Analysis of the Bioactive Compounds and Physiological Activities of Commonly Consumed Noni Juice in Republic of Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation, °Brix, pH and Lyophilization Yield
2.3. Analysis of Bioactive Compounds Using HPLC-PDA Method
2.4. Total Phenolic, Flavonoid, and Proanthocyanidin Contents
2.5. Antioxidant Activity
2.5.1. DPPH Radical Scavenging Activity
2.5.2. ABTS Radical Scavenging Activity
2.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.4. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.5.5. Reducing Power Assay
2.5.6. Superoxide Dismutase (SOD)-like Activity
2.6. Cell Viability, Lipid Accumulation, and ROS Production in MDI- and BPA-Induced 3T3-L1 Adipocytes
2.7. Evaluation of the Anti-Muscle Atrophy Effects in C2C12 Cells
2.8. Measurements of Immunoenhancing and Anti-Inflammatory Effects in RAW 264.7 Cells
2.9. Measurement of Protective Effects in UVB-Induced HDF Cells
2.10. Statistical Analysis
3. Results
3.1. Quantification of Bioactive Compounds in Noni Juice by HPLC-PDA
3.2. °Brix, pH, Lyophilization Yield, and Content of Total Phenolics, Flavonoids, and Proanthocyanidins in Noni Juice
3.3. Effect of Noni Juice on Antioxidant Activity
3.4. Effects of Noni Juice on Anti-Obesity and Anti-Obesogenic Activities
3.5. Anti-Muscle Atrophy Effects of Noni Juice in C2C12 Myotubes
3.6. Evaluation of Immunoenhancing and Anti-Inflammatory Effects of Noni Juice in RAW 264.7 Cells
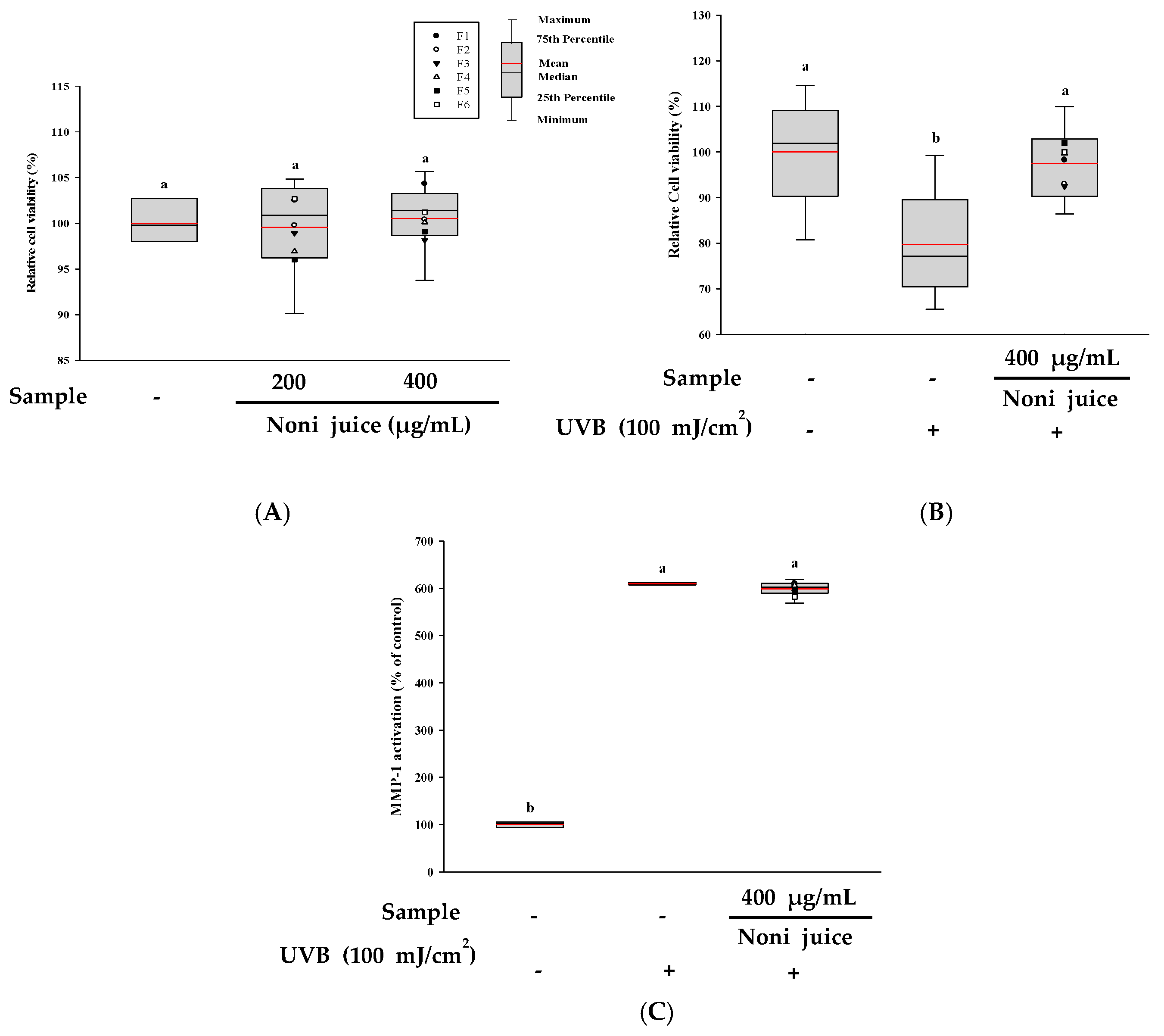
3.7. Cytoprotective Properties of Noni Juice on HDF Cells Exposed to UVB Irradiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GAE | Gallic acid equivalents |
| CE | Catechin equivalents |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt |
| ROS | R eactive oxygen species |
| NO | Nitric oxide |
| DAA | Deacetylasperulosidic acid |
| Asp | Asperuloside |
| AA | Asperulosidic acid |
| Sco | Scopoletin |
| HPLC | High-performance liquid chromatography |
| PDA | Photodiode array |
| LPS | Lipopolysaccharides |
