A Comprehensive Investigation of Lipid Profile During the Solid-State Fermentation of Rice by Monascus purpureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Raw and Fermented Samples
2.3. Lipids Extraction Procedures
2.4. LC–MS Analysis
2.5. Quality Assurance
2.6. Statistical Analysis
3. Results and Discussion
3.1. Optimization of the Lipid Extraction
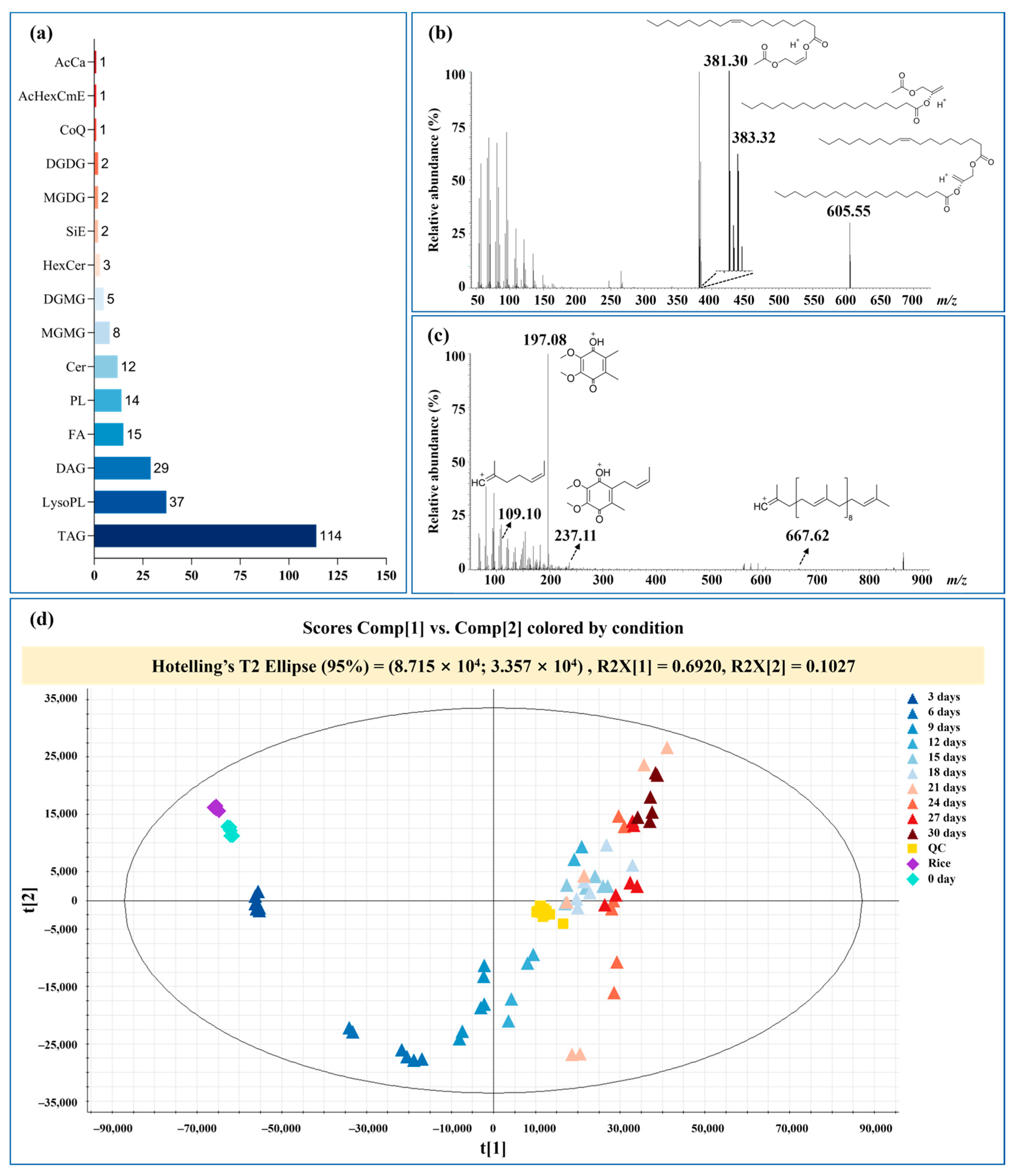
3.2. Lipid Identification by UHPLC–Q–Orbitrap–MS
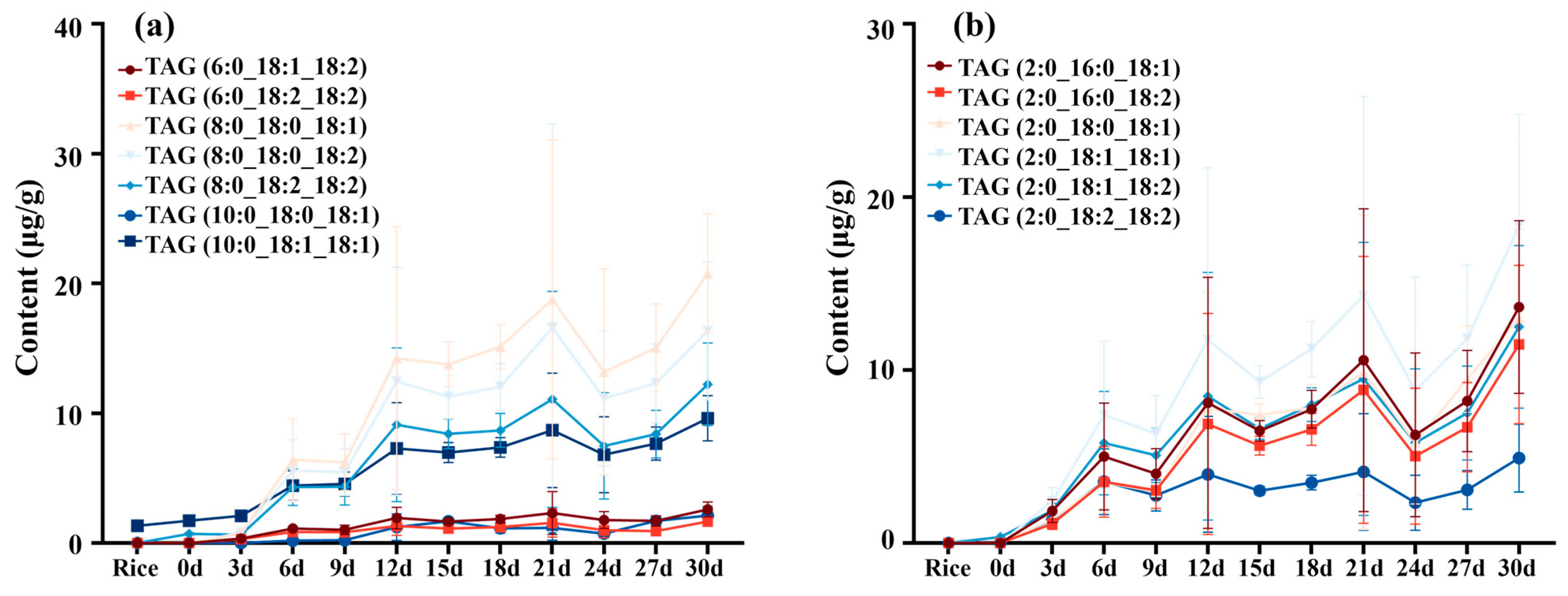
3.3. TAG Profile Changed in Fermentation Process
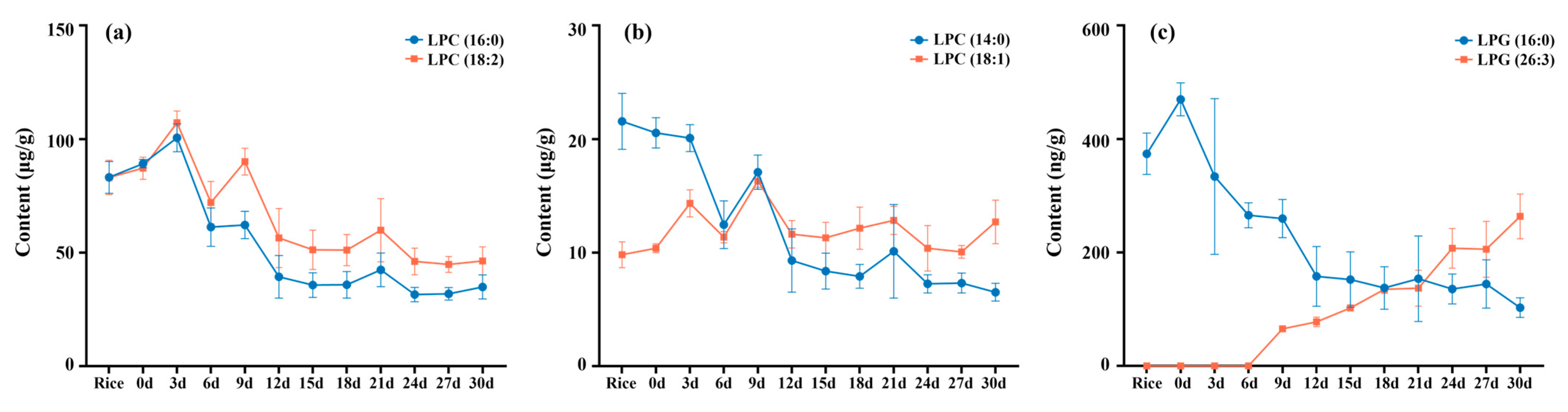
3.4. Phospholipids Changed in Fermentation Process
3.5. Other Lipids Changed in Fermentation Process
3.6. Lipid Biosynthesis Regulation for Monascus-Fermented Rice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AcCa | Acylcarnitine |
| AcHexCmE | Acyl hexosyl campesterol ester |
| Cer | Ceramide |
| CoQ | Coenzyme Q |
| DAG | Diacylglycerol |
| DGDG | Digalactosyldiacylglycerol |
| DGMG | Digalactosylmonoacylglycerol |
| FA | Fatty acid |
| HexCer | Hexosylceramide |
| LCT | Long-chain triacylglycerol |
| LPC | Lysophosphatidylcholine |
| LPE | Lysophosphatidylethanolamine |
| LPG | Lysophosphatidylglycerol |
| LPI | Lysophosphatidylinositol |
| MLCT | Medium- and long-chain triacylglycerol |
| MCT | Medium-chain triacylglycerol |
| MTBE | Methyl-tert-butyl ether |
| MGDG | Monogalactosyldiacylglycerol |
| MGMG | Monogalactosylmonoacylglycerol |
| NIFDC | National Institutes for Food and Drug Control |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PG | Phosphatidylglycerol |
| PI | Phosphatidylinositol |
| PL | Phospholipid |
| SCFA | Short-chain fatty acid |
| SiE | Sitosterol ester |
| TAG | Triacylglycerol |
References
- Cicero, A.F.G.; Fogacci, F.; Stoian, A.P.; Toth, P.P. Red Yeast Rice for the Improvement of Lipid Profiles in Mild-to-Moderate Hypercholesterolemia: A Narrative Review. Nutrients 2023, 15, 2288. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Catapano, A.L.; Cicero, A.F.G.; Escobar, C.; Foger, B.; Katsiki, N.; Latkovskis, G.; Rakowski, M.; Reiner, Z.; Sahebkar, A.; et al. Red yeast rice for dyslipidaemias and cardiovascular risk reduction: A position paper of the International Lipid Expert Panel. Pharmacol. Res. 2022, 183, 106370. [Google Scholar] [CrossRef] [PubMed]
