High-Throughput Screening of Industrial Brewing Yeast with Lower Synthetic Level of Acetaldehyde During Beer Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
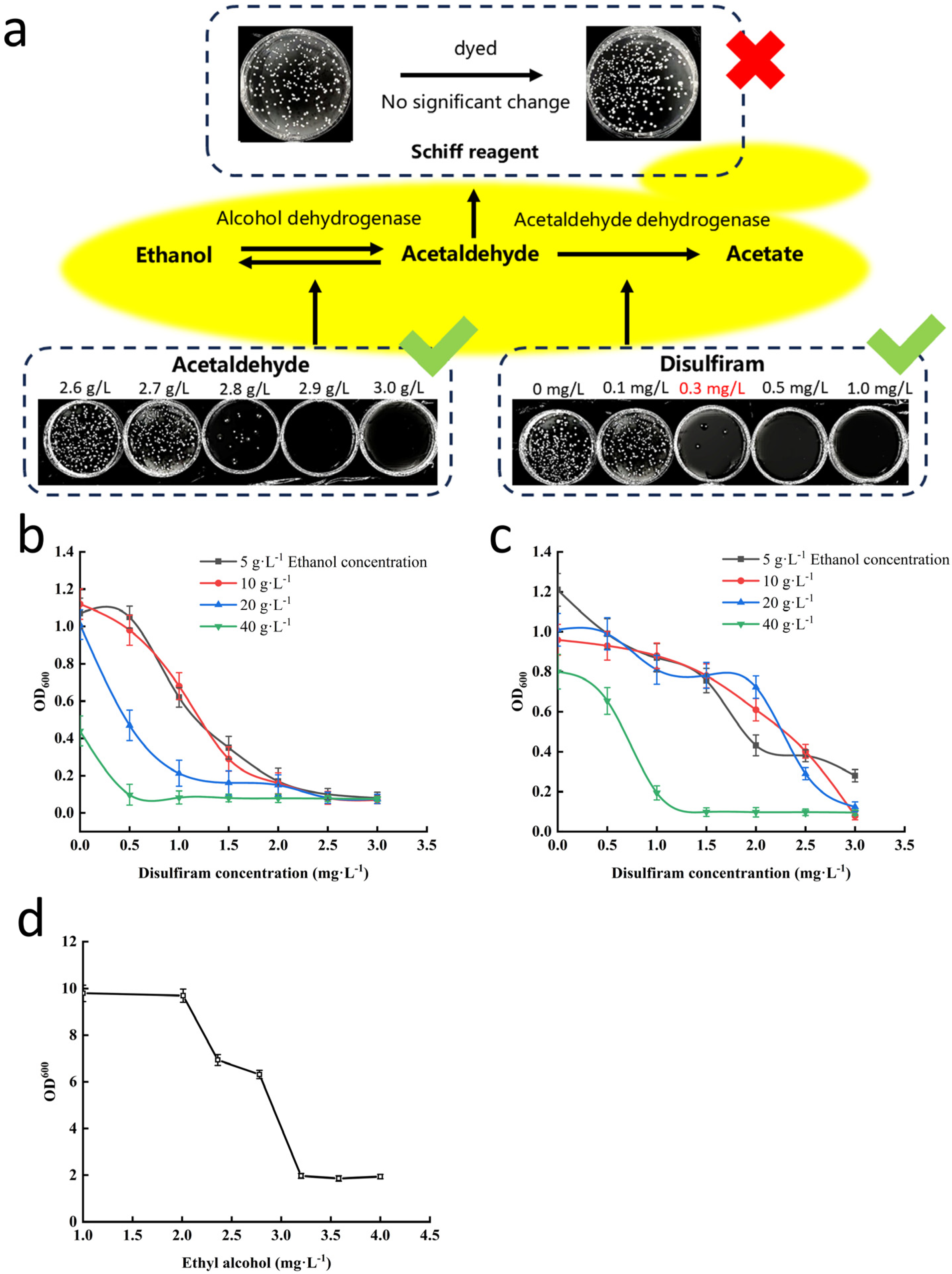
2.2. Establishment of a High-Throughput Screening Method Strategy for Yeast Strains with Low Acetaldehyde Production
2.3. Measurement of Acetaldehyde Content in Mutant Strains with Low Acetaldehyde Production Using 3-Methyl-2-Benzothiazolone Hydrazone
2.4. Simulated Beer Fermentation
2.5. Determination of Acetaldehyde Content Using Headspace Gas Chromatography
2.6. Determination of Mutant Strain Phenotypic Stability
2.7. Determination of Fermentation Performance of Different Yeast Strains
2.8. Determination of Biological Characteristics
2.9. Data and Statistical Analysis
3. Results
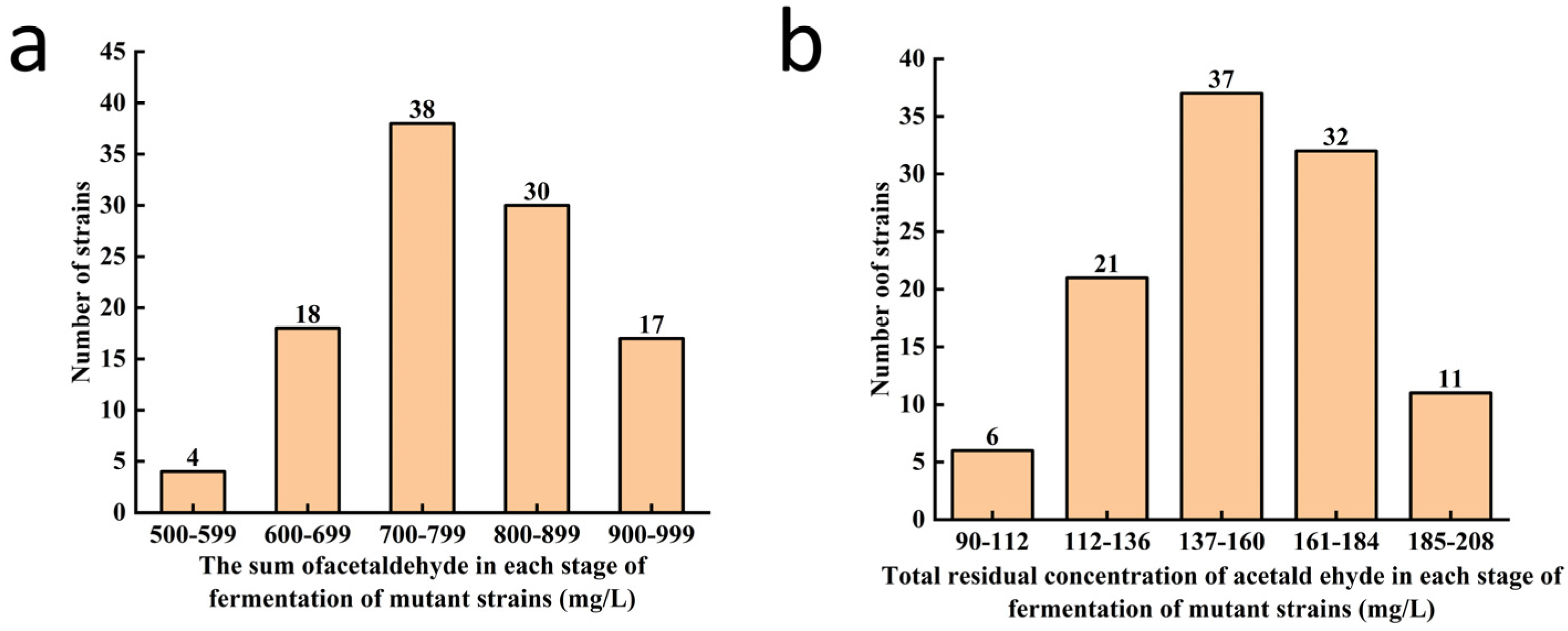
3.1. Primary Screening of Beer Yeast Mutant Strains for Low Acetaldehyde Production
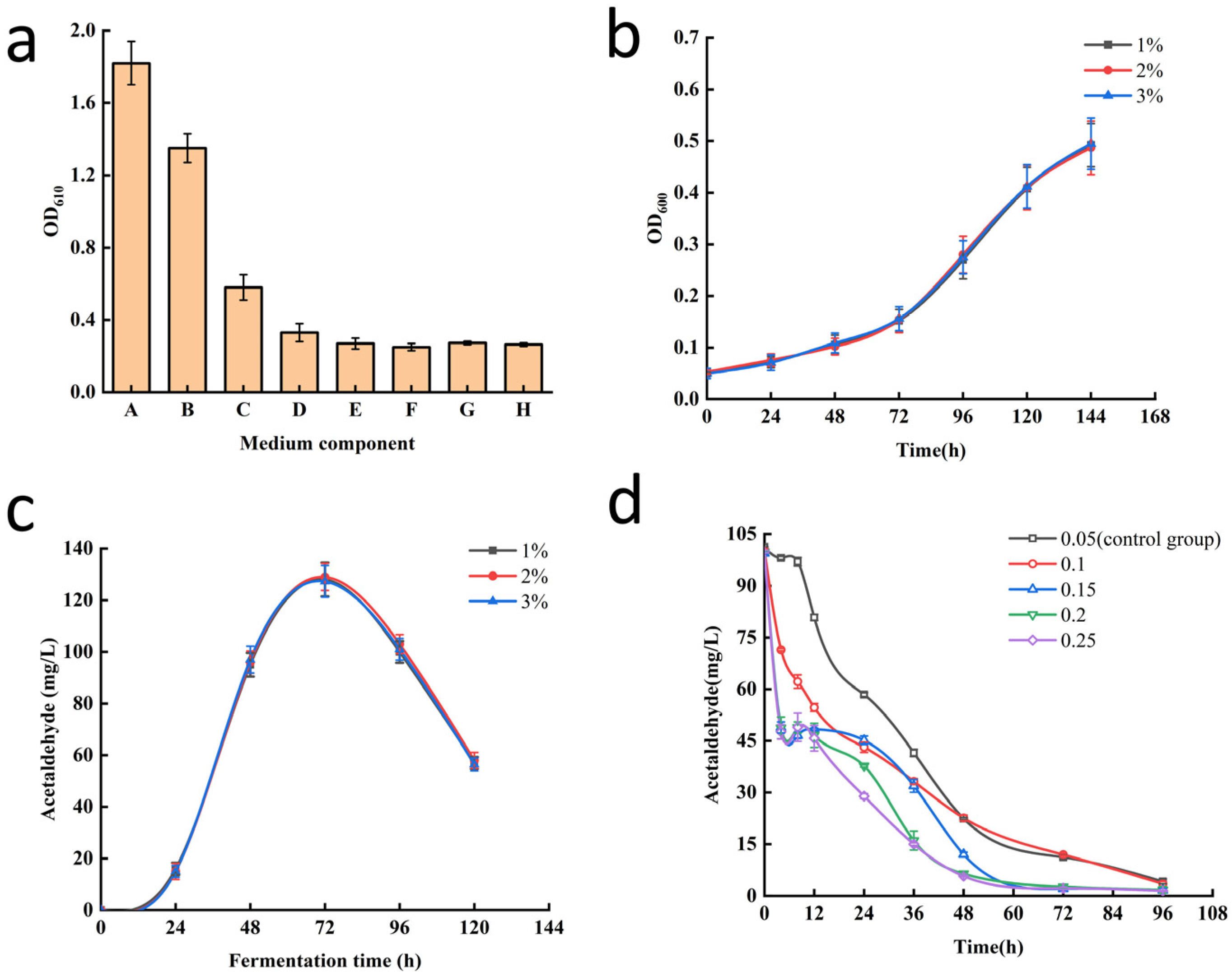
3.2. Optimization of Secondary Screening Conditions
3.3. Screening of Low-Acetaldehyde Yeast Mutants Obtained by Primary Screening for Low Acetaldehyde Biosynthetic Rate
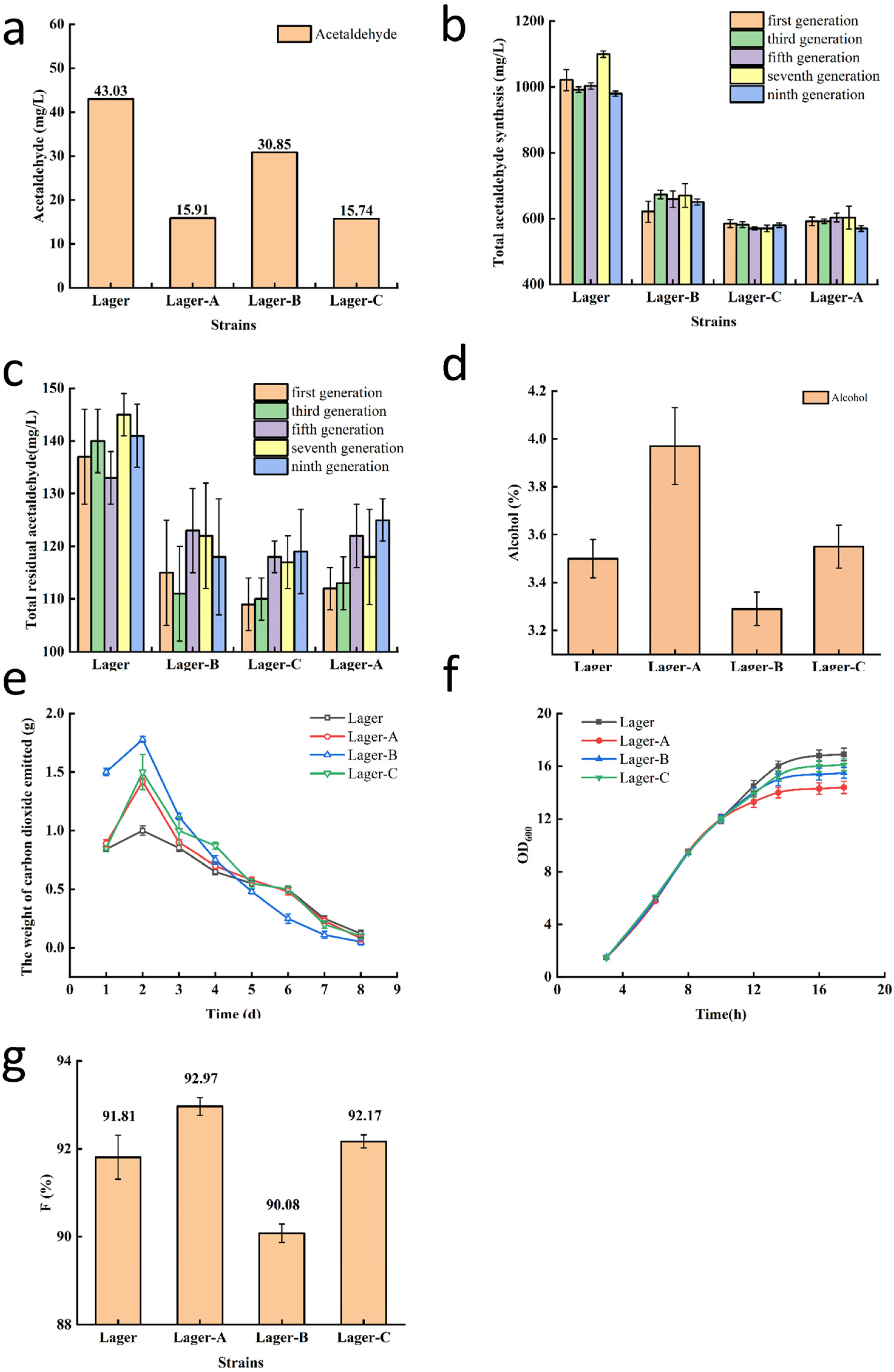
3.4. Effect of Industrial Yeast Strains with Low Acetaldehyde Production on Beer Fermentation
3.5. Fermentation Performance of Low Acetaldehyde Producing Mutant Industrial Yeast Strains Lager-A and Lager-C
