Green Optimization of Sesame Seed Oil Extraction via Pulsed Electric Field and Ultrasound Bath: Yield, Antioxidant Activity, Oxidative Stability, and Functional Food Potential
Abstract
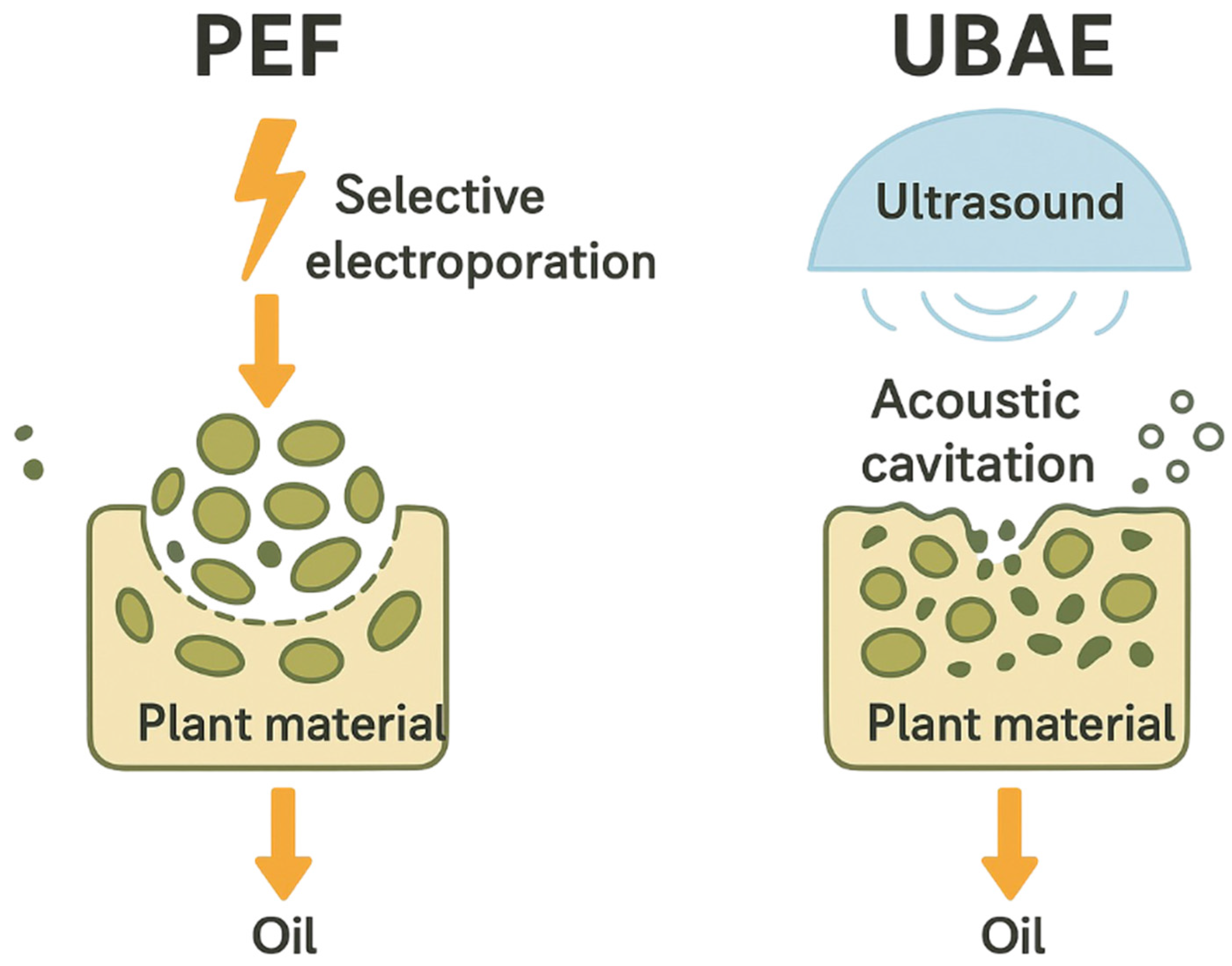
1. Introduction
2. Materials and Methods
2.1. Sesame Seeds Material
2.2. Chemicals and Reagents
2.3. Experimental Design
2.4. Determinations
2.4.1. Oil Content
2.4.2. DPPH• Antiradical Activity
2.4.3. Conjugated Dienes and Trienes
2.5. Benchmarking Method Specifications
2.5.1. Peroxide Value (PV)
2.5.2. Thiobarbituric Acid Reactive Substances (TBARS)
2.5.3. p-Anisidine Value (p-AV)
2.5.4. Totox Value
2.5.5. Fatty Acids Profile
2.5.6. ATR–FTIR Spectroscopy
2.5.7. Specific Energy Consumption for the PEF and UBAE Treatments
2.6. Statistical Analysis
3. Results and Discussion
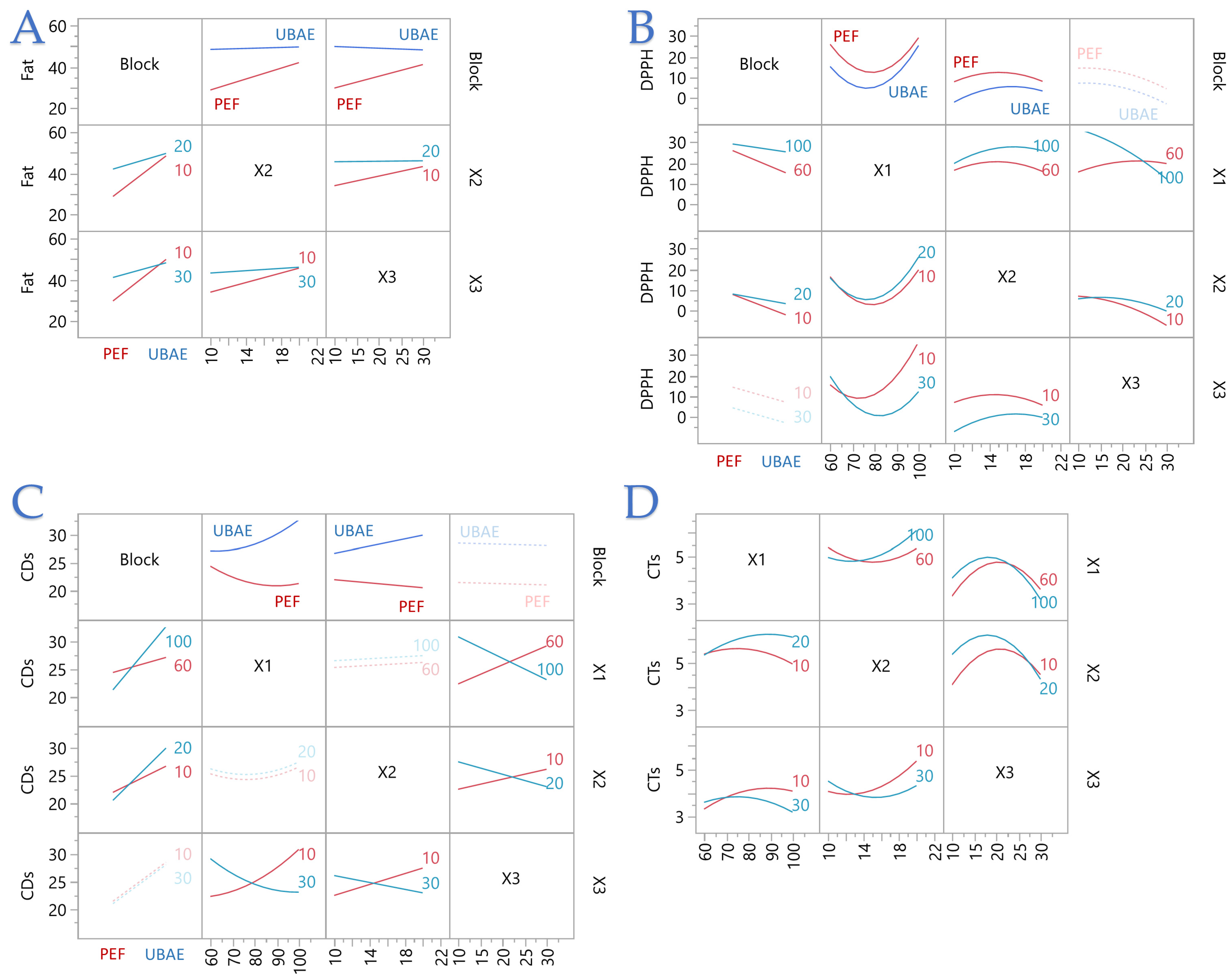
3.1. RSM Overview Across Design Space and Yield–Antioxidant Trade-Offs
3.2. Model Analysis and Key Coefficients
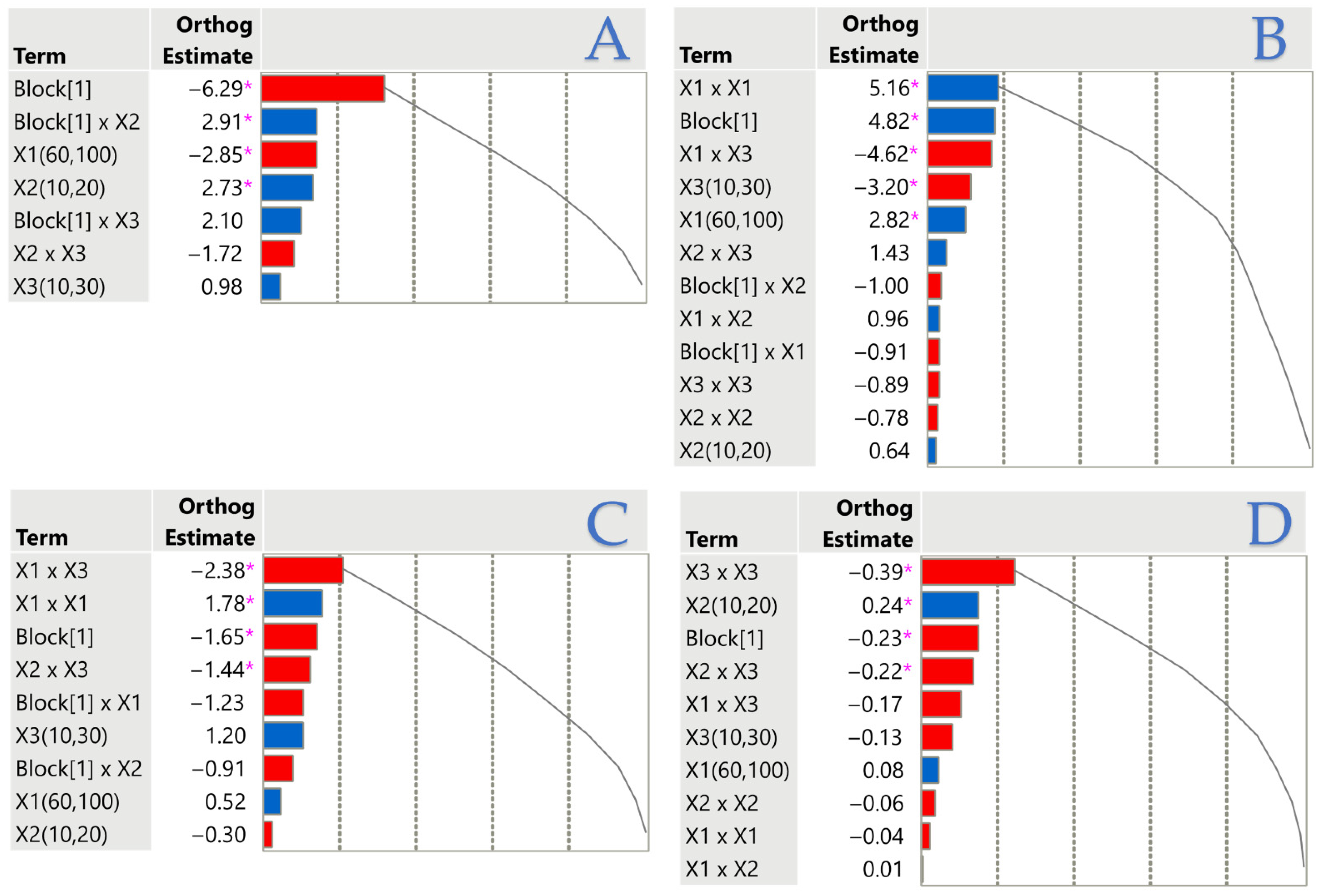
3.3. Factor Importance via Pareto Plots
3.4. Oxidative Stability Patterns from Principal Component Analysis (PCA)
3.5. Integrated Optimization Through Partial Least Squares (PLS)
3.6. Benchmarking Against Conventional Methods
3.6.1. Fat Yield, Antioxidant Activity, and Oxidation Indices
3.6.2. Fatty Acid Composition and Nutritional Quality Indices
3.6.3. ATR–FTIR Spectra Analysis
3.6.4. Scalability and Energy Consumption for the Optimal PEF and UBAE Conditions
3.7. Human Health Relevance and Future Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Atherogenicity Index |
| ANOVA | Analysis of Variance |
| ATR-FTIR | Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy |
| BIC | Bayesian Information Criterion |
| CDs | Conjugated Dienes |
| COX | Calculated Oxidizability Value |
| CTs | Conjugated Trienes |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl (radical scavenging assay) |
| DSD | Definitive Screening Design |
| FA | Fatty Acids |
| FAMEs | Fatty Acid Methyl Esters |
| FID | Flame Ionization Detector |
| GC-FID | Gas Chromatography with Flame Ionization Detection |
| H/H | Hypocholesterolemic/Hypercholesterolemic Ratio |
| HPI | Health-Promoting Index |
| MDAE | Malondialdehyde Equivalents |
| MUFA | Monounsaturated Fatty Acids |
| OLS | Ordinary Least Squares |
| p-AV | p-Anisidine Value |
| PCA | Principal Component Analysis |
| PEF | Pulsed Electric Field |
| PLS | Partial Least Squares |
| PUFA | Polyunsaturated Fatty Acids |
| PV | Peroxide Value |
| RSM | Response Surface Methodology |
| SFA | Saturated Fatty Acids |
| TBARS | Thiobarbituric Acid Reactive Substances |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| TI | Thrombogenicity Index |
| Totox | Total Oxidation Value |
| UAE | Ultrasound-Assisted Extraction |
| UBAE | Ultrasound Bath-Assisted Extraction |
| UFA | Unsaturated Fatty Acids |
| VIP | Variable Importance in Projection |
| ω-3/ω-6 | Omega-3/Omega-6 Fatty Acids |
References
- Zech-Matterne, V.; Tengberg, M.; Van Andringa, W. Sesamum indicum L. (sesame) in 2nd century BC Pompeii, southwest Italy, and a review of early sesame finds in Asia and Europe. Veg. Hist. Archaeobotany 2015, 24, 673–681. [Google Scholar] [CrossRef]
