Self-Enhanced Near-Infrared Copper Nanoscale Electrochemiluminescence Probe for the Sensitive Detection of Ciprofloxacin in Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
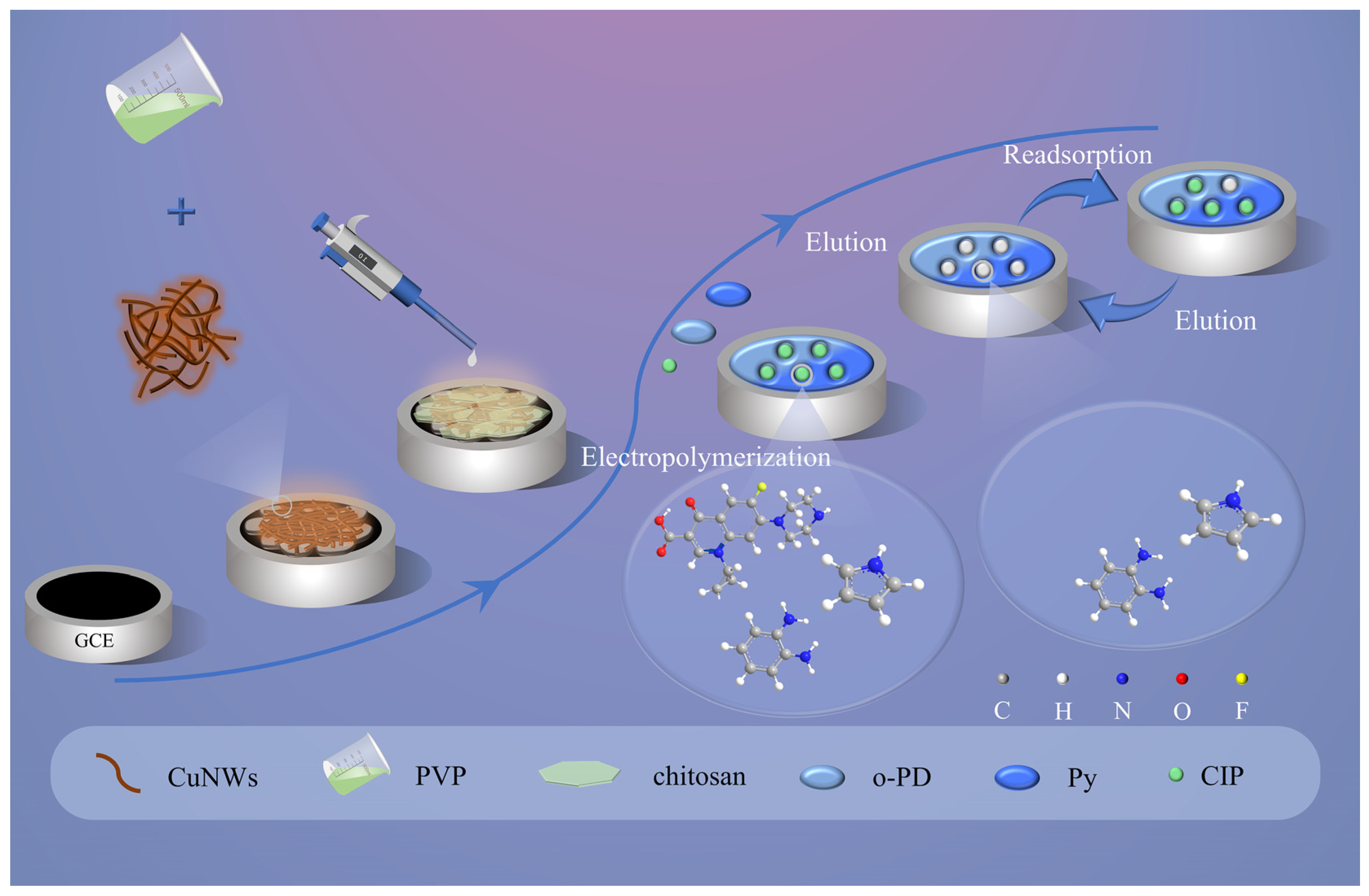
2.2. Synthesis of CuNWs@PVP
2.3. Fabrication Process of CuNWs@PVP/GCE
2.4. Preparation of MIP/CuNWs@PVP/GCE and NIPCuNWs@PVP/GCE
2.5. Testing Conditions and Instruments
2.6. Real Sample Preparation
3. Results
3.1. Morphology Characterization of MIP/CuNWs@PVP/GCE
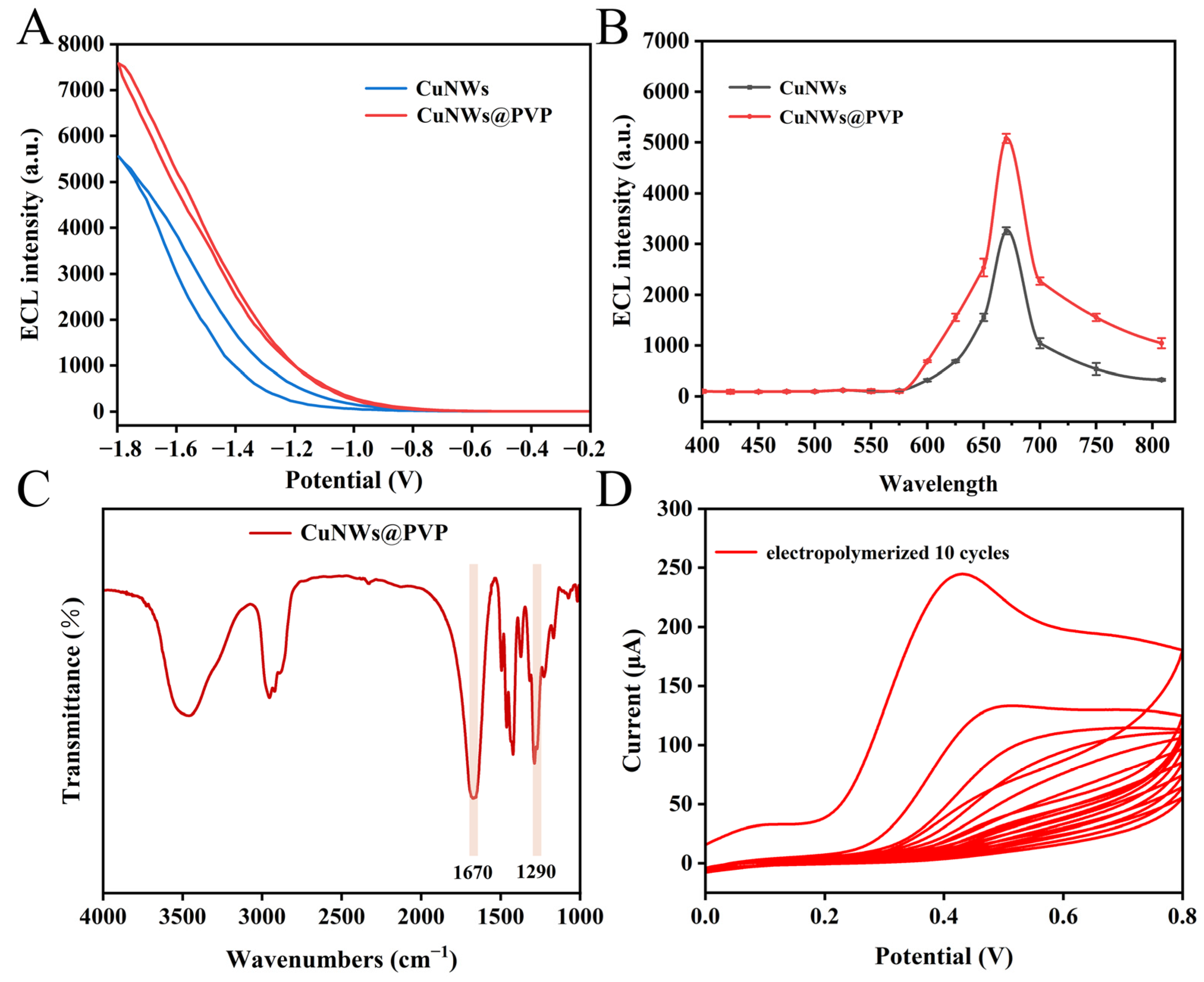
3.2. NIR ECL and Fourier Transform Infrared Spectroscopy Characterization
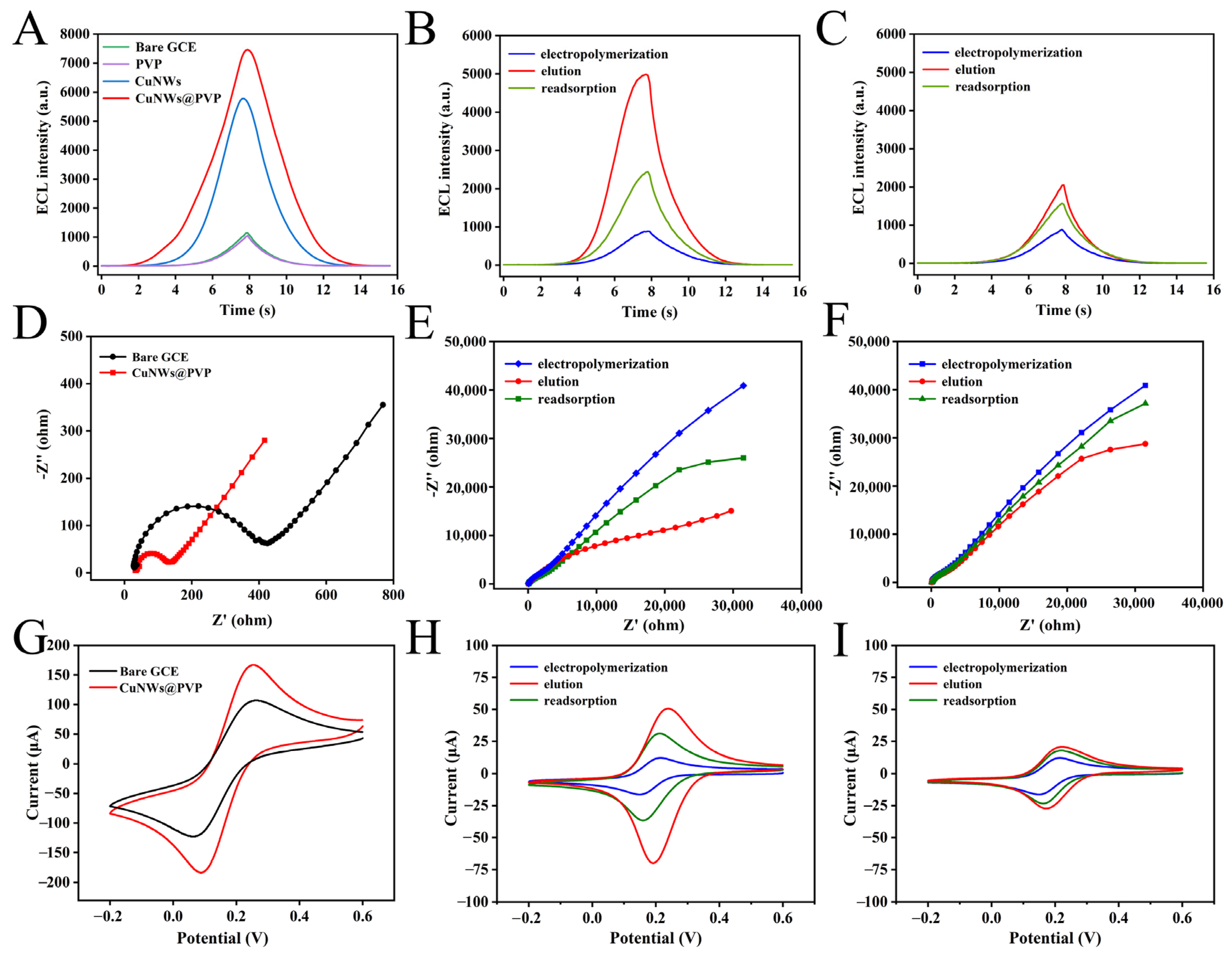
3.3. Characterization of MIP/CuNWs@PVP/GCE
3.4. MIP/CuNWs@PVP/GCE Response Behavior to CIP
3.5. Condition Optimization of MIP/CuNWs@PVP/GCE
3.6. Analysis of MIECLS Application Performance
3.7. Stability, Selectivity, Reproducibility, and Regeneration Analysis of MIECLS
3.8. Assessment of MIECLS Performance in Actual Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tan, L.; Luo, J.; Liu, Q.; Wang, M.; Li, Y.; Li, J.; Yu, J.; Dong, C.; Xu, T.; Ye, W. Machine learning-aided quantitative detection of two mixed antibiotics in a narrow concentration range based on oxygen incorporation-induced 3D SERS substrates. Sens. Actuators B Chem. 2024, 423, 136741. [Google Scholar] [CrossRef]
- Lu, Z.; Gong, Y.; Shen, C.; Chen, H.; Zhu, W.; Liu, T.; Wu, C.; Sun, M.; Su, G.; Wang, X.; et al. Portable, intelligent MIECL sensing platform for ciprofloxacin detection using a fast convolutional neural networks-assisted Tb@ Lu2O3 nanoemitter. Food Chem. 2024, 444, 138656. [Google Scholar] [CrossRef] [PubMed]