- Benjian, C.; Xiaodan, H.; Huiting, P.; Yishi, L.I.; Yongtao, C.; Huanlin, W.U.; Danping, X.U. Effectiveness and safety of red yeast rice predominated by monacolin K beta-hydroxy acid form for hyperlipidemia treatment and management. J. Tradit. Chin. Med. 2022, 42, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Luo, J.; Ma, Z.; Sun, Q.; Wu, C.; Li, X. Quality and Authenticity Control of Functional Red Yeast Rice-A Review. Molecules 2019, 24, 1944. [Google Scholar] [CrossRef]
- Fukami, H.; Higa, Y.; Hisano, T.; Asano, K.; Hirata, T.; Nishibe, S. A Review of Red Yeast Rice, a Traditional Fermented Food in Japan and East Asia: Its Characteristic Ingredients and Application in the Maintenance and Improvement of Health in Lipid Metabolism and the Circulatory System. Molecules 2021, 26, 1619. [Google Scholar] [CrossRef]
- Liu, B.Y.; Xu, F.; Bai, J.; Yan, D.J.; Zhang, L.; Zhang, D.; Hu, Y.C. Six new monacolin analogs from red yeast rice. Chin. J. Nat. Med. 2019, 17, 394–400. [Google Scholar] [CrossRef]
- Yuan, X.; Gao, S.; Tan, Y.; Cao, J.; Yang, S.; Zheng, B. Production of red yeast rice rich in monacolin K by variable temperature solid fermentation of Monascus purpureus. RSC Adv. 2023, 13, 27303–27308. [Google Scholar] [CrossRef]
- Chaudhary, V.; Katyal, P.; Poonia, A.K.; Kaur, J.; Puniya, A.K.; Panwar, H. Natural pigment from Monascus: The production and therapeutic significance. J. Appl. Microbiol. 2022, 133, 18–38. [Google Scholar] [CrossRef]
- Yang, H.; Pan, R.; Wang, J.; Zheng, L.; Li, Z.; Guo, Q.; Wang, C. Modulation of the Gut Microbiota and Liver Transcriptome by Red Yeast Rice and Monascus Pigment Fermented by Purple Monascus SHM1105 in Rats Fed with a High-Fat Diet. Front. Pharmacol. 2020, 11, 599760. [Google Scholar] [CrossRef]
- Jiang, Z.; Duan, Y.; Yin, Q.; Zhang, J.; Chen, J.; Lan, J.; Xiao, C.; Tang, X.; Wang, X.; Zuo, Y. Study on the effect of ascorbic acid on the biosynthesis of pigment and citrinin in red yeast rice based on comparative transcriptomics. Front. Microbiol. 2024, 15, 1460690. [Google Scholar] [CrossRef]
- Qi, L.; Chen, Z.; Wang, D.; Wang, L.; Soliman, M.M.; El-Bahy, S.M.; Guo, Z.; El-Bahy, Z.M.; Zhang, M.; Hu, P.; et al. Structural characterization of red yeast rice-derived polysaccharide and its promotion of lipid metabolism and gut function in high-fat diet-induced mice. Int. J. Biol. Macromol. 2024, 282, 136744. