Effect of Polyphenols Extracted from Rosa roxburghii Tartt Pomace with Different Particle Sizes on Quality and Biological Activity of Noodles: A View of Molecular Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
2.2. The Preparation of Rosa roxburghii Pomace with Different Particle Sizes
2.3. The Preparation of Phenolic Extract
2.4. The Preparation of Noodles
2.5. Total Phenolic Content (TPC)
2.6. HPLC Analysis of Phenolic Components
2.7. Color
2.8. Texture
2.9. Boiling Properties
2.10. Antioxidant Activity
2.11. Starch Digestion
2.11.1. Total Starch Content
2.11.2. The Simulated Digestion In Vitro
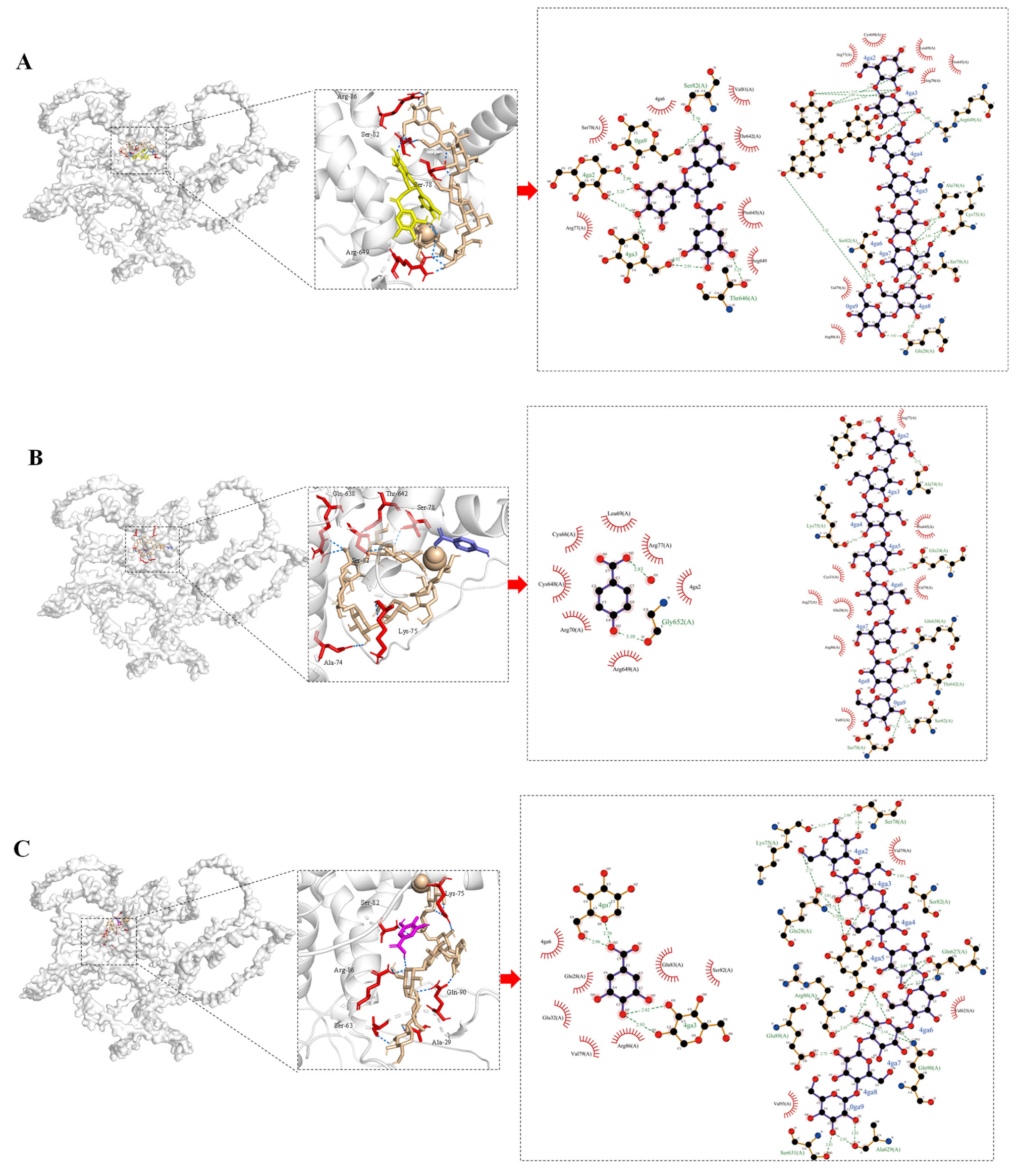
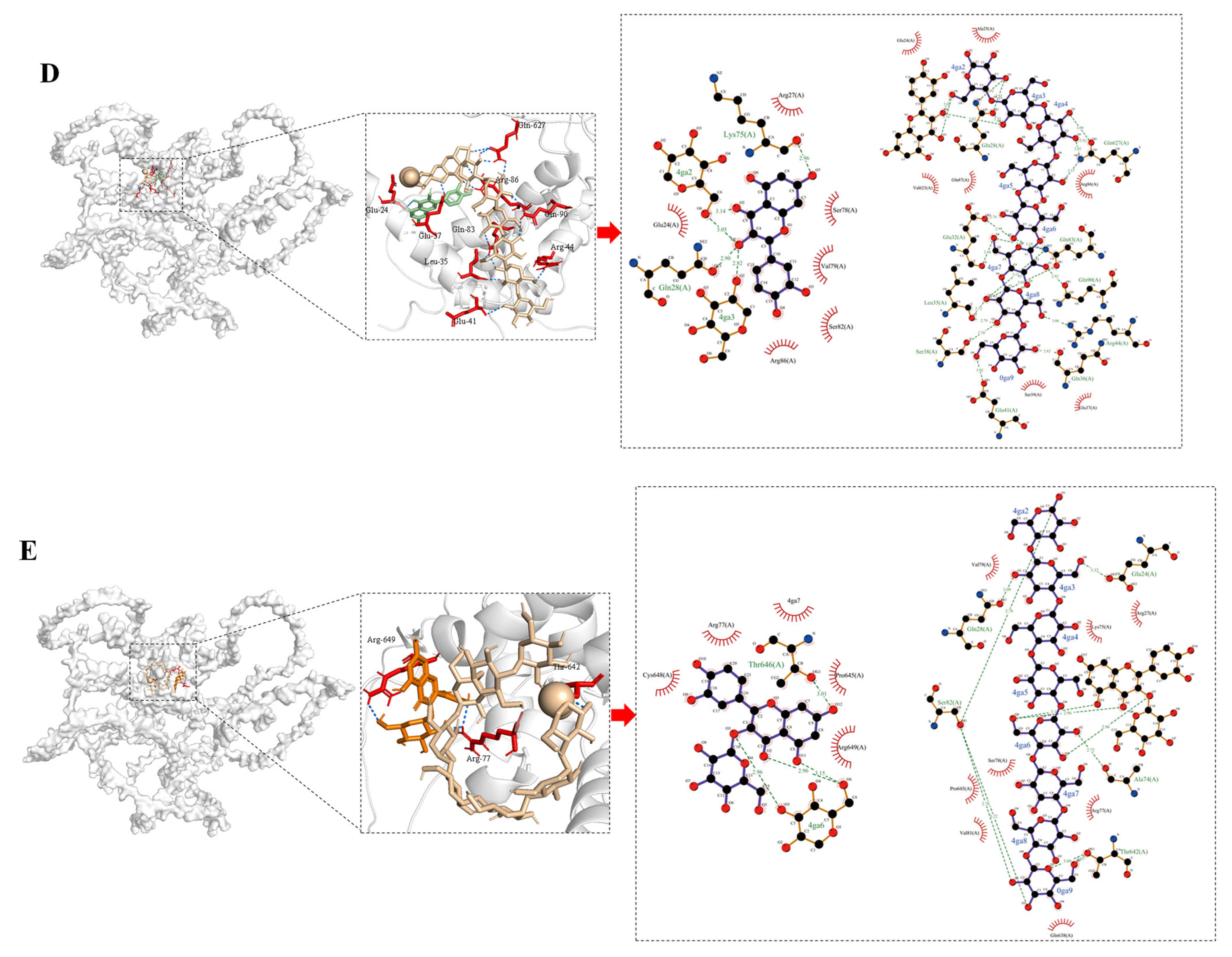
2.12. Molecular Docking
2.13. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Content (TPC) of Noodles
3.1.1. TPC of the Fresh Noodles
3.1.2. TPC of the Boiled Noodles
3.2. Individual Phenolic Content of Noodles
3.3. Color of Noodles
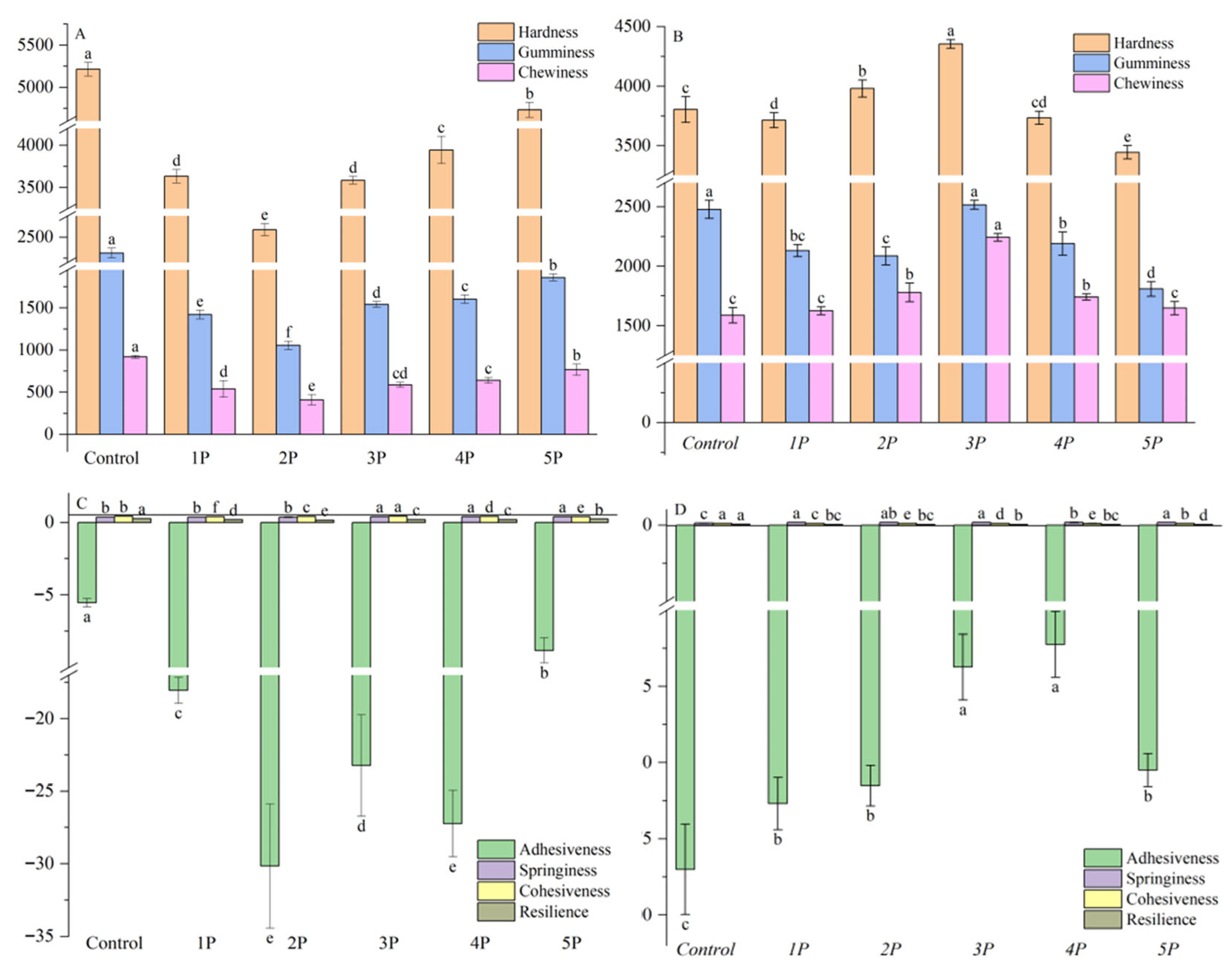
3.4. Texture Profile
3.5. Cooking Properties
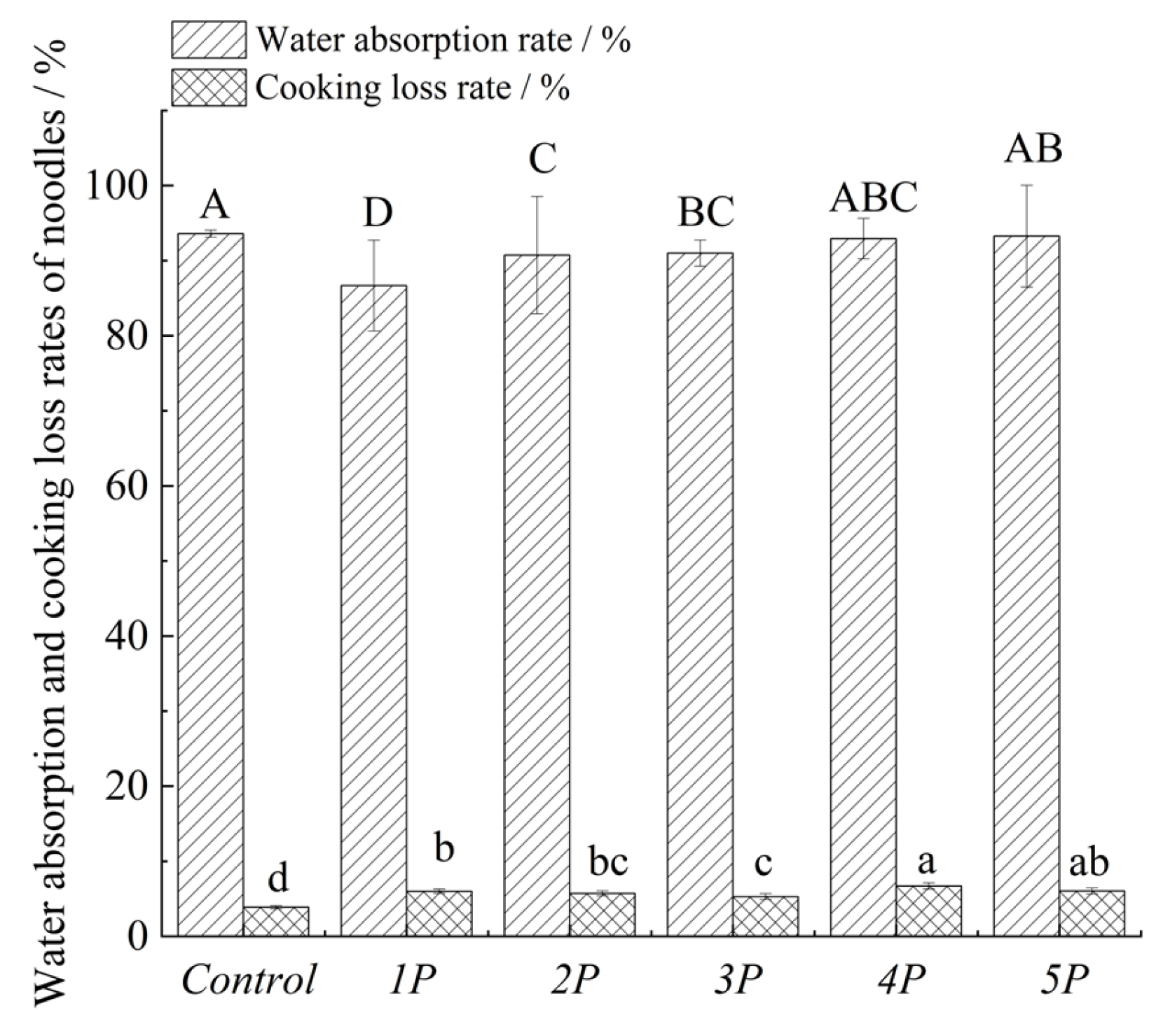
3.5.1. Water Absorption Rate
3.5.2. Cooking Loss (CL)
3.6. Antioxidant Ability
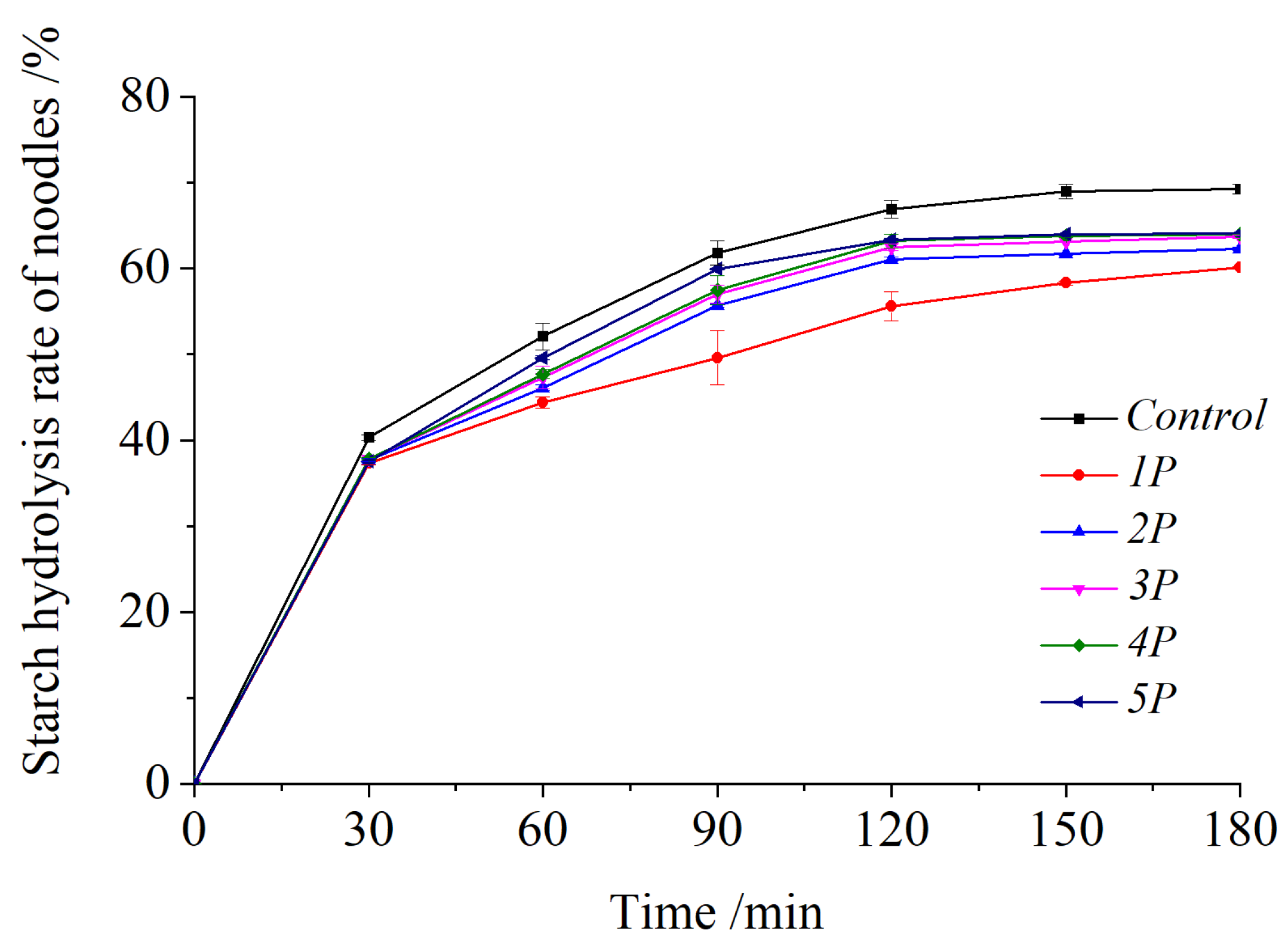
3.7. Starch Digestion Rate
3.8. Correlation Analysis
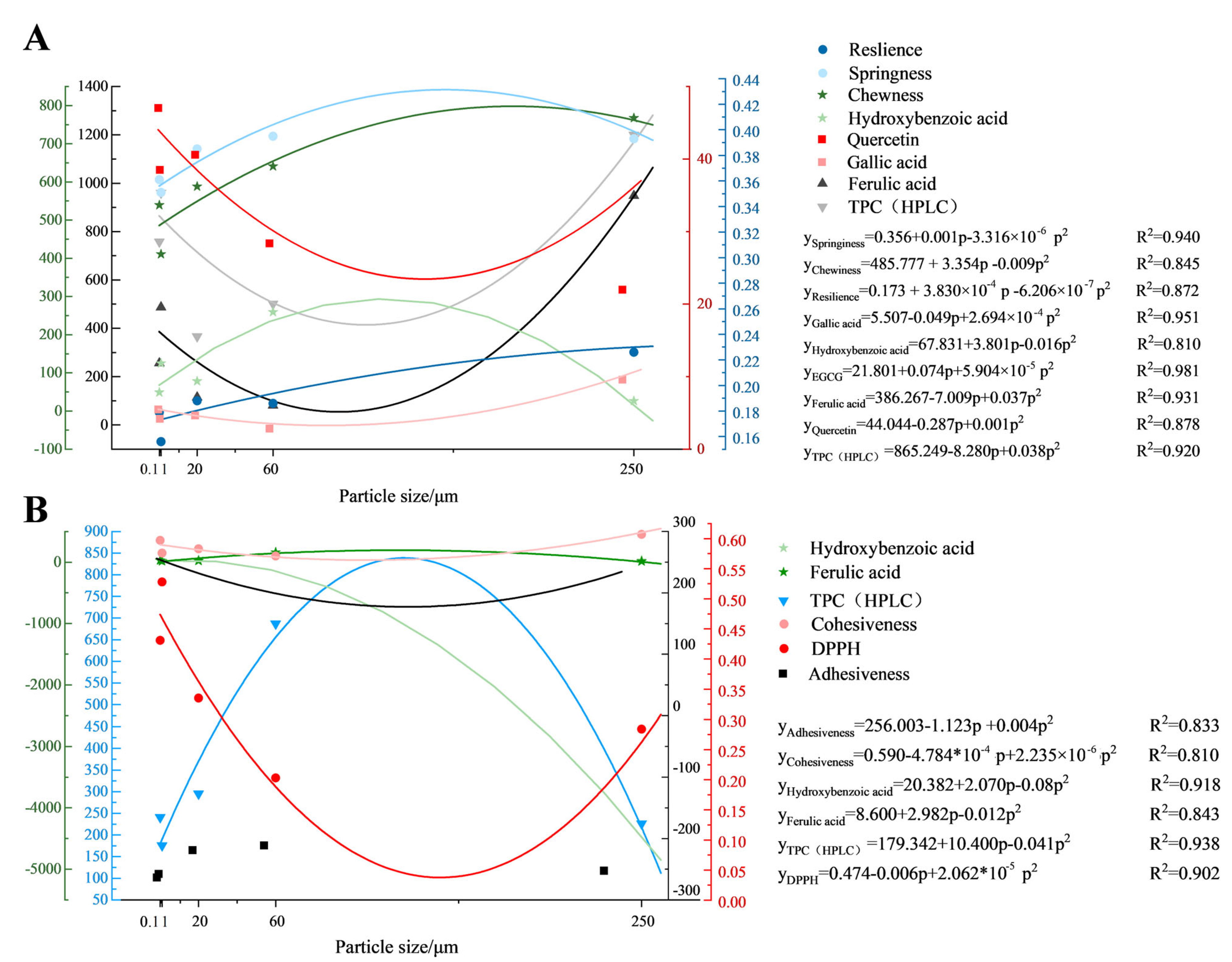
3.9. Modeling Quality Parameter Dynamics in Noodles via Mathematical Fitting
3.10. Molecular Docking Analysis of Interactions Between Phenolic Compounds and Glutenin/Starch
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, A.; Sarsaiya, S.; Gong, Q.; Wu, Q.; Shi, J. Chemical diversity, traditional uses, and bioactivities of Rosa roxburghii Tratt: A comprehensive review. Pharmacol. Ther. 2024, 259, 108657. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Li, C.; Chen, Q.; Xie, X.; Fu, X.; Chen, C.; Huang, Q.; Huang, Z.; Dong, H. Identification of polyphenols from Rosa roxburghii Tratt pomace and evaluation of in vitro and in vivo antioxidant activity. Food Chem. 2022, 377, 131922. [Google Scholar] [CrossRef]
- Zhou, X.; Qin, Y.; Wang, Y.; Wang, Y.; Qin, Z. Phytochemical profile and antioxidant characteristics of bound and free phenolics from Rosa roxburghii Tratt. Food Biosci. 2024, 57, 103576. [Google Scholar] [CrossRef]
- Zhou, D.; Zhong, J.; Huang, Y.; Cheng, Y. Effect of free and bound polyphenols from Rosa roxburghii Tratt distiller’s grains on moderating fecal microbiota. Food Chem. X 2023, 19, 100747. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Tang, L.; Zhang, M.; Wang, P.; Sun, X.; Shang, L.; Wang, Q.; Zhao, Y.; Meng, D.; et al. Analysis of the effects of Rosa roxburghii Tratt fruit polyphenols on immune function in mice through gut microbiota and metabolomics: An in vivo preclinical trial study. J. Funct. Foods 2023, 102, 105464. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Zhang, D.; Farha, A.K.; Yang, X.; Li, H.-B.; Kong, K.-W.; Zhang, J.-R.; Chan, C.-L.; Lu, W.-Y.; Corke, H.; et al. Phytochemicals, essential oils, and bioactivities of an underutilized wild fruit Cili (Rosa roxburghii). Ind. Crops Prod. 2020, 143, 111928. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Li, H.; Deng, Z.; Tsao, R. A review on insoluble-bound phenolics in plant-based food matrix and their contribution to human health with future perspectives. Trends Food Sci. Technol. 2020, 105, 347–362. [Google Scholar] [CrossRef]
- Su, J.; Fu, X.; Huang, Q.; Liu, G.; Li, C. Phytochemical profile, bioactivity and prebiotic potential of bound polyphenols released from Rosa roxburghii fruit pomace dietary fiber during in vitro digestion and fermentation. Food Funct. 2022, 13, 8880–8891. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, M.; Bhandari, B.; Yang, Z.X. Micronization and nanosizing of particles for an enhanced quality of food: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Jin, X.; Gao, J.; Qiu, Z.; Ying, J.; Wang, Y.; Dong, Z.; Zhou, W. Impact of wheat bran micronization on dough properties and bread quality: Part I—Bran functionality and dough properties. Food Chem. 2021, 353, 129407. [Google Scholar] [CrossRef]