- Wei, P.; Zhao, F.; Wang, Z.; Wang, Q.; Chai, X.; Hou, G.; Meng, Q. Sesame (Sesamum indicum L.): A Comprehensive Review of Nutritional Value, Phytochemical Composition, Health Benefits, Development of Food, and Industrial Applications. Nutrients 2022, 14, 4079. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.A.; Kancharla, P.K.; Chandra, K.; Sodhi, Y.S.; Arumugam, N. Assessment of variability in lignan and fatty acid content in the germplasm of Sesamum indicum L. J. Food Sci. Technol. 2019, 56, 976–986. [Google Scholar] [CrossRef]
- Xu, F.; Zhou, R.; Dossou, S.S.K.; Song, S.; Wang, L. Fine Mapping of a Major Pleiotropic QTL Associated with Sesamin and Sesamolin Variation in Sesame (Sesamum indicum L.). Plants 2021, 10, 1343. [Google Scholar] [CrossRef]
- Ruankham, W.; Suwanjang, W.; Wongchitrat, P.; Prachayasittikul, V.; Prachayasittikul, S.; Phopin, K. Sesamin and sesamol attenuate H2O2-induced oxidative stress on human neuronal cells via the SIRT1-SIRT3-FOXO3a signaling pathway. Nutr. Neurosci. 2021, 24, 90–101. [Google Scholar] [CrossRef]
- Oboulbiga, E.B.; Douamba, Z.; Compaoré-Sérémé, D.; Semporé, J.N.; Dabo, R.; Semde, Z.; Tapsoba, F.W.-B.; Hama-Ba, F.; Songré-Ouattara, L.T.; Parkouda, C.; et al. Physicochemical, potential nutritional, antioxidant and health properties of sesame seed oil: A review. Front. Nutr. 2023, 10, 1127926. [Google Scholar] [CrossRef]
- Mostashari, P.; Mousavi Khaneghah, A. Sesame Seeds: A Nutrient-Rich Superfood. Foods 2024, 13, 1153. [Google Scholar] [CrossRef]
- Kancharla, P.K.; Arumugam, N. Variation of Oil, Sesamin, and Sesamolin Content in the Germplasm of the Ancient Oilseed Crop Sesamum indicum L. J. Am. Oil Chem. Soc. 2020, 97, 475–483. [Google Scholar] [CrossRef]
- Hadipour, E.; Emami, S.A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Effects of sesame (Sesamum indicum L.) and bioactive compounds (sesamin and sesamolin) on inflammation and atherosclerosis: A review. Food Sci. Nutr. 2023, 11, 3729–3757. [Google Scholar] [CrossRef]
- Woo, M.; Han, S.; Song, Y.O. Sesame Oil Attenuates Renal Oxidative Stress Induced by a High Fat Diet. Prev. Nutr. Food Sci. 2019, 24, 114–120. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Zhang, W.; Qi, W.; Lu, C.; Huang, H.; Yang, Z.; Liu, B.; Zhang, L. Corrigendum to “Sesamin suppresses NSCLC cell proliferation through cyclin D1 inhibition-dependent cell cycle arrest via Akt/p53 pathway” [Toxicology and applied pharmacology, 387 (2020) 114848]. Toxicol. Appl. Pharmacol. 2020, 400, 115048. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Eltayeb, A.E.; Abu-Yousef, I.A.; Yousef, S.M. Hypolipidemic and Anti-Atherogenic Effects of Sesamol and Possible Mechanisms of Action: A Comprehensive Review. Molecules 2023, 28, 3567. [Google Scholar] [CrossRef]
- Kheirati Rounizi, S.; Akrami Mohajeri, F.; Moshtaghi Broujeni, H.; Pourramezani, F.; Jambarsang, S.; Kiani, H.; Khalili Sadrabad, E. The chemical composition and heavy metal content of sesame oil produced by different methods: A risk assessment study. Food Sci. Nutr. 2021, 9, 2886–2893. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.C.A.; Macedo, M.L.R.; Munhoz, C.L.; Silva, M.C.B.L.; Viana, L.H.; Filiu, W.; Hiane, P.A. Assessment of nutritional properties of sesame and flaxseed oil using quality indexes. Acta Hortic. 2018, 1198, 115–124. [Google Scholar] [CrossRef]