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaczyński, P.; Iwaniuk, P.; Hrynko, I.; Luniewski, S.; Lozowicka, B. The effect of the multi-stage process of wheat beer brewing on the behavior of pesticides according to their physicochemical properties. Food Control 2024, 160, 110356. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Worch, T.; Phelps, T.; Jin, D.; Cardello, A.V. Effects of “craft” vs. “traditional” labels to beer consumers with different flavor preferences: A comprehensive multi-response approach. Food Qual. Prefer. 2021, 87, 104043. [Google Scholar] [CrossRef]
- Kucharczyk, K.; Tuszyński, T.; Żyła, K.; Puchalski, C. The effect of yeast generations on fermentation, maturation and volatile compounds of beer. Czech J. Food Sci. 2020, 38, 144–150. [Google Scholar] [CrossRef]
- Reichel, S.; Carvalho, D.O.; Santos, J.R.; Bednar, P.; Rodrigues, J.A.; Guido, L.F. Profiling the volatile carbonyl compounds of barley and malt samples using a low-pressure assisted extraction system. Food Control 2021, 121, 107568. [Google Scholar] [CrossRef]
- Shin, K.-S.; Lee, J.-H. Acetaldehyde contents and quality characteristics of commercial alcoholic beverages. Food Sci. Biotechnol. 2019, 28, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, K.C.; Souza-Silva, E.A.; Assumpcao, C.F. Carbonyl compounds and furan derivatives with toxic potential evaluated in the brewing stages of craft beer. Food Addit. Contam. Part A-Chem. Anal. Control. Expo. Risk Assess. 2020, 37, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, J.; Liu, C.; Li, Y.; Li, Q. Domesticating brewing yeast for decreasing acetaldehyde production and improving beer flavor stability. Eur. Food Res. Technol. 2014, 238, 347–355. [Google Scholar] [CrossRef]
