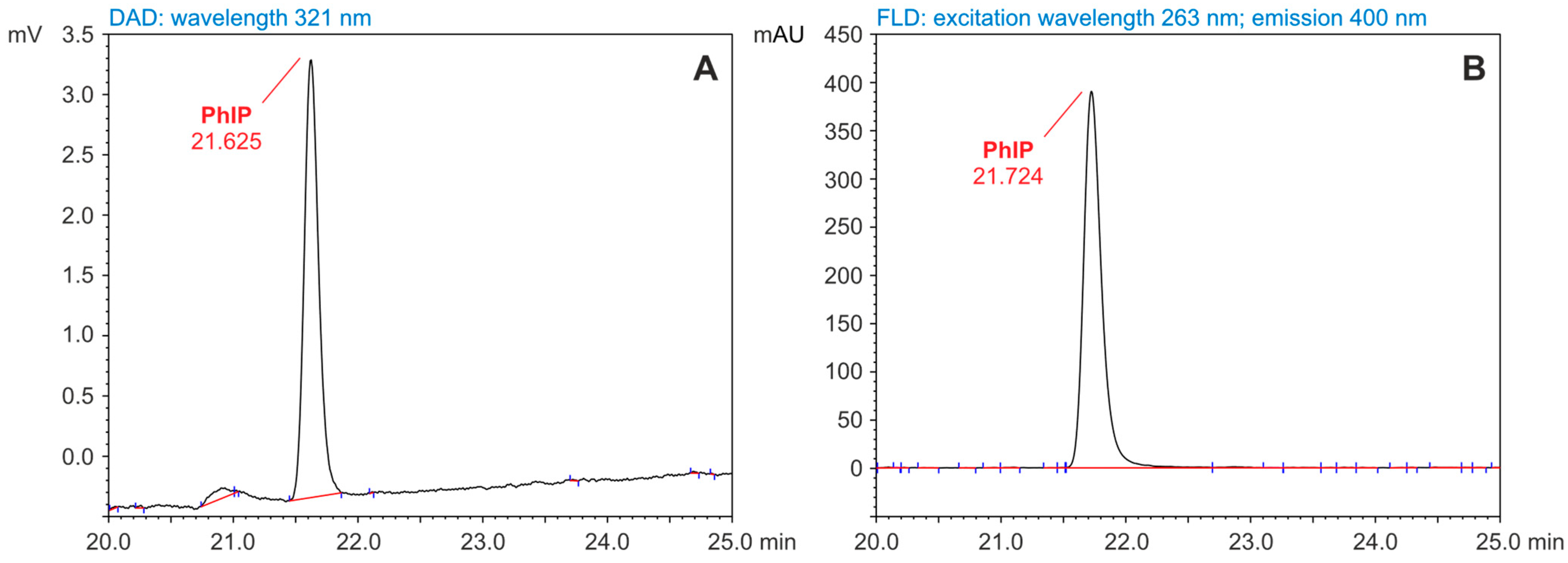
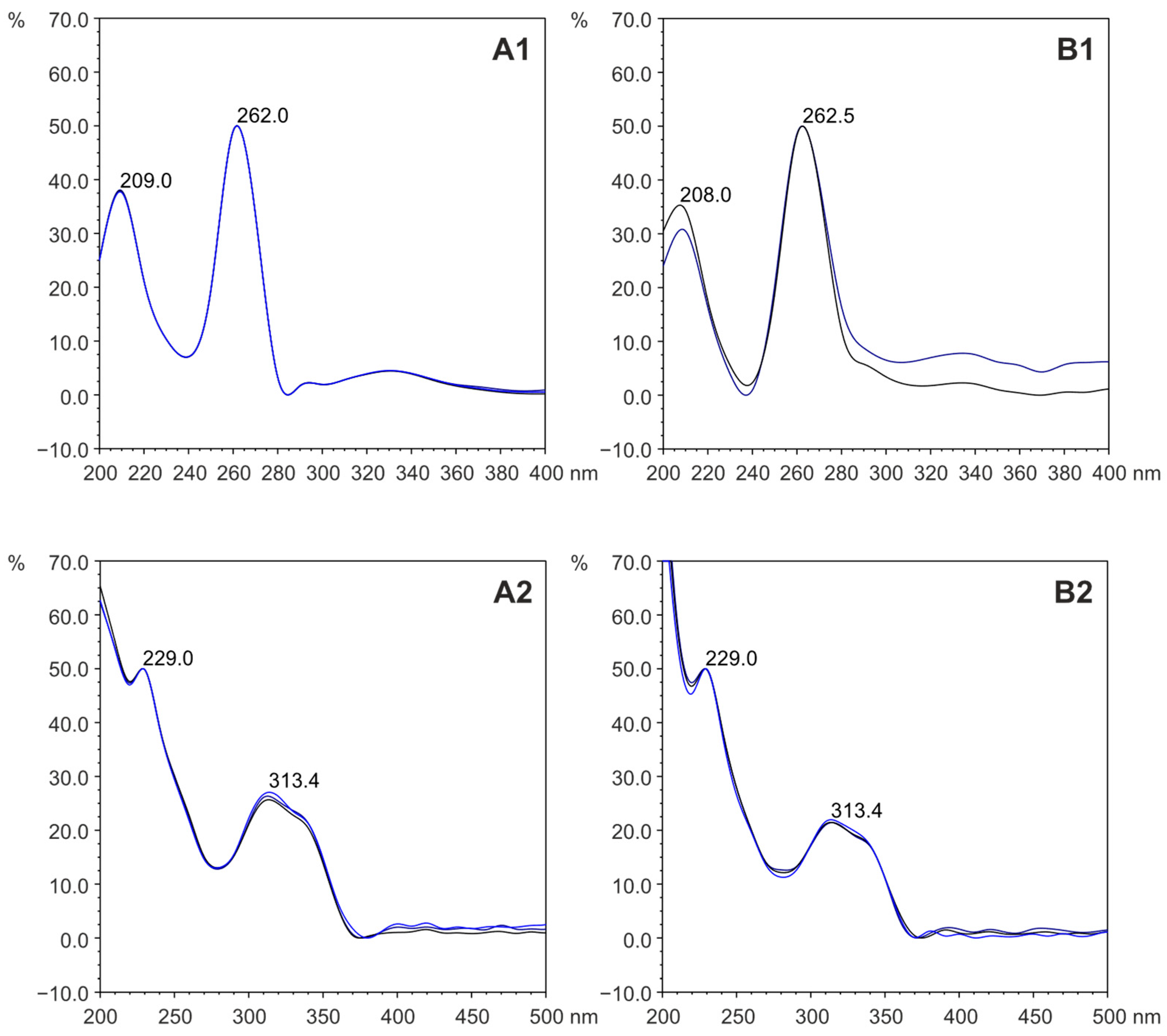
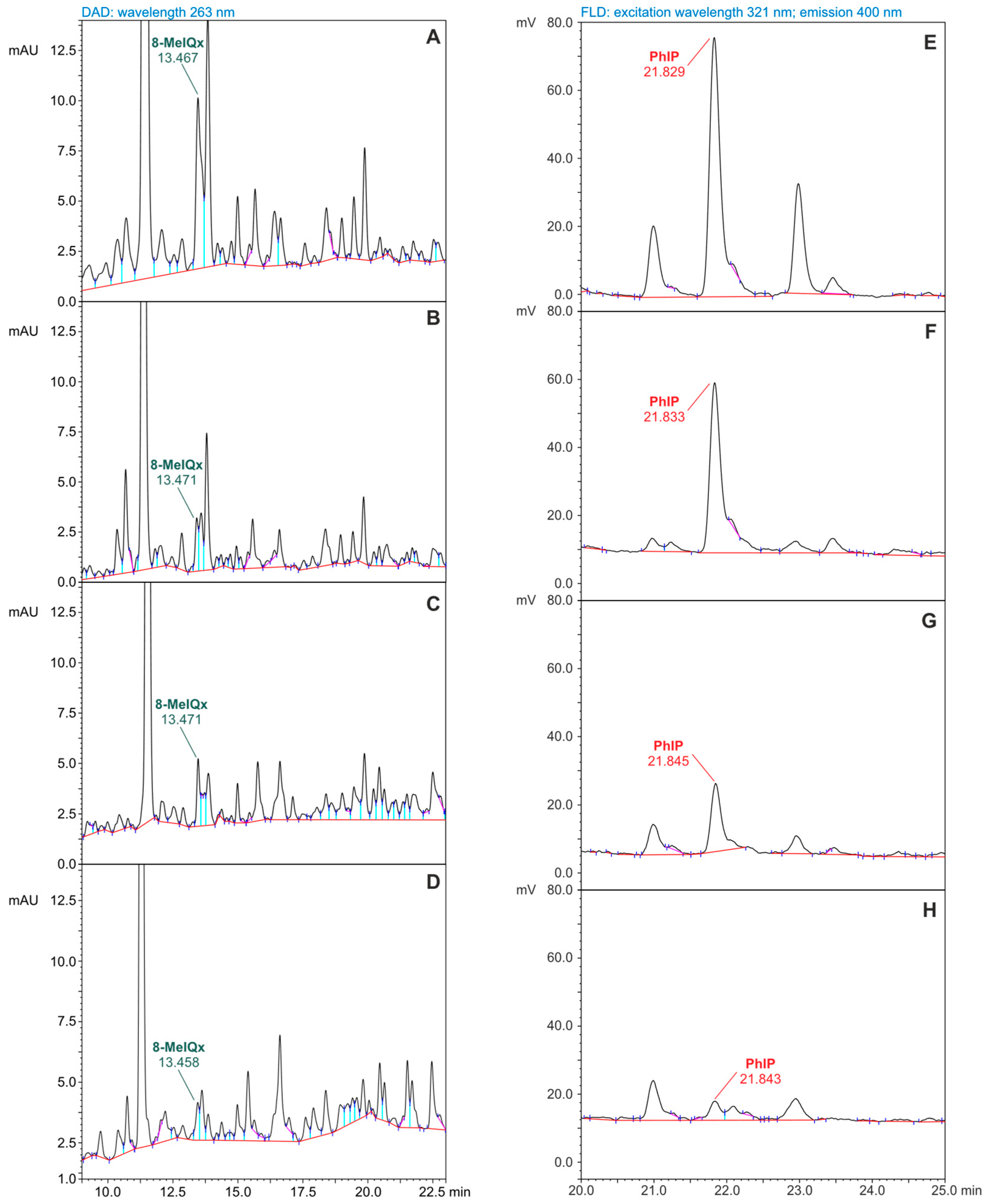
3.1. HAAs Concentrations Determination by HPLC-DAD-FLD
3.2. Results of Concentration Determination for Heterocyclic Aromatic Amines in Meat Samples
4. Discussion
4.1. Influence of the Preparation Method of Meat Dishes on Their Content of Heterocyclic Aromatic Amines
4.2. Effect of Dried Fruits on HAAs Formation in Thermally Treated Meat
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Red and Processed Meat in the Context of Health and the Environment: Many Shades of Red and Green: Information Brief; WHO: Geneva, Switzerland, 2023.