- El Riachy, M.; Hamade, A.; Ayoub, R.; Dandachi, F.; Chalak, L. Oil Content, Fatty Acid and Phenolic Profiles of Some Olive Varieties Growing in Lebanon. Front. Nutr. 2019, 6, 94. [Google Scholar] [CrossRef]
- Hussain, S.A.; Hameed, A.; Ajmal, I.; Nosheen, S.; Suleria, H.A.R.; Song, Y. Effects of sesame seed extract as a natural antioxidant on the oxidative stability of sunflower oil. J. Food Sci. Technol. 2018, 55, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Özkılıç, S.Y.; Arslan, D. Acidic and enzymatic pre-treatment effects on cold-pressed pumpkin, terebinth and flaxseed oils. Grasas Aceites 2022, 73, e462. [Google Scholar] [CrossRef]
- Atamyradova, N.; Özkılıç, S.Y.; Arslan, D. Blanching of olive fruits before storage at different conditions: Effects on oil yield, lipase activity and oxidation. J. Agric. Food Res. 2024, 18, 101509. [Google Scholar] [CrossRef]
- Langyan, S.; Yadava, P.; Sharma, S.; Gupta, N.C.; Bansal, R.; Yadav, R.; Kalia, S.; Kumar, A. Food and nutraceutical functions of sesame oil: An underutilized crop for nutritional and health benefits. Food Chem. 2022, 389, 132990. [Google Scholar] [CrossRef]
- Imran, M.; Khan, M.K.; Ali, M.; Nadeem, M.; Mushtaq, Z.; Ahmad, M.H.; Arshad, M.S.; Ahmad, N.; Rahim, M.A. Cold Pressed Sesame (Sesamum Indicum) Oil. In Cold Pressed Oils; Ramadan, M.F., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 105–111. ISBN 978-0-12-818188-1. [Google Scholar]
- Luengo, E.; Condón-Abanto, S.; Álvarez, I.; Raso, J. Effect of Pulsed Electric Field Treatments on Permeabilization and Extraction of Pigments from Chlorella vulgaris. J. Membr. Biol. 2014, 247, 1269–1277. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Bouzoubaa, Z.; Asdadi, A.; El Yadini, A.; Charrouf, Z. Chemical characterization and oxidative stability of seeds and oil of sesame grown in Morocco. J. Saudi Soc. Agric. Sci. 2017, 16, 105–111. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, Q.; Dossou, S.S.K.; Zhou, R.; Zhao, Y.; Zhou, W.; Zhang, Y.; Li, D.; You, J.; Wang, L. Genome-Wide Association Study Uncovers Loci and Candidate Genes Underlying Phytosterol Variation in Sesame (Sesamum indicum L.). Agriculture 2022, 12, 392. [Google Scholar] [CrossRef]
- Morris, J.B.; Wang, M.L.; Tonnis, B.D. Variability for oil, protein, lignan, tocopherol, and fatty acid concentrations in eight sesame (Sesamum indicum L.) genotypes. Ind. Crops Prod. 2021, 164, 113355. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Kalompatsios, D.; Mantiniotou, M.; Lalas, S.I. Investigation into the Reduction of Palm Oil in Foods by Blended Vegetable Oils through Response Surface Methodology and Oxidative Stability Tests. Antioxidants 2024, 13, 929. [Google Scholar] [CrossRef]
- Pegg, R.B. Measurement of Primary Lipid Oxidation Products. Curr. Protoc. Food Anal. Chem. 2001, D2.1.1–D2.1.15. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Utilization of Blackthorn Plums (Prunus spinosa) and Sweet Cherry (Prunus avium) Kernel Oil: Assessment of Chemical Composition, Antioxidant Activity, and Oxidative Stability. Biomass 2024, 4, 49–64. [Google Scholar] [CrossRef]
