Fabrication of a Chitosan–Gelatin/Polylactic Acid Bilayer Active Film Loaded with Tannic Acid for Enhancing Shelf-Life of Refrigerated Baby Clams
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Raw Material
2.2. Preparation of PLA/CSFG Bilayer Films Loaded with TA at Various Concentrations
2.3. Characterization of Active Bilayer Films
2.3.1. Thickness, Color, and Light Barrier Properties
2.3.2. Mechanical Characteristics and Water Vapor Permeability (WVP)
2.3.3. Attenuated Total Reflection–Fourier Transform Infrared (ATR-FTIR) Spectroscopic Spectra
2.3.4. Antioxidant Activities
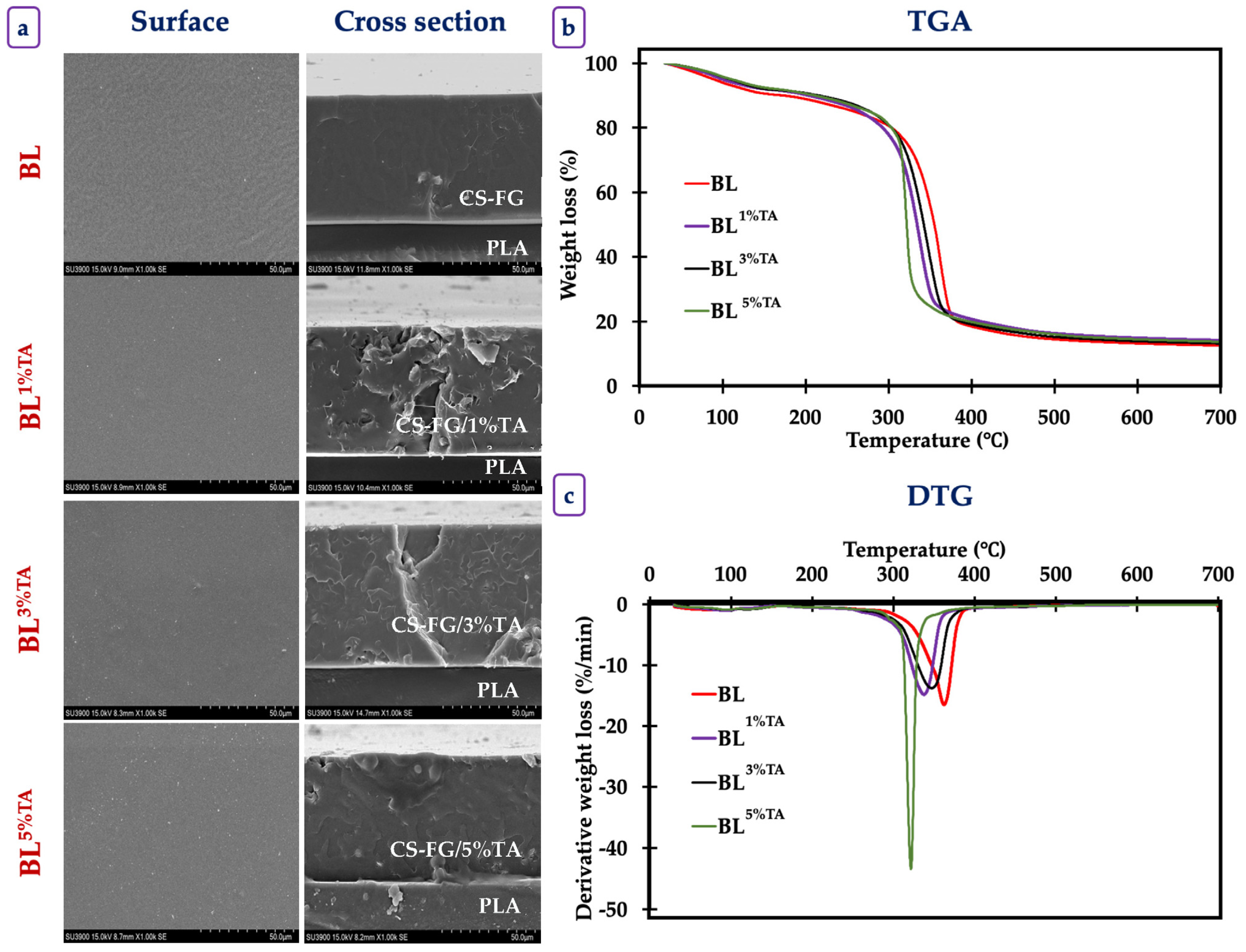
2.3.5. Scanning Electron Microscope (SEM) Images
2.3.6. Thermogravimetric Property
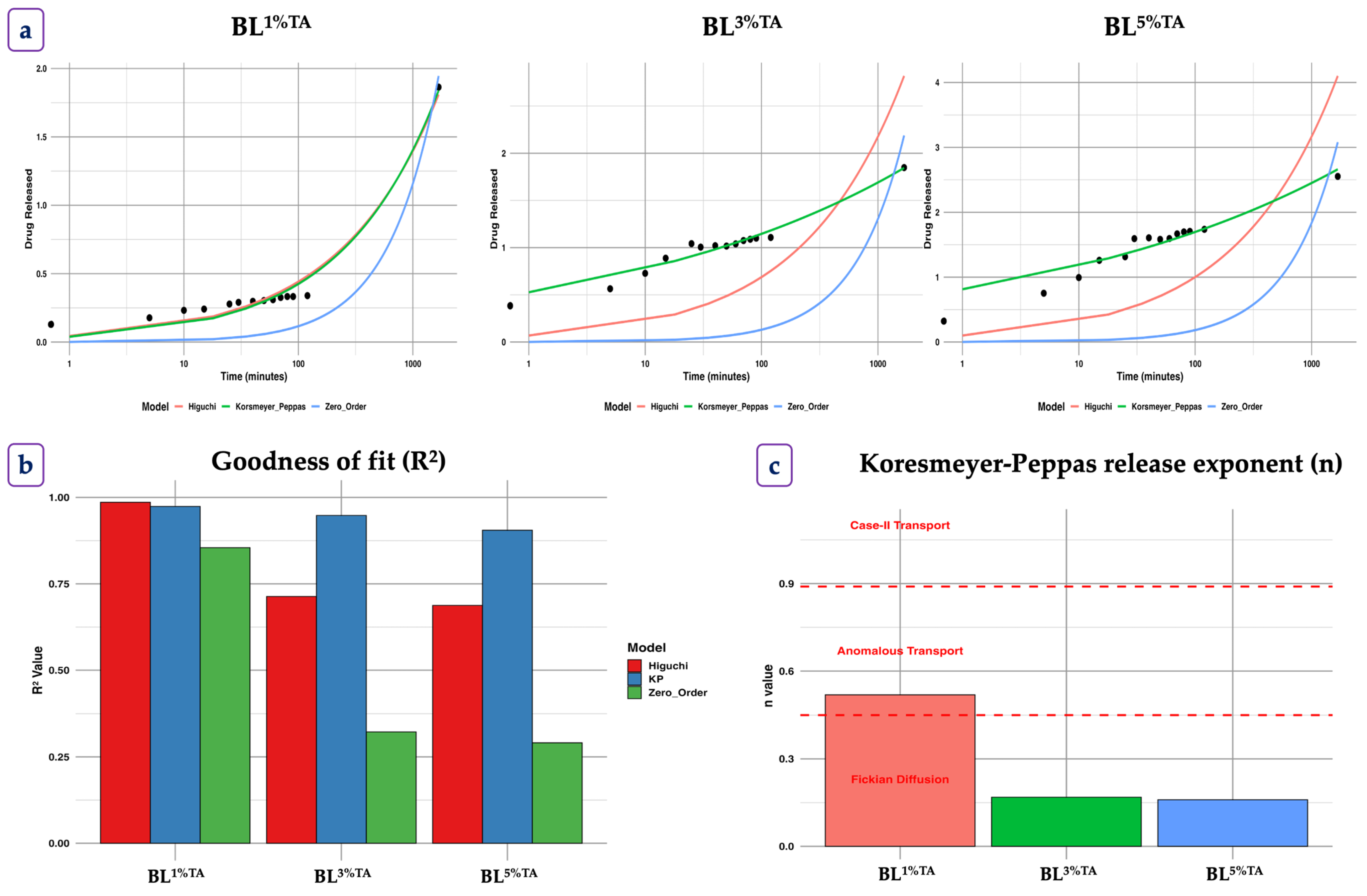
2.3.7. Release Profile and Kinetics
2.4. Preparation of Pouch for Packaging Baby Clams
2.5. Quality Assessment of BC-EP
2.6. Next-Generation Sequencing
2.7. Data Analysis
3. Results and Discussion
3.1. Effects of Different Levels of TA on Properties of Bilayer Films
3.1.1. Film Thickness, Mechanical, and Barrier Properties
3.1.2. Color, Appearance, and Optical Properties
3.1.3. FTIR Spectra and Microstructure
3.1.4. Thermogravimetric Property of the Films
3.1.5. Antioxidant Activities of the Films
3.1.6. Release Profile of TA from the CS-FG Layer of the Bilayer Film
3.2. Quality Changes in Baby Clam Edible Portions Packaged in Pouches Made of Bilayer Films Loaded with TA at Various Concentrations During Refrigerated Storage
3.2.1. Chemical Changes
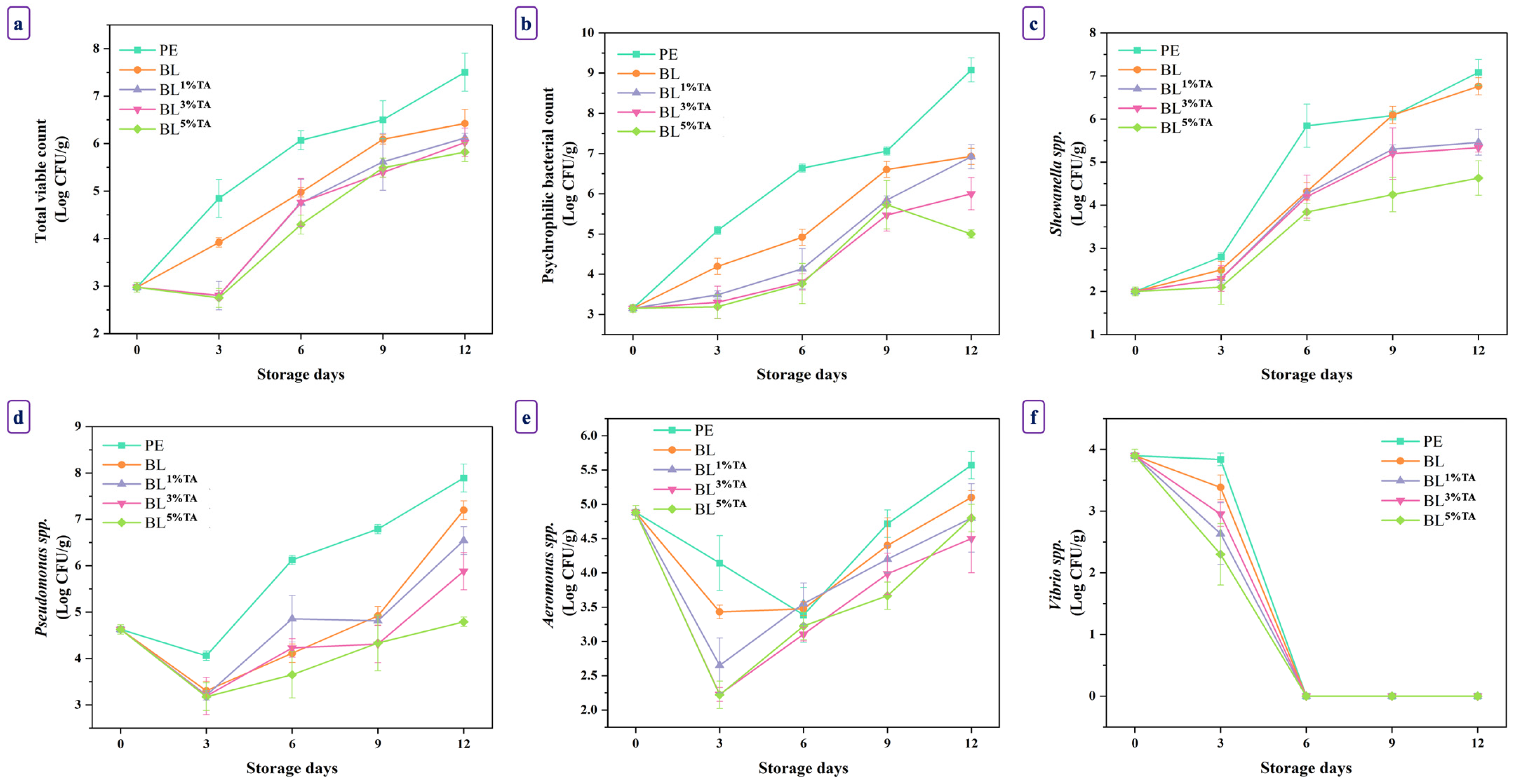
3.2.2. Microbiological Changes
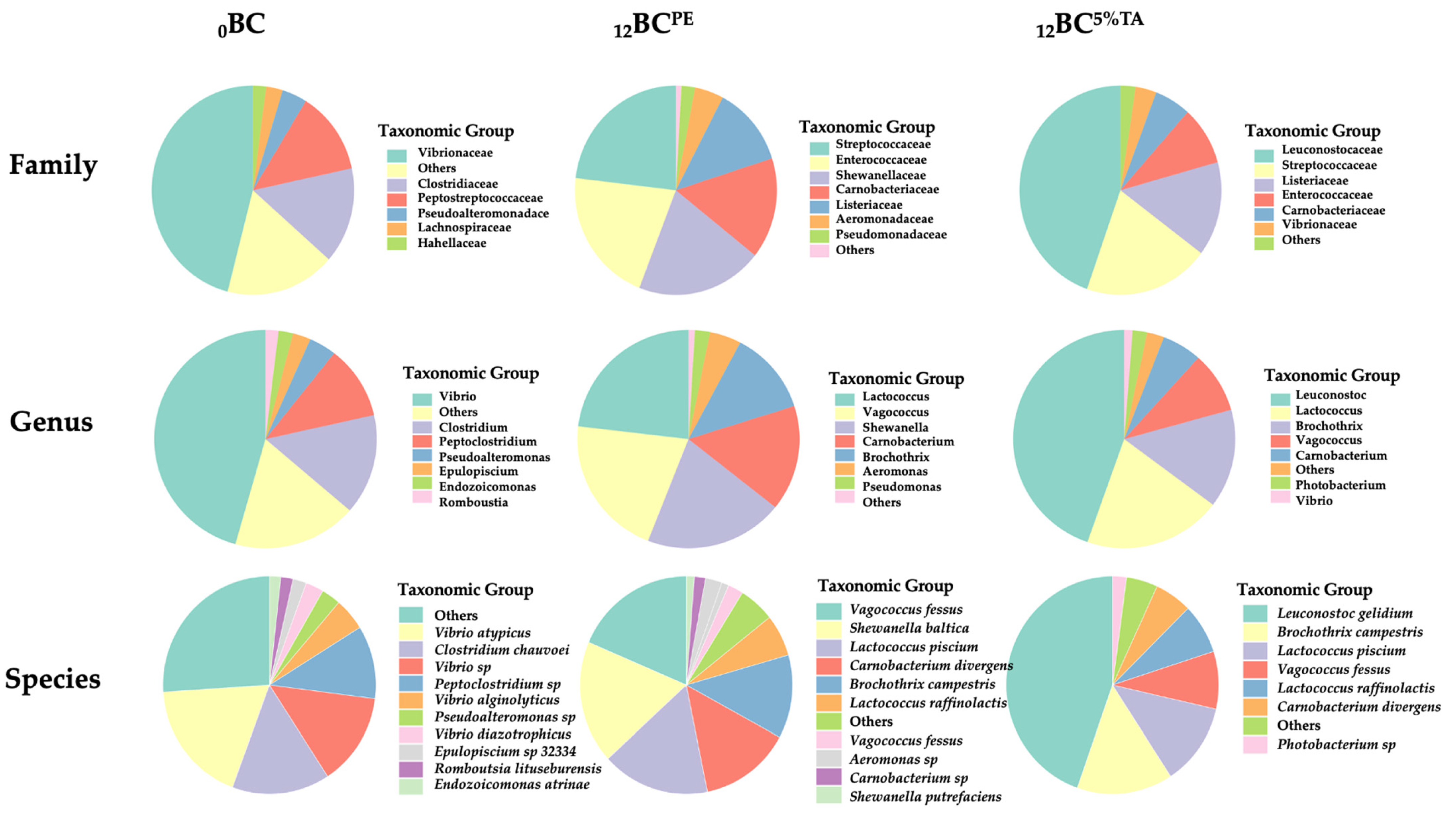
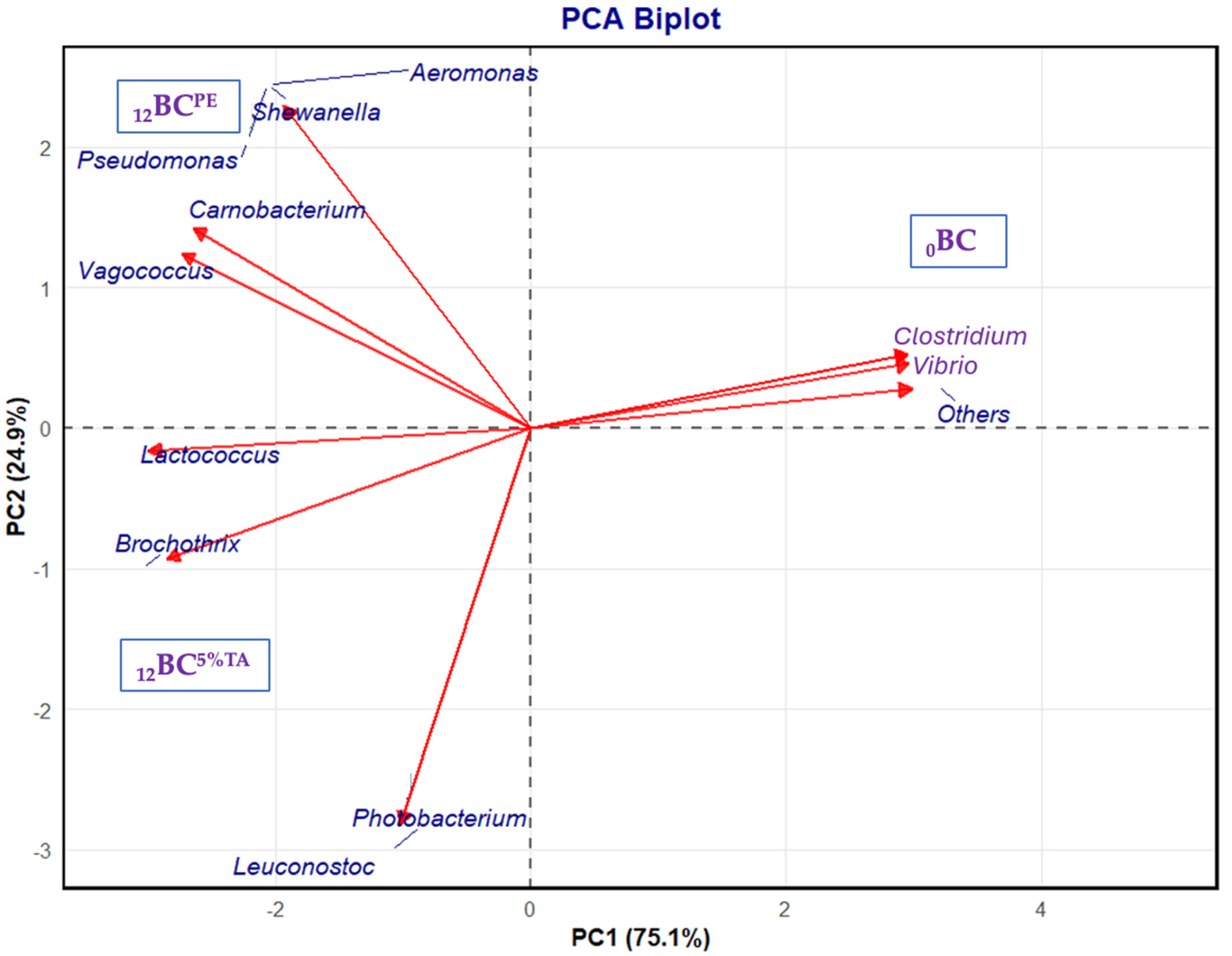
3.2.3. Bacterial Diversity of Fresh and Refrigerated BC-EP Packaged in Bilayer Pouches Containing 5% Tannic Acid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, H.; Shao, C.; Ma, Y.; Zhang, Y.; Lu, P. Development of active and intelligent pH food packaging composite films incorporated with litchi shell extract as an indicator. Int. J. Biol. Macromol. 2023, 226, 77–89. [Google Scholar] [CrossRef]