| IBMX | 3-isobutyl-1-methylxanthine |
| BPA | Bisphenol A |
| DEX | Dexamethasone |
| NBT | Nitroblue tetrazolium |
| ORO | Oil red O |
| QE | Quercetin equivalents |
| FRAP | Ferric-reducing antioxidant power |
| ORAC | Oxygen radical absorbance capacity |
| SOD | Superoxide dismutase |
| UVB | Ultraviolet B |
| MMP-1 | Matrix metalloproteinase-1 |
| GAR | Garcinia cambogia |
| ECM | Extracellular matrix |
References
- Westendorf, J.; Mettlich, C. The benefits of noni juice: An epidemiological evaluation in Europe. J. Med. Food Plants 2009, 1, 64–79. [Google Scholar]
- West, B.J.; Deng, S.; Isami, F.; Uwaya, A.; Jensen, C.J. The potential health benefits of noni juice: A review of human intervention studies. Foods 2018, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Kenganora, M.; Manjula, S.N. Health benefits of Morinda citrifolia (Noni): A review. Pharmacogn. J. 2016, 8, 321–334. [Google Scholar] [CrossRef]
- Kim, Y.; Pyeon, J.; Lee, J.Y.; Kim, E.M.; La, I.J.; Lee, O.H.; Kim, K.; Sung, J.; Kim, Y. Chemical fingerprint analysis of fermented Morinda citrifolia L.(Noni) juice by UHPLC Q-TOF/MS combined with chemometric analysis. Appl. Biol. Chem. 2024, 67, 59. [Google Scholar] [CrossRef]
- Potterat, O.; Von Felten, R.; Dalsgaard, P.W.; Hamburger, M. Identification of TLC markers and quantification by HPLC-MS of various constituents in noni fruit powder and commercial noni-derived products. J. Agric. Food Chem. 2007, 55, 7489–7494. [Google Scholar] [CrossRef]
- Choi, S.I.; Kwon, H.Y.; La, I.J.; Jo, Y.H.; Han, X.; Men, X.; Lee, S.J.; Kim, Y.D.; Seong, G.S.; Lee, O.H. Development and validation of an analytical method for deacetylasperulosidic acid, asperulosidic acid, scopolin, asperuloside and scopoletin in fermented Morinda citrifolia L.(Noni). Separations 2021, 8, 80. [Google Scholar] [CrossRef]
- Ikeda, R.; Wada, M.; Nishigaki, T.; Nakashima, K. Quantification of coumarin derivatives in Noni (Morinda citrifolia) and their contribution of quenching effect on reactive oxygen species. Food Chem. 2009, 113, 1169–1172. [Google Scholar] [CrossRef]