- International Agency for Research on Cancer (IARC). Red meat and processed meat. In Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Publication: Lyon, France, 2018; p. 114. [Google Scholar]
- Pei-Tjun, E.H.; Liang, Z.; Zhang, P.; Fang, Z. Formation mechanisms, detection methods and mitigation strategies of acrylamide, polycyclic aromatic hydrocarbons and heterocyclic amines infood products. Food Cont. 2024, 158, 110236. [Google Scholar]
- Dybing, E.; O’Brien, J.; Renwick, A.; Sanner, T. Risk assessment of dietary exposures to compounds that are genotoxic and carcinogenic—An overview. Toxicol. Lett. 2008, 180, 110–117. [Google Scholar] [CrossRef]
- Chiavarini, M.; Bertarelli, G.; Minelli, L. Dietary intake of meat cooking-related mutagens (HCAs) and risk of colorectal adenoma and cancer: A systematic review and metaanalysis. Nutrients 2017, 9, 514. [Google Scholar] [CrossRef] [PubMed]
- Oz, E.; Aoudeh, E.; Murkovic, M.; Toldra, F.; Gomez-Zavaglia, A.; Brennan, C.; Proestos, C.; Zeng, M.; Oz, F. Heterocyclic aromatic amines in meat: Formation mechanisms, toxicological implications, occurrence, risk evaluation. Meat Sci. 2023, 205, 109312. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Xian, Y.; Li, H.; Bai, W.; Zeng, X. Potential carcinogenic heterocyclic aromatic amines (HAAs) in foodstuffs: Formation, extraction, analytical methods and mitigation strategies. Compr. Rev. in Food Sci. and Food Saf. 2020, 19, 36. [Google Scholar] [CrossRef]
- Kulczyński, B.; Sidor, A.; Gramza-Michałowska, A. Characteristics of selected antioxidative and bioactive compounds in meat and animal origin products. Antioxidants 2019, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Polak, M.L.; Demšar, L.; Zahija, I.; Polak, T. Influence of temperature on the formation of heterocyclic aromatic amines in pork steaks. Czech J. Food Sci. 2020, 38, 248–254. [Google Scholar] [CrossRef]
- Knize, M.G.; Dolbeare, F.A.; Carroll, K.L.; Moore, D.H.; Felton, J.S. Effect of cooking time and temperature on the heterocyclic amine content of fried beef patties. Food Chem. Toxicol. 1994, 32, 595–603. [Google Scholar] [CrossRef]
- Barzegar, F.; Kamankesh, M.; Mohammadi, A. Heterocyclic aromatic amines in cooked food: A review on formation, health risk-toxicology and their analytical techniques. Food Chem. 2019, 280, 240–254. [Google Scholar] [CrossRef]
- Gibis, M. Heterocyclic aromatic amines in cooked meat products: Causes, formation, occurrence, and risk assessment. Compr. Rev. Food Sci. Food Saf. 2016, 15, 269–302. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Hidalgo, F.J. Formation of heterocyclic aromatic amines with the structure of aminoimidazoazarenes in food products. Food Chem. 2020, 313, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, H.R.; Akhtar, S.; Ismail, T.; Sestili, P.; Lorenzo, J.M.; Ranjha, M.; Jooste, L.; Hano, C.; Aadil, R.M. Heterocyclic aromatic amines in meat: Formation, isolation, risk assessment and inhibitory effect of plant extracts. Foods 2021, 10, 1466. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. In Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Publications: Lyon, France, 1993; p. 56. [Google Scholar]
- Jägerstad, M.; Skog, K.; Arvidsson, P.; Solyakov, A. Chemistry, formation and occurrence of genotoxic heterocyclic amines identified in model systems and cooked foods. Z. Lebensm. Unters. For. 1998, 207, 419–427. [Google Scholar] [CrossRef]
- Guo, Z.; Feng, X.; He, G.; Yang, H.; Zhong, T.; Xiao, Y.; Yu, X. Using bioactive compounds to mitigate the formation of typical chemical contaminants generated during the thermal processing of different food matrices. Compr. Rev. Food Sci. Food Saf. 2024, 23, 13409. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.M.C.; Cunha, S.C.; Faria, M.A.; Ferreira, I.M. Domestic Cooking of muscle foods: Impact on composition of nutrients and contaminants. Compr. Rev. Food Sci. Food Saf. 2018, 17, 255–509. [Google Scholar] [CrossRef] [PubMed]
- Neves, T.; Cunha, D.; Rosso, V.; Domene, S. Effects of seasoning on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in meats: A metaanalysis. Compr. Rev. Food Sci. Food Saf. 2021, 20, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Meurillon, M.; Engel, E. Mitigation strategies to reduce the impact of heterocyclic aro-matic amines in proteinaceous foods. Trends Food Sci. Technol. 2016, 50, 70–84. [Google Scholar] [CrossRef]