- Pu, Q.; Wang, H.-T.; Pan, T.; Li, H.; Su, J.-Q. Enhanced removal of ciprofloxacin and reduction of antibiotic resistance genes by earthworm Metaphire vulgaris in soil. Sci. Total Environ. 2020, 742, 140409. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wang, Y.; Lin, H.; Sun, Y.; Pan, Y.; Qiao, J.-Q.; Lian, H.-Z.; Xu, C.-X. Residue screening and analysis of enrofloxacin and its metabolites in real aquatic products based on ultrahigh-performance liquid chromatography coupled with high resolution mass spectrometry. Food Chem. 2022, 404, 134757. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Müller-Bötticher, L.; Liu, C.; Popp, J.; Fischer, D.; Cialla-May, D. Raman-based detection of ciprofloxacin and its degradation in pharmaceutical formulations. Talanta 2022, 250, 123719. [Google Scholar] [CrossRef]
- Jia, H.; Li, J.; Yang, L.; Fan, D.; Kuang, X.; Sun, X.; Wei, Q.; Ju, H. Hollow double-shell CuCO2O4@ Cu2O heterostructures as a highly efficient coreaction accelerator for amplifying NIR electrochemiluminescence of gold nanoclusters in immunoassay. Anal. Chem. 2022, 94, 7132–7139. [Google Scholar] [CrossRef]
- Wei, M.; Du, X.; Jiang, D.; Zhang, Y.; Shan, X.; Wang, W.; Shiigi, H.; Chen, Z. Highly efficient near-infrared electrochemiluminescence resonance energy transfer system for biosensing: Nonmetallic plasmon Mediated well-matched energy donor-acceptor pair. Biosens. Bioelectron. 2023, 236, 115420. [Google Scholar] [CrossRef]
- Yang, Y.-T.; Guo, Y.-Z.; Shen, Z.-C.; Liu, J.-L.; Yuan, R.; Chai, Y.-Q. AgAuS quantum dots as a highly efficient near-infrared electrochemiluminescence emitter for the ultrasensitive detection of microRNA. Anal. Chem. 2023, 95, 9314–9322. [Google Scholar] [CrossRef]
- Bagchi, B.; Datta, P.; Fernandez, C.S.; Gupta, P.; Jaufuraully, S.; David, A.L.; Siassakos, D.; Desjardins, A.; Tiwari, M.K. Flexible triboelectric nanogenerators using transparent copper nanowire electrodes: Energy harvesting, sensing human activities and material recognition. Mater. Horiz. 2023, 10, 3124–3134. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, A.; Liu, M.; Tang, S.; Wang, W.; Liang, A. Self-enhanced luminescence of copper nanowires combined with pH-sensitive glycosyl imprinted polymers: Electrochemiluminescence for high-sensitivity detection of sialic acid. Sens. Actuators B Chem. 2024, 410, 135730. [Google Scholar] [CrossRef]