- Ahmad, A.N.; Daud, Z.A.M.; Ismail, A. Review on potential therapeutic effect of Morinda citrifolia L. Curr. Opin. Food Sci. 2016, 8, 62–67. [Google Scholar] [CrossRef]
- Mani, J.S.; Johnson, J.B.; Naiker, M. The phytochemistry and anticarcinogenic activity of noni juice. Eng. Proc. 2021, 11, 16. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, P.; Hu, B.; Xu, L.; Liu, S.; Yu, H.; Guo, Y.; Xie, Y.; Yao, W.; Qian, H. Correlation analysis reveals the intensified fermentation via Lactobacillus plantarum improved the flavor of fermented noni juice. Food Biosci. 2021, 43, 101234. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, L.; Shen, R.; Guo, S.; Peng, X. Identification of the influential odorants for the unpleasant rancid smell of ripe noni fruit (Morinda citrifolia). Int. J. Food Sci. Technol. 2022, 57, 2277–2284. [Google Scholar] [CrossRef]
- West, B.J. Consumer Perceptions of Noni Juice Health Benefits during a 90-Day In-Home Use Test. Health 2023, 15, 266–279. [Google Scholar] [CrossRef]
- Wang, M.Y.; West, B.J.; Jensen, C.J.; Nowicki, D.; Su, C.; Palu, A.K.; Anderson, G. Morinda citrifolia (Noni): A literature review and recent advances in Noni research. Acta Pharmacol. Sin. 2002, 23, 1127–1141. [Google Scholar]
- Yang, J.; Paulino, R.; Janke-Stedronsky, S.; Abawi, F. Free-radical-scavenging activity and total phenols of noni (Morinda citrifolia L.) juice and powder in processing and storage. Food Chem. 2007, 102, 302–308. [Google Scholar] [CrossRef]
- Potterat, O.; Hamburger, M. Morinda citrifolia (Noni) fruit-phytochemistry, pharmacology, safety. Planta Med. 2007, 73, 191–199. [Google Scholar] [CrossRef]
- Almeida, É.S.; de Oliveira, D.; Hotza, D. Properties and applications of Morinda citrifolia (noni): A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 883–909. [Google Scholar] [CrossRef] [PubMed]