- Meurillon, M.; Angénieux, M.; Mercier, F.; Blinet, P.; Chaloin, L.; Chevolleau, S.; Debrauwer, L.; Engel, E. Mitigation of heterocyclic aromatic amines in cooked meat. Part I: Informed selection of antioxidants based on molecular modeling. Food Chem. 2020, 30, 33. [Google Scholar] [CrossRef]
- Bulanda, S.; Janoszka, B. Consumption of thermally processed meat containing carcinogenic compounds (polycyclic aromatic hydrocarbons and heterocyclic aromatic amines) versus a risk of some cancers in humans and the possibility of reducing their formation by natural food additives—A literature review. Int. J. Environ. Res. Public Health 2022, 19, 4781. [Google Scholar] [CrossRef]
- Tengilimoglu-Metin, M.; Hamzalioglu, A.; Gokmen, V.; Kizil, M. Inhibitory effect of hawthorn extract on heterocyclic aromatic amine formation in beef and chicken breast meat. Food Res. Int. 2017, 99, 586–595. [Google Scholar] [CrossRef]
- Cao, H.; Chen, B.; Inbaraj, B.; Chen, L.; Alvarez-Rivera, G.; Cifuentes, A.; Zhang, N.; Yang, D.; Simal-Gandara, J.; Wang, M. Preventive potential and mechanism of dietary polyphenols on the formation of heterocyclic aromatic amines. Food Front. 2020, 1, 134–151. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Cheng, Z.; Song, B.; Shu, C.; Chen, Y.; Chen, W.; Yang, S.; Yang, Y.; Tian, J. Flavonoids mitigation of typical food thermal processing contaminants:Potential mechanisms and analytical strategies. Food Chem. 2023, 416, 135793. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Kuhnle, G.; Cheng, Q. The effect of common spices and meat type on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in deep-fried meatballs. Food Control 2018, 92, 399–411. [Google Scholar]
- Khan, I.A.; Luo, J.; Shi, H.; Zou, Y.; Khan, A.; Zhu, Z.; Xu, W.; Wang, D.; Huang, M. Mitigation of heterocyclic amines by phenolic compounds in allspice and perilla frutescens seed extract: The correlation between antioxidant capacities and mitigating activities. Food Chem. 2022, 368, 130845. [Google Scholar] [CrossRef] [PubMed]
- Śnieżek, E.; Szumska, M.; Nowak, A.; Muzyka, R.; Janoszka, B. Application of high-performance liquid chromatography with fluorescence detection for non-polar heterocyclic aromatic amines and acridine derivatives determination in pork loin roasted in a roasting bag. Foods 2022, 11, 3385. [Google Scholar] [CrossRef]
- Khan, M.R. Influence of food condiments on the formation of carcinogenic heterocyclic amines in cooked chicken and determination by LC-MS/MS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 307–314. [Google Scholar] [CrossRef]
- Lai, Y.; Inbaraj, B.; Chen, B. Effects of Oil and Processing conditions on formation of heterocyclic amines and polycyclic aromatic hydrocarbons in pork fiber. Foods 2023, 12, 3504. [Google Scholar] [CrossRef]
- Yang, D.; He, Z.; Gao, D.; Qin, F.; Deng, S.; Wang, P.; Xu, X.; Chen, J.; Zenget, M. Effects of smoking or baking procedures during sausage processing on the formation of heterocyclic amines measured using UPLC-MS/MS. Food Chem. 2019, 276, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Omidi, N.; Barzegar, F.; Abedi, A.S.; Kamankesh, M.; Ghanati, K.; Mohammadi, A. Response surface methodology of quantitative of heterocyclic aromatic amines in fried fish using efficient microextraction method coupled with high-performance liquid chromatography: Central composite design. J. Chromatogr. Sci. 2021, 59, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Jamali, M.A.; Zhang, Y.; Teng, H.; Li, S.; Wang, F.; Peng, Z. Inhibitory effect of rosa rugosa tea extract on the formation of heterocyclic amines in meat patties at different temperatures. Molecules 2016, 21, 173. [Google Scholar] [CrossRef]
- Gross, G.A.; Grüter, A. Quantitation of mutagenic/carcinogenic heterocyclic aromatic amines in food products. J. Chromatogr. 1992, 592, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Wang, J.; Zhang, M. Inhibitory effects of Sichuan pepper (Zanthoxylum bungeanum) and sanshoamide extract on heterocyclic amine formation in grilled ground beef patties. Food Chem. 2018, 239, 111–118. [Google Scholar] [CrossRef]