- Brewer, L.R.; Kubola, J.; Siriamornpun, S.; Herald, T.J.; Shi, Y.-C. Wheat bran particle size influence on phytochemical extractability and antioxidant properties. Food Chem. 2014, 152, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Noort, M.W.J.; van Haaster, D.; Hemery, Y.; Schols, H.A.; Hamer, R.J. The effect of particle size of wheat bran fractions on bread quality—Evidence for fibre–protein interactions. J. Cereal Sci. 2010, 52, 59–64. [Google Scholar] [CrossRef]
- Du, B.; Meenu, M.; Xu, B. Insights into Improvement of Physiochemical and Biological Properties of Dietary Fibers from Different Sources via Micron Technology. Food Rev. Int. 2020, 36, 367–383. [Google Scholar] [CrossRef]
- Jakobek, L.; Matic, P. Non-covalent dietary fiber—Polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Ngo, T.V.; Kusumawardani, S.; Kunyanee, K.; Luangsakul, N. Polyphenol-Modified Starches and Their Applications in the Food Industry: Recent Updates and Future Directions. Foods 2022, 11, 3384. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.; Xu, H.; Zhou, Q.; He, J.; Zhu, B.; Li, S.; Wang, M. A review of green methods used in starch–polyphenol interactions: Physicochemical and digestion aspects. Food Funct. 2023, 14, 8071–8100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Huang, J.; Wang, D.; Wan, X.; Wang, Y. Covalent polyphenols-proteins interactions in food processing: Formation mechanisms, quantification methods, bioactive effects, and applications. Front. Nutr. 2024, 11, 1371401. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Y.; Wang, A.; Wang, J.; Wu, X.; Wu, Y.; Fu, Y.; Sun, H. Insights into interactions between food polyphenols and proteins: An updated overview. J. Food Process. Preserv. 2022, 46, e16597. [Google Scholar] [CrossRef]
- Bindes, M.M.M.; Cardoso, V.L.; Reis, M.H.M.; Boffito, D.C. Maximisation of the polyphenols extraction yield from green tea leaves and sequential clarification. J. Food Eng. 2019, 241, 97–104. [Google Scholar] [CrossRef]
- Chen, Q.; Su, J.; Zhang, Y.; Li, C.; Zhu, S. Phytochemical Profile and Bioactivity of Bound Polyphenols Released from Rosa roxburghii Fruit Pomace Dietary Fiber by Solid-State Fermentation with Aspergillus niger. Molecules 2024, 29, 1689. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Han, L.; Shi, B. Microwave-assisted extraction of flavonoids from Radix astragali. Sep. Purif. Technol. 2008, 62, 614–618. [Google Scholar] [CrossRef]
- Zhao, Q.; Tian, H.; Chen, L.; Zeng, M.; Qin, F.; Wang, Z.; He, Z.; Chen, J. Interactions between soluble soybean polysaccharide and starch during the gelatinization and retrogradation: Effects of selected starch varieties. Food Hydrocoll. 2021, 118, 106765. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Deng, N.; Deng, Z.; Tang, C.; Liu, C.; Luo, S.; Chen, T.; Hu, X. Formation, structure and properties of the starch-polyphenol inclusion complex: A review. Trends Food Sci. Technol. 2021, 112, 667–675. [Google Scholar] [CrossRef]
