The Impact of Controlled Fermentation Temperature on Chemical Composition and Sensory Properties of Cacao
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
2.3. Collection and Preparation of Samples
2.4. Extraction and Analysis of Cacao Phenolic-Rich Extract
2.4.1. Ultra-Performance Liquid Chromatography (UPLC-DAD-RI)
2.4.2. Ultraviolet–Visible (UV–Vis) Spectroscopy
2.5. Method’s Validation
2.5.1. Linearity
2.5.2. Limits of Detection (LOD) and Quantification (LOQ)
2.5.3. Precision
2.6. Method’s Application
Phenolic Compounds of Cacao Nibs with Temperature-Controlled Fermentation
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results
3.1. Method’s Validation
3.1.1. Linearity, LOD, and LOQ
3.1.2. Precision
3.2. Method’s Application
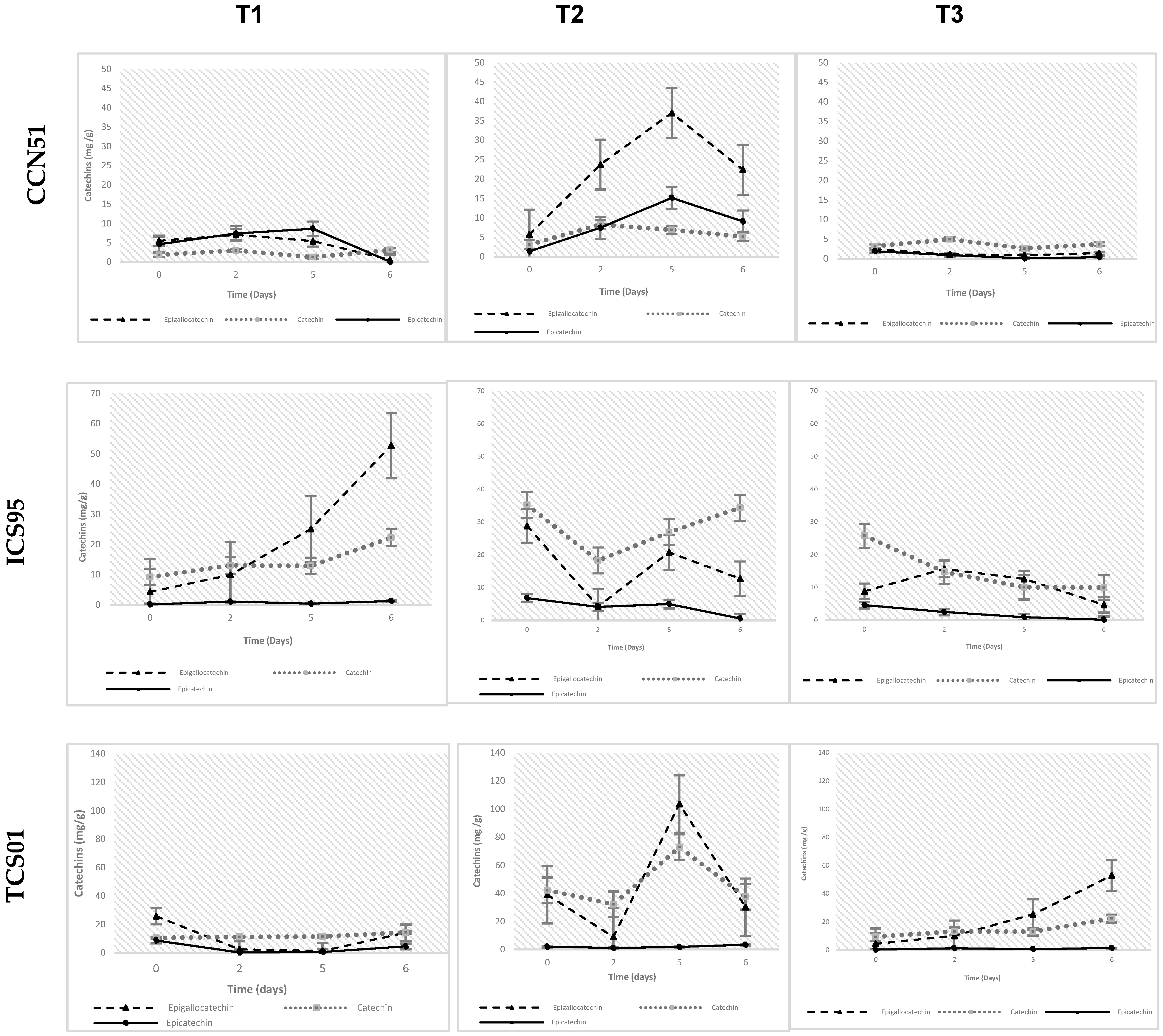
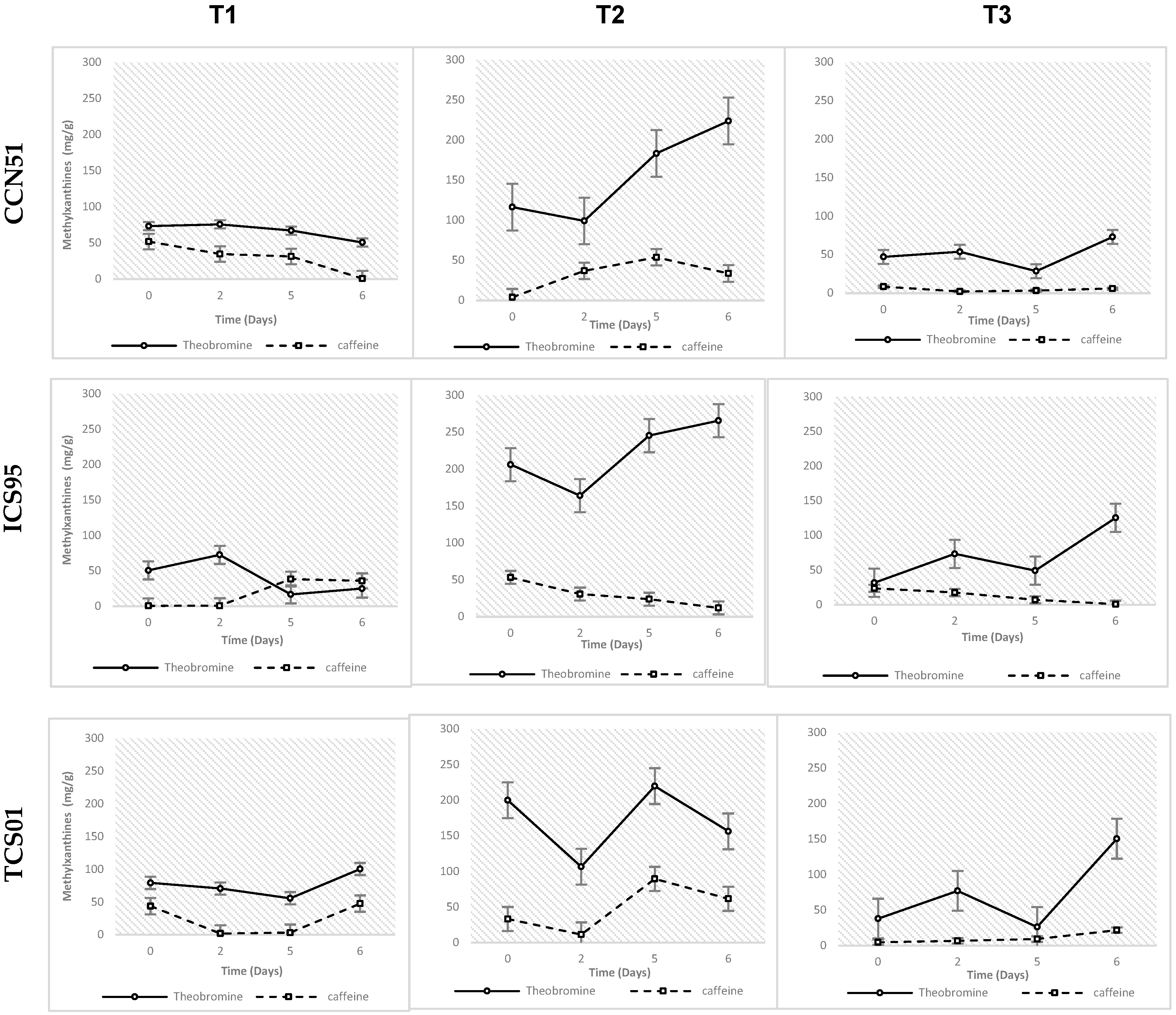