- Li, Z.; Ye, B.; Fang, J.; Li, M.; Xiong, Y.; Xiong, P.; Zhou, Y.; Guo, Z.; Zhong, H.; Liu, Z. Engineering the functional surface of carbon dots for antibacterial, bacterial bioimaging and sensing applications. New J. Chem. 2024, 48, 6020–6038. [Google Scholar] [CrossRef]
- Palamae, S.; Saetang, J.; Yingkajorn, M.; Zhao, Y.; Zhang, B.; Kim, T.J.; Benjakul, S. Quality changes, diversity and virulence of Aeromonas spp. in the refrigerated Meretrix meretrix and Paphia undulata clams. LWT 2025, 228, 118046. [Google Scholar] [CrossRef]
- Karanth, S.; Feng, S.; Patra, D.; Pradhan, K.A. Linking microbial contamination to food spoilage and food waste: The role of smart packaging, spoilage risk assessments, and date labeling. Front. Microbiol. 2023, 14, 1198124. [Google Scholar] [CrossRef]
- Versino, F.; Ortega, F.; Monroy, Y.; Rivero, S.; López, O.V.; García, M.A. Sustainable and bio-based food packaging: A review on past and current design innovations. Foods 2023, 12, 1057. [Google Scholar] [CrossRef]
- Koc, F.E.; Altıncekic, T.G. Investigation of gelatin/chitosan as potential biodegradable polymer films on swelling behavior and methylene blue release kinetics. Polym. Bull. 2021, 78, 3383–3398. [Google Scholar] [CrossRef]
- Ponnusamy, A.; Niluswan, K.; Prodpran, T.; Kim, J.T.; Rhim, J.-W.; Benjakul, S. Storage stability of Asian seabass oil-in-water Pickering emulsion packed in pouches made from electrospun and solvent casted bilayer films from poly lactic acid/chitosan-gelatin blend containing epigallocatechin gallate. Int. J. Biol. Macromol. 2024, 265, 130760. [Google Scholar] [CrossRef]
- Ponnusamy, A.; Rajasekaran, B.; Tagrida, M.; Prodpran, T.; Kim, J.T.; Benjakul, S. Bilayer polylactic acid and chitosan/gelatin film containing epigallocatechin gallate prepared through solvent casting and electrospinning: Properties, bioactivities and release kinetics. J. Polym. Environ. 2024, 32, 260–276. [Google Scholar] [CrossRef]
- Fu, X.; Yuan, S.; Yang, F.; Yu, H.; Xie, Y.; Guo, Y.; Yao, W. Characterization of the interaction between boscalid and tannic acid and its effect on the antioxidant properties of tannic acid. J. Food Sci. 2023, 88, 1325–1335. [Google Scholar] [CrossRef]