- Adom, K.K.; Sorrells, M.E.; Liu, R.H. Phytochemical Profiles and Antioxidant Activity of Wheat Varieties. J. Agric. Food Chem. 2003, 51, 7825–7834. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant Activity of Grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Ojukwu, M.; Tan, H.L.; Murad, M.; Nafchi, A.M.; Easa, A.M. Improvement of cooking and textural properties of rice flour-soy protein isolate noodles stabilised with microbial transglutaminase and glucono-δ-lactone and dried using superheated steam. Food Sci. Technol. Int. 2022, 29, 799–808. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Guan, E.; Zhang, Y.; Wang, X. Physicochemical properties of wheat granular flour and quality characteristics of the corresponding fresh noodles as affected by particle size. LWT 2024, 204, 116439. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, W.; Zhang, Z.; Shen, S.; Lu, G.; Wu, W. Effect of Maltodextrin on the Physicochemical Properties and Cooking Performance of Sweet Potato Starch Noodles. Foods 2022, 11, 4082. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, J.; Li, K.; Bai, J.; Zhang, X.; Lu, Y.; Sun, X.; Li, W. The Rheological Performance and Structure of Wheat/Acorn Composite Dough and the Quality and In Vitro Digestibility of Its Noodles. Foods 2021, 10, 2727. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Trujillo, N.; Monribot-Villanueva, J.L.; Alvarado-Olivarez, M.; Luna-Solano, G.; Guerrero-Analco, J.A.; Jiménez-Fernández, M. Phenolic profile and antioxidative properties of pulp and seeds of Randia monantha Benth. Ind. Crops Prod. 2018, 124, 53–58. [Google Scholar] [CrossRef]
- GB 5009.9-2016; National Food Safety Standard—Determination of Moisture in Food. Standardization Administration of the People’s Republic of China: Beijing, China, 2016.
- Sharavathy, M.K.; Urooj, A.; Puttaraj, S. Nutritionally important starch fractions in cereal based Indian food preparations. Food Chem. 2001, 75, 241–247. [Google Scholar] [CrossRef]
- Bi, S.; Pan, X.; Zhang, W.; Ma, Z.; Lao, F.; Shen, Q.; Wu, J. Non-covalent interactions of selected flavors with pea protein: Role of molecular structure of flavor compounds. Food Chem. 2022, 389, 133044. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Sefrin Speroni, C.; Rigo Guerra, D.; Beutinger Bender, A.B.; Stiebe, J.; Ballus, C.A.; Picolli da Silva, L.; Lozano-Sánchez, J.; Emanuelli, T. Micronization increases the bioaccessibility of polyphenols from granulometrically separated olive pomace fractions. Food Chem. 2021, 344, 128689. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, D.; Liu, Y.; Zha, L.; Chen, D.; Guo, H. Physicochemical properties and anthocyanin bioaccessibility of downy rose-myrtle powder prepared by superfine grinding. Int. J. Food Prop. 2019, 22, 2022–2032. [Google Scholar] [CrossRef]
- Verardo, V.; Arráez-Román, D.; Segura-Carretero, A.; Marconi, E.; Fernández-Gutiérrez, A.; Caboni, M.F. Determination of Free and Bound Phenolic Compounds in Buckwheat Spaghetti by RP-HPLC-ESI-TOF-MS: Effect of Thermal Processing from Farm to Fork. J. Agric. Food Chem. 2011, 59, 7700–7707. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Lucini, L.; Chiodelli, G.; Giuberti, G.; Montesano, D.; Masoero, F.; Trevisan, M. Impact of boiling on free and bound phenolic profile and antioxidant activity of commercial gluten-free pasta. Food Res. Int. 2017, 100, 69–77. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, S.; Lin, H.; Chen, L.; Qin, S.; Wu, W.; Zheng, B.; Guo, Z. Physicochemical properties and digestion of the lotus seed starch-green tea polyphenol complex under ultrasound-microwave synergistic interaction. Ultrason. Sonochem. 2019, 52, 50–61. [Google Scholar] [CrossRef]
- Li, C.; Chen, L.; McClements, D.J.; Peng, X.; Xu, Z.; Meng, M.; Ji, H.; Qiu, C.; Long, J.; Jin, Z. Encapsulation of polyphenols in protein-based nanoparticles: Preparation, properties, and applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 11341–11355. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Gawlik-Dziki, U.; Świeca, M. Influence of Phenolic-Food Matrix Interactions on In Vitro Bioaccessibility of Selected Phenolic Compounds and Nutrients Digestibility in Fortified White Bean Paste. Antioxidants 2021, 10, 1825. [Google Scholar] [CrossRef]