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xie, L.; Gu, H.; Luo, J.; Li, X. Ultrasonic extraction, structural characterization, and antioxidant activity of oligosaccharides from red yeast rice. Food Sci. Nutr. 2022, 10, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.Y.; Chen, S.H.; Lin, Y.S.; Wu, H.P.; Chao, P.M. An antidiabetic nutraceutical combination of red yeast rice (Monascus purpureus), bitter gourd (Momordica charantia), and chromium alleviates dedifferentiation of pancreatic beta cells in db/db mice. Food Sci. Nutr. 2020, 8, 6718–6726. [Google Scholar] [CrossRef] [PubMed]
- Long, P.; Zhu, L.; Lai, H.; Xu, S.; Dong, X.; Shao, Y.; Wang, L.; Cheng, S.; Liu, G.; He, J.; et al. Monascus Red Pigment Liposomes: Microstructural Characteristics, Stability, and Anticancer Activity. Foods 2023, 12, 447. [Google Scholar] [CrossRef]
- Choe, D.; Song, S.M.; Shin, C.S.; Johnston, T.V.; Ahn, H.J.; Kim, D.; Ku, S. Production and Characterization of Anti-Inflammatory Monascus Pigment Derivatives. Foods 2020, 9, 858. [Google Scholar] [CrossRef]
- Adin, S.N.; Gupta, I.; Panda, B.P.; Mujeeb, M. Monascin and ankaflavin-Biosynthesis from Monascus purpureus, production methods, pharmacological properties: A review. Biotechnol. Appl. Biochem. 2023, 70, 137–147. [Google Scholar] [CrossRef]
- Farawahida, A.H.; Palmer, J.; Flint, S. Monascus spp. and citrinin: Identification, selection of Monascus spp. isolates, occurrence, detection and reduction of citrinin during the fermentation of red fermented rice. Int. J. Food Microbiol. 2022, 379, 109829. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, D.; Fogliano, V.; Pellegrini, N. Rice varieties with a high endosperm lipid content have reduced starch digestibility and increased gamma-oryzanol bioaccessibility. Food Funct. 2021, 12, 11547–11556. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, X.; Shang, B.; Hong, Y.; Sun, H. Analysis of lipidomics profile of rice and changes during storage by UPLC-Q-extractive orbitrap mass spectrometry. Food Res. Int. 2021, 142, 110214. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, X.; Liu, H.; Zhang, D.; Wang, Q.; Liu, J.; Sun, H. A panel of lipid markers for rice discrimination of Wuchang Daohuaxiang in China. Food Res. Int. 2022, 159, 111511. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, S.; Wang, Q.; Shang, B.; Liu, J.; Xing, X.; Hong, Y.; Liu, H.; Duan, X.; Sun, H. Lipidomics and volatilomics reveal the changes in lipids and their volatile oxidative degradation products of brown rice during accelerated aging. Food Chem. 2023, 421, 136157. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, D.; Wang, Q.; Shang, B.; Liu, J.; Xing, X.; Hong, Y.; Duan, X.; Sun, H. Shotgun lipidomics reveals the changes in phospholipids of brown rice during accelerated aging. Food Res. Int. 2023, 171, 113073. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Zhao, Y.; Xu, X.; Li, Y.; Lv, H. Untargeted Lipidomics Reveal Quality Changes in High-Moisture Japonica Brown Rice at Different Storage Temperatures. Foods 2023, 12, 4218. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhou, B.; Cheng, L.; Huang, J.; Zou, P.; Zeng, Y.; Huang, S.; Chen, T.; Liu, C.; Wu, J. Pre-fermentation of rice flour for improving the cooking quality of extruded instant rice. Food Chem. 2022, 386, 132757. [Google Scholar] [CrossRef]
- Stone, S.J. Mechanisms of intestinal triacylglycerol synthesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159151. [Google Scholar] [CrossRef]
- Xu, C.; Shanklin, J. Triacylglycerol Metabolism, Function, and Accumulation in Plant Vegetative Tissues. Annu. Rev. Plant Biol. 2016, 67, 179–206. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Liu, R.; Chang, M.; Wei, W.; Jin, Q.; Wang, X. Reviews of medium- and long-chain triglyceride with respect to nutritional benefits and digestion and absorption behavior. Food Res. Int. 2022, 155, 111058. [Google Scholar] [CrossRef]
- Yu, J.; Lu, H.; Zhang, X.; Tang, J.; Liu, Z.; Jin, Q.; Wei, W.; Wang, X. The triacylglycerol structures are key factors influencing lipid digestion in preterm formulas during in vitro digestion. Food Chem. 2024, 443, 138546. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Tang, T.K.; Chan, E.S.; Phuah, E.T.; Lai, O.M.; Tan, C.P.; Wang, Y.; Ab Karim, N.A.; Mat Dian, N.H.; Tan, J.S. Medium chain triglyceride and medium-and long chain triglyceride: Metabolism, production, health impacts and its applications—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 4169–4185. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, C.; Jin, J.; Jin, Q.; Akoh, C.C.; Wei, W.; Wang, X. Medium- and Long-Chain Triacylglycerol: Preparation, Health Benefits, and Food Utilization. Annu. Rev. Food Sci. Technol. 2024, 15, 381–408. [Google Scholar] [CrossRef]