- Chan-Blanco, Y.; Vaillant, F.; Perez, A.M.; Reynes, M.; Brillouet, J.M.; Brat, P. The noni fruit (Morinda citrifolia L.): A review of agricultural research, nutritional and therapeutic properties. J. Food Compos. Anal. 2006, 19, 645–654. [Google Scholar] [CrossRef]
- Guo, M.; Mao, B.; Sadiq, F.A.; Hao, Y.; Cui, S.; Yi, M.; Hong, Q.; Lee, Y.K.; Zhao, J. Effects of noni fruit and fermented noni juice against acute alcohol induced liver injury in mice. J. Funct. Foods. 2020, 70, 103995. [Google Scholar] [CrossRef]
- Yang, S.C.; Chen, T.I.; Li, K.Y.; Tsai, T.C. Change in phenolic compound content, reductive capacity and ACE inhibitory activity in noni juice during traditional fermentation. J. Food Drug Anal. 2007, 15, 10. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef]
- Nerurkar, P.V.; Hwang, P.W.; Saksa, E. Anti-diabetic potential of noni: The Yin and the Yang. Molecules 2015, 20, 17684–17719. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Vermaak, I.; Viljoen, A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia 2015, 102, 134–148. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Chen, Y.; Guo, J.; Li, C.; Zhang, J. Metatranscriptomic approach reveals the functional and enzyme dynamics of core microbes during noni fruit fermentation. Food Res. Int. 2021, 141, 109999. [Google Scholar] [CrossRef]
- Ma, D.L.; Chen, M.; Su, C.X.; West, B.J. In vivo antioxidant activity of deacetylasperulosidic acid in noni. J. Anal. Methods Chem. 2013, 2013, 804504. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gu, C.; Zhu, K.; Xu, F.; Feng, Z.; Zhang, Y. Insight into the effects of different ripeness levels on the quality and flavor chemistry of Noni fruit (Morinda citrifolia L.). Food Chem. 2024, 434, 137408. [Google Scholar] [CrossRef] [PubMed]
- Dussossoy, E.; Brat, P.; Bony, E.; Boudard, F.; Poucheret, P.; Mertz, C.; Giaimis, J.; Michel, A. Characterization, anti-oxidative and anti-inflammatory effects of Costa Rican noni juice (Morinda citrifolia L.). J. Ethnopharmacol. 2011, 133, 108–115. [Google Scholar] [CrossRef]