- Dirk; Lachenmeier, W.; Sohnius, E.-M. The role of acetaldehyde outside ethanol metabolism in the carcinogenicity of alcoholic beverages: Evidence from a large chemical survey. Food Chem. Toxicol. 2008, 46, 2903–2911. [Google Scholar] [CrossRef]
- Wang, J.J.; Shen, N.; Yin, H.; Liu, C.; Li, Y.; Li, Q. Development of industrial brewing yeast with low acetaldehyde production and improved flavor stability. Appl. Biochem. Biotechnol. 2013, 169, 1016–1025. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Wang, J.J.; Liu, X.F. Recombinant industrial brewing yeast strains with ADH2 interruption using self-cloning GSH1+CUP1 cassette. FEMS Yeast Res. 2009, 9, 574–581. [Google Scholar] [CrossRef][Green Version]
- Zhang, Q.; Jin, Y.-L.; Fang, Y.; Zhao, H. Adaptive evolution and selection of stress-resistant Saccharomyces cerevisiae for very high-gravity bioethanol fermentation. Electron. J. Biotechnol. 2019, 41, 88–94. [Google Scholar] [CrossRef]
- LaCroix, R.A.; Palsson, B.O.; Feist, A.M. A model for designing adaptive laboratory evolution experiments. Appl. Environ. Microbiol. 2017, 83, e03115–e03116. [Google Scholar] [CrossRef]
- Cui, L.Y.; Wang, S.S.; Guan, C.G.; Liang, W.F.; Xue, Z.L.; Zhang, C.; Xing, X.H. Breeding of methanol-tolerant Methylobacterium extorquens AM1 by atmospheric and room temperature plasma mutagenesis combined with adaptive laboratory evolution. Biotechnol. J. 2018, 13, 1700679. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wang, Y.; Chi, S.; Yan, J.; Guan, G.; Hui, B.; Li, Y. Reduction of fatty acid flux results in enhancement of astaxanthin synthesis in a mutant strain of Phaffia rhodozyma. J. Ind. Microbiol. Biotechnol. 2010, 37, 595–602. [Google Scholar] [CrossRef]