- Gumus, D.; Kizil, M. Comparison of the reducing effects of blueberry and propolis extracts on heterocyclic aromatic amines formation in pan fried beef. Meat Sci. 2022, 186, 108746. [Google Scholar] [CrossRef]
- Khan, M.R.; Busquets, R.; Azam, M. Blueberry, raspberry, and strawberry extracts reduce the formation of carcinogenic het-erocyclic amines in fried camel, beef and chicken meats. Food Control 2021, 123, 107852. [Google Scholar] [CrossRef]
- Lai, Y.; Lee, Y.T.; Inbaraj, B.S.; Chen, B.H. Formation and inhibition of heterocyclic amines and polycyclic aromatic hydrocarbons in ground pork during marinating. Foods 2022, 11, 3080. [Google Scholar] [CrossRef] [PubMed]
- Sabally, K.; Sleno, L.; Jauffrit, J.A.; Iskandar, M.M.; Kubow, S. Inhibitory effects of apple peel polyphenol extract on the for-mation of heterocyclic amines in pan fried beef patties. Meat Sci. 2016, 117, 57–62. [Google Scholar] [CrossRef]
- Rounds, L.; Havens, C.; Feinstein, Y.; Friedman, M.; Ravishankar, S. Concentration-dependent inhibition of Escherichia coli O157:H7 and heterocyclic amines in heated ground beef patties by apple and olive extracts, onion powder and clove bud oil. Meat Sci. 2013, 94, 461–467. [Google Scholar] [CrossRef]
- Keşkekoğlu, H.; Üren, A. Inhibitory effects of pomegranate seed extract on the formation of heterocyclic aromatic amines in beef and chicken meatballs after cooking by four different methods. Meat Sci. 2014, 96, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, G.; Mo, L.; Li, M.; Luo, J.; Shen, Q.; Quan, W. Citrus peel extracts: Effective inhibitors of heterocyclic amines and advanced glycation end products in grilled pork meat patties. Foods 2024, 13, 114. [Google Scholar] [CrossRef]
- Janoszka, B. Heterocyclic amines and azaarenes in pan-fried meat and its gravy fried without additives and in the presence of onion and garlic. Food Chem. 2010, 120, 463–473. [Google Scholar] [CrossRef]
- Bulanda, S.; Janoszka, B. Polycyclic aromatic hydrocarbons (PAHs) in roasted pork meat and the effect of dried fruits on PAH content. Int. J. Environ. Res. Public Health 2023, 20, 4922. [Google Scholar] [CrossRef]
- Konieczka, P.; Namieśnik, J. Evaluation and Quality Control of Analytical Measurement Results, 1st ed.; PWN: Warsaw, Poland, 2017; pp. 225–300. [Google Scholar]
- Gibis, M.; Loeffler, M. Effect of creatine and glucose on formation of heterocyclic amines in grilled chicken breasts. Foods 2019, 8, 616. [Google Scholar] [CrossRef]
- The Commission of the European Communities. European Commission European Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- Iammarino, M.; Marino, R.; Nardelli, V.; Ingegno, M.; Albenzio, M. Red meat heating processes, toxic compounds production and nutritional parameters changes: What about risk–benefit? Foods 2024, 13, 445. [Google Scholar] [CrossRef]
- National Toxicology Program, Department of Health and Human Services. Report on Carcinogens, RoC Profile: Heterocyclic Amines (Selected), 15th Edition. 2021. Available online: https://ntp.niehs.nih.gov/sites/default/files/ntp/roc/content/profiles/heterocyclicamines.pdf (accessed on 1 February 2025).
- Gibis, M.; Weiss, J. Impact of Precursors Creatine, Creatinine, and Glucose on the Formation of Heterocyclic Aromatic Amines in Grilled Patties of Various Animal Species. J. Food Sci. 2015, 80, C2430–C2439. [Google Scholar] [CrossRef] [PubMed]
- Savas, A.; Oz, E.; Oz, F. Is oven bag really advantageous in terms of heterocyclic aromatic amines and bisphenol-A? Chicken meat perspective. Food Chem. 2021, 355, 129646. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Huang, Z.; Kong, B.; Liu, Q.; Wang, H.; Xu, M.; Xia, X. Dynamic changes in the qualities and heterocyclic aromatic amines of roasted pork induced by frying temperature and time. Meat Sci. 2021, 176, 108457. [Google Scholar] [CrossRef] [PubMed]
- European Union 2023. Commission Regulation (EU) 2023/915 of April 25, 2023, on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006, Off. J. Eur. Union L 119/103. Available online: http://data.europa.eu/eli/reg/2023/915/oj (accessed on 31 December 2024).