- Sarıtaş, S.; Portocarrero, A.C.M.; Miranda López, J.M.; Lombardo, M.; Koch, W.; Raposo, A.; El-Seedi, H.R.; de Brito Alves, J.L.; Esatbeyoglu, T.; Karav, S.; et al. The impact of fermentation on the antioxidant activity of food products. Molecules 2024, 29, 3941. [Google Scholar] [CrossRef]
- Nowak, D.; Gośliński, M.; Kłębukowska, L. Antioxidant and antimicrobial properties of selected fruit juices. Plant Foods Hum. Nutr. 2022, 77, 427–435. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.; Elkhayat, E.S.; El Dine, R.S. Natural anti-obesity agents. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 269–284. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in metabolic diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef]
- Mamun, M.A.A.; Rakib, A.; Mandal, M.; Kumar, S.; Singla, B.; Singh, U.P. Polyphenols: Role in modulating immune function and obesity. Biomolecules 2024, 14, 221. [Google Scholar] [CrossRef]
- Inada, A.C.; Figueiredo, P.S.; Santos-Eichler, R.A.D.; Freitas, K.D.C.; Hiane, P.A.; Castro, A.P.D.; Guimarães, R.D.C.A. Morinda citrifolia Linn.(Noni) and its potential in obesity-related metabolic dysfunction. Nutrients 2017, 9, 540. [Google Scholar] [CrossRef]
- Nishioka, A.; Nerurkar, P. Effects of Morinda citrifolia (noni) on obesity and glucose tolerance in C57BL/6 mice. FASEB J. 2007, 21, A982. [Google Scholar] [CrossRef]
- Shoeb, A.; Alwar, M.C.; Shenoy, P.J.; Gokul, P. Effect of Morinda citrifolia (Noni) fruit juice on high fat diet induced dyslipidemia in rats. J. Clin. Diagn. Res. 2016, 10, FF06. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Wang, S.; Wang, J.; Su, C.; Zhang, L.; Li, C.; Liu, S. Phenolics from noni (Morinda citrifolia L.) fruit alleviate obesity in high fat diet-fed mice via modulating the gut microbiota and mitigating intestinal damage. Food Chem. 2023, 402, 134232. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Shirwaikar, A.; Kamariya, Y.; Patel, B.; Nanda, S.; Parmar, V.; Khan, S. Methanol extract of the fruits of Morinda citrifolia Linn., restores bone loss in ovariectomized rats. Int. J. Pharmacol. 2011, 7, 446–454. [Google Scholar] [CrossRef]
