Dual-Modified A- and B-Type Wheat Starch–PCL Composite Films: Antibacterial and HACCP-Oriented Biodegradable Packaging from Kazakhstani Resources
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Modification
2.3. Determination of Acetyl Group Content and Degree of Substitution
2.4. Determination of Solubility and Swelling Capacity
2.5. Determination of Physicochemical Properties
2.6. Scanning Electron Microscopy (SEM)
2.7. Film Preparation and Optimization
2.8. Evaluation of Film Properties
2.9. Antibacterial Activity Test
2.10. Evaluation of Biodegradability (Soil Burial and Controlled Composting)
2.11. Statistical Analysis
3. Results
3.1. Physicochemical Properties of Native and Modified Starches
3.1.1. Physicochemical Properties of Native and Modified Starches
3.1.2. Solubility, Swelling Power, and Degree of Substitution
3.2. Plasticization and Mechanical Properties of Acetylated Starch Films (Casting Stage)
3.3. Composite Formulations and Mechanical Properties of PCL–Starch Films (Extrusion Stage)
3.4. Optical Transparency of Composite Films
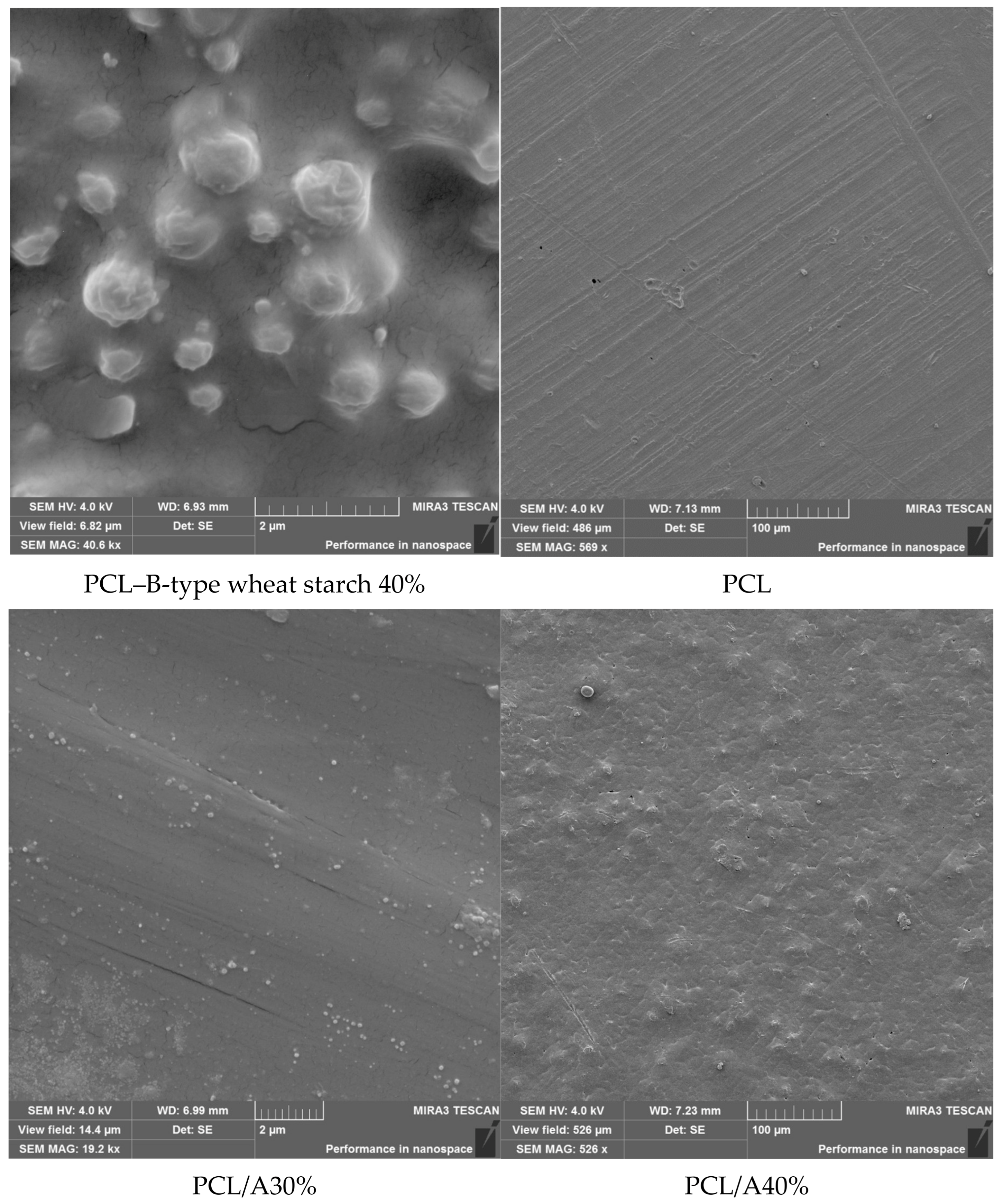
3.5. Morphological Characterization of Composite Films
3.6. Thermal Degradation Profiles
3.7. Water-Resistance Properties
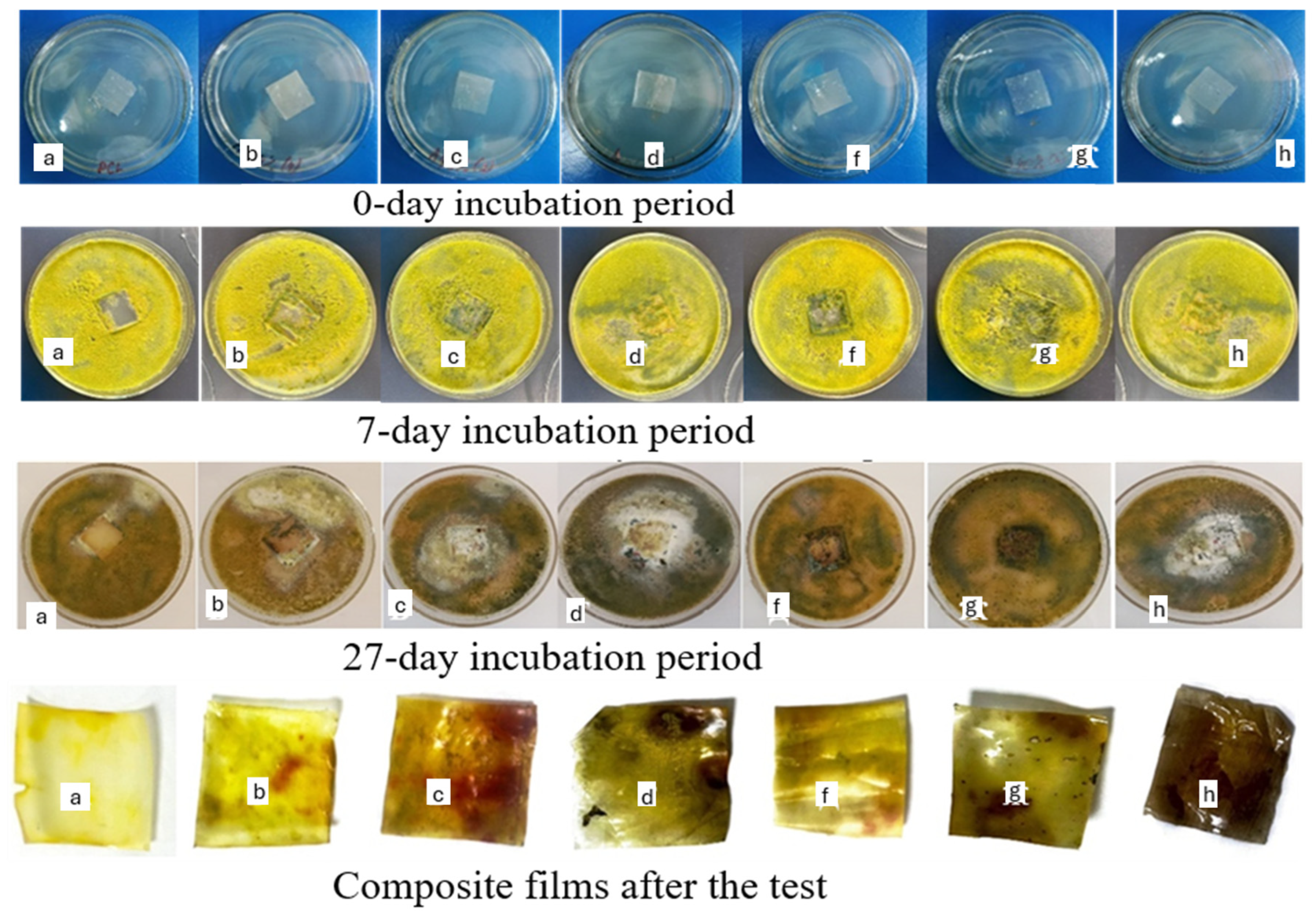
3.8. Antibacterial Activity of Composite Films
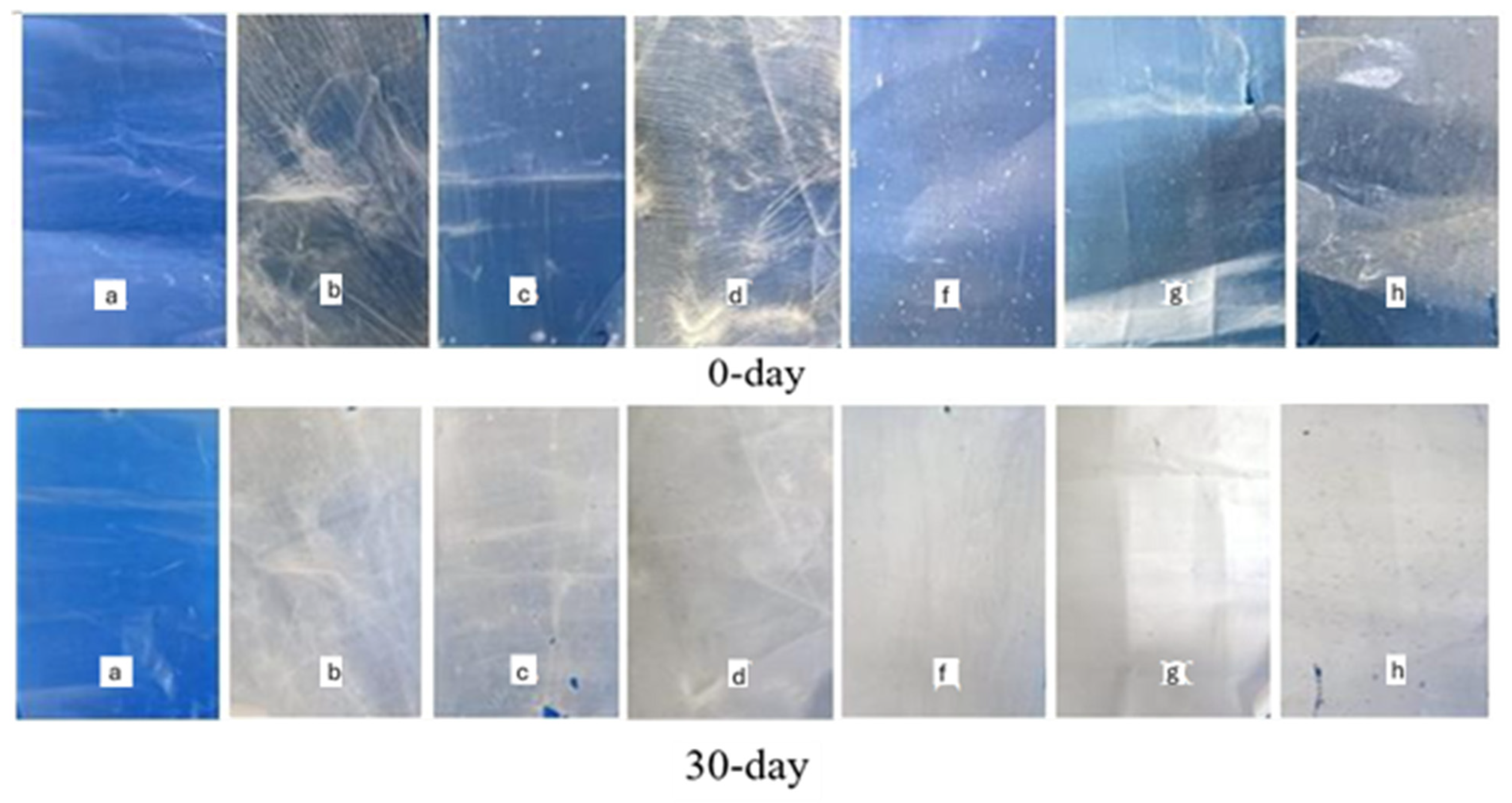
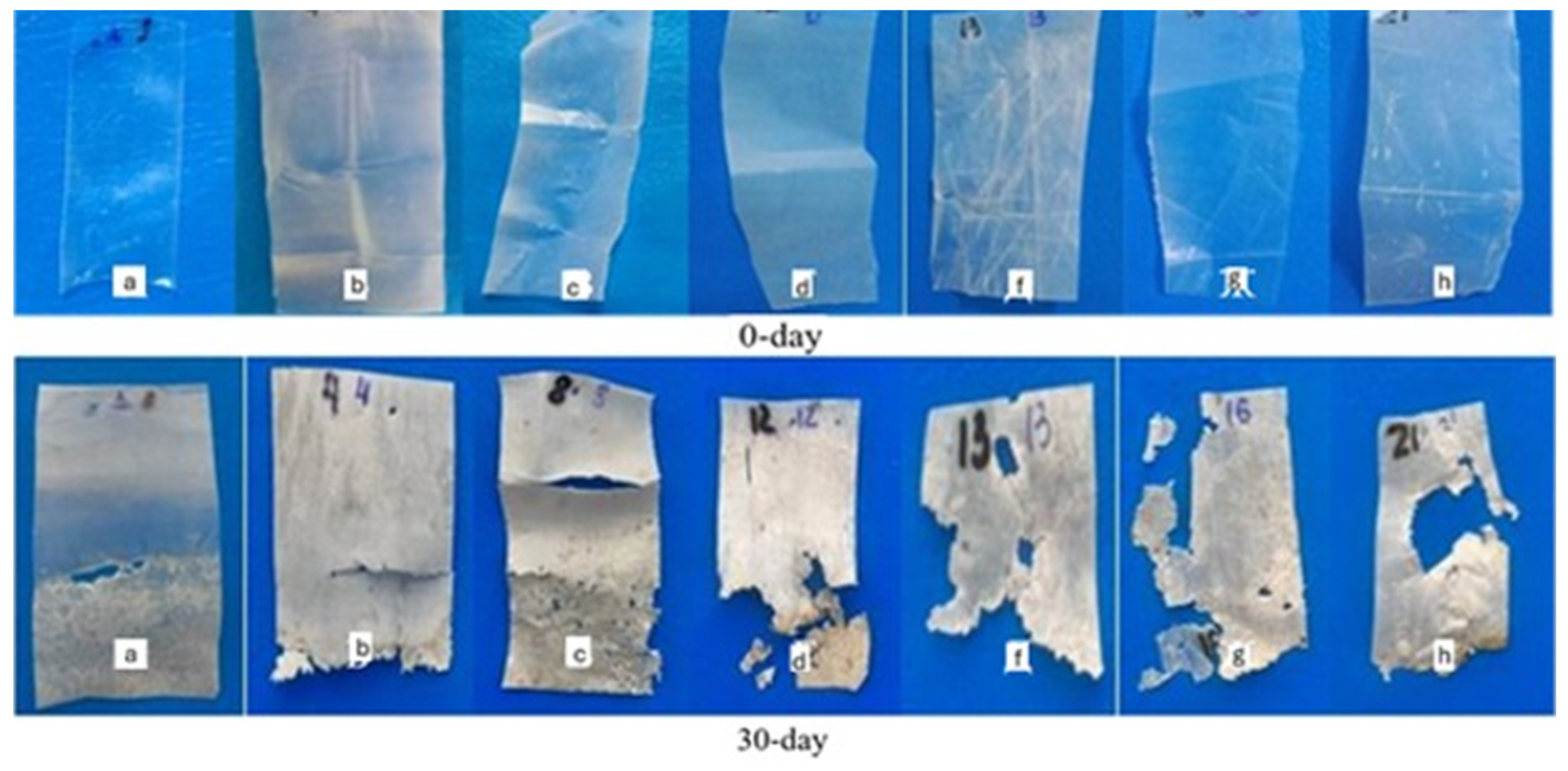
3.9. Biodegradation Properties of Composite Films Under Soil and Composting Conditions
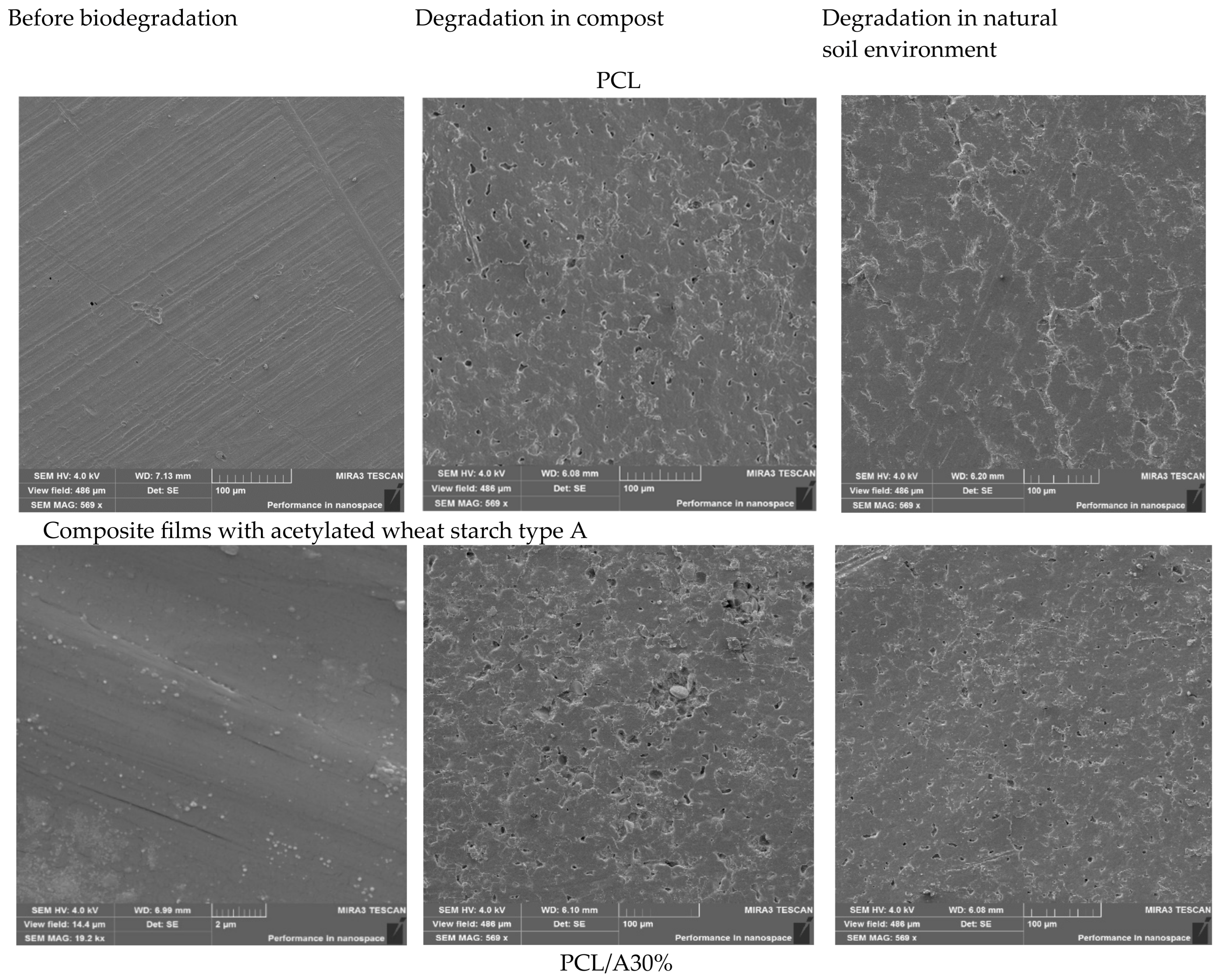
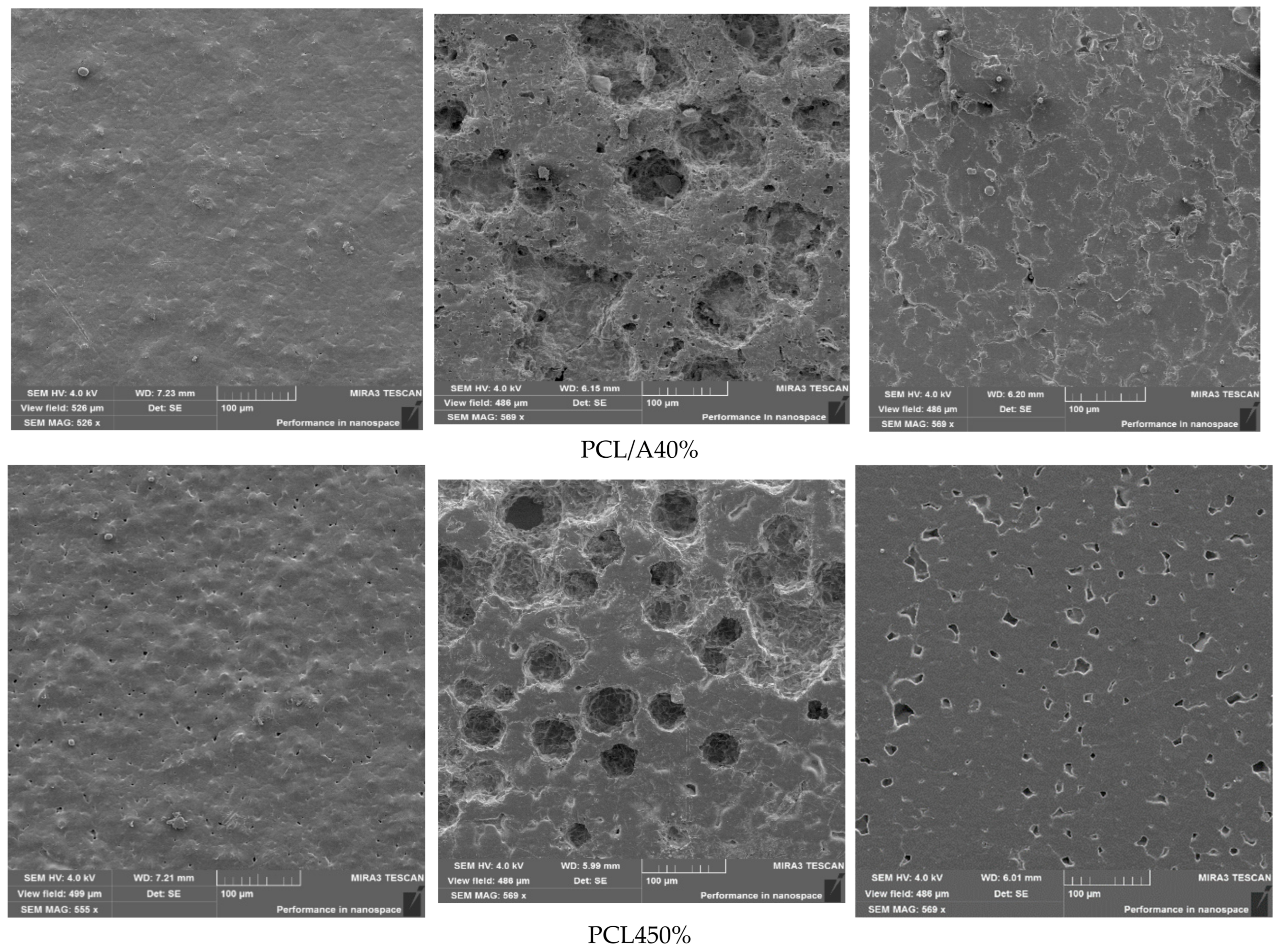
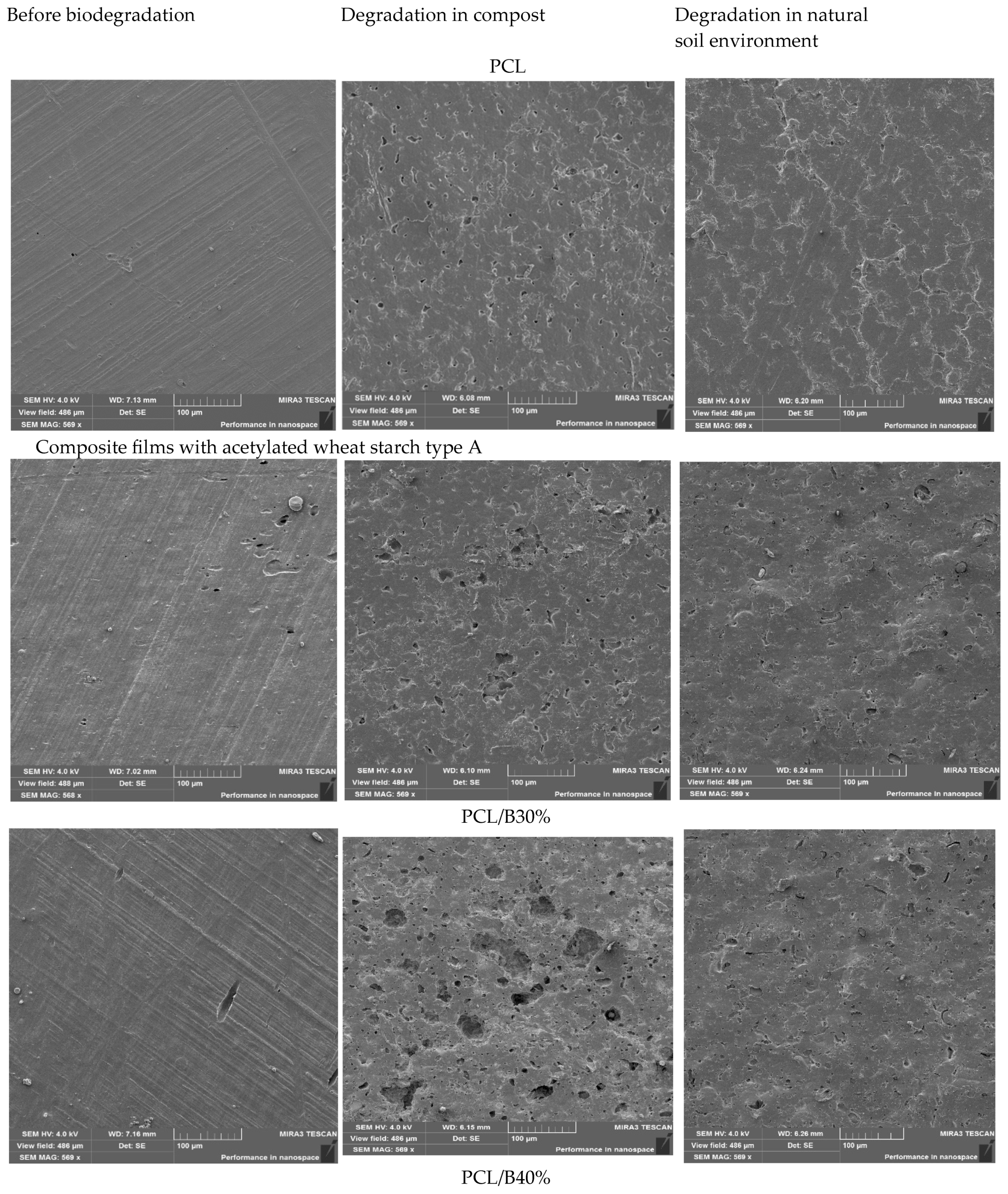
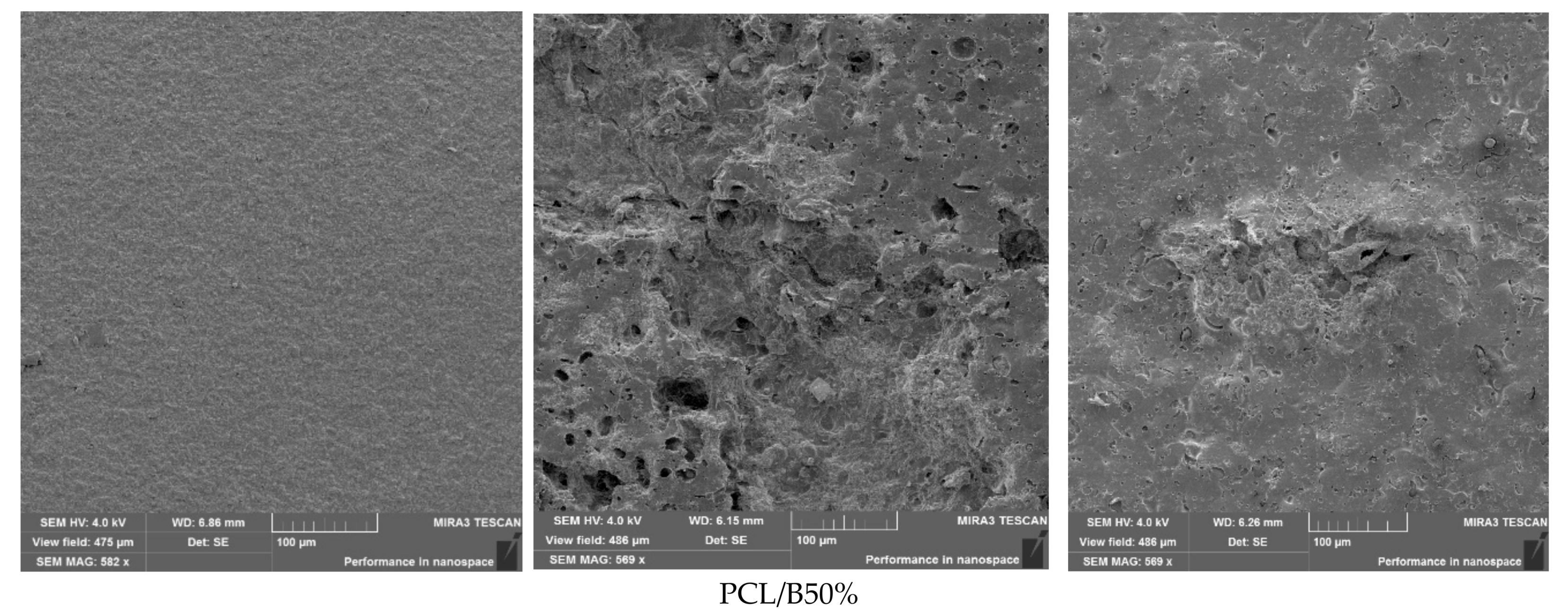
3.10. Morphological Characterization of Films After Biodegradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCL | Polycaprolactone |
| WVP | Water Vapor Permeability |
| SEM | Scanning Electron Microscopy |
| DSC | Differential Scanning Calorimetry |
| TGA | Thermogravimetric Analysis |
| FTIR | Fourier Transform Infrared Spectroscopy |
| CFU | Colony Forming Units |
| RH | Relative Humidity |
| ISO | International Organization for Standardization |
| A-type starch | Acetylated wheat starch (type A) |
| B-type starch | Acetylated wheat starch (type B) |
| ASTM | American Society for Testing and Materials |
| SD | Standard Deviation |
| ANOVA | Analysis of Variance |
| HACCP | Hazard Analysis Critical Control Point |
| WAC | Water absorption capacity |
| OAC | Oil absorption capacity |
| WS | Water Solubility |
| SP | Swelling Power |
| DS | Degree of Substitution |
| UV–Vis | Ultraviolet–Visible Spectroscopy |
| EDX | Energy Dispersive X-ray Spectroscopy |
References