- Wolk, A. Potential health hazards of eating red meat. J. Intern. Med. 2017, 281, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhang, D.; Liu, H.; Wang, Z.; Hui, T. Chlorogenic acid and epicatechin: An efficient inhibitor of heterocyclic amines in charcoal roasted lamb meats. Food Chem. 2022, 368, 130865. [Google Scholar] [CrossRef]
- Średnicka-Tober, D.; Kazimierczak, R.; Ponder, A.; Hallmann, E. Biologically active compounds in selected organic and conventionally produced dried fruits. Foods 2020, 9, 1005. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y.; He, Z.; Qin, F. Discrimination and investigation of inhibitory patterns of flavonoids and phenolic acids on heterocyclic amine formation in chemical model systems by UPLC-MS profiling and chemometrics. Eur. Food Res. Technol. 2016, 242, 313–319. [Google Scholar] [CrossRef]
- Zhao, L.; Pan, F.; Li, Y.; Hao, S.; Mehmood, A.; Wang, Y.; Wang, C. Structure characteristics of flavonoids for heterocyclic aromatic amines inhibition using quantitative structure–activity relationship modeling. J. Food Biochem. 2020, 44, e13390. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Xu, X.; Yang, X.; Hu, X.; Chen, F.; Zhu, Y. Polyphenols as reactive carbonyl substances regulators: A comprehensive review of thermal processing hazards mitigation. Food Res. Int. 2025, 200, 115515. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhang, S.; Wang, M.; Chen, J.; Zheng, Z.P. Inhibitory effects of selected dietary flavonoids on the formation of total heterocyclic amines and 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP) in roast beef patties and in chemical model. Food Funct. 2016, 7, 1057–1066. [Google Scholar] [CrossRef]
- Kalemba-Drożdż, M.; Cierniak, A.; Cichoń, I. Berry fruit juices protect lymphocytes against DNA damage and ROS formation induced with heterocyclic aromatic amine PhIP. J. Berry Res. 2020, 10, 95–113. [Google Scholar] [CrossRef]
- Liao, G.; Xu, X.; Zhou, G. Effects of Cooked Temperatures and Addition of Antioxidants on Formation of Heterocyclic Aromatic Amines in Pork Floss. J. Food Process. Preserv. 2009, 33, 159–175. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yim, D.G.; Lee, D.Y.; Kim, O.Y.; Kang, H.J.; Kim, H.S.; Jang, A.; Park, T.S.; Jin, S.K.; Hur, S.J. Overview of the effect of natural products on reduction of potential carcinogenic substances in meat products. Trends Food Sci. Technol. 2020, 99, 568–579. [Google Scholar] [CrossRef]
- Shin, H.S.; Strasburg, G.M.; Ustunol, Z. Influence of different unifloral honeys on heterocyclic aromatic amine formation and overall mutagenicity in fried ground-beef patties. J. Food Sci. 2003, 68, 810–815. [Google Scholar] [CrossRef]
- Jung, K.; Lee, K.; Park, J.; Dong, A.; Shin, H.S. Influence of fructooligosaccharides and garlic on formation of heterocyclic amines in fried ground beef patties. Food Sci. Biotechnol. 2010, 19, 1159–1164. [Google Scholar] [CrossRef]
- Stacewicz-Sapuntzakis, M. Dried plums and their products: Composition and health effects—An updated review. Crit. Rev. Food Sci. Nutr. 2013, 53, 1277–1302. [Google Scholar] [CrossRef]