- Luo, Z.; Du, P.; Guo, Z.; Song, M.; Li, B.; Cai, Z.; Ge, F. Construction of a Reciprocal-Supporting Phenol-amine@ CuNW Network for Antisedimentation Conductive Ink. ACS Appl. Mater. Interfaces 2023, 15, 27422–27433. [Google Scholar] [CrossRef]
- Baek, I.-G.; Nyamaa, O.; Kim, J.-S.; Goo, K.-M.; Kim, K.-S.; Nam, T.-H.; Yang, J.-H.; Noh, J.-P. Enhancing the electrochemical performance of free-standing electrodes using multi-walled carbon nanotubes functionalized with PVP/SDBS mixed dispersant. J. Energy Storage 2024, 103, 114362. [Google Scholar] [CrossRef]
- Zacahua-Tlacuatl, G.; Ramírez-Meneses, E.; Manzo-Robledo, A.; Torres-Huerta, A.; Betancourt, I.; Philippot, K.; Ibrahim, M.; Domínguez-Crespo, M. Improved methanol electro-oxidation reaction on PdRh-PVP/C electrodes. Int. J. Hydrogen Energy 2022, 48, 8450–8464. [Google Scholar] [CrossRef]
- Wang, X.; Huang, W.; Li, X.; Li, X.; Chai, X.; Zhou, Y.; Zhong, J.; Zhou, R. Preparation of mixed matrix membranes with PVP-induced fluorinated Zr-MOF for high-efficiency hydrogen purification under high humidity conditions. J. Membr. Sci. 2024, 713, 123351. [Google Scholar] [CrossRef]
- Zhao, S.; Han, F.; Li, J.; Meng, X.; Huang, W.; Cao, D.; Zhang, G.; Sun, R.; Wong, C. Advancements in copper nanowires: Synthesis, purification, assemblies, surface modification, and applications. Small 2018, 14, 1800047. [Google Scholar] [CrossRef]