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A.; Chemistry, S. Sustainability of biodegradable plastics: New problem or solution to solve the global plastic pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Huang, W.; Wen, X.; Tang, W. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ. Pollut. 2020, 263, 114469. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Biodegradable plastic applications towards sustainability: A recent innovations in the green product. Clean. Eng. Technol. 2022, 6, 100404. [Google Scholar] [CrossRef]
- Kumari, S.; Rao, A.; Kaur, M.; Dhania, G. Petroleum-Based Plastics Versus Bio-Based Plastics: A Review. Nat. Environ. Pollut. Technol. 2023, 22, 1111–1124. [Google Scholar] [CrossRef]
- Elkaliny, N.E.; Alzamel, N.M.; Moussa, S.H.; Elodamy, N.I.; Madkor, E.A.; Ibrahim, E.M.; Elshobary, M.E.; Ismail, G.A. Macroalgae bioplastics: A sustainable shift to mitigate the ecological impact of petroleum-based plastics. Polymers 2024, 16, 1246. [Google Scholar] [CrossRef] [PubMed]
- Abrisham, M.; Noroozi, M.; Panahi-Sarmad, M.; Arjmand, M.; Goodarzi, V.; Shakeri, Y.; Golbaten-Mofrad, H.; Dehghan, P.; Sahzabi, A.S.; Sadri, M. The role of polycaprolactone-triol (PCL-T) in biomedical applications: A state-of-the-art review. Eur. Polym. J. 2020, 131, 109701. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, L.; McClements, D.J.; Yang, T.; Zhang, Z.; Ren, F.; Miao, M.; Tian, Y.; Jin, Z. Starch-based biodegradable packaging materials: A review of their preparation, characterization and diverse applications in the food industry. Trends Food Sci. Technol. 2021, 114, 70–82. [Google Scholar] [CrossRef]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bártolo, P.J.; Granja, P.L. Electrospun polycaprolactone (PCL) degradation: An in vitro and in vivo study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef]
- Ilyas, R.; Zuhri, M.; Norrrahim, M.N.F.; Misenan, M.S.M.; Jenol, M.A.; Samsudin, S.A.; Nurazzi, N.; Asyraf, M.; Supian, A.; Bangar, S.P. Natural fiber-reinforced polycaprolactone green and hybrid biocomposites for various advanced applications. Polymers 2022, 14, 182. [Google Scholar] [CrossRef]
- Kayan, G.Ö.; Kayan, A. Polycaprolactone composites/blends and their applications especially in water treatment. ChemEngineering 2023, 7, 104. [Google Scholar] [CrossRef]
- Sudheer, S.; Bandyopadhyay, S.; Bhat, R. Sustainable polysaccharide and protein hydrogel-based packaging materials for food products: A review. Int. J. Biol. Macromol. 2023, 248, 125845. [Google Scholar] [CrossRef]
- Galgano, F.; Condelli, N.; Favati, F.; Di Bianco, V.; Perretti, G.; Caruso, M.C. Biodegradable packaging and edible coating for fresh-cut fruits and vegetables. Ital. J. Food Sci. 2015, 27, 1–20. [Google Scholar]
- Rahmat, A.R.; Rahman, W.A.W.A.; Sin, L.T.; Yussuf, A.A. Approaches to improve compatibility of starch filled polymer system: A review. Mater. Sci. Eng. C 2009, 29, 2370–2377. [Google Scholar] [CrossRef]