- Saraswathi, C.D.; Prakash, W.S.; Kunal, P.W. Anti-arthritic activity of Morinda citrifolia L. fruit juice in Complete Freund’s Adjuvant induced arthritic rats. J. Pharm. Res. 2012, 5, 1236–1239. [Google Scholar]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, X.; Wang, Y.; Yan, Y.; Wang, Y.; Su, M.; Lv, H.; Li, K.; Hao, X.; Xing, X.; et al. Application of lipopolysaccharide in establishing inflammatory models. Int. J. Biol. Macromol. 2024, 279, 135371. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Choi, S.I.; La, I.J.; Han, X.; Men, X.; Lee, S.J.; Oh, G.; Kwon, H.Y.; Kim, Y.D.; Seong, G.S.; Kim, S.H.; et al. Immunomodulatory effect of polysaccharide from fermented Morinda citrifolia L. (Noni) on RAW 264.7 macrophage and Balb/c mice. Foods 2022, 11, 1925. [Google Scholar] [CrossRef]
- Son, W.C.; Yun, J.W.; Kim, B.H. Adipose-derived mesenchymal stem cells reduce MMP-1 expression in UV-irradiated human dermal fibroblasts: Therapeutic potential in skin wrinkling. Biosci. Biotechnol. Biochem. 2015, 79, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Choi, M. Anti-inflammatory and anti-aging effects of hydroxytyrosol on human dermal fibroblasts (HDFs). Biomed. Dermatol. 2018, 2, 21. [Google Scholar] [CrossRef]
- Kim, K.; Kim, C.E.; Baek, D.J.; Park, E.Y.; Oh, Y.S. Prevention of UVB-Induced Photoaging by an Ethyl Acetate Fraction from Allomyrina dichotoma Larvae and Its Potential Mechanisms in Human Dermal Fibroblasts. Int. J. Mol. Sci. 2024, 25, 7850. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Noh, E.M.; Kwon, K.B.; Lee, Y.S.; Chu, J.P.; Kim, E.J.; Park, Y.S.; Kim, B.S.; Kim, J.S. Radix clematidis extract inhibits UVB-induced MMP expression by suppressing the NF-κB pathway in human dermal fibroblasts. Int. J. Mol. Med. 2009, 23, 679–684. [Google Scholar] [CrossRef]
- West, B.J. The influence of Morinda citrifolia (noni) fruit juice on collagen deposition in the skin: A minireview. J. Biosci. Med. 2018, 6, 1–10. [Google Scholar] [CrossRef]