- Qiu, C.; Zhao, M.; Decker, E.A.; McClements, D.J. Influence of Protein Type on Oxidation and Digestibility of Fish Oil-in-Water Emulsions: Gliadin, Caseinate, and Whey Protein. Food Chem. 2015, 175, 249–257. [Google Scholar] [CrossRef]
- ISO 6885:2016; Animal and Vegetable Fats and Oils—Determination of Anisidine Value. International Organization for Standardization (ISO): Geneva, Switzerland, 2016.
- Galanakis, C.M.; Tsatalas, P.; Charalambous, Z.; Galanakis, I.M. Polyphenols recovered from olive mill wastewater as natural preservatives in extra virgin olive oils and refined olive kernel oils. Environ. Technol. Innov. 2018, 10, 62–70. [Google Scholar] [CrossRef]
- European Union Commission. COMMISSION REGULATION (EC) No 796/2002 of 6 May 2002 Amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Pomace Oil and on the Relevant Methods of Analysis and the Additional Notes in the Annex to Council Regulation (EEC) No 2658/87 on the Tariff and Statistical Nomenclature and on the Common Customs Tariff; European Union Commission: Brussels, Belgium, 2002. [Google Scholar]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Process. Process Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Khadhraoui, B.; Ummat, V.; Tiwari, B.K.; Fabiano-Tixier, A.S.; Chemat, F. Review of ultrasound combinations with hybrid and innovative techniques for extraction and processing of food and natural products. Ultrason. Sonochem. 2021, 76, 105625. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.J.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “Green” and Novel Strategies for the Extraction of Bioactive Added Value Compounds from Citrus Wastes—A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Should the in vitro colorimetric assays in antioxidant and lipid oxidation evaluation be abandoned? A critical review focusing on bioactive molecule screening assays in in vitro and in vivo models. J. Food Bioact. 2020, 9, 23–35. [Google Scholar] [CrossRef]
- Hamitri-Guerfi, F.; Ouahrani, S.; Benbouriche, A.; Bey, M.B.; Boulekbache-Makhlouf, L.; Madani, K. Impact of the extraction method on physico-chemical proprieties, phytochemicals and biological activity of sesame seeds oil. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Technol. 2020, 44, 82–103. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Iwamoto, S. Bioactive compounds from by-products of rice cultivation and rice processing: Extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2019, 86, 109–117. [Google Scholar] [CrossRef]
- Gerstenmeyer, E.; Reimer, S.; Berghofer, E.; Schwartz, H.; Sontag, G. Effect of thermal heating on some lignans in flax seeds, sesame seeds and rye. Food Chem. 2013, 138, 1847–1855. [Google Scholar] [CrossRef]
- Elouafy, Y.; El Yadini, A.; El Moudden, H.; Harhar, H.; Alshahrani, M.M.; Awadh, A.A.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A.; Tabyaoui, M. Influence of the Extraction Method on the Quality and Chemical Composition of Walnut (Juglans regia L.) Oil. Molecules 2022, 27, 7681. [Google Scholar] [CrossRef]
- The Effect of the Type of Extraction Process and Different Stages of Purification on the Composition of Sesame Oil. Available online: https://www.ijcce.ac.ir/article_711197.html (accessed on 21 September 2025).