- Ojogbo, E.; Ogunsona, E.O.; Mekonnen, T.H. Chemical and physical modifications of starch for renewable polymeric materials. Mater. Today Sustain. 2020, 7, 100028. [Google Scholar] [CrossRef]
- Wang, X.; Huang, L.; Zhang, C.; Deng, Y.; Xie, P.; Liu, L.; Cheng, J. Research advances in chemical modifications of starch for hydrophobicity and its applications: A review. Carbohydr. Polym. 2020, 240, 116292. [Google Scholar] [CrossRef] [PubMed]
- Muratkhan, M.; Zhainagul, K.; Yermekov, Y.; Svetlana, K.; Toimbayeva, D.; Temirova, I.; Amirsana, K.; Khamitova, D.; Zharykbasov, Y.; Sugirbay, A. Comparable Analysis of Natural and Modified Starches from Kazakhstan: Physicochemical Properties, Applications, and Insights on Biodegradable Films. Appl. Sci. 2025, 15, 3938. [Google Scholar] [CrossRef]
- Muratkhan, M.; Zhainagul, K.; Svetlana, K.; Toimbayeva, D.; Temirova, I.; Tazhina, S.; Khamitova, D.; Saule, S.; Tultabayeva, T.; Bulashev, B. Development and Optimization of Edible Antimicrobial Films Based on Dry Heat–Modified Starches from Kazakhstan. Foods 2025, 14, 2001. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Amalia, R.; Retnowati, D.S.; Budiyati, C.S.; Ratnawati, R. Preparation and physicochemical characterization of modified (acetylated) Gadung (Dioscorea hispida Dennst) flours. Sci. Study Res. Chem. Chem. Eng. 2014, 15, 135. [Google Scholar]
- Zhang, X.; Xi, C.; Guo, S.; Yan, M.; Lu, Y.; Sun, Z.; Ge, X.; Shen, H.; Ospankulova, G.; Muratkhan, M.; et al. Electron beam pre-irradiation enhances substitution degree, and physicochemical and functional properties of carboxymethyl peanut shell nanocellulose. Ind. Crops Prod. 2024, 209, 118035. [Google Scholar] [CrossRef]
- Wurzburg, O.B. Cross-linked Starches. In Modified Starches: Properties and Uses; Wurzburg, O.B., Ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 41–53. [Google Scholar]
- Ulfa, G.; Putri, W.; Fibrianto, K.; Prihatiningtyas, R.; Widjanarko, S. The influence of temperature in swelling power, solubility, and water binding capacity of pregelatinised sweet potato starch. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Changsha, China, 18–20 September 2020; p. 012036. [Google Scholar]
- GOST 26312.7-84; Grain and Products of Its Processing—Methods for Determination of Moisture Content. Gosstandart: Moscow, Russia, 1984.
- ISO 712:2009; Cereals and Cereal Products—Determination of Moisture Content—Reference Method. International Organization for Standardization: Geneva, Switzerland, 2009.
- GOST 26312.10-84; Grain and Products of Its Processing—Methods for Determination of Ash Content. Gosstandart: Moscow, Russia, 1984.
- ISO 3593:1981; Starch—Determination of Ash Content. International Organization for Standardization: Geneva, Switzerland, 1981.