- Tang, P.Q.; Zhang, S.Y.; Meng, L.H.; Wang, Z.J.; Yang, Y.L.; Shen, X.C.; Tang, X.Z. Effects of different content of EGCG or caffeic acid addition on the structure, cooking, antioxidant characteristics and in vitro starch digestibility of extruded buckwheat noodles. Int. J. Biol. Macromol. 2023, 252, 10. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef] [PubMed]
- Talcott, S.T.; Brenes, C.H.; Pires, D.M.; Del Pozo-Insfran, D. Phytochemical Stability and Color Retention of Copigmented and Processed Muscadine Grape Juice. J. Agric. Food Chem. 2003, 51, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Lee, M.-J.; Hou, Z.; Ho, C.-T.; Yang, C.S. Stability of Tea Polyphenol (−)-Epigallocatechin-3-gallate and Formation of Dimers and Epimers under Common Experimental Conditions. J. Agric. Food Chem. 2005, 53, 9478–9484. [Google Scholar] [CrossRef]
- Wang, L.-F.; Kim, D.-M.; Lee, C.Y. Effects of Heat Processing and Storage on Flavanols and Sensory Qualities of Green Tea Beverage. J. Agric. Food Chem. 2000, 48, 4227–4232. [Google Scholar] [CrossRef]
- Miyazawa, T.; Nakagawa, K. Structure-related Emission Spectrometric Analysis of the Chemiluminescence of Catechins, Theaflavins and Anthocyanins. Biosci. Biotechnol. Biochem. 1998, 62, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Sugiura, K.; Kawahara, R.; Fujita, T.; Making, M.; Kamiya, M.; Tsuyumu, S. Formation of Radicals and Chemiluminescence during the Autoxidation of Tea Catechins. Agric. Biol. Chem. 1991, 55, 2717–2723. [Google Scholar] [CrossRef][Green Version]
- Benitez, F.J.; Real, F.J.; Acero, J.L.; Leal, A.I.; Garcia, C. Gallic acid degradation in aqueous solutions by UV/H2O2 treatment, Fenton’s reagent and the photo-Fenton system. J. Hazard. Mater. 2005, 126, 31–39. [Google Scholar] [CrossRef]
- Poojary, M.M.; Zhang, W.; Olesen, S.B.; Rauh, V.; Lund, M.N. Green Tea Extract Decreases Arg-Derived Advanced Glycation Endproducts but Not Lys-Derived AGEs in UHT Milk during 1-Year Storage. J. Agric. Food Chem. 2020, 68, 14261–14273. [Google Scholar] [CrossRef]
- Xu, L.; Song, X.; Yao, D.; Wang, C.; Yao, X.; Li, Z. Dynamic migration of phenolics in microwaved combined cooked sorghum: Focus on the polyphenols interact with starch/protein. Food Chem. X 2025, 27, 102342. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Ren, F.; Zhu, X.; Han, Z.; Jia, Y.; Liu, X.; Chen, B.; Liu, H. The interaction between starch and phenolic acids: Effects on starch physicochemical properties, digestibility and phenolic acids stability. Food Funct. 2025, 16, 4202–4225. [Google Scholar] [CrossRef]
- Xu, J.; Bock, J.E.; Stone, D. Quality and textural analysis of noodles enriched with apple pomace. J. Food Process. Preserv. 2020, 44, e14579. [Google Scholar] [CrossRef]
- Yu, K.; Huang, X.; He, W.; Wu, D.; Du, C. Kinetics of polyphenol losses during cooking of dried green tea noodles as influenced by microwave treatment of dough. LWT 2023, 180, 114675. [Google Scholar] [CrossRef]
- Fuerst, E.P.; Anderson, J.V.; Morris, C.F. Delineating the role of polyphenol oxidase in the darkening of alkaline wheat noodles. J. Agric. Food Chem. 2006, 54, 2378–2384. [Google Scholar] [CrossRef]
- Ma, Y.; Hong, T.T.; Xu, D.; Wu, F.F.; Xu, X.M. Inhibition of PPO-related browning in fresh noodles: A combination of chemical and heat treatment. Food Chem. 2023, 404, 9. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.H.; Sosulski, F.W.; Tyler, R.T. Wet-milling, composition and functional properties of starch and protein isolated from buckwheat groats. Food Res. Int. 1997, 30, 493–502. [Google Scholar] [CrossRef]