- Navaei, F.; Zandi, M.; Ganjloo, A.; Dardmeh, N. Fabrication of magnetic nanoparticle-enhanced Bi-layer films: Fe3O4-loaded electrospun PVA/Gelatin nanofibers on a balangu seed mucilage-gelatin base. Ind. Crops Prod. 2025, 229, 121037. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Z.; Shi, J.; Zou, X.; Zhai, X.; Huang, X.; Li, Z.; Holmes, M.; Daglia, M.; Xiao, J. Physical properties and bioactivities of chitosan/gelatin-based films loaded with tannic acid and its application on the preservation of fresh-cut apples. LWT 2021, 144, 111223. [Google Scholar] [CrossRef]
- Ponnusamy, A.; Khan, A.; Prodpran, T.; Kim, T.J.; Benjakul, S.; Rhim, J.-W. Active packaging film based on chitosan/gelatin blend incorporated with mango peel carbon dots: Properties and shelf life extension of minced pork. Int. J. Biol. Macromol. 2024, 288, 138692. [Google Scholar] [CrossRef] [PubMed]
- Sutharsan, J.; Boyer, A.C.; Zhao, J. Biological properties of chitosan edible films incorporated with different classes of flavonoids and their role in preserving the quality of chilled beef. Food Hydrocoll. 2023, 139, 108508. [Google Scholar] [CrossRef]
- Lee, J.S.; Gwak, A.M.; Chathuranga, K.; Lee, S.J.; Koo, J.; Park, H.W. Multifunctional chitosan/tannic acid composite films with improved anti-UV, antioxidant, and antimicrobial properties for active food packaging. Food Hydrocoll. 2023, 136, 108249. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Wang, R.; Ge, Y.; Wang, L. Tannic acid crosslinked chitosan/gelatin/SiO2 biopolymer film with superhydrophobic, antioxidant and UV resistance properties for prematuring fruit packaging. Int. J. Biol. Macromol. 2024, 275, 133368. [Google Scholar] [CrossRef]
- Khadsai, S.; Janmanee, R.; Sam-Ang, P.; Nuanchawee, Y.; Rakitikul, W.; Mankhong, W.; Likittrakulwong, W.; Ninjiaranai, P. Influence of crosslinking concentration on the properties of biodegradable modified cassava starch-based films for packaging applications. Polymers 2024, 16, 1647. [Google Scholar] [CrossRef]