- GOST 26312.15-84; Grain and Products of Its Processing—Methods for Determination of pH Value. Gosstandart: Moscow, Russia, 1984.
- GOST R 55865-2013; Grain and Cereal Products—Methods for Determining Bulk Density. Standartinform: Moscow, Russia, 2013.
- GOST R 55486-2013; Grain and Products of Its Processing—Methods for Determining Water and Oil Absorption Capacity. Standartinform: Moscow, Russia, 2013.
- Liang, W.; Ge, X.; Lin, Q.; Niu, L.; Zhao, W.; Muratkhan, M.; Li, W. Ternary composite degradable plastics based on Alpinia galanga essential oil Pickering emulsion templates: A potential multifunctional active packaging. Int. J. Biol. Macromol. 2024, 257, 128580. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, W.; Liu, X.; Zheng, J.; Sun, Z.; Ge, X.; Shen, H.; Ospankulova, G.; Muratkhan, M.; Li, W. Investigating the role and mechanism of water in E-beam modified sweet potato starch: Multi-scale structure, physicochemical properties, and in vitro digestibility. Food Hydrocoll. 2023, 137, 108433. [Google Scholar] [CrossRef]
- ASTM D882–12; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM E96–00; Standard Test Method for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2000.
- ISO 22196:2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. International Organization for Standardization: Geneva, Switzerland, 2011.
- ISO 17556:2019; Plastics—Determination of the Ultimate Aerobic Biodegradability in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbon Dioxide Evolved. International Organization for Standardization: Geneva, Switzerland, 2019.
- ASTM D5338–15; Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials Under Controlled Composting Conditions. ASTM International: West Conshohocken, PA, USA, 2015.
- ISO EN 846:2019; Plastics—Evaluation of the Action of Microorganisms. International Organization for Standardization: Geneva, Switzerland, 2019.
- ASTM D570–98(2010); Standard Test Method for Water Absorption of Plastics. ASTM International: West Conshohocken, PA, USA, 2010.
- Fatima, S.; Khan, M.R.; Ahmad, I.; Sadiq, M.B. Recent advances in modified starch based biodegradable food packaging: A review. Heliyon 2024, 10, e27453. [Google Scholar] [CrossRef]
- Dong, M.; Mastroianni, G.; Bilotti, E.; Zhang, H.; Papageorgiou, D.G. Biodegradable starch-based nanocomposite films with exceptional water and oxygen barrier properties. ACS Sustain. Chem. Eng. 2024, 12, 11056–11066. [Google Scholar] [CrossRef]
- Fredriksson, H.; Silverio, J.; Andersson, R.; Eliasson, A.-C.; Åman, P. The influence of amylose and amylopectin characteristics on gelatinization and retrogradation properties of different starches. Carbohydr. Polym. 1998, 35, 119–134. [Google Scholar] [CrossRef]