- Skog, K.; Jagerstad, M. Effects of monosaccharides and disaccharides on the formation of food mutagens in model systems. Mutat. Res. 1990, 230, 263–272. [Google Scholar] [CrossRef]

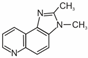
| Structure | Name (Abbreviation) | Structure | Name (Abbreviation) |
|---|---|---|---|
 | 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) |  | 2-amino-3,4-dimethylimiazo[4,5-f]quinoline (MeIQ) |
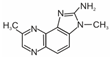 | 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (8-MeIQx) |  | 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) |
 | 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (4,8-DiMeIQx) |
| Parameter | Compound 1 | ||||
|---|---|---|---|---|---|
| IQ | MeIQ | 8-MeIQx | 4,8-DiMeIQx | PhIP | |
| Wavelength used for HPLC-DAD 2 detection and determination | λ UV 4 = 263 nm (detection and determination) | λ UV 4 = 263 nm (detection) | |||
| Retention time (min) | 10.192 | 11.197 | 13.467 | 16.383 | 21.625 |
| Wavelengths used for HPLC-FLD 3 determination | N/A 7 | λ Ex 5 = 263 nm λ Em 6 = 400 nm | |||
| Retention time (min) | N/A | 21.724 | |||
| HAA 1 | Detection Method | Range of Calibration Curve (ng/mL) | Regression Coefficient r | LOQ 3 (ng/mL) | LOD 2 (ng/g) 4 | LOQ 3 (ng/g) 4 |
|---|---|---|---|---|---|---|
| IQ | UV-DAD 263 nm | 100–2500 | 0.9999 | 100 | 0.3 | 1.0 |
| MeIQ | UV-DAD 263 nm | 100–2500 | 0.9999 | 100 | 0.3 | 1.0 |
| 8-MeIQx | UV-DAD 263 nm | 100–2500 | 0.9998 | 60 | 0.2 | 0.7 |
| 4,8-DiMeIQx | UV-DAD 263 nm | 100–2500 | 0.9998 | 60 | 0.2 | 0.7 |
| PhIP | FLD λ Ex = 263 nm λ Em = 400 nm | 20–2500 | 0.9999 | 6.0 | 0.02 | 0.07 |
| Concentration 1 (ng/g) and Inhibition (%) in Meat Samples | ||||
|---|---|---|---|---|
| Compound 3 | Without Additives | With Prunes | With Apricots | With Cranberries |
| 8-MeIQx | 3.53 ± 0.55 a,2 | 1.85 ± 0.25 b (47.5%) | 2.44 ± 0.36 b,c (30.9%) | 1.18 ± 0.16 b,d (66.6%) |
| PhIP | 2.36 ± 0.16 a | 1.55 ± 0.19 b (34.3%) | 0.71 ± 0.07 c (69.9%) | 0.20 ± 0.015 d (91.5%) |
| 8-MeIQx + PhIP | 5.89 | 3.4 (42.3%) | 3.15 (46.5%) | 1.95 (76.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulanda, S.; Szumska, M.; Nowak, A.; Janoszka, B.; Damasiewicz-Bodzek, A. Determination of Polar Heterocyclic Aromatic Amines in Meat Thermally Treated in a Roasting Bag with Dried Fruits. Foods 2025, 14, 559. https://doi.org/10.3390/foods14040559
Bulanda S, Szumska M, Nowak A, Janoszka B, Damasiewicz-Bodzek A. Determination of Polar Heterocyclic Aromatic Amines in Meat Thermally Treated in a Roasting Bag with Dried Fruits. Foods. 2025; 14(4):559. https://doi.org/10.3390/foods14040559
Chicago/Turabian StyleBulanda, Sylwia, Magdalena Szumska, Agnieszka Nowak, Beata Janoszka, and Aleksandra Damasiewicz-Bodzek. 2025. "Determination of Polar Heterocyclic Aromatic Amines in Meat Thermally Treated in a Roasting Bag with Dried Fruits" Foods 14, no. 4: 559. https://doi.org/10.3390/foods14040559
APA StyleBulanda, S., Szumska, M., Nowak, A., Janoszka, B., & Damasiewicz-Bodzek, A. (2025). Determination of Polar Heterocyclic Aromatic Amines in Meat Thermally Treated in a Roasting Bag with Dried Fruits. Foods, 14(4), 559. https://doi.org/10.3390/foods14040559