- Yuan, T.; Geng, Z.; Dai, X.; Zhang, X.; Wei, W.; Wang, X.; Jin, Q. Triacylglycerol Containing Medium-Chain Fatty Acids: Comparison of Human Milk and Infant Formulas on Lipolysis during In Vitro Digestion. J. Agric. Food Chem. 2020, 68, 4187–4195. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Akoh, C.C. Enzymatic Synthesis and Characterization of MLM-type Structured Lipid Using Grapeseed Oil and Capric Acid. J. Oleo Sci. 2024, 73, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wu, Q.; Yao, Q.; Jiang, K.; Yu, J.; Tang, Q. Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res. Rev. 2022, 81, 101706. [Google Scholar] [CrossRef]
- Liang, J.X.; Zhang, Q.Q.; Huang, Y.F.; Pang, H.Q.; Liu, X.G.; Gao, W.; Li, P.; Yang, H. Comprehensive chemical profiling of monascus-fermented rice product and screening of lipid-lowering compounds other than monacolins. J. Ethnopharmacol. 2019, 238, 111879. [Google Scholar] [CrossRef]
- Yuan, T.; Cheng, X.; Shen, L.; Liu, Z.; Ye, X.; Yan, Z.; Wei, W.; Wang, X. Novel Human Milk Fat Substitutes Based on Medium- and Long-Chain Triacylglycerol Regulate Thermogenesis, Lipid Metabolism, and Gut Microbiota Diversity in C57BL/6J Mice. J. Agric. Food Chem. 2024, 72, 6213–6225. [Google Scholar] [CrossRef]
- Du, Y.X.; Chen, S.N.; Zhu, H.L.; Niu, X.; Li, J.; Fan, Y.W.; Deng, Z.Y. Consumption of Interesterified Medium- and Long-Chain Triacylglycerols Improves Lipid Metabolism and Reduces Inflammation in High-Fat Diet-Induced Obese Rats. J. Agric. Food Chem. 2020, 68, 8255–8262. [Google Scholar] [CrossRef]
- Ikeda, T.; Nishida, A.; Yamano, M.; Kimura, I. Short-chain fatty acid receptors and gut microbiota as therapeutic targets in metabolic, immune, and neurological diseases. Pharmacol. Ther. 2022, 239, 108273. [Google Scholar] [CrossRef]
- Wang, S.; Chao, C.; Cai, J.; Niu, B.; Copeland, L.; Wang, S. Starch-lipid and starch-lipid-protein complexes: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1056–1079. [Google Scholar] [CrossRef]
- Liu, L.; Waters, D.L.; Rose, T.J.; Bao, J.; King, G.J. Phospholipids in rice: Significance in grain quality and health benefits: A review. Food Chem. 2013, 139, 1133–1145. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhao, X.B.; Ha, W.; Zhang, Y.D.; Shi, Y.P. Spatial distribution analysis of phospholipids in rice by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry imaging. J. Chromatogr. A 2021, 1651, 462302. [Google Scholar] [CrossRef]