- Heidarbeigi, K.; Naderi, M.; Khoubakht, G.; Tabatabaeefar, A.; Kheiralipour, K.; Karimi, M. The effect of moisture content on physical properties of wheat. Pak. J. Nutr. 2009, 8, 90–95. [Google Scholar]
- Ghanem, A.F.; Yassin, M.A.; Rabie, A.M.; Gouanvé, F.; Espuche, E.; Abdel Rehim, M.H. Investigation of water sorption, gas barrier and antimicrobial properties of polycaprolactone films contain modified graphene. J. Mater. Sci. 2021, 56, 497–512. [Google Scholar] [CrossRef]
- He, C.; Zhang, R.; Fan, R.; Yu, J.; Hu, J.; Peng, Q.; Han, L.; Wang, M. Starch modification by low-dose electron beam irradiation: A comprehensive study between buckwheat starch, potato starch and pea starch. Int. J. Biol. Macromol. 2024, 283, 137810. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, W.; Liu, X.; Zhao, W.; Liang, W.; Zheng, Y.; Li, W. Effects of electron beam irradiation pretreatment on the substitution degree, multiscale structure and physicochemical properties of OSA-esterified rice starch. Food Biosci. 2023, 56, 103136. [Google Scholar] [CrossRef]
- Ačkar, Đ.; Babić, J.; Jozinović, A.; Miličević, B.; Jokić, S.; Miličević, R.; Rajič, M.; Šubarić, D. Starch modification by organic acids and their derivatives: A review. Molecules 2015, 20, 19554–19570. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Chen, Y.; Chen, Z.; Yang, Y.; Wang, Y. Preparation and characteristics of starch esters and its effects on dough physicochemical properties. J. Food Qual. 2018, 2018, 1395978. [Google Scholar] [CrossRef]
- Hong, J.; Zeng, X.-A.; Brennan, C.S.; Brennan, M.; Han, Z. Recent advances in techniques for starch esters and the applications: A review. Foods 2016, 5, 50. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Contreras, J.; Talens, P.; Chiralt, A. Physical and structural properties and thermal behaviour of starch-poly (ɛ-caprolactone) blend films for food packaging. Food Packag. Shelf Life 2015, 5, 10–20. [Google Scholar]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on tensile, thermal and barrier properties of biodegradable films based on sugar palm (Arenga pinnata) starch. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Effects of controlled storage on thermal, mechanical and barrier properties of plasticized films from different starch sources. J. Food Eng. 2006, 75, 453–460. [Google Scholar] [CrossRef]
- Avella, M.; De Vlieger, J.J.; Errico, M.E.; Fischer, S.; Vacca, P.; Volpe, M.G. Biodegradable starch/clay nanocomposite films for food packaging applications. Food Chem. 2005, 93, 467–474. [Google Scholar] [CrossRef]