- Wu, J.-H.; Liao, J.-H.; Hu, T.-G.; Zong, M.-H.; Wen, P.; Wu, H. Fabrication of multifunctional ethyl cellulose/gelatin-based composite nanofilm for the pork preservation and freshness monitoring. Int. J. Biol. Macromol. 2024, 265, 130813. [Google Scholar] [CrossRef]
- Morais, Q.K.S.; Felipe, H.K.; Silva, S.D.D.J.; Santos, C.N.; Araújo, F.D.S.M.; Vieira, F.P.P.; Gusmão, S.A.T.; Gouveia, S.D. Development of Nile tilapia gelatin films with grape pomace extract for fish fillet storage: Hydrophobic, functional properties, and biodegradability. Food Biosci. 2025, 68, 106675. [Google Scholar] [CrossRef]
- Rioux, B.; Combes, J.; Woolley, M.J.; Rodrigues, N.D.N.; Mention, M.M.; Stavros, G.V.; Allais, F. From biomass-derived p-hydroxycinnamic acids to novel sustainable and non-toxic phenolics-based uv-filters: A multidisciplinary journey. Front. Chem. 2022, 10, 886367. [Google Scholar] [CrossRef]
- Flórez, M.; Cazón, P.; Vázquez, M. Characterization of active films of chitosan containing nettle Urtica dioica L. extract: Spectral and water properties, microstructure, and antioxidant activity. Int. J. Biol. Macromol. 2023, 253, 127318. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Bamunuarachchi, I.N.; Tabassum, N.; Kim, Y.-M. Caffeic acid and its derivatives: Antimicrobial drugs toward microbial pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, J.; Xing, L.; Zhang, W. Applications and effects of ultrasound assisted emulsification in the production of food emulsions: A review. Trends Food Sci. Technol. 2021, 110, 493–512. [Google Scholar] [CrossRef]
- Alobad, Z.K.; Albozahid, M.; Naji, H.Z.; Alraheem, H.S.; Saiani, A. Influence of hard segments content on thermal, morphological and mechanical properties of homo and co-polyurethanes: A comparative study. Arch. Mater. Sci. Eng. 2021, 1, 5–16. [Google Scholar] [CrossRef]
- Al-Musawi, H.M.; Al-Sudani, T.B.; Fadhil, N.A.S.; Al-Bahrani, H.M.; Ghorbani, M.; Maleki, F.; Moghadam, M.F. Tannic acid-reinforced soy protein/oxidized tragacanth gum-based multifunctional hemostatic film for regulation of wound healing. Int. J. Biol. Macromol. 2024, 280, 135750. [Google Scholar] [CrossRef] [PubMed]