- AOCS. Official Method Ba 3-38: Oil Content. In Official Methods and Recommended Practices of the AOCS, 6th ed.; AOCS Press: Urbana, IL, USA, 2009; Available online: https://www.scribd.com/document/425054524/oil-content (accessed on 19 October 2025).
- Dharmender; Mandal, S.; Mani, I.; Parray, R.A.; Malkani, P.; Kumar, R. Comparative evaluation of sesame oil obtained with microwave-assisted enzymatic extraction and conventional solvent extraction. Int. J. Adv. Biochem. Res. 2024, 8, 216–220. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef]
- Ponphaiboon, J.; Krongrawa, W.; Aung, W.W.; Chinatangkul, N.; Limmatvapirat, S.; Limmatvapirat, C. Advances in Natural Product Extraction Techniques, Electrospun Fiber Fabrication, and the Integration of Experimental Design: A Comprehensive Review. Molecules 2023, 28, 5163. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Kowalczyk, A.; Sarritzu, E.; Cabras, P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007, 103, 1494–1501. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kalompatsios, D.; Mantiniotou, M.; Bozinou, E.; Lalas, S.I. Pulsed Electric Field Applications for the Extraction of Bioactive Compounds from Food Waste and By-Products: A Critical Review. Biomass 2023, 3, 367–401. [Google Scholar] [CrossRef]
- Puértolas, E.; Barba, F.J. Electrotechnologies applied to valorization of by-products from food industry: Main findings, energy and economic cost of their industrialization. Food Bioprod. Process. 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Boussetta, N.; Tessaro, I.C.; Marczak, L.D.F.; Vorobiev, E. Application of pulsed electric fields and high voltage electrical discharges for oil extraction from sesame seeds. J. Food Eng. 2015, 153, 20–27. [Google Scholar] [CrossRef]
- Guderjan, M.; Töpfl, S.; Angersbach, A.; Knorr, D. Impact of pulsed electric field treatment on the recovery and quality of plant oils. J. Food Eng. 2005, 67, 281–287. [Google Scholar] [CrossRef]
- Guderjan, M.; Elez-Martínez, P.; Knorr, D. Application of pulsed electric fields at oil yield and content of functional food ingredients at the production of rapeseed oil. Innov. Food Sci. Emerg. Technol. 2007, 8, 55–62. [Google Scholar] [CrossRef]
- Bahramian, B.; Kheirati Ronizi, Z.; Hashemi, M.; Rezaie, M.; Tavassoli, M. The effect of ultrasound process on the extraction yield and the quality of edible oils. Appl. Food Res. 2025, 5, 101268. [Google Scholar] [CrossRef]
- Samaram, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M.; Bordbar, S.; Serjouie, A. Optimisation of ultrasound-assisted extraction of oil from papaya seed by response surface methodology: Oil recovery, radical scavenging antioxidant activity, and oxidation stability. Food Chem. 2015, 172, 7–17. [Google Scholar] [CrossRef]
- Krishnan, V.C.A.; Kuriakose, S.; Rawson, A. Ultrasound Assisted Extraction of Oil from Rice Bran: A Response Surface Methodology Approach. J. Food Process. Technol. 2015, 6, 2. [Google Scholar] [CrossRef]
- Xu, J.; Chen, S.; Hu, Q. Antioxidant activity of brown pigment and extracts from black sesame seed (Sesamum indicum L.). Food Chem. 2005, 91, 79–83. [Google Scholar] [CrossRef]
- Abirached, C.; Bonifacino, C.; Dutto, E.; Velazco, L.; Jorge, F.; Vieitez, I. Study of sesame seeds antioxidant and emulsifying properties: Original high-quality research paper. J. Supercrit. Fluids 2020, 166, 104994. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, C.; Wang, B.; Yagoub, A.E.-G.A.; Ma, H.; Zhang, X.; Wu, M. Study of ultrasonic cavitation during extraction of the peanut oil at varying frequencies. Ultrason. Sonochem. 2017, 37, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Moradi, N.; Rahimi, M.; Moeini, A.; Parsamoghadam, M.A. Impact of ultrasound on oil yield and content of functional food ingredients at the oil extraction from sunflower. Sep. Sci. Technol. 2018, 53, 261–276. [Google Scholar] [CrossRef]
- Gila, A.; Sánchez-Ortiz, A.; Jiménez, A.; Beltrán, G. The ultrasound application does not affect to the thermal properties and chemical composition of virgin olive oils. Ultrason. Sonochem. 2021, 70, 105320. [Google Scholar] [CrossRef]
- Shalmashi, A. Ultrasound-Assisted Extraction of Oil from Tea Seeds. J. Food Lipids 2009, 16, 465–474. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs: II. Fatty acid composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Chen, S.; Bobe, G.; Zimmerman, S.; Hammond, E.G.; Luhman, C.M.; Boylston, T.D.; Freeman, A.E.; Beitz, D.C. Physical and Sensory Properties of Dairy Products from Cows with Various Milk Fatty Acid Compositions. J. Agric. Food Chem. 2004, 52, 3422–3428. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ksibi, N.; Wroniak, M.; Lefek, M.; Ratusz, K. Oxidative Stability Analysis of Selected Oils from Unconventional Raw Materials Using Rancimat Apparatus. Appl. Sci. 2022, 12, 10355. [Google Scholar] [CrossRef]
- Gao, X.; Xiao, Y.; Li, W.; Xu, L.; Yuan, J. Synergistic Effects of Antioxidant Blends: A Comparative Study on Oxidative Stability of Lipids in Feed Matrices. Antioxidants 2025, 14, 981. [Google Scholar] [CrossRef] [PubMed]
- Damerau, A.; Ahonen, E.; Kortesniemi, M.; Puganen, A.; Tarvainen, M.; Linderborg, K.M. Evaluation of the composition and oxidative status of omega-3 fatty acid supplements on the Finnish market using NMR and SPME-GC–MS in comparison with conventional methods. Food Chem. 2020, 330, 127194. [Google Scholar] [CrossRef] [PubMed]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef]
- Peake, C.G.; Odgers-Jewell, K.; De Sousa, C.J.; English, C.J.; Ingabire, A.; Mayr, H.L.; Reidlinger, D.P. Association between dietary inflammatory or oxidative stress indices and biomarkers in cardiometabolic and related conditions: A systematic literature review. Br. J. Nutr. 2025, 133, 1090–1106. [Google Scholar] [CrossRef]
- Nuñez-Selles, A.J.; Nuñez-Musa, R.A.; Guillen-Marmolejos, R.A. Linking oxidative stress biomarkers to disease progression and antioxidant therapy in hypertension and diabetes mellitus. Front. Mol. Biosci. 2025, 12, 1611842. [Google Scholar] [CrossRef]
- Myszko, M.; Bychowski, J.; Skrzydlewska, E.; Łuczaj, W. The Dual Role of Oxidative Stress in Atherosclerosis and Coronary Artery Disease: Pathological Mechanisms and Diagnostic Potential. Antioxidants 2025, 14, 275. [Google Scholar] [CrossRef]
- Dunford, N.T. Edible Oil Quality. Robert M. Kerr Food & Agricultural Products Center Food Technology Fact Sheet 405-744-6071. References-Scientific Research Publishing. 2016. Available online: https://www.scirp.org/reference/referencespapers?referenceid=3688793 (accessed on 20 September 2025).