- Nie, M.; Liu, T.; Qiu, X.; Yang, J.; Liu, J.; Ren, J.; Zhou, B. Regulation mechanism of lipids for extracellular yellow pigments production by Monascus purpureus BWY-5. Appl. Microbiol. Biotechnol. 2023, 107, 5191–5208. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Morbini, M.; Rosticci, M.; D’Addato, S.; Grandi, E.; Borghi, C. Middle-Term Dietary Supplementation with Red Yeast Rice Plus Coenzyme Q10 Improves Lipid Pattern, Endothelial Reactivity and Arterial Stiffness in Moderately Hypercholesterolemic Subjects. Ann. Nutr. Metab. 2016, 68, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, A.; Longo, S.; Gnoni, G.V.; Giudetti, A.M. Carnitine in Human Muscle Bioenergetics: Can Carnitine Supplementation Improve Physical Exercise? Molecules 2020, 25, 182. [Google Scholar] [CrossRef] [PubMed]
- Pavesi, C.; Flon, V.; Mann, S.; Leleu, S.; Prado, S.; Franck, X. Biosynthesis of azaphilones: A review. Nat. Prod. Rep. 2021, 38, 1058–1071. [Google Scholar] [CrossRef]
- Jiang, X.; Hong, X.; Wang, Z.; Liu, J.; Zhong, H.; Ren, J.; Zhou, B. Phospholipid biosynthesis regulation for improving pigment production by Monascus in response to ammonium chloride stress. Appl. Environ. Microbiol. 2024, 90, e0114624. [Google Scholar] [CrossRef]
- Yi, X.; Han, J.; Xu, X.; Wang, Y.; Zhang, M.; Zhu, J.; He, Y. Taurine-mediated gene transcription and cell membrane permeability reinforced co-production of bioethanol and Monascus azaphilone pigments for a newly isolated Monascus purpureus. Biotechnol. Biofuels Bioprod. 2024, 17, 59. [Google Scholar] [CrossRef]

| No. | Lipid Species | Formula | RT (min) | Proposed Ion | Observed m/z | Theoretical m/z | Fragmental Ions (Measured) |
|---|---|---|---|---|---|---|---|
| 1 | TAG (O-14:2_4:0_18:2) | C39H68O5 | 16.72 | [M + NH4]+ | 634.5385 | 634.5405 | 337.2734, 599.5027, 617.5138 |
| 2 | TAG (2:0_18:2_18:2) | C41H70O6 | 17.11 | [M + NH4]+ | 676.5496 | 676.5511 | 379.2839, 599.5024 |
| 3 | TAG (2:0_16:0_18:2) | C39H70O6 | 17.92 | [M + NH4]+ | 652.5495 | 652.5511 | 379.2839, 355.2839, 575.5029 |
| 4 | TAG (2:0_18:1_18:2) | C41H72O6 | 17.94 | [M + NH4]+ | 678.5649 | 678.5667 | 381.2995, 379.2841, 601.5186 |
| 5 | TAG (O-13:1_5:0_18:1) | C39H72O5 | 18.38 | [M + NH4]+ | 638.5699 | 638.5718 | 339.2892, 603.5346 |
| 6 | TAG (6:0_18:2_18:2) | C45H78O6 | 18.79 | [M + NH4]+ | 732.6116 | 732.6137 | 435.3466, 599.5028 |