- Morris, C.F. Determinants of wheat noodle color. J. Sci. Food Agric. 2018, 98, 5171–5180. [Google Scholar] [CrossRef]
- Yao, M.; Li, M.; Dhital, S.; Tian, Y.; Guo, B. Texture and digestion of noodles with varied gluten contents and cooking time: The view from protein matrix and inner structure. Food Chem. 2020, 315, 126230. [Google Scholar] [CrossRef]
- Girard, A.L.; Awika, J.M. Effects of edible plant polyphenols on gluten protein functionality and potential applications of polyphenol–gluten interactions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2164–2199. [Google Scholar] [CrossRef] [PubMed]
- Han, C.W.; Ma, M.; Zhang, H.H.; Li, M.; Sun, Q.J. Progressive study of the effect of superfine green tea, soluble tea, and tea polyphenols on the physico-chemical and structural properties of wheat gluten in noodle system. Food Chem. 2020, 308, 125676. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.W.; Tang, N.; Zhao, B.; Zhang, Y.Q.; Guo, B.L.; Wei, Y.M. Study on the water state, mobility and textural property of Chinese noodles during boiling. Int. J. Food Sci. Technol. 2020, 55, 1716–1724. [Google Scholar] [CrossRef]
- Mariana, S.; Federico, L.; Ruiz, A.L.T.G. Nanocomplex formation between β-lactoglobulin or caseinomacropeptide and green tea polyphenols: Impact on protein gelation and polyphenols antiproliferative activity. J. Funct. Foods 2012, 4, 800–809. [Google Scholar] [CrossRef]
- Han, L.; Peng, X.; Cheng, Y.; Zhu, Y.; Huang, Y.; Zhang, S.; Qi, B. Effects of catechin types found in tea polyphenols on the structural and functional properties of soybean protein isolate–catechin covalent complexes. LWT 2023, 173, 114336. [Google Scholar] [CrossRef]
- Mei, Z.; Wang, W.; Feng, X.; Liu, M.; Peng, S.; Chen, L.; Chen, H.; Lin, S. Effect of soluble oat β-glucan and tea polyphenols on the rheological properties and microstructure of wheat dough. LWT 2024, 198, 116004. [Google Scholar] [CrossRef]
- Lv, Y.; Li, M.; Pan, J.; Zhang, S.; Jiang, Y.; Liu, J.; Zhu, Y.; Zhang, H. Interactions between tea products and wheat starch during retrogradation. Food Biosci. 2020, 34, 100523. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Jia, Y.; Zhang, H.; Ren, F. Formation and Application of Starch-Polyphenol Complexes: Influencing Factors and Rapid Screening Based on Chemometrics. Foods 2024, 13, 1557. [Google Scholar] [CrossRef]
- Xue, H.; Feng, J.; Tang, Y.; Wang, X.; Tang, J.; Cai, X.; Zhong, H. Research progress on the interaction of the polyphenol–protein–polysaccharide ternary systems. Chem. Biol. Technol. Agric. 2024, 11, 95. [Google Scholar] [CrossRef]
- Chen, N.; Gao, H.X.; He, Q.; Yu, Z.L.; Zeng, W.C. Interaction and action mechanism of starch with different phenolic compounds. Int. J. Food Sci. Nutr. 2020, 71, 726–737. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Rabalski, I. Changes in Phenolic Acids and Antioxidant Properties during Baking of Bread and Muffin Made from Blends of Hairless Canary Seed, Wheat, and Corn. Antioxidants 2022, 11, 1059. [Google Scholar] [CrossRef] [PubMed]
- Grussu, D.; Stewart, D.; McDougall, G.J. Berry Polyphenols Inhibit α-Amylase in Vitro: Identifying Active Components in Rowanberry and Raspberry. J. Agric. Food Chem. 2011, 59, 2324–2331. [Google Scholar] [CrossRef]
- Barros, F.; Awika, J.M.; Rooney, L.W. Interaction of Tannins and Other Sorghum Phenolic Compounds with Starch and Effects on in Vitro Starch Digestibility. J. Agric. Food Chem. 2012, 60, 11609–11617. [Google Scholar] [CrossRef]