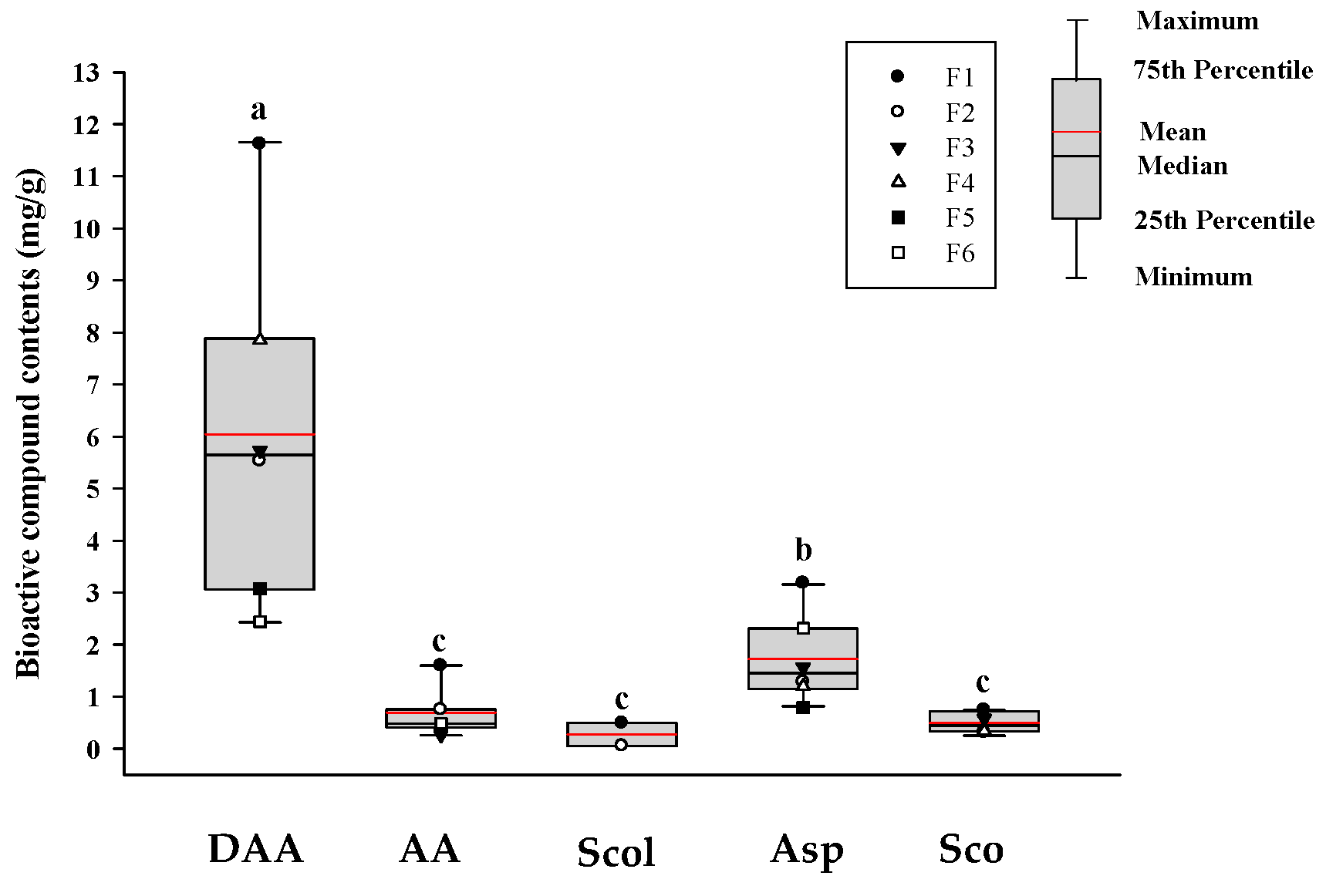
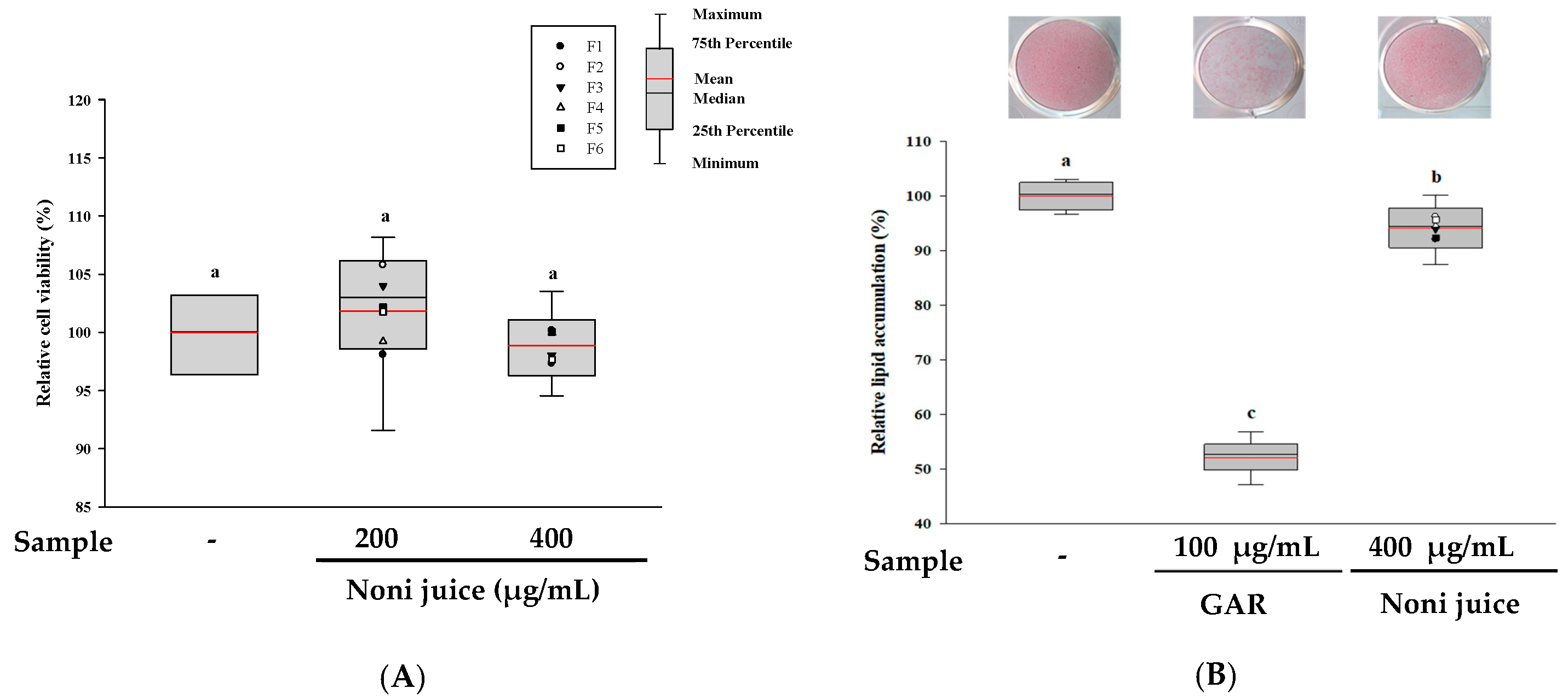
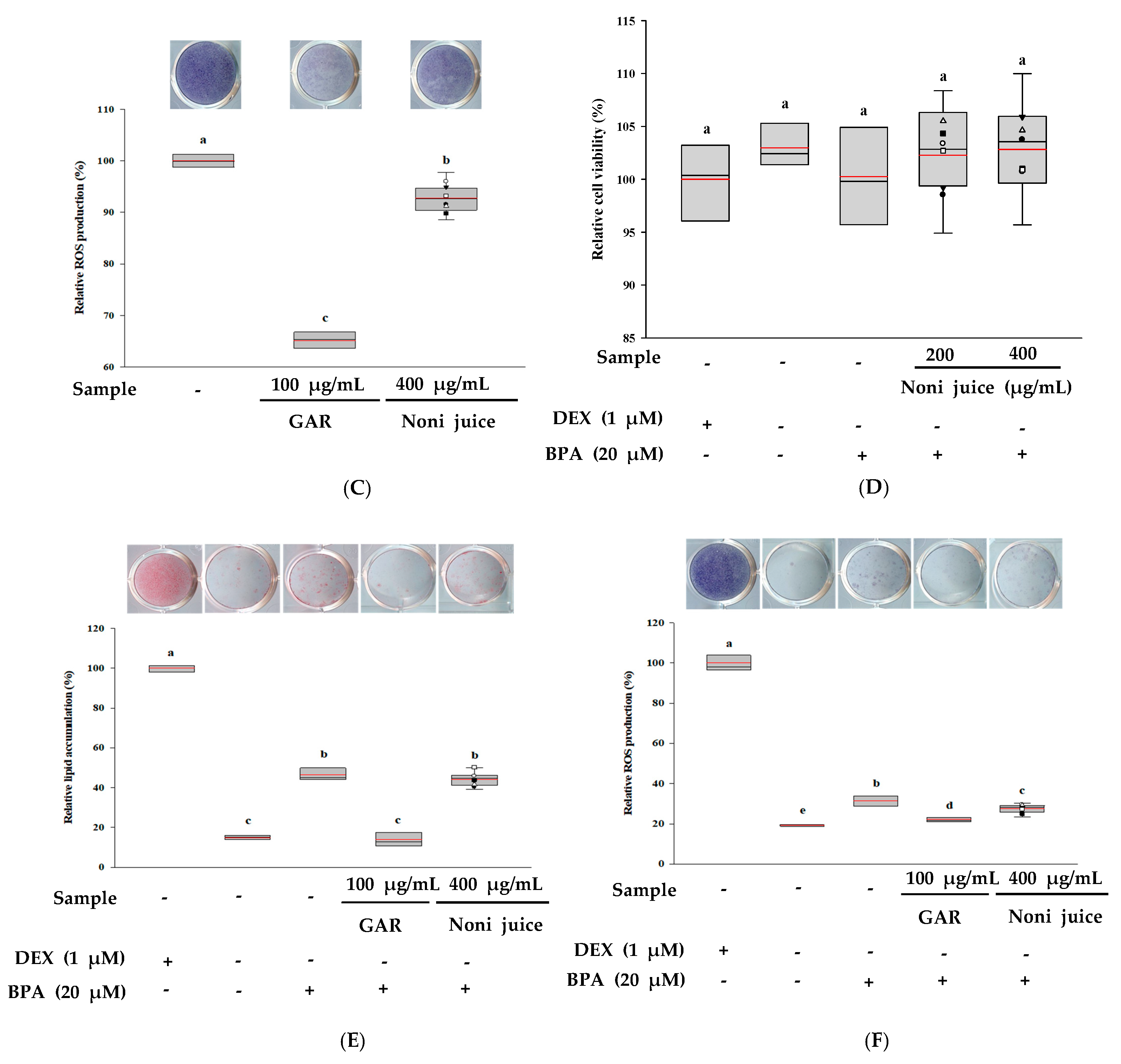

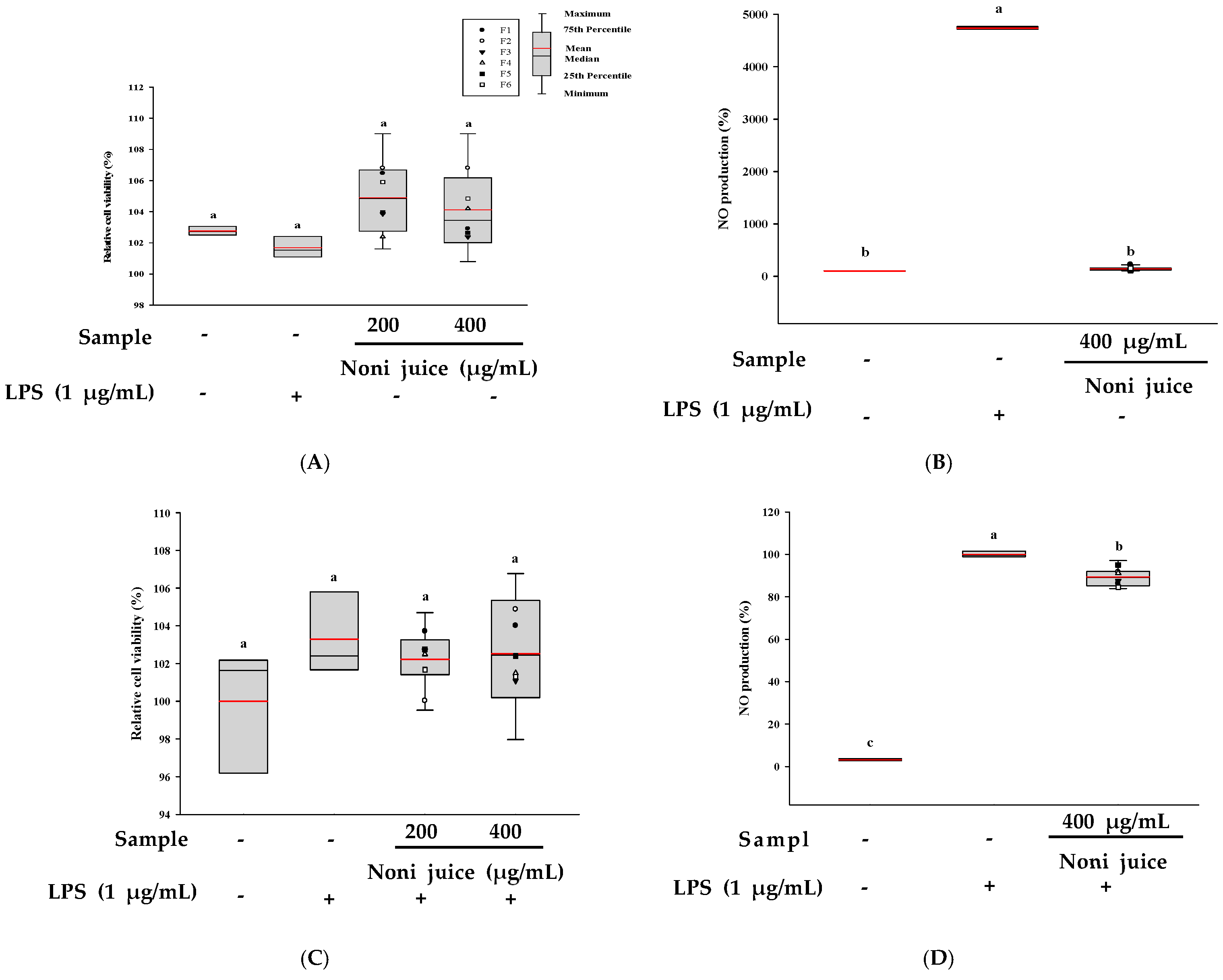
| Sample | Main Ingredients | Manufacturer 1 |
|---|---|---|
| F1 | Fermented and ripened noni juice. | NST Bio |
| F2 | Fermented and ripened noni juice, coconut sugar, calamondin juice. | NST Bio |
| F3 | Fermented and ripened noni juice, fructooligosaccharides, aronia juice concentrate, fermented noni concentrate, bilberry concentrate. | NST Bio |
| F4 | Fermented and ripened noni juice, fructooligosaccharides, seven berry concentrate mix, fermented noni concentrate. | NST Bio |
| F5 | Noni fruit puree, blueberry juice concentrate, grape juice concentrate. | Morinda |
| F6 | Noni fruit puree, cornelian cherry puree, cornelian cherry reconstituted juice, olive leaf extract. | Morinda |
| Noni Juice | °Brix (%) | pH | Lyophilization Yield (%) | Total Phenolic Content (mg GAE 1/g) | Total Flavonoid Content (mg QE 2/g) | Total Proanthocyanidin Content (mg CE 3/g) |
|---|---|---|---|---|---|---|
| F1 | 8.80 ± 0.08 e | 3.88 ± 0.01 b | 7.74 ± 0.05 d | 7.50 ± 0.01 b | 1.28 ± 0.04 c | 11.96 ± 0.34 b |
| F2 | 15.78 ± 0.09 c | 3.97 ± 0.01 a | 16.11 ± 0.04 b | 4.58 ± 0.07 f | 1.09 ± 0.03 d | 3.95 ± 0.54 d |
| F3 | 19.57 ± 0.19 b | 3.77 ± 0.01 c | 20.26 ± 0.29 a | 7.38 ± 0.02 c | 1.60 ± 0.08 b | 20.61 ± 0.33 a |
| F4 | 15.80 ± 0.00 c | 3.65 ± 0.00 d | 16.30 ± 0.41 b | 5.24 ± 0.01 d | 1.24 ± 0.06 c | 6.98 ± 0.05 c |
| F5 | 11.10 ± 0.00 d | 3.22 ± 0.00 f | 11.38 ± 0.23 c | 5.16 ± 0.01 e | 1.63 ± 0.05 b | 6.54 ± 0.05 c |
| F6 | 19.9 ± 0.00 a | 3.28 ± 0.01 e | 20.70 ± 0.36 a | 8.45 ± 0.03 a | 2.00 ± 0.06 a | 1.80 ± 0.18 e |
| Mean ± SD | 15.16 ± 4.08 | 3.63 ± 0.29 | 15.42 ± 4.62 | 6.39 ± 1.45 | 1.47 ± 0.31 | 8.64 ± 6.20 |
| Noni Juice | DPPH Radical Scavenging (%) | ABTS Radical Scavenging (%) | FRAP Activity (O.D. 593 nm) | Reducing Power (O.D. 700 nm) | SOD-Like Activity (Unit/mg) | ORAC Value (μM TE 1/mg) |
|---|---|---|---|---|---|---|