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable bio-composites from renewable resources: Opportunities and challenges in the green materials world. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J. Properties of biodegradable thermoplastic pea starch/carboxymethyl cellulose and pea starch/microcrystalline cellulose composites. Carbohydr. Polym. 2008, 72, 369–375. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- López, O.V.; García, M.A.; Zaritzky, N.E. Film forming capacity of chemically modified corn starches. Carbohydr. Polym. 2008, 73, 573–581. [Google Scholar] [CrossRef]
- Reddy, J.P.; Rhim, J.-W. Characterization of bionanocomposite films prepared with agar and paper-mulberry pulp nanocellulose. Carbohydr. Polym. 2014, 110, 480–488. [Google Scholar] [CrossRef]
- Chivrac, F.; Pollet, E.; Dole, P.; Avérous, L. Starch-based nano-biocomposites: Plasticizer impact on the montmorillonite exfoliation process. Carbohydr. Polym. 2010, 79, 941–947. [Google Scholar] [CrossRef]
- Thuwall, M.; Boldizar, A.; Rigdahl, M. Extrusion processing of high amylose potato starch materials. Carbohydr. Polym. 2006, 65, 441–446. [Google Scholar] [CrossRef]
- de Menezes, B.R.C.; Rodrigues, K.F.; da Silva Fonseca, B.C.; Ribas, R.G.; do Amaral Montanheiro, T.L.; Thim, G.P. Recent advances in the use of carbon nanotubes as smart biomaterials. J. Mater. Chem. B 2019, 7, 1343–1360. [Google Scholar] [CrossRef]
- Ribba, L.; Lopretti, M.; de Oca-Vasquez, G.M.; Batista, D.; Goyanes, S.; Vega-Baudrit, J.R. Biodegradable plastics in aquatic ecosystems: Latest findings, research gaps, and recommendations. Environ. Res. Lett. 2022, 17, 033003. [Google Scholar] [CrossRef]
- Chen, P.; Tian, H.; Zhang, L.; Chang, P.R. Structure and properties of soy protein plastics with ε-caprolactone/glycerol as binary plasticizers. Ind. Eng. Chem. Res. 2008, 47, 9389–9395. [Google Scholar] [CrossRef]
- Chivrac, F.; Pollet, E.; Avérous, L. Progress in nano-biocomposites based on polysaccharides and nanoclays. Mater. Sci. Eng. R Rep. 2009, 67, 1–17. [Google Scholar] [CrossRef]
- Bodîrlău, R.; Teacă, C.-A.; Spiridon, I.; Tudorachi, N. Effects of chemical modification on the structure and mechanical properties of starch-based biofilms. Monatshefte Fuer Chem. Chem. Mon. 2012, 143, 335–343. [Google Scholar] [CrossRef]
- Chen, Z.; Gengenbach, U.; Gananian, S.; Moser, D.; Reichert, K.-M.; Huang, L.; Koker, L. Solution Processable Barrier Films Using A Filled Polymer to Encapsulate Flexible Printed Electronics. IEEE J. Flex. Electron. 2024, 4, 4–11. [Google Scholar] [CrossRef]
- Wojtowicz, A.; Janssen, L.P.; Moscicki, L. Blends of natural and synthetic polymers. In Thermoplastic Starch; Janssen, L.P.B.M., Mościcki, L., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2009; pp. 35–53. [Google Scholar]
- Siracusa, V.; Rocculi, P.; Romani, S.; Dalla Rosa, M. Biodegradable Polymers for Food Packaging: A Review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]