- Sherratt, S.C.R.; Lero, M.; Mason, R.P. Are dietary fish oil supplements appropriate for dyslipidemia management? A review of the evidence. Curr. Opin. Lipidol. 2020, 31, 94–100. [Google Scholar] [CrossRef]
- Vekic, J.; Stefanovic, A.; Zeljkovic, A. Obesity and Dyslipidemia: A Review of Current Evidence. Curr. Obes. Rep. 2023, 12, 207–222. [Google Scholar] [CrossRef]
- Hajri, T.; Ouguerram, K.; Fungwe, T.V. Impact of Lipid Oxidation Products on Inflammation and Insulin Resistance: A Focus on Mechanisms of Action. Cell Biochem. Biophys. 2025, 1–21. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, G.; Zhang, M.; Zhou, D.; Zhu, B. Potential adverse health effects of dietary lipid oxidation products. J. Food Bioact. 2021, 15, 51–62. [Google Scholar] [CrossRef]
- Valaitienė, J.; Laučytė-Cibulskienė, A. Oxidative Stress and Its Biomarkers in Cardiovascular Diseases. Artery Res. 2024, 30, 18. [Google Scholar] [CrossRef]
- Sule, R.O.; Rivera, G.D.T.; Vaidya, T.; Gartrell, E.; Gomes, A.V. Environmental Toxins and Oxidative Stress: The Link to Cardiovascular Diseases. Antioxidants 2025, 14, 604. [Google Scholar] [CrossRef] [PubMed]
- Ricker, M.A.; Haas, W.C. Anti-Inflammatory Diet in Clinical Practice: A Review. Nutr. Clin. Pract. 2017, 32, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Frydrych, A.; Kulita, K.; Jurowski, K.; Piekoszewski, W. Lipids in Clinical Nutrition and Health: Narrative Review and Dietary Recommendations. Foods 2025, 14, 473. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef]
- Tamminen, J.; Holappa, J.; Vladimirovich Gradov, D.; Koiranen, T. Scaling up continuous ultrasound-assisted extractor for plant extracts by using spinach leaves as a test material. Ultrason. Sonochem. 2022, 90, 106171. [Google Scholar] [CrossRef]
- Taha, A.; Casanova, F.; Šimonis, P.; Stankevič, V.; Gomaa, M.A.E.; Stirkė, A. Pulsed Electric Field: Fundamentals and Effects on the Structural and Techno-Functional Properties of Dairy and Plant Proteins. Foods 2022, 11, 1556. [Google Scholar] [CrossRef]
- Golberg, A.; Sack, M.; Teissie, J.; Pataro, G.; Pliquett, U.; Saulis, G.; Stefan, T.; Miklavcic, D.; Vorobiev, E.; Frey, W. Energy-efficient biomass processing with pulsed electric fields for bioeconomy and sustainable development. Biotechnol. Biofuels 2016, 9, 94. [Google Scholar] [CrossRef]
- Choo, W.-S.; Birch, J.; Dufour, J.-P. Physicochemical and quality characteristics of cold-pressed flaxseed oils. J. Food Compos. Anal. 2007, 20, 202–211. [Google Scholar] [CrossRef]
- Azadmard-Damirchi, S.; Habibi-Nodeh, F.; Hesari, J.; Nemati, M.; Achachlouei, B.F. Effect of pretreatment with microwaves on oxidative stability and nutraceuticals content of oil from rapeseed. Food Chem. 2010, 121, 1211–1215. [Google Scholar] [CrossRef]
- Namiki, M. Nutraceutical Functions of Sesame: A Review. Crit. Rev. Food Sci. Nutr. 2007, 47, 651–673. [Google Scholar] [CrossRef]
- Dossou, S.S.K.; Xu, F.; Dossa, K.; Zhou, R.; Zhao, Y.; Wang, L. Antioxidant lignans sesamin and sesamolin in sesame (Sesamum indicum L.): A comprehensive review and future prospects. J. Integr. Agric. 2023, 22, 14–30. [Google Scholar] [CrossRef]




| Independent Variables | Coded Units | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Energy power * (E, %) | X1 | 60 | 80 | 100 |
| Liquid-to-solid ratio (R, mL/g) | X2 | 10 | 15 | 20 |
| Extraction time (t, min) | X3 | 10 | 20 | 30 |
| Design Point | Independent Variables | Actual Responses * | ||||||
|---|---|---|---|---|---|---|---|---|