Fermentation Temperature and Variation of Phenolic Compound Concentration
4. Discussion
4.1. Effect of Temperature Fermentation on Phenolic Compound Concentration
4.2. Correlation Between Phenolic Compounds Concentration and Sensory Profile
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mougang, N.N.; Tene, S.T.; Zokou, R.; Kohole, H.A.F.; Solefack, E.N.; Mboukap, A.N.; Abaoabo, A.A.F.; Womeni, H.M. Influence of fermentation time, drying time and temperature on cocoa pods (Theobroma cacao L.) marketability. Appl. Food Res. 2024, 4, 100460. [Google Scholar] [CrossRef]
- Cortez, D.; Quispe-Sanchez, L.; Mestanza, M.; Oliva-Cruz, M.; Yoplac, I.; Torres, C.; Chavez, S.G. Changes in bioactive compounds during fermentation of cocoa (Theobroma cacao) harvested in Amazonas-Peru. Curr. Res. Food Sci. 2023, 6, 100494. [Google Scholar] [CrossRef]
- Ali, M.; Benfante, V.; Stefano, A.; Yezzi, A.; Di Raimondo, D.; Tuttolomondo, A.; Comelli, A. Anti-Arthritic and Anti-Cancer Activities of Polyphenols: A Review of the Most Recent In Vitro Assays. Life 2023, 13, 361. [Google Scholar] [CrossRef] [PubMed]
- Peñarrieta, J.M.; Tejeda, L.; Mollinedo, P.; Vila, J.L.; Bravo, J.A. Phenolic Compounds in Food. 2014. Available online: http://www.bolivianchemistryjournal.org (accessed on 10 January 2025).
- Vázquez-Ovando, A.; Ovando-Medina, I.; Adriano-Anaya, L.; Betancur-Ancona, D.; Salvador-Figueroa, M. Alcaloides y Polifenoles del Cacao, Mecanismos que Regulan su Biosíntesis y sus Implicaciones en el Sabor y Aroma. Chiapas. 2016. Available online: https://ve.scielo.org/pdf/alan/v66n3/art10.pdf (accessed on 19 March 2025).