| Starch Type | Modification | Moisture (%) | Ash (%) | pH | Bulk Density (g/mL) | WAC (g/g) | OAC (g/g) |
|---|---|---|---|---|---|---|---|
| Wheat A | Native | 10.5 ± 0.2 b | 0.21 ± 0.01 b | 6.72 ± 0.05 b | 0.61 ± 0.01 a | 0.84 ± 0.03 b | 0.72 ± 0.04 b |
| Wheat A | Dual-modified | 9.9 ± 0.1 a | 0.18 ± 0.01 a | 6.55 ± 0.04 a | 0.58 ± 0.02 a | 1.22 ± 0.05 a | 0.91 ± 0.06 a |
| Wheat B | Native | 11.4 ± 0.3 b | 0.24 ± 0.01 b | 6.54 ± 0.06 b | 0.57 ± 0.01 a | 0.89 ± 0.04 b | 0.69 ± 0.05 b |
| Wheat B | Dual-modified | 10.2 ± 0.2 a | 0.19 ± 0.02 a | 6.41 ± 0.05 a | 0.55 ± 0.01 a | 1.15 ± 0.06 a | 0.87 ± 0.04 a |
| Starch Type | Modification | WS (g/100 g) | SP (%) | DS |
|---|---|---|---|---|
| Wheat A | Dual-modified | 3.28 ± 0.13 b | 5.88 ± 0.01 b | 1.95 ± 0.04 a |
| Wheat B | Dual-modified | 3.28 ± 0.13 b | 7.76 ± 0.01 a | 1.08 ± 0.03 b |
| Corn | Dual-modified | 8.09 ± 0.11 a | 5.93 ± 0.01 b | 0.88 ± 0.02 c |
| Starch Type | Plasticizer | 10% | 20% | 30% | 40% |
|---|---|---|---|---|---|
| Wheat A | PVA | 31.53 ±6.69 d | 28.56 ±5.78 c | 23.23 ±6.34 b | 15.10 ±5.56 a |
| Wheat A | Glycerol | 41.57 ±4.36 a | 38.74 ±3.45 a | 31.68 ±5.67 c | 22.85 ±4.87 b |
| Wheat B | PVA | 35.66 ±4.79 a | 32.42 ±4.48 a | 28.35 ±5.22 ab | 23.13 ±4.85 b |
| Wheat B | Glycerol | 48.32 ±4.27 a | 43.53 ±4.43 a | 38.37 ±5.44 ab | 34.73 ±5.22 b |
| Corn | PVA | 33.27 ±3.69 a | 31.36 ±4.12 a | 26.34 ±3.95 c | 18.61 ±4.23 b |
| Corn | Glycerol | 44.24 ±3.83 a | 41.88 ±4.36 a | 35.46 ±4.76 b | 27.47 ±4.56 c |
| Starch (%) | Glycerol (%) | CaCO3 (%) | Measured MPa | Predicted MPa |
|---|---|---|---|---|
| 20 | 10 | 5 | 26.47 ± 0.27 | 26.48 |
| 40 | 10 | 5 | 21.35 ± 0.17 | 21.56 |
| 60 | 10 | 5 | 16.23 ± 0.10 | 16.34 |
| Sample | Transparency (%) |
|---|---|
| Neat PCL | 91.3 ± 1.2 a |
| PCL/A30% + 10% glycerol | 74.6 ± 2.1 b |
| PCL/A40% + 10% glycerol | 62.7 ± 1.8 c |
| PCL/A50% + 10% glycerol | 48.9 ± 2.5 d |
| PCL/B30% + 10% glycerol | 69.2 ± 1.9 b |
| PCL/B40% + 10% glycerol | 55.8 ± 2.2 c |
| PCL/B50% + 10% glycerol | 41.5 ± 2.7 d |
| Sample Composition | Film Thickness (μm) | Adsorption Rate (m/min) | WVP (g·mm/m2·day·kPa) |
|---|---|---|---|
| PCL (control) | 86 ± 7 d | 4.66 ± 0.44 c | 11 ± 0.27 c |
| PCL/A30% | 208 ± 27 c | 11.08 ± 0.34 b | 15 ± 0.54 c |
| PCL/A40% | 422 ± 5 b | 16.11 ± 0.43 a | 23 ± 0.79 c |
| PCL/A50% | 815 ± 13 a | 17.60 ± 0.34 a | 190 ± 7.22 b |
| PCL/B30% | 109 ± 32 d | 9.68 ± 0.24 c | 21 ± 0.61 c |
| PCL/B40% | 268 ± 3 c | 6.09 ± 0.40 c | 106 ± 4.68 b |
| PCL/B50% | 668 ± 3 b | 13.86 ± 0.46 b | 215 ± 8.66 a |
| Sample | E. coli | S. aureus |
|---|---|---|
| PCL | 6.0 ± 0.5 a | 6.2 ± 0.3 a |
| PCL/A30% | 9.1 ± 0.4 b | 10.3 ± 0.5 b |
| PCL/A40% | 11.8 ± 0.3 c | 12.5 ± 0.6 c |
| PCL/A50% | 14.6 ± 0.2 d | 15.3 ± 0.4 d |
| PCL/B30% | 7.8 ± 0.4 ab | 8.5 ± 0.5 ab |