- Sun, L.; Song, Y.; Chen, Y.; Ma, Y.; Fu, M.; Liu, X. The galloyl moiety enhances the inhibitory activity of catechins and theaflavins against α-glucosidase by increasing the polyphenol-enzyme binding interactions. Food Funct. 2021, 12, 215–229. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.; Sineiro, J.; Rosell, C. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2021, 372, 131231. [Google Scholar] [CrossRef] [PubMed]
- Kandil, A.; Li, J.; Vasanthan, T.; Bressler, D.C. Phenolic Acids in Some Cereal Grains and Their Inhibitory Effect on Starch Liquefaction and Saccharification. J. Agric. Food Chem. 2012, 60, 8444–8449. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.W.M.; Zhang, G. Interaction between amylose and tea polyphenols modulates the postprandial glycemic response to high-amylose maize starch. J. Agric. Food Chem. 2013, 61, 8608–8615. [Google Scholar] [CrossRef]
- Bordenave, N.H.B.R.; Ferruzzi, M.G. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014, 5, 18–34. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Lucini, L. Interactions between phenolic compounds, amylolytic enzymes and starch: An updated overview. Curr. Opin. Food Sci. 2020, 31, 102–113. [Google Scholar] [CrossRef]
- Mitra, S.; Tareq, A.M.; Das, R.; Emran, T.B.; Nainu, F.; Chakraborty, A.J.; Ahmad, I.; Tallei, T.E.; Idris, A.M.; Simal-Gandara, J. Polyphenols: A first evidence in the synergism and bioactivities. Food Rev. Int. 2023, 39, 4419–4441. [Google Scholar] [CrossRef]
- Xin, D.; Xinyue, Z.; Fang, W.; Qin, L.; Qing, X.; Yingqiong, Z.; Yuxiao, L. Effects of in vitro simulated digestion on antioxidant components and activities of walnut shell and distraction wood. Food Mach. 2024, 40, 22. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, S.; Meng, D.; Zhang, J.; Lai, C.; Zhang, M.; Sun, X.; Yu, H.; Wang, P.; Gao, X. Optimization of Rosa roxburghii Tratt pomace fermentation process and the effects of mono- and mixed culture fermentation on its chemical composition. Front. Nutr. 2024, 11, 1494678. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, J.; Bao, M.-F.; Yang, L.; Cai, Z.-L.; Schinnerl, J.; Cai, X.-H. Fruits from Rosa roxburghii: A Valuable Bioresource of Potent Radical Scavengers and Novel Ursane-Type Triterpenoids. ACS Omega 2024, 9, 38023–38031. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Wang, P.; Zhao, Y.; Zhu, Y.; Xiao, X. The Effect of Protein-Starch Interaction on the Structure and Properties of Starch, and Its Application in Flour Products. Foods 2025, 14, 778. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Y.; Yang, Y.; Zhao, J.; Zhang, Y.; Li, L.; Wang, Q.; Ming, J. Interaction between wheat gliadin and quercetin under different pH conditions analyzed by multi-spectroscopy methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117937. [Google Scholar] [CrossRef]
- Shahidi, F.; Dissanayaka, C.S. Phenolic-protein interactions: Insight from in-silico analyses—A review. Food Prod. Process. Nutr. 2023, 5, 2. [Google Scholar] [CrossRef]
- Mao, S.; Ren, Y.; Ye, X.; Kong, X.; Tian, J. Regulating the physicochemical, structural characteristics and digestibility of potato starch by complexing with different phenolic acids. Int. J. Biol. Macromol. 2023, 253, 127474. [Google Scholar] [CrossRef] [PubMed]
- Kurczab, R.; Śliwa, P.; Rataj, K.; Kafel, R.; Bojarski, A.J. Salt Bridge in Ligand–Protein Complexes—Systematic Theoretical and Statistical Investigations. J. Chem. Inf. Model. 2018, 58, 2224–2238. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Woo, S.M.; Siu, T.; Cortopassi, W.A.; Duarte, F.; Paton, R.S. Cation–π interactions in protein–ligand binding: Theory and data-mining reveal different roles for lysine and arginine. Chem. Sci. 2018, 9, 2655–2665. [Google Scholar] [CrossRef]