| Block (Technique) | X1 (E, %) | X2 (R, mL/g) | X3 (t, min) | Fat | DPPH | CDs | CTs | |
| 1 | 1 (PEF) | −1 (60) | 1 (20) | 0 (20) | 45 ± 3.2 | 20.7 ± 1.2 | 24.3 ± 0.9 | 4.8 ± 0.4 |
| 2 | 1 (PEF) | 1 (100) | 1 (20) | −1 (10) | 35.3 ± 1.6 | 33.7 ± 1.9 | 26.9 ± 1.6 | 4.8 ± 0.3 |
| 3 | 1 (PEF) | 0 (80) | −1 (10) | −1 (10) | 21.7 ± 0.8 | 12.7 ± 0.4 | 19.2 ± 0.4 | 3.5 ± 0.3 |
| 4 | 2 (UBAE) | −1 (60) | 0 (15) | −1 (10) | 45.6 ± 3.4 | 13.1 ± 0.3 | 27.3 ± 1.9 | 4.6 ± 0.2 |
| 5 | 2 (UBAE) | 1 (100) | 1 (20) | 1 (30) | 36.6 ± 0.7 | 12.9 ± 0.7 | 30.3 ± 1 | 5 ± 0.3 |
| 6 | 1 (PEF) | 1 (100) | −1 (10) | 0 (20) | 30.5 ± 1.6 | 23.6 ± 1.5 | 23.1 ± 1.5 | 4.1 ± 0.1 |
| 7 | 1 (PEF) | −1 (60) | −1 (10) | 1 (30) | 37.9 ± 2 | 20 ± 1.2 | 31.9 ± 1 | 3.9 ± 0.2 |
| 8 | 2 (UBAE) | −1 (60) | −1 (10) | −1 (10) | 50.5 ± 3.8 | 5.2 ± 0.3 | 19 ± 0.6 | 4 ± 0.2 |
| 9 | 2 (UBAE) | 0 (80) | 0 (15) | 0 (20) | 53.4 ± 1.5 | 6.9 ± 0.4 | 31.5 ± 1.4 | 6.2 ± 0.4 |
| 10 | 1 (PEF) | 0 (80) | 0 (15) | 0 (20) | 32.9 ± 2.1 | 9.2 ± 0.6 | 18.7 ± 0.5 | 4.2 ± 0.2 |
| 11 | 2 (UBAE) | 1 (100) | −1 (10) | 1 (30) | 45.1 ± 2.9 | 0.1 ± 0 | 28.5 ± 1 | 4.6 ± 0.2 |
| 12 | 1 (PEF) | 1 (100) | 1 (20) | −1 (10) | 35.9 ± 0.8 | 33.5 ± 2 | 26.3 ± 0.7 | 4.9 ± 0.4 |
| 13 | 2 (UBAE) | 1 (100) | 0 (15) | 1 (30) | 52.7 ± 2.1 | 8.9 ± 0.5 | 26.7 ± 2 | 3.6 ± 0.1 |
| 14 | 1 (PEF) | 0 (80) | 1 (20) | 1 (30) | 47.8 ± 2.6 | 2.8 ± 0.1 | 18.3 ± 0.8 | 3.5 ± 0.1 |
| 15 | 2 (UBAE) | −1 (60) | 1 (20) | 1 (30) | 50.4 ± 2.4 | 12.5 ± 0.7 | 28.6 ± 1.4 | 4.5 ± 0.3 |
| 16 | 2 (UBAE) | −1 (60) | 1 (20) | −1 (10) | 59 ± 1.8 | 3.1 ± 0.1 | 24.5 ± 0.6 | 4.7 ± 0.2 |
| 17 | 1 (PEF) | −1 (60) | −1 (10) | 1 (30) | 37 ± 2.7 | 20.4 ± 1.4 | 30.1 ± 1.3 | 3.8 ± 0.2 |
| 18 | 2 (UBAE) | 1 (100) | −1 (10) | −1 (10) | 44 ± 1 | 27.1 ± 0.7 | 32.1 ± 2.1 | 4.5 ± 0.2 |
| Responses | Independent Variables | Desirability | Stepwise Regression | |||
|---|---|---|---|---|---|---|
| Block (Technique) | X1 (E, %) | X2 (R, mL/g) | X3 (t, min) | |||
| Fat content (%) | UBAE | 60 | 20 | 30 | 0.6978 | 48.36 ± 6.59 |
| DPPH (μmol TEAC/kg oil) | PEF | 100 | 17 | 10 | 0.9923 | 36.02 ± 5.92 |
| CDs (mmol/kg oil) | UBAE | 60 | 10 | 10 | 0.8341 | 19.78 ± 4.78 |
| CTs (mmol/kg oil) | UBAE | 60 | 12 | 10 | 0.8885 | 3.34 ± 0.75 |
| Responses | Fat | DPPH | CDs | CTs |
|---|---|---|---|---|
| Block 1 | ||||
| Fat | - | −0.100 | 0.223 | 0.237 |
| DPPH | - | 0.609 | 0.742 | |
| CDs | - | 0.233 | ||
| CTs | - | |||
| Block 2 | ||||
| Fat | - | −0.478 | −0.470 | 0.179 |
| DPPH | - | 0.456 | −0.192 | |
| CDs | - | 0.178 | ||
| CTs | - | |||
| Responses | PLS-Predicted | PEF | UBAE | Cold-Pressed | Soxhlet | Formula (Section) |
|---|---|---|---|---|---|---|
| Fat content (%) | 44.21 | 45.65 ± 2.65 b | 50.41 ± 1.61 a,b | 51.78 ± 2.12 a | 55.65 ± 1.5 a | 2.4.1 |
| DPPH (μmol TEAC/kg oil) | 20.28 | 19.49 ± 1.35 a | 12.67 ± 0.84 b | 18.66 ± 1.16 a | 6.03 ± 0.18 c | 2.4.2 |
| CDs (mmol/kg oil) | 27.52 | 19.13 ± 1.24 c | 22.41 ± 0.94 b | 14.25 ± 0.68 d | 26.04 ± 1.41 a | 2.4.3 |
| CTs (mmol/kg oil) | 3.80 | 2.95 ± 0.18 b | 3.21 ± 0.19 b | 2.16 ± 0.11 c | 3.9 ± 0.25 a | 2.4.3 |
| PV (mmol H2O2/kg oil) | 114.07 ± 6.84 c | 160.85 ± 11.9 b | 92.08 ± 4.24 c | 281.69 ± 9.01 a | 2.5.1 | |
| TBARS (mmol MDAE/kg oil) | 0.3 ± 0.01 c | 0.69 ± 0.04 b | 0.08 ± 0 d | 2.01 ± 0.13 a | 2.5.2 | |
| p-AV | 0.5 ± 0.03 c | 0.66 ± 0.04 b | 0.41 ± 0.02 c | 2.3 ± 0.09 a | 2.5.3 | |
| Totox value | 228.65 ± 13.72 c | 322.35 ± 23.84 b | 184.58 ± 8.49 c | 565.67 ± 18.11 a | 2.5.4 |
| Samples | PEF (%) | UBAE (%) | Cold-Pressed (%) | Soxhlet (%) |