- Schaich, K.M.; Tian, X.; Xie, J. Reprint of “Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays”. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, D.; Singh, P.V. Modulation of the chain-breaking antioxidant activity of phenolic organochalcogens with various co-antioxidants at various pH values. Org. Biomol. Chem. 2023, 21, 1316–1327. [Google Scholar] [CrossRef]
- Khan, A.; Ezati, P.; Rhim, J.-W. Chitosan/gelatin-based multifunctional film integrated with green tea carbon dots to extend the shelf life of pork. Food Packag. Shelf Life 2023, 37, 101075. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernandez-Lopez, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of antibacterial and antioxidant properties of chitosan edible films incorporated with maqui berry (Aristotelia chilensis). LWT-Food Sci. Technol. 2015, 64, 1057–1062. [Google Scholar] [CrossRef]
- Zarandona, I.; Minh, N.C.; Trung, T.S.; de la Caba, K.; Guerrero, P. Evaluation of bioactive release kinetics from crosslinked chitosan films with Aloe vera. Int. J. Biol. Macromol. 2021, 182, 1331–1338. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Ge, H.; Chen, L.; Wang, J.; Zhang, S.; Guo, Y.; Li, Z.; Feng, X. Physical properties and antioxidant capacity of chitosan/epigallocatechin-3-gallate films reinforced with nano-bacterial cellulose. Carbohydr. Polym. 2018, 179, 207–220. [Google Scholar] [CrossRef]
- Sharma, R.; Dhamodharan, R. Tannic acid crosslinked chitosan-guar gum composite films for packaging application. Int. J. Biol. Macromol. 2024, 260, 129317. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Caturla, Y.M.; Margalho, P.L.; Graça, S.J.; Pia, K.R.A.; Xavier, L.V.; Noronha, F.M.; Cabral, L.; Lemos-Junior, J.F.W.; Castillo, J.C.C.; Sant’Ana, S.A. Bacterial dynamics and volatile metabolome changes of vacuum-packaged beef with different pH during chilled storage. Int. J. Food Microbiol. 2025, 427, 110955. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials—A minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef]