- Melo, T.S.; Pires, T.C.; Engelmann, J.V.P.; Monteiro, A.L.O.; Maciel, L.F.; Bispo, E.d.S. Evaluation of the content of bioactive compounds in cocoa beans during the fermentation process. J. Food Sci. Technol. 2021, 58, 1947–1957. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.d.l.L.; Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; Guerrero-Muñoz, N.; Villegas-Aguilar, M.d.C.; Pimentel-Moral, S.; Ramos-Escudero, F.; Segura-Carretero, A. LC-MS and spectrophotometric approaches for evaluation of bioactive compounds from Peru cocoa by-products for commercial applications. Molecules 2020, 25, 3177. [Google Scholar] [CrossRef] [PubMed]
- Maleyki, M.J.A.; Ismail, A. Antioxidant properties of cocoa powder. J. Food Biochem. 2010, 34, 111–128. [Google Scholar] [CrossRef]
- Payne, M.J.; Hurst, W.J.; Miller, K.B.; Rank, C.; Stuart, D.A. Impact of Fermentation, Drying, Roasting, and Dutch Processing on Epicatechin and Catechin Content of Cacao Beans and Cocoa Ingredients. J. Agric. Food Chem. 2010, 58, 10518–10527. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Inda, B.; Jiménez-Moreno, N.; Esparza, I.; Ancín-Azpilicueta, C. Coffee and Cocoa By-Products as Valuable Sources of Bioactive Compounds: The Influence of Ethanol on Extraction. Antioxidants 2025, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Stark, T.; Bareuther, S.; Hofmann, T. Molecular definition of the taste of roasted cocoa nibs (Theobroma cacao) by means of quantitative studies and sensory experiments. J. Agric. Food Chem. 2006, 54, 5530–5539. [Google Scholar] [CrossRef] [PubMed]
- Peláez, P.; Bardón, I.; Camasca, P. Methylxanthine and catechin content of fresh and fermented cocoa beans, dried cocoa beans, and cocoa liquor. Sci. Agropecu. 2016, 7, 355–365. [Google Scholar] [CrossRef]
- Camu, N.; De Winter, T.; Addo, S.K.; Takrama, J.S.; Bernaert, H.; De Vuyst, L. Fermentation of cocoa beans: Influence of microbial activities and polyphenol concentrations on the flavour of chocolate. J. Sci. Food Agric. 2008, 88, 2288–2297. [Google Scholar] [CrossRef]
- Calvo, A.M.; Botina, B.L.; García, M.C.; Cardona, W.A.; Montenegro, A.C.; Criollo, J. Dynamics of cocoa fermentation and its effect on quality. Sci. Rep. 2021, 11, 16746. [Google Scholar] [CrossRef]
- Camargo, I.D.; Rodriguez-Silva, L.G.; Carreño-Olejua, R.; Montenegro, A.C.; Quintana-Fuentes, L.F. High temperature and nib acidification during cacao-controlled fermentation improve cadmium transfer from nibs to testa and the liquor’s flavor. Sci. Rep. 2024, 14, 12254. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Rocha, F.; Cala, M.P.; Mejía, J.L.A.; Rodríguez-López, C.M.; Chica, M.J.; Olarte, H.H.; Fernández-Niño, M.; Barrios, A.F.G. Dissecting fine-flavor cocoa bean fermentation through metabolomics analysis to break down the current metabolic paradigm. Sci. Rep. 2021, 11, 21904. [Google Scholar] [CrossRef] [PubMed]
- Goya, L.; Kongor, J.E.; de Pascual-Teresa, S. From Cocoa to Chocolate: Effect of Processing on Flavanols and Methylxanthines and Their Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 14365. [Google Scholar] [CrossRef] [PubMed]