- Yu, M.; Zhu, S.; Zhong, F.; Zhang, S.; Du, C.; Li, Y. Insight into the multi-scale structure changes and mechanism of corn starch modulated by different structural phenolic acids during retrogradation. Food Hydrocoll. 2022, 128, 107581. [Google Scholar] [CrossRef]
- Wei, X.; Xie, H.; Hu, Z.; Zeng, X.; Dong, H.; Liu, X.; Bai, W. Multiscale structure changes and mechanism of polyphenol-amylose complexes modulated by polyphenolic structures. Int. J. Biol. Macromol. 2024, 262, 130086. [Google Scholar] [CrossRef]
- He, W.-J.; Chen, N.; Yu, Z.-L.; Sun, Q.; He, Q.; Zeng, W.-C. Gliadin interacted with tea polyphenols: Potential application and action mechanism. Int. J. Food Sci. Nutr. 2022, 73, 786–799. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Duan, W.; Zhang, J.; Huang, Y.; Zhang, Y.; Sun, B. Characterization and molecular docking study of taste peptides from chicken soup by sensory analysis combined with nano-LC-Q-TOF-MS/MS. Food Chem. 2022, 383, 132455. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhang, X.; Zhang, Z.; Cai, F.; Yu, H.; Lin, D.; Liu, H.; Zhao, Q. Isolation and characterization of novel umami peptides from bay scallop (Argopecten irradians) and molecular docking to the T1R1/T1R3 taste receptor. LWT 2024, 207, 116645. [Google Scholar] [CrossRef]





| Noodle Sample Label | Descriptions | |
|---|---|---|
| Fresh | Boiled | |
| 0 | 0 | Without phenolic extract addition |
| 1P | 1P | Added phenolic extract from pomace (particle size: 0.1–1 μm) |
| 2P | 2P | Added phenolic extract from pomace (particle size: 1–10 μm) |
| 3P | 3P | Added phenolic extract from pomace (particle size: 20–40 μm) |
| 4P | 4P | Added phenolic extract from pomace (particle size: 60–80 μm) |
| 5P | 5P | Added phenolic extract from pomace (particle size: 250 μm) |
| Samples | Total Polyphenols Content (mg GAE/g DW) | Retention Rate (%) | |
|---|---|---|---|
| Fresh | Boiled | ||
| 1P/1P | 6.34 ± 0.15 b | 1.06 ± 0.06 a | 16.72 ± 1.09 a |
| 2P/2P | 7.87 ± 0.16 a | 0.67 ± 0.05 bc | 8.51 ± 0.45 e |
| 3P/3P | 5.80 ± 0.17 c | 0.70 ± 0.06 b | 12.07 ± 1.29 b |
| 4P/4P | 5.53 ± 0.05 d | 0.64 ± 0.07 c | 11.03 ± 0.76 d |
| 5P/5P | 4.77 ± 0.07 e | 0.55 ± 0.03 d | 11.50 ± 0.40 c |
| Phenols | Status | Control/ Control | 1P/1P | 2P/2P | 3P/3P | 4P/4P | 5P/5P |
|---|---|---|---|---|---|---|---|
| GA | F | -- | 5.48 ± 1.52 b | 4.19 ± 2.31 bc | 4.64 ± 0.60 bc | 2.84 ± 0.46 c | 9.58 ± 0.67 a |
| B | -- | 8.12 ± 3.73 a | 4.47 ± 2.01 b | 4.47 ± 0.23 b | 6.87 ± 1.24 ab | 5.06 ± 0.23 ab | |
| HBA | F | -- | 48.66 ± 0.35 d | 124.79 ± 33.74 b | 78.26 ± 6.03 c | 259.13 ± 0.75 a | 26.35 ± 0.24 d |
| B | -- | 27.35 ± 9.29 b | 28.40 ± 5.39 b | 38.04 ± 4.24 b | 123.54 ± 2.01 a | 30.82 ± 7.28 b | |
| EGCG | F | -- | 23.61 ± 3.59 bc | 19.98 ± 1.72 c | 27.70 ± 0.77 b | -- | 44.10 ± 2.22 a |
| B | -- | -- | -- | 35.75 ± 0.43 a | -- | 35.19 ± 1.31 a | |
| EC | F | -- | 211.67 ± 16.00 a | 55.85 ± 21.03 c | 4.25 ± 2.05 d | 57.83 ± 5.82 c | 90.04 ± 20.57 b |
| B | -- | 22.56 ± 10.37 b | 11.20 ± 8.26 b | 40.65 ± 18.82 b | 18.29 ± 11.28 b | 70.45 ± 20.73 a | |
| IQ | F | -- | 15.08 ± 0.82 b | 189.03 ± 68.10 a | 54.58 ± 35.50 b | 58.33 ± 12.71 b | 44.60 ± 22.59 b |
| B | -- | 40.28 ± 5.73 c | 71.73 ± 1.80 b | 111.20 ± 10.61 a | 102.22 ± 0.98 a | 30.80 ± 14.22 c | |
| FA | F | -- | 255.36 ± 7.25 c | 486.82 ± 77.68 b | 114.29 ± 9.95 d | 80.72 ± 23.82 d | 948.01 ± 3.66 a |
| B | -- | 30.98 ± 7.97 b | 15.30 ± 3.39 bc | 21.63 ± 6.79 bc | 161.73 ± 14.46 a | 14.22 ± 3.40 c | |
| Rutin | F | -- | 150.74 ± 30.21 a | 40.80 ± 6.76 bc | 41.96 ± 3.82 b | 13.91 ± 3.05 d | 14.96 ± 6.64 cd |
| B | -- | 89.53 ± 8.92 b | 17.30 ± 11.33 c | 19.62 ± 4.99 c | 248.76 ± 38.73 a | 15.18 ± 8.02 c | |
| Quercetin | F | -- | 47.02 ± 0.74 a | 38.49 ± 7.22 a | 40.59 ± 6.82 a | 28.37 ± 7.19 b | 22.00 ± 0.90 b |