| PCL/B40% | 9.4 ± 0.3 b | 10.1 ± 0.4 b |
| PCL/B50% | 11.2 ± 0.3 c | 12.0 ± 0.5 c |
| Sample | Natural Soil (%) | Compost Soil (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 10 | Day 20 | Day 30 | Day 0 | Day 5 | Day 10 | Day 30 | |
| PCL | 100 ± 0.00 a | 99.35 ± 0.01 a | 99.19 ± 0.02 a | 98.95 ± 0.02 a | 100 ± 0.00 a | 96.33 ± 0.01 a | 94.69 ± 0.02 a | 92.15 ± 0.03 a |
| PCL/A30% | 100 ± 0.00 a | 99.40 ± 0.02 a | 99.22 ± 0.08 a | 99.02 ± 0.03 a | 100 ± 0.00 a | 75.24 ± 0.11 b | 64.08 ± 0.13 b | 42.67 ± 0.18 b |
| PCL/A40% | 100 ± 0.00 a | 99.18 ± 0.04 a | 98.82 ± 0.08 a | 98.40 ± 0.05 a | 100 ± 0.00 a | 82.56 ± 0.02 b | 62.18 ± 0.10 b | 40.12 ± 0.15 b |
| PCL/A50% | 100 ± 0.00 a | 98.11 ± 0.08 a | 97.99 ± 0.07 a | 97.91 ± 0.06 a | 100 ± 0.00 a | 76.88 ± 0.07 b | 56.25 ± 0.06 b | 33.54 ± 0.14 b |
| PCL/B30% | 100 ± 0.00 a | 89.82 ± 0.02 b | 89.65 ± 0.05 b | 89.42 ± 0.01 b | 100 ± 0.00 a | 84.31 ± 0.06 b | 73.35 ± 0.07 b | 49.12 ± 0.17 b |
| PCL/B40% | 100 ± 0.00 a | 82.64 ± 0.04 b | 82.40 ± 0.01 b | 82.14 ± 0.03 b | 100 ± 0.00 a | 75.83 ± 0.09 b | 58.72 ± 0.12 b | 29.86 ± 0.16 c |
| PCL/B50% | 100 ± 0.00 a | 75.65 ± 0.08 c | 75.49 ± 0.08 c | 75.37 ± 0.03 c | 100 ± 0.00 a | 71.67 ± 0.06 c | 55.29 ± 0.09 c | 25.42 ± 0.13 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ospankulova, G.; Saduakhasova, S.; Kamanova, S.; Toimbayeva, D.; Temirova, I.; Kakimova, Z.; Yermekov, Y.; Bulashev, B.; Tamara, T.; Muratkhan, M. Dual-Modified A- and B-Type Wheat Starch–PCL Composite Films: Antibacterial and HACCP-Oriented Biodegradable Packaging from Kazakhstani Resources. Foods 2025, 14, 3730. https://doi.org/10.3390/foods14213730
Ospankulova G, Saduakhasova S, Kamanova S, Toimbayeva D, Temirova I, Kakimova Z, Yermekov Y, Bulashev B, Tamara T, Muratkhan M. Dual-Modified A- and B-Type Wheat Starch–PCL Composite Films: Antibacterial and HACCP-Oriented Biodegradable Packaging from Kazakhstani Resources. Foods. 2025; 14(21):3730. https://doi.org/10.3390/foods14213730
Chicago/Turabian StyleOspankulova, Gulnazym, Saule Saduakhasova, Svetlana Kamanova, Dana Toimbayeva, Indira Temirova, Zhainagul Kakimova, Yernaz Yermekov, Berdibek Bulashev, Tultabayeva Tamara, and Marat Muratkhan. 2025. "Dual-Modified A- and B-Type Wheat Starch–PCL Composite Films: Antibacterial and HACCP-Oriented Biodegradable Packaging from Kazakhstani Resources" Foods 14, no. 21: 3730. https://doi.org/10.3390/foods14213730
APA StyleOspankulova, G., Saduakhasova, S., Kamanova, S., Toimbayeva, D., Temirova, I., Kakimova, Z., Yermekov, Y., Bulashev, B., Tamara, T., & Muratkhan, M. (2025). Dual-Modified A- and B-Type Wheat Starch–PCL Composite Films: Antibacterial and HACCP-Oriented Biodegradable Packaging from Kazakhstani Resources. Foods, 14(21), 3730. https://doi.org/10.3390/foods14213730