| 7 | TAG (2:0_16:0_18:1) | C39H72O6 | 18.80 | [M + NH4]+ | 654.5652 | 654.5667 | 381.2995, 355.2838, 577.5183 |
| 8 | TAG (2:0_18:1_18:1) | C41H74O6 | 18.84 | [M + NH4]+ | 680.5808 | 680.5824 | 381.2995, 603.5334 |
| 9 | TAG (6:0_18:1_18:2) | C45H80O6 | 19.32 | [M + NH4]+ | 734.6268 | 734.6293 | 437.3624, 435.3468, 601.5186 |
| 10 | TAG (8:0_18:2_18:2) | C47H82O6 | 19.37 | [M + NH4]+ | 760.6427 | 760.6450 | 463.3776, 599.5026 |
| 11 | TAG (2:0_18:0_18:1) | C41H76O6 | 19.41 | [M + NH4]+ | 682.5964 | 682.5980 | 381.2997, 383.3152, 605.5497 |
| 12 | TAG (O-15:0_18:0_3:0) | C39H76O5 | 19.57 | [M + NH4]+ | 642.6016 | 642.6031 | 341.3048, 607.5656 |
| 13 | TAG (8:0_16:0_18:2) | C45H82O6 | 19.65 | [M + NH4]+ | 736.6432 | 736.6450 | 463.3775, 439.3776, 575.5028 |
| 14 | TAG (8:0_18:1_18:2) | C47H84O6 | 19.70 | [M + NH4]+ | 762.6589 | 762.6606 | 465.3937, 463.3778, 601.5184 |
| 15 | TAG (8:0_18:0_18:2) | C47H86O6 | 19.88 | [M + NH4]+ | 764.6741 | 764.6763 | 603.5343, 467.4092, 463.3776 |
| 16 | TAG (10:0_18:1_18:1) | C49H90O6 | 20.05 | [M + NH4]+ | 792.7055 | 792.7076 | 493.4249, 603.5344 |
| 17 | TAG (8:0_18:0_18:1) | C47H88O6 | 20.05 | [M + NH4]+ | 766.6901 | 766.6919 | 605.5496, 467.4090, 465.3934 |
| 18 | TAG (10:0_18:0_18:1) | C49H92O6 | 20.31 | [M + NH4]+ | 794.7205 | 794.7232 | 495.4403, 493.4248, 605.5496 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, L.; Cao, Y.; Yuan, J.; Feng, R.; Pan, H.; Mao, X.; Ji, S.; Hu, Q.; Zhou, H. A Comprehensive Investigation of Lipid Profile During the Solid-State Fermentation of Rice by Monascus purpureus. Foods 2025, 14, 537. https://doi.org/10.3390/foods14030537
Lan L, Cao Y, Yuan J, Feng R, Pan H, Mao X, Ji S, Hu Q, Zhou H. A Comprehensive Investigation of Lipid Profile During the Solid-State Fermentation of Rice by Monascus purpureus. Foods. 2025; 14(3):537. https://doi.org/10.3390/foods14030537
Chicago/Turabian StyleLan, Lan, Yimin Cao, Jiajia Yuan, Rui Feng, Huiqin Pan, Xiuhong Mao, Shen Ji, Qing Hu, and Heng Zhou. 2025. "A Comprehensive Investigation of Lipid Profile During the Solid-State Fermentation of Rice by Monascus purpureus" Foods 14, no. 3: 537. https://doi.org/10.3390/foods14030537
APA StyleLan, L., Cao, Y., Yuan, J., Feng, R., Pan, H., Mao, X., Ji, S., Hu, Q., & Zhou, H. (2025). A Comprehensive Investigation of Lipid Profile During the Solid-State Fermentation of Rice by Monascus purpureus. Foods, 14(3), 537. https://doi.org/10.3390/foods14030537