- Mulyawanti, I. Pengaruh Jenis Fermentor Terhadap Mutu Biji Kakao Kering Non Fermentasi. J. Penelit. Pascapanen Pertan. 2018, 15, 91–98. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products—An Overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Mulyawanti, I.; Budijanto, S.; Yasni, S. Stability of anthocyanin during processing, storage and simulated digestion of purple sweet potato pasta. Indones. J. Agric. Sci. 2018, 19, 1. [Google Scholar] [CrossRef]
- Kongor, J.E.; Hinneh, M.; Van de Walle, D.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—A review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Balcázar-Zumaeta, C.R.; Fernández-Romero, E.; Lopes, A.S.; Ferreira, N.R.; Chagas-Júnior, G.C.A.; Yoplac, I.; López-Trigoso, H.A.; Tuesta-Occ, M.L.; Maldonado-Ramirez, I.; Maicelo-Quintana, J.L.; et al. Amino acid profile behavior during the fermentation of Criollo cocoa beans. Food Chem. X 2024, 22, 101486. [Google Scholar] [CrossRef]
- Luna, F.; Crouzillat, D.; Cirou, L.; Bucheli, P. Chemical Composition and Flavor of Ecuadorian Cocoa Liquor. J. Agric. Food Chem. 2002, 50, 3527–3532. [Google Scholar] [CrossRef] [PubMed]
- Febrianto, N.A.; Zhu, F. Changes in the Composition of Methylxanthines, Polyphenols, and Volatiles and Sensory Profiles of Cocoa Beans from the Sul 1 Genotype Affected by Fermentation. J. Agric. Food Chem. 2020, 68, 8658–8675. [Google Scholar] [CrossRef] [PubMed]
- Misnawi; Jinap, S.; Jamilah, B.; Nazamid, S. Sensory properties of cocoa liquor as affected by polyphenol concentration and duration of roasting. Food Qual. Prefer. 2004, 15, 403–409. [Google Scholar] [CrossRef]
- Miller, K.B.; Stuart, D.A.; Smith, N.L.; Lee, C.Y.; McHale, N.L.; Flanagan, J.A.; Ou, B.; Hurst, W.J. Antioxidant Activity and Polyphenol and Procyanidin Contents of Selected Commercially Available Cocoa-Containing and Chocolate Products in the United States. J. Agric. Food Chem. 2006, 54, 4062–4068. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, N.; Whitworth, M.B.; Fowler, M.S.; Fisk, I.D. Hyperspectral imaging for non-destructive prediction of fermentation index, polyphenol content and antioxidant activity in single cocoa beans. Food Chem. 2018, 258, 343–351. [Google Scholar] [CrossRef]
- do Carmo Brito, B.D.N.; Chisté, R.C.; da Silva Pena, R.; Gloria, M.B.A.; Lopes, A.S. Bioactive amines and phenolic compounds in cocoa beans are affected by fermentation. Food Chem. 2017, 228, 484–490. [Google Scholar] [CrossRef]
- Schwan, R.F.; Vinícius, G.; Pereira, M. Microbial Activities During Cocoa Fermentation. 2014. Available online: https://www.researchgate.net/publication/285267847 (accessed on 12 February 2025).
- dos Santos, R.M.; Silva, N.M.d.J.; Moura, F.G.; Lourenço, L.d.F.H.; de Souza, J.N.S.; de Lima, C.L.S. Analysis of the Sensory Profile and Physical and Physicochemical Characteristics of Amazonian Cocoa (Theobroma cacao L.) Beans Produced in Different Regions. Foods 2024, 13, 2171. [Google Scholar] [CrossRef]
- Pedan, V.; Fischer, N.; Rohn, S. An online NP-HPLC-DPPH method for the determination of the antioxidant activity of condensed polyphenols in cocoa. Food Res. Int. 2016, 89, 890–900. [Google Scholar] [CrossRef]
- Trognitz, B.; Cros, E.; Assemat, S.; Davrieux, F.; Forestier-Chiron, N.; Ayestas, E.; Kuant, A.; Scheldeman, X.; Hermann, M. Diversity of Cacao Trees in Waslala, Nicaragua: Associations between Genotype Spectra, Product Quality and Yield Potential. PLoS ONE 2013, 8, e54079. [Google Scholar] [CrossRef] [PubMed]