- Tian, J. Determination of several flavours in beer with headspace sampling–gas chromatography. Food Chem. 2010, 123, 1318–1321. [Google Scholar] [CrossRef]
- Gurdo, N.; Poisson, N.G.F.; Juárez, A.B.; de Molina, R.M.C.; Galvagno, M.A. Improved robustness of an ethanologenic yeast strain through adaptive evolution in acetic acid is associated with its enzymatic antioxidant ability. J. Appl. Microbiol. 2018, 125, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Trueba, P.B.; Jaskula-Goiris, B.; Ditrych, M.; Filipowska, W.; De Brabanter, J.; De Rouck, G.; Aerts, G.; De Cooman, L.; De Clippeleer, J. Monitoring the evolution of free and cysteinylated aldehydes from malt to fresh and forced aged beer. Food Res. Int. 2021, 140, 110049. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, K.C.; Souza-Silva, É.A.; Assumpção, C.F.; Zini, C.A.; Welke, J.E. Validation of an analytical method using HS-SPME-GC/MS-SIM to assess the exposure risk to carbonyl compounds and furan derivatives through beer consumption. Food Addit. Contam. Part A 2019, 36, 1808–1821. [Google Scholar] [CrossRef]
- Lv, Y.; Gu, Y.; Xu, J.; Zhou, J.; Xu, P. Coupling metabolic addiction with negative autoregulation to improve strain stability and pathway yield. Metab. Eng. 2020, 61, 79–88. [Google Scholar] [CrossRef]
- Einfalt, D. Barley-sorghum craft beer production with Saccharomyces cerevisiae, Torulaspora delbrueckii and Metschnikowia pulcherrima yeast strains. Eur. Food Res. Technol. 2021, 247, 385–393. [Google Scholar] [CrossRef]