|---|---|---|---|---|
| C16:0 | 8.68 ± 0.49 a | 8.7 ± 0.45 a | 8.65 ± 0.38 a | 8.77 ± 0.5 a |
| C18:0 | 13.6 ± 0.31 a | 13.24 ± 0.53 a | 13.98 ± 0.95 a | 14.19 ± 1.05 a |
| C22:0 | 0.38 ± 0.01 b | 0.38 ± 0.03 b | 0.19 ± 0 c | 0.46 ± 0.02 a |
| ∑ SFA | 22.66 ± 0.81 a | 22.32 ± 1.01 a | 22.82 ± 1.34 a | 23.42 ± 1.57 a |
| C18:1 | 44.29 ± 2.26 a | 44.43 ± 2.18 a | 44.24 ± 1.28 a | 43.83 ± 2.37 a |
| ∑ MUFA | 44.29 ± 2.26 a | 44.43 ± 2.18 a | 44.24 ± 1.28 a | 43.83 ± 2.37 a |
| C18:2 (ω-6) | 31.99 ± 1.41 a | 32.18 ± 1.54 a | 31.92 ± 1.69 a | 31.72 ± 1.43 a |
| C18:3 (ω-3) | 1.06 ± 0.06 a | 1.07 ± 0.07 a | 1.02 ± 0.03 a | 1.03 ± 0.02 a |
| ∑ PUFA | 33.05 ± 1.47 a | 33.25 ± 1.61 a | 32.94 ± 1.73 a | 32.75 ± 1.45 a |
| ∑ UFA | 77.34 ± 3.72 a | 77.68 ± 3.79 a | 77.18 ± 3.01 a | 76.58 ± 3.82 a |
| PUFA:SFA ratio | 1.46 ± 0.01 a | 1.49 ± 0 a | 1.44 ± 0.01 a,b | 1.4 ± 0.03 b |
| MUFA:PUFA ratio | 1.34 ± 0.01 a | 1.34 ± 0 a | 1.34 ± 0.03 a | 1.34 ± 0.01 a |
| ω-6/ω-3 ratio | 30.19 ± 0.33 a | 30.09 ± 0.42 a | 31.28 ± 0.63 a | 30.79 ± 0.65 a |
| AI | 0.11 ± 0 b | 0.11 ± 0 b | 0.11 ± 0 b | 0.11 ± 0 a |
| TI | 0.54 ± 0.01 a,b | 0.53 ± 0 b | 0.55 ± 0.01 a,b | 0.56 ± 0.01 a |
| H/H | 8.91 ± 0.07 a | 8.93 ± 0.03 a | 8.92 ± 0.04 a | 8.73 ± 0.06 b |
| HPI | 8.91 ± 0.07 a | 8.93 ± 0.03 a | 8.92 ± 0.04 a | 8.73 ± 0.06 b |
| COX | 3.97 ± 0.18 a | 3.99 ± 0.2 a | 3.95 ± 0.19 a | 3.93 ± 0.18 a |
| Wavenumbers (cm−1) | PEF | UBAE | Cold-Pressed | Soxhlet |
|---|---|---|---|---|
| 3005 | 0.137 ± 0.009 | 0.143 ± 0.007 | 0.146 ± 0.004 | 0.144 ± 0.009 |
| 2922 | 0.998 ± 0.052 | 1.014 ± 0.068 | 1.034 ± 0.022 | 1.015 ± 0.048 |
| 2853 | 0.714 ± 0.045 | 0.732 ± 0.045 | 0.762 ± 0.018 | 0.732 ± 0.032 |
| 1746 | 1.117 ± 0.067 | 1.161 ± 0.086 | 1.11 ± 0.064 | 1.129 ± 0.078 |
| 988 | 0.18 ± 0.007 | 0.195 ± 0.012 | 0.184 ± 0.013 | 0.187 ± 0.006 |
| 966 | 0.184 ± 0.009 | 0.194 ± 0.008 | 0.194 ± 0.013 | 0.195 ± 0.014 |
| 912 | 0.147 ± 0.004 | 0.154 ± 0.004 | 0.156 ± 0.004 | 0.157 ± 0.007 |
| 721 | 0.553 ± 0.032 | 0.572 ± 0.017 | 0.6 ± 0.016 | 0.585 ± 0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanasiadis, V.; Giannopoulou, M.; Sarlami, G.; Bozinou, E.; Varagiannis, P.; Lalas, S.I. Green Optimization of Sesame Seed Oil Extraction via Pulsed Electric Field and Ultrasound Bath: Yield, Antioxidant Activity, Oxidative Stability, and Functional Food Potential. Foods 2025, 14, 3653. https://doi.org/10.3390/foods14213653
Athanasiadis V, Giannopoulou M, Sarlami G, Bozinou E, Varagiannis P, Lalas SI. Green Optimization of Sesame Seed Oil Extraction via Pulsed Electric Field and Ultrasound Bath: Yield, Antioxidant Activity, Oxidative Stability, and Functional Food Potential. Foods. 2025; 14(21):3653. https://doi.org/10.3390/foods14213653
Chicago/Turabian StyleAthanasiadis, Vassilis, Marianna Giannopoulou, Georgia Sarlami, Eleni Bozinou, Panagiotis Varagiannis, and Stavros I. Lalas. 2025. "Green Optimization of Sesame Seed Oil Extraction via Pulsed Electric Field and Ultrasound Bath: Yield, Antioxidant Activity, Oxidative Stability, and Functional Food Potential" Foods 14, no. 21: 3653. https://doi.org/10.3390/foods14213653
APA StyleAthanasiadis, V., Giannopoulou, M., Sarlami, G., Bozinou, E., Varagiannis, P., & Lalas, S. I. (2025). Green Optimization of Sesame Seed Oil Extraction via Pulsed Electric Field and Ultrasound Bath: Yield, Antioxidant Activity, Oxidative Stability, and Functional Food Potential. Foods, 14(21), 3653. https://doi.org/10.3390/foods14213653