- Zhen, J.; Ma, F.; Yan, J.; Lin, R.; Yan, M.; Wu, Y. Synthesis of metal-organic framework hybrid nanocomposites based on MOFs@ C3N4 with high selective separation ability for luteolin. Sep. Purif. Technol. 2023, 335, 126139. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Dang, Y.; Chen, Z.; Zhang, R.; Li, Y.; Ye, B.-C. A robust electrochemical sensing of molecularly imprinted polymer prepared by using bifunctional monomer and its application in detection of cypermethrin. Biosens. Bioelectron. 2018, 127, 207–214. [Google Scholar] [CrossRef]
- He, Y.; Luo, L.; Li, L.; You, T.; Chen, X. Synergistic signal−amplification effect of silver nanowires and bifunctional monomers on molecularly imprinted electrochemical sensor for diuron analysis. Biosens. Bioelectron. 2024, 262, 116570. [Google Scholar] [CrossRef]
- Chinese Ministry of Agriculture. Bulletin No. 1025-14-2008; Chinese Ministry of Agriculture: Beijing, China, 2008. [Google Scholar]
- Yang, H.; Zhou, J.; Duan, Z.; Lu, B.; Deng, B.; Xu, W. Preparation of structural color on cotton fabric with high color fastness through multiple hydrogen bonds between polyphenol hydroxyl and lactam. ACS Appl. Mater. Interfaces 2022, 14, 3244–3254. [Google Scholar] [CrossRef]
- He, J.; Li, J. Core-satellite nanocomposites via PEI-induced self-assembly for enhanced SERS sensing of thiram and ciprofloxacin. J. Saudi Chem. Soc. 2023, 28, 101790. [Google Scholar] [CrossRef]
- Li, J.; Tang, X.; Guo, X.; Han, W.; Wang, S.; Zha, F.; Tian, H.; Chang, Y. CeO2/NH2-MIL-88B (Fe) composites with peroxidase-like activity for colorimetric detection and photo-enzymatic synergetic degradation of ciprofloxacin hydrochloride. J. Environ. Chem. Eng. 2024, 12, 114734. [Google Scholar] [CrossRef]
- Pavão e Pavão, D.; Nascimento Botelho, C.; Nunes Fernandes, R.; Costa dos Santos, C.; Santos Damos, F.; de Cássia Silva Luz, R. A Simple, Cost-effective, and Environmentally Friendly Method for Determination of Ciprofloxacin in Drugs and Urine Samples Based on Electrogenerated Chemiluminescence. Electroanalysis 2020, 32, 1498–1506. [Google Scholar] [CrossRef]