- Oomuro, M.; Watanabe, D.; Sugimoto, Y.; Kato, T.; Motoyama, Y.; Watanabe, T.; Takagi, H. Accumulation of intracellular S-adenosylmethionine increases the fermentation rate of bottom-fermenting brewer’s yeast during high-gravity brewing. J. Biosci. Bioeng. 2018, 126, 736–741. [Google Scholar] [CrossRef]
- Sung, C.; Kim, S.; Oh, C.; Yang, S.; Han, B.; Mo, E. Taraxerone enhances alcohol oxidation via increases of alcohol dehyderogenase (ADH) and acetaldehyde dehydrogenase (ALDH) activities and gene expressions. Food Chem. Toxicol. 2012, 50, 2508–2514. [Google Scholar] [CrossRef] [PubMed]
- Sariki, S.K.; Kumawat, R.; Singh, V.; Tomar, R.S. Flocculation of Saccharomyces cerevisiae is dependent on activation of Slt2 and Rlm1 regulated by the cell wall integrity pathway. Mol. Microbiol. 2019, 112, 1350–1369. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Liu, M.; Deng, Y. Reduced acetaldehyde production by genome shuffling of an industrial brewing yeast strain. J. Inst. Brew. 2017, 123, 527–532. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, J.; Zhu, Y.; Wang, L.; Qi, B. Endogenic oxidative stress response contributes to glutathione over-accumulation in mutant Saccharomyces cerevisiae Y518. Appl. Environ. Microbiol. 2015, 99, 7069–7078. [Google Scholar] [CrossRef]
- Akamatsu, K.; Shikazono, N.; Saito, T. New method for estimating clustering of DNA lesions induced by physical/chemical mutagens using fluorescence anisotropy. Anal. Biochem. 2017, 536, 78–89. [Google Scholar] [CrossRef]