| B | -- | 22.04 ± 0.38 a | 27.23 ± 4.79 a | 23.76 ± 4.14 a | 23.51 ± 1.56 a | 24.28 ± 4.14 a | |
| Sum. | F | -- | 757.61 ± 49.49 c | 959.95 ± 117.78 b | 366.24 ± 23.65 e | 501.12 ± 40.24 d | 1199.64 ± 40.24 a |
| B | -- | 240.85 ± 24.73 c | 175.62 ± 5.81 d | 295.11 ± 17.63 b | 687.17 ± 18.54 a | 226.00 ± 22.10 c |
| Samples | L* | a* | b* | ΔE | |
|---|---|---|---|---|---|
| Fresh | 0 | 81.13 ± 0.49 a | 1.73 ± 0.28 g | 15.62 ± 0.22 bc | -- |
| 1P | 69.95 ± 0.36 d | 7.72 ± 0.22 f | 29.05 ± 0.67 a | 18.48 ± 0.58 c | |
| 2P | 70.63 ± 0.30 c | 8.47 ± 0.4 e | 28.44 ± 0.3 a | 17.89 ± 0.26 c | |
| 3P | 68.72 ± 0.25 e | 9.64 ± 0.71 c | 29.62 ± 1.23 a | 20.33 ± 0.96 a | |
| 4P | 69.81 ± 0.44 d | 9.02 ± 0.19 d | 29.48 ± 0.85 a | 19.32 ± 0.72 b | |
| 5P | 69.9 ± 0.61 d | 8.15 ± 0.09 e | 28.66 ± 0.77 a | 18.38 ± 0.49 c | |
| Boiled | 0 | 76.66 ± 0.35 b | 0.91 ± 0.19 h | 14.05 ± 0.57 c | -- |
| 1P | 58.24 ± 0.12 gh | 10.68 ± 0.17 a | 30.31 ± 0.52 a | 26.44 ± 0.34 ab | |
| 2P | 57.8 ± 0.55 hi | 10.25 ± 0.43 b | 30.1 ± 1.49 a | 26.48 ± 1.35 ab | |
| 3P | 60.45 ± 0.69 f | 10.66 ± 0.33 a | 29.97 ± 0.93 a | 24.73 ± 1.01 ab | |
| 4P | 57.59 ± 0.46 i | 10.96 ± 0.36 a | 31.86 ± 0.81 a | 27.96 ± 0.94 a | |
| 5P | 58.74 ± 0.61 g | 11.01 ± 0.29 a | 20.1 ± 12.18 b | 23.78 ± 4.06 b |
| Receptors | Ligands | Acting Forces | The Binding Sites | Minimum Binding Energy (kal/mol) |
|---|---|---|---|---|
| Glutenin | Starch, EGCG | Hydrogen bonds | Ser-82 *, Arg-86 *, Arg-77 *, Arg-649 *#, Ser-78 *, Cys-66 * | −8.1 |
| Hydrophobic interactions | Arg-77 #, Pro-645 #, Val-81 #, Thr-642 # | |||
| Starch, hydroxybenzoic acid | Hydrogen bonds | Gln-638 *, Thr-642 *, Arg-86, Glu-24 *, Arg-27 *, Lys-75 *, Ser-78 *, Ser-82 *, Arg-77 *#, Gly-652 # | −7.2 | |
| Hydrophobic interactions | Arg-70# | |||
| Starch, gallic acid | Hydrophobic interactions | Ser-78 *, Gln-627 *, Gln-90 *, Arg-86 *#, Gln-28 *, Ala-629 *, Ser-631 *, Gln-83 # | −7.9 | |
| π–cation interactions | Arg-86 # | |||
| Salt bridges | Arg-86 # | |||
| Starch, quercetin | Hydrogen bonds | Leu-35 *, Arg-44 *, Gln-83 *, Gln-90 *, Gln-627 *, Gln-28 *, Arg-86 *, Glu-37 *, Glu-24 # | −8.4 | |
| Hydrophobic interactions | Gln-28 #,Val-79 # | |||
| Starch, isoquercitrin | Hydrogen bonds | Ser-82 *, Thr-646 *, Glu-24 *, Gln-638 *, Arg-649 #, Arg-77 # | −7.4 | |
| Hydrophobic interactions | Pro-645 # | |||
| π–cation interactions | Arg-649 # | |||
| Salt bridges | Arg-649 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.; Huang, J.; Zhao, J.; Lei, X.; Ming, J.; Li, F. Effect of Polyphenols Extracted from Rosa roxburghii Tartt Pomace with Different Particle Sizes on Quality and Biological Activity of Noodles: A View of Molecular Interaction. Foods 2025, 14, 3679. https://doi.org/10.3390/foods14213679
Lin K, Huang J, Zhao J, Lei X, Ming J, Li F. Effect of Polyphenols Extracted from Rosa roxburghii Tartt Pomace with Different Particle Sizes on Quality and Biological Activity of Noodles: A View of Molecular Interaction. Foods. 2025; 14(21):3679. https://doi.org/10.3390/foods14213679
Chicago/Turabian StyleLin, Keying, Junjie Huang, Jichun Zhao, Xiaojuan Lei, Jian Ming, and Fuhua Li. 2025. "Effect of Polyphenols Extracted from Rosa roxburghii Tartt Pomace with Different Particle Sizes on Quality and Biological Activity of Noodles: A View of Molecular Interaction" Foods 14, no. 21: 3679. https://doi.org/10.3390/foods14213679
APA StyleLin, K., Huang, J., Zhao, J., Lei, X., Ming, J., & Li, F. (2025). Effect of Polyphenols Extracted from Rosa roxburghii Tartt Pomace with Different Particle Sizes on Quality and Biological Activity of Noodles: A View of Molecular Interaction. Foods, 14(21), 3679. https://doi.org/10.3390/foods14213679