- Mohebi, E.; Shahbazi, Y. Application of chitosan and gelatin based active packaging films for peeled shrimp preservation: A novel functional wrapping design. LWT-Food Sci. Technol. 2017, 76, 108–116. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F.; Olgunoglu, A.I.; Kuley, E. Bacteriological and biochemical assessment of marinating cephalopods, crustaceans and gastropoda during 24 weeks of storage. Int. J. Food Sci. Nutr. 2008, 59, 465–476. [Google Scholar] [CrossRef]
- Bonilla, J.; Poloni, T.; Lourenço, V.R.; Sobral, J.A.P. Antioxidant potential of eugenol and ginger essential oils with gelatin/chitosan films. Food Biosci. 2018, 23, 107–114. [Google Scholar] [CrossRef]
- De Leon, J.A.D.; Borges, C.R. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. J. Vis. Exp. 2020, 159, e61122. [Google Scholar] [CrossRef]
- Berizi, E.; Hosseinzadeh, S.; Shekarforoush, S.S.; Barbieri, G. Microbial, chemical, textural and sensory properties of coated rainbow trout by chitosan combined with pomegranate peel extract during frozen storage. Int. J. Biol. Macromol. 2018, 106, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, X.; Liao, W.; Wang, Q.; Xia, W. Chitosan/bacterial cellulose films incorporated with tea polyphenol nanoliposomes for silver carp preservation. Carbohydr. Polym. 2022, 297, 120048. [Google Scholar] [CrossRef]
- Ding, J.; Dwibedi, V.; Huang, H.; Ge, Y.; Li, Y.; Li, Q.; Sun, T. Preparation and antibacterial mechanism of cinnamaldehyde/tea polyphenol/polylactic acid coaxial nanofiber films with zinc oxide sol to Shewanella putrefaciens. Int. J. Biol. Macromol. 2023, 237, 123932. [Google Scholar] [CrossRef]
- Wen, M.; Tao, R.; Jiang, J.; Chen, Y.; Wang, Y. Characterization and anti-Vibrio activity of citral-chitosan Schiff base complexed tannic acid-zinc slow-release bacteriostatic formulations. LWT 2025, 224, 117780. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Hong, S.-I.; Ha, C.-S. Tensile, water vapor barrier and antimicrobial properties of PLA/nanoclay composite films. LWT-Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Chang, C.C.; Trinh, M.B.; Mekonnen, H.T. Robust multiphase and multilayer starch/polymer (TPS/PBAT) film with simultaneous oxygen/moisture barrier properties. J. Colloid Interface Sci. 2021, 593, 290–303. [Google Scholar] [CrossRef]