- Kozaeva, E.; Mol, V.; Nikel, P.I.; Nielsen, A.T. High-throughput colorimetric assays optimized for detection of ketones and aldehydes produced by microbial cell factories. Microb. Biotechnol. 2022, 15, 2426–2438. [Google Scholar] [CrossRef]
| Method | Detection Limit (mg∙L−1) | Accuracy (%) | Linearity Range (mg∙L−1) | Apparent Color of Detection Limit | Correlation Coefficient | Linear Equation |
|---|---|---|---|---|---|---|
| a | 500 | 0.34–1.8 | 400–1800 | Almost colorless | 0.997 | y = 15060.9x + 336.3 |
| b | 120 | 0.27–0.62 | 600–1800 | Almost colorless | 0.996 | y = 3412.6x + 306.5 |
| c | 0.15 | 0.18–0.66 | 0.1–600 | Light blue | 0.999 | y = 28.92x + 0.73 |
| Starting Strain | Mutation Method | Screening Plate and Adaptive Evolution Solution | Re-Screening Strategy | Transformation Efficiency (%) | Serial Number |
|---|---|---|---|---|---|
| Lager497 | 60Coγ | High concentration of acetaldehyde | Strong metabolism | 34.2 | A |
| Low synthesis | 51.1 | C | |||
| Ethanol-disulfiram | Strong metabolism | 10.3 | B | ||
| Low synthesis | 43.4 | B |
| Strain | Ethanol Dehydrogenase I Activity | Ethanol Dehydrogenase II Activity | Acetaldehyde Dehydrogenase Activity |
|---|---|---|---|
| Blanks (water) | 0.11 | 0.11 | 0.17 |
| Lager | 1.77 | 2.01 | 7.15 |
| Lager-A | 2.94 | 1.13 | 7.70 |
| Lager-B | 1.58 | 2.98 | 7.47 |
| Lager-C | 2.04 | 1.36 | 8.77 |
| Strain | Lager | Lager-A | Lager-B | Lager-C |
|---|---|---|---|---|
| Ethyl acetate | 9.16 ± 0.88 a | 10.91 ± 0.58 c | 8.67 ± 0.39 c | 8.03 ± 1.55 c |
| Isoamyl acetate | 0.40 ± 0.02 b | 0.50 ± 0.03 c | 0.38 ± 0.01 c | 0.41 ± 0.03 c |