- James, J.E. Critical Review of Dietary Caffeine and Blood Pressure: A Relationship That Should Be Taken More Seriously. Psychosom. Med. 2004, 66, 63–71. [Google Scholar] [CrossRef] [PubMed]



| Hour | T3 | T1 | T2 |
|---|---|---|---|
| 0 | 35 | 35 | 35 |
| 24 | 40 | 37 | 38 |
| 48 | 44 | 40 | 42 |
| 72 | 46 | 44 | 44 |
| 96 | 48 | 44 | 46 |
| 120 | 47 | 44 | 46 |
| 144 | 47 | 44 | 46 |
| Phenolic Compound | Theobromine | Caffeine | Catechin | Epicatechin | Epigallocatechin | Total Phenols |
|---|---|---|---|---|---|---|
| Standard | Theobromine | Caffeine | (+)-catechin | (-)-epicatechin | (-)-epigallocatechin | Tannic Acid |
| Working range (ug-mL−1) | 0–50 | 0–50 | 0–50 | 0–50 | 0–50 | 0–100 |
| LOD (ug-mL−1) | 0.0031 | 0.0011 | 0.0012 | 0.0006 | 0.0147 | 1.83 |
| LOQ (ug-mL−1) | 0.0105 | 0.0037 | 0.0039 | 0.0020 | 0.0491 | 6.09 |
| Regression equation | y = mx + b | y = mx + b | y = mx + b | y = mx + b | y = mx + b | y = mx + b |
| Correlation coefficient ® | 0.9965 | 0.9998 | 0.9994 | 0.9991 | 0.9969 | 0.9940 |
| Slope (m) | 24,624 | 53,575 | 16,674 | 16,858 | 787.04 | 0.0146 |
| Intercept (b) | 14,265 | −526.4 | 1717.8 | 1690.1 | 382.5 | 0.3211 |
| Phenolic Compound | Theobromine | Caffeine | Catechin | Epicatechin | Epigallocatechin | Total Phenols |
|---|---|---|---|---|---|---|
| Standard | Theobromine | Caffeine | (+)-catechin | (-)-epicatechin | (-)-epigallocatechin | Tannic Acid |
| Intermediate accuracy (RSD) a | 2.49 | 0.72 | 12.17 | 0.94 | 246.3 | 0.044 |
| CV (%) | 4.54 | 5.40 | 7.29 | 5.85 | 4.05 | 3.05 |
| Factor | Theobromine | Caffeine | Catechin | Epicatechin | Epigallocatechin | Total Phenols | |
|---|---|---|---|---|---|---|---|
| Temperature (Treatment) | T1 | 61.507 ± 11.295 a | 24.242 ± 6.433 | 9.204 ± 2.476 | 4.240 ± 0.990 | 6.051 ± 3.749 | 56.247 ± 3.197 |
| T2 | 182.173 ± 11.295 b | 36.960 ± 6.433 | 26.879 ± 2.476 | 4.794 ± 0.990 | 28.064 ± 3.749 | 53.493 ± 3.197 | |
| T3 | 64.505 ± 11.295 c | 9.317 ± 6.433 | 11.023 ± 2.476 | 1.177 ± 0.990 | 11.620 ± 3.749 | 44.862 ± 3.197 | |
| p-value | <0.0001 | 0.013 | <0.0001 | 0.025 | <0.0001 | 0.037 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo, A.M.; Montenegro, A.C.; Monroy, D.M.; Rodriguez-Silva, L.G.; Carreño-Olejua, A.R.; Camargo, I.D. The Impact of Controlled Fermentation Temperature on Chemical Composition and Sensory Properties of Cacao. Foods 2025, 14, 1441. https://doi.org/10.3390/foods14091441
Calvo AM, Montenegro AC, Monroy DM, Rodriguez-Silva LG, Carreño-Olejua AR, Camargo ID. The Impact of Controlled Fermentation Temperature on Chemical Composition and Sensory Properties of Cacao. Foods. 2025; 14(9):1441. https://doi.org/10.3390/foods14091441
Chicago/Turabian StyleCalvo, Ana M., Andrea C. Montenegro, Diana M. Monroy, Lucero G. Rodriguez-Silva, Ariel R. Carreño-Olejua, and Ivan D. Camargo. 2025. "The Impact of Controlled Fermentation Temperature on Chemical Composition and Sensory Properties of Cacao" Foods 14, no. 9: 1441. https://doi.org/10.3390/foods14091441
APA StyleCalvo, A. M., Montenegro, A. C., Monroy, D. M., Rodriguez-Silva, L. G., Carreño-Olejua, A. R., & Camargo, I. D. (2025). The Impact of Controlled Fermentation Temperature on Chemical Composition and Sensory Properties of Cacao. Foods, 14(9), 1441. https://doi.org/10.3390/foods14091441