- Lou, X.; Zhai, D.; Yang, H. Changes of metabolite profiles of fish models inoculated with Shewanella baltica during spoilage. Food Control 2021, 123, 107697. [Google Scholar] [CrossRef]
- Syropoulou, F.; Parlapani, F.F.; Bosmali, I.; Madesis, P.; Boziaris, S.I. HRM and 16S rRNA gene sequencing reveal the cultivable microbiota of the European sea bass during ice storage. Int. J. Food Microbiol. 2020, 327, 108658. [Google Scholar] [CrossRef]
- Palamae, S.; Mittal, A.; Zhang, B.; Benjakul, S. Chitooligosaccharide-catechin conjugate and high-pressure processing: Microbial control and quality preservation of baby clam during refrigerated storage. Food Biosci. 2024, 62, 105493. [Google Scholar] [CrossRef]


| Film | Thickness (µm) | TS (MPa) | EAB (%) | WVP * |
|---|---|---|---|---|
| BL | 99.96 ± 5.07 a | 17.27 ± 1.35 c | 85.03 ± 4.86 a | 2.52 ± 0.10 a |
| BL1%TA | 101.78 ± 2.57 a | 17.89 ± 1.41 c | 46.73 ± 4.44 b | 2.43 ± 0.01 a |
| BL3%TA | 103.42 ± 8.08 a | 21.63 ± 1.08 b | 39.97 ± 2.19 c | 2.35 ± 0.29 a |
| BL5%TA | 103.50 ± 7.16 a | 27.57 ± 1.29 a | 38.18 ± 3.07 c | 2.30 ± 0.02 a |
| Film | L* | a* | b* | ∆E |
|---|---|---|---|---|
| BL | 89.59 ± 0.06 a | −1.53 ± 0.02 d | 3.17 ± 0.09 d | 4.25 ± 0.08 d |
| BL1%TA | 82.20 ± 1.01 b | 2.08 ± 0.16 c | 5.04 ± 0.26 c | 11.82 ± 0.77 c |
| BL3%TA | 80.23 ± 0.59 c | 3.65 ± 0.22 b | 8.33 ± 0.50 b | 15.64 ± 0.80 b |
| BL5%TA | 78.63 ± 0.14 d | 4.49 ± 0.15 a | 10.80 ± 0.24 a | 18.48 ± 0.15 a |
| Film | First Weight Loss | Second Weight Loss | Third Weight Loss | |||
|---|---|---|---|---|---|---|
| Δw (%) | Td (°C) | Δw (%) | Td (°C) | Δw (%) | Td (°C) | |
| BL | 6.98 | 30–112 | 2.73 | 112–163 | 68.45 | 163–410 |
| BL1%TA | 6.13 | 30–114 | 2.01 | 114–158 | 66.71 | 158–391 |
| BL3%TA | 4.93 | 10–108 | 3.35 | 108–165 | 69.89 | 165–409 |
| BL5%TA | 4.89 | 30–109 | 3.12 | 109–165 | 65.05 | 165–345 |
| Film | ABTS-RSA * | DPPH-RSA * | FRAP * | MCA ** |
|---|---|---|---|---|