| N-propanol | 14.15 ± 1.23 a | 17.98 ± 0.84 c | 13.16 ± 0.63 c | 13.44 ± 2.08 c |
| Isobutanol | 14.97 ± 0.89 c | 13.89 ± 0.55 c | 13.69 ± 0.99 c | 11.08 ± 1.18 c |
| Isoamyl alcohol | 57.13 ± 3.89 c | 61.96 ± 2.51 c | 54.93 ± 4.60 c | 53.62 ± 5.46 c |
| Alcohol | 3.5 ± 0.08 a | 3.97 ± 0.16 c | 3.29 ± 0.07 a | 3.55 ± 0.09 c |
| Rate of change in alcohol ratio (%) | - | −8.85 | 0.16 | 2.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Sun, K.; Hou, X.; Wan, X.; Ding, J.; Li, J.; Chen, J.; Du, G.; Zhao, X.; Yin, H. High-Throughput Screening of Industrial Brewing Yeast with Lower Synthetic Level of Acetaldehyde During Beer Production. Foods 2025, 14, 3762. https://doi.org/10.3390/foods14213762
Han S, Sun K, Hou X, Wan X, Ding J, Li J, Chen J, Du G, Zhao X, Yin H. High-Throughput Screening of Industrial Brewing Yeast with Lower Synthetic Level of Acetaldehyde During Beer Production. Foods. 2025; 14(21):3762. https://doi.org/10.3390/foods14213762
Chicago/Turabian StyleHan, Shuangxin, Kecheng Sun, Xiaoping Hou, Xiujuan Wan, Jiahui Ding, Jianghua Li, Jian Chen, Guocheng Du, Xinrui Zhao, and Hua Yin. 2025. "High-Throughput Screening of Industrial Brewing Yeast with Lower Synthetic Level of Acetaldehyde During Beer Production" Foods 14, no. 21: 3762. https://doi.org/10.3390/foods14213762
APA StyleHan, S., Sun, K., Hou, X., Wan, X., Ding, J., Li, J., Chen, J., Du, G., Zhao, X., & Yin, H. (2025). High-Throughput Screening of Industrial Brewing Yeast with Lower Synthetic Level of Acetaldehyde During Beer Production. Foods, 14(21), 3762. https://doi.org/10.3390/foods14213762