| F1 | 45.96 ± 0.61 c | 57.10 ± 0.10 c | 0.24 ± 0.00 c | 0.17 ± 0.00 b | 4.01 ± 0.01 d | 1.13 ± 0.06 c |
| F2 | 26.44 ± 0.26 f | 53.20 ± 0.76 e | 0.16 ± 0.00 f | 0.12 ± 0.00 e | 2.23 ± 0.03 e | 0.79 ± 0.00 d |
| F3 | 50.89 ± 0.07 b | 58.27 ± 0.52 b | 0.27 ± 0.00 b | 0.17 ± 0.00 b | 4.83 ± 0.07 b | 2.18 ± 0.13 a |
| F4 | 33.25 ± 0.41 e | 52.99 ± 0.38 e | 0.18 ± 0.00 e | 0.12 ± 0.00 d | 4.04 ± 0.05 d | 0.71 ± 0.09 d |
| F5 | 35.64 ± 1.76 d | 54.21 ± 0.18 d | 0.20 ± 0.00 d | 0.13 ± 0.00 c | 4.43 ± 0.01 c | 1.16 ± 0.02 c |
| F6 | 71.98 ± 0.42 a | 59.71 ± 0.10 a | 0.34 ± 0.00 a | 0.25 ± 0.00 a | 5.05 ± 0.04 a | 1.49 ± 0.08 b |
| Mean ± SD | 44.03 ± 14.88 | 55.91 ± 2.62 | 0.23 ± 0.62 | 0.16 ± 0.05 | 4.10 ± 0.92 | 1.24 ± 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Kim, M.-H.; Oh, G.; Im, J.-H.; Lim, J.-S.; Seong, Y.-S.; Lee, J.-Y.; Park, E.Y.; Lee, D.S.; La, I.-J.; et al. Analysis of the Bioactive Compounds and Physiological Activities of Commonly Consumed Noni Juice in Republic of Korea. Foods 2025, 14, 3732. https://doi.org/10.3390/foods14213732
Fu X, Kim M-H, Oh G, Im J-H, Lim J-S, Seong Y-S, Lee J-Y, Park EY, Lee DS, La I-J, et al. Analysis of the Bioactive Compounds and Physiological Activities of Commonly Consumed Noni Juice in Republic of Korea. Foods. 2025; 14(21):3732. https://doi.org/10.3390/foods14213732
Chicago/Turabian StyleFu, Xiaolu, Min-Hye Kim, Geon Oh, Ji-Hyun Im, June-Seok Lim, Yeon-Seok Seong, Jae-Yeon Lee, Eun Young Park, Do Sang Lee, Im-Joung La, and et al. 2025. "Analysis of the Bioactive Compounds and Physiological Activities of Commonly Consumed Noni Juice in Republic of Korea" Foods 14, no. 21: 3732. https://doi.org/10.3390/foods14213732
APA StyleFu, X., Kim, M.-H., Oh, G., Im, J.-H., Lim, J.-S., Seong, Y.-S., Lee, J.-Y., Park, E. Y., Lee, D. S., La, I.-J., & Lee, O.-H. (2025). Analysis of the Bioactive Compounds and Physiological Activities of Commonly Consumed Noni Juice in Republic of Korea. Foods, 14(21), 3732. https://doi.org/10.3390/foods14213732