| BL | - | - | 19.74 ± 0.23 d | 0.29 ± 0.04 d |
| BL1%TA | 74.97 ± 9.31 c | 4.83 ± 0.01 c | 23.51 ± 0.00 c | 2.58 ± 0.29 c |
| BL3%TA | 280.13 ± 2.12 b | 7.18 ± 0.11 b | 44.56 ± 0.15 b | 4.57 ± 0.35 b |
| BL5%TA | 739.96 ± 0.85 a | 8.43 ± 0.01 a | 78.78 ± 0.15 a | 5.32 ± 0.01 a |
| Film Type | Observed Species | PD Whole Tree | Chao | Fisher- Alpha | Shannon | Simpson | Simpson Reciprocal | Simpson’s Evenness |
|---|---|---|---|---|---|---|---|---|
| 0BC | 463.40 ± 2.63 | 26.02 ± 0.16 | 471.04 ± 4.15 | 84.75 ± 0.59 | 5.75 ± 0.02 | 0.94 ± 0.00 | 17.95 ± 0.21 | 0.04 ± 0.00 |
| 12BCPE | 98.20 ± 0.92 | 6.73 ± 0.14 | 99.08 ± 1.60 | 13.44 ± 0.15 | 4.13 ± 0.01 | 0.91 ± 0.00 | 10.74 ± 0.06 | 0.11 ± 0.00 |
| 12BC5%TA | 73.70 ± 0.48 | 7.02 ± 0.01 | 73.99 ± 0.82 | 9.65 ± 0.07 | 2.88 ± 0.01 | 0.75 ± 0.00 | 4.05 ± 0.04 | 0.05 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponnusamy, A.; Palamae, S.; Prodpran, T.; Kim, J.T.; Zhang, B.; Ma, L.; Benjakul, S. Fabrication of a Chitosan–Gelatin/Polylactic Acid Bilayer Active Film Loaded with Tannic Acid for Enhancing Shelf-Life of Refrigerated Baby Clams. Foods 2025, 14, 3934. https://doi.org/10.3390/foods14223934
Ponnusamy A, Palamae S, Prodpran T, Kim JT, Zhang B, Ma L, Benjakul S. Fabrication of a Chitosan–Gelatin/Polylactic Acid Bilayer Active Film Loaded with Tannic Acid for Enhancing Shelf-Life of Refrigerated Baby Clams. Foods. 2025; 14(22):3934. https://doi.org/10.3390/foods14223934
Chicago/Turabian StylePonnusamy, Arunachalasivamani, Suriya Palamae, Thummanoon Prodpran, Jun Tae Kim, Bin Zhang, Lukai Ma, and Soottawat Benjakul. 2025. "Fabrication of a Chitosan–Gelatin/Polylactic Acid Bilayer Active Film Loaded with Tannic Acid for Enhancing Shelf-Life of Refrigerated Baby Clams" Foods 14, no. 22: 3934. https://doi.org/10.3390/foods14223934
APA StylePonnusamy, A., Palamae, S., Prodpran, T., Kim, J. T., Zhang, B., Ma, L., & Benjakul, S. (2025). Fabrication of a Chitosan–Gelatin/Polylactic Acid Bilayer Active Film Loaded with Tannic Acid for Enhancing Shelf-Life of Refrigerated Baby Clams. Foods, 14(22), 3934. https://doi.org/10.3390/foods14223934