- Matsunaga, T.; Kondo, T.; Osasa, T.; Kotsugai, A.; Shitanda, I.; Hoshi, Y.; Itagaki, M.; Aikawa, T.; Tojo, T.; Yuasa, M. Sensitive electrochemical detection of ciprofloxacin at screen-printed diamond electrodes. Carbon 2020, 159, 247–254. [Google Scholar] [CrossRef]
- Pollap, A.; Baran, K.; Kuszewska, N.; Kochana, J. Electrochemical sensing of ciprofloxacin and paracetamol in environmental water using titanium sol based sensor. J. Electroanal. Chem. 2020, 878, 114574. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, C.; Zhang, Y.; Zhang, G.; Shuang, S. Dual-emission ratio fluorescent probe based on carbon dots and copper-silver bimetallic nanoclusters for visual and fluorescent detection of ciprofloxacin. Microchem. J. 2024, 202, 110810. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, J.; Lin, D.; Han, L.; Li, B.; Chen, J.; Wu, Y.; Huang, Y.; Chen, L.; Wang, X. Visual detection of ciprofloxacin using a multi-emission ratiometric fluorescence sensor based on the molecularly imprinted polymers doped with terbium (III) ions. Talanta 2025, 286, 127537. [Google Scholar] [CrossRef]
- Vella, J.; Busuttil, F.; Bartolo, N.S.; Sammut, C.; Ferrito, V.; Serracino-Inglott, A.; Azzopardi, L.M.; LaFerla, G. A simple HPLC–UV method for the determination of ciprofloxacin in human plasma. J. Chromatogr. B 2015, 989, 80–85. [Google Scholar] [CrossRef]
- Szerkus, O.; Jacyna, J.; Gibas, A.; Sieczkowski, M.; Siluk, D.; Matuszewski, M.; Kaliszan, R.; Markuszewski, M. Robust HPLC–MS/MS method for levofloxacin and ciprofloxacin determination in human prostate tissue. J. Pharm. Biomed. Anal. 2017, 132, 173–183. [Google Scholar] [CrossRef]



| Spiked (μg kg−1) | Found (μg kg−1) | Recovery (%) (Mean ± SD) (n = 3) | |
|---|---|---|---|
| Pork | 0 | Nd a | –– a |
| 20 | 17.08 | 85.44 ± 1.32 | |
| 30 | 27.00 | 90.03 ± 2.15 | |
| 40 | 36.62 | 91.56 ± 1.89 | |
| Fish | 0 | Nd a | –– a |
| 20 | 16.88 | 84.39 ± 1.45 | |
| 30 | 27.74 | 92.48 ± 0.79 | |
| 40 | 36.44 | 91.09 ± 1.37 |
| Methods | Linear Range (M) | LOD (M) | Reference |
|---|---|---|---|
| SERS a | 1.00 × 10−8–1.00 × 10−4 | 5.20 × 10−9 | [21] |
| Colorimetric | 5.00 × 10−5–2.00 × 10−4 | 2.90 × 10−8 | [22] |
| Electrochemiluminescence | 5.00 × 10−7–2.00 × 10−4 | 5.00 × 10−7 | [23] |
| Electrochemical | 1.00 × 10−5–3.00 × 10−3 | 5.88 × 10−7 | [24] |
| Electrochemical | 1.00 × 10−6–3.00 × 10−5 | 1.08 × 10−7 | [25] |
| Fluorescence | 5.00 × 10−6–2.00 × 10−4 | 3.30 × 10−6 | [26] |
| Fluorescence | 8.00 × 10−8–5.00 × 10−5 | 1.80 × 10−8 | [27] |
| HPLC-UV b | 1.50 × 10−7–2.40 × 10−5 | 3.00 × 10−8 | [28] |
| HPLC-MS/MS c | 3.00 × 10−6–2.00 × 10−4 | 2.50 × 10−7 | [29] |
| Electrochemiluminescence | 5.00 × 10−9–5.00 × 10−5 | 2.59 × 10−9 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Qin, Y.; Mei, X.; Cai, L.; Hao, W.; Fang, G. Self-Enhanced Near-Infrared Copper Nanoscale Electrochemiluminescence Probe for the Sensitive Detection of Ciprofloxacin in Foods. Foods 2025, 14, 538. https://doi.org/10.3390/foods14030538
Wu J, Qin Y, Mei X, Cai L, Hao W, Fang G. Self-Enhanced Near-Infrared Copper Nanoscale Electrochemiluminescence Probe for the Sensitive Detection of Ciprofloxacin in Foods. Foods. 2025; 14(3):538. https://doi.org/10.3390/foods14030538
Chicago/Turabian StyleWu, Jie, Yuanjie Qin, Xiaoxin Mei, Lin Cai, Wen Hao, and Guozhen Fang. 2025. "Self-Enhanced Near-Infrared Copper Nanoscale Electrochemiluminescence Probe for the Sensitive Detection of Ciprofloxacin in Foods" Foods 14, no. 3: 538. https://doi.org/10.3390/foods14030538
APA StyleWu, J., Qin, Y., Mei, X., Cai, L., Hao, W., & Fang, G. (2025). Self-Enhanced Near-Infrared Copper Nanoscale Electrochemiluminescence Probe for the Sensitive Detection of Ciprofloxacin in Foods. Foods, 14(3), 538. https://doi.org/10.3390/foods14030538





