Harnessing Edible Insect Bioactives for Gut Health: A Comprehensive Review on Chitin-Derived Prebiotics and Peptidomic Insights from the Black Soldier Fly
Abstract
1. Introduction
2. Methodology
3. Edible Insects as Novel Functional Foods
3.1. Historical and Cultural Background of Entomophagy
3.2. Nutritional Composition of Edible Insects
3.3. Overview of Major Bioactive Classes Identified in Insects
4. Black Soldier Fly (H. illucens): Biology, Production, and Safety
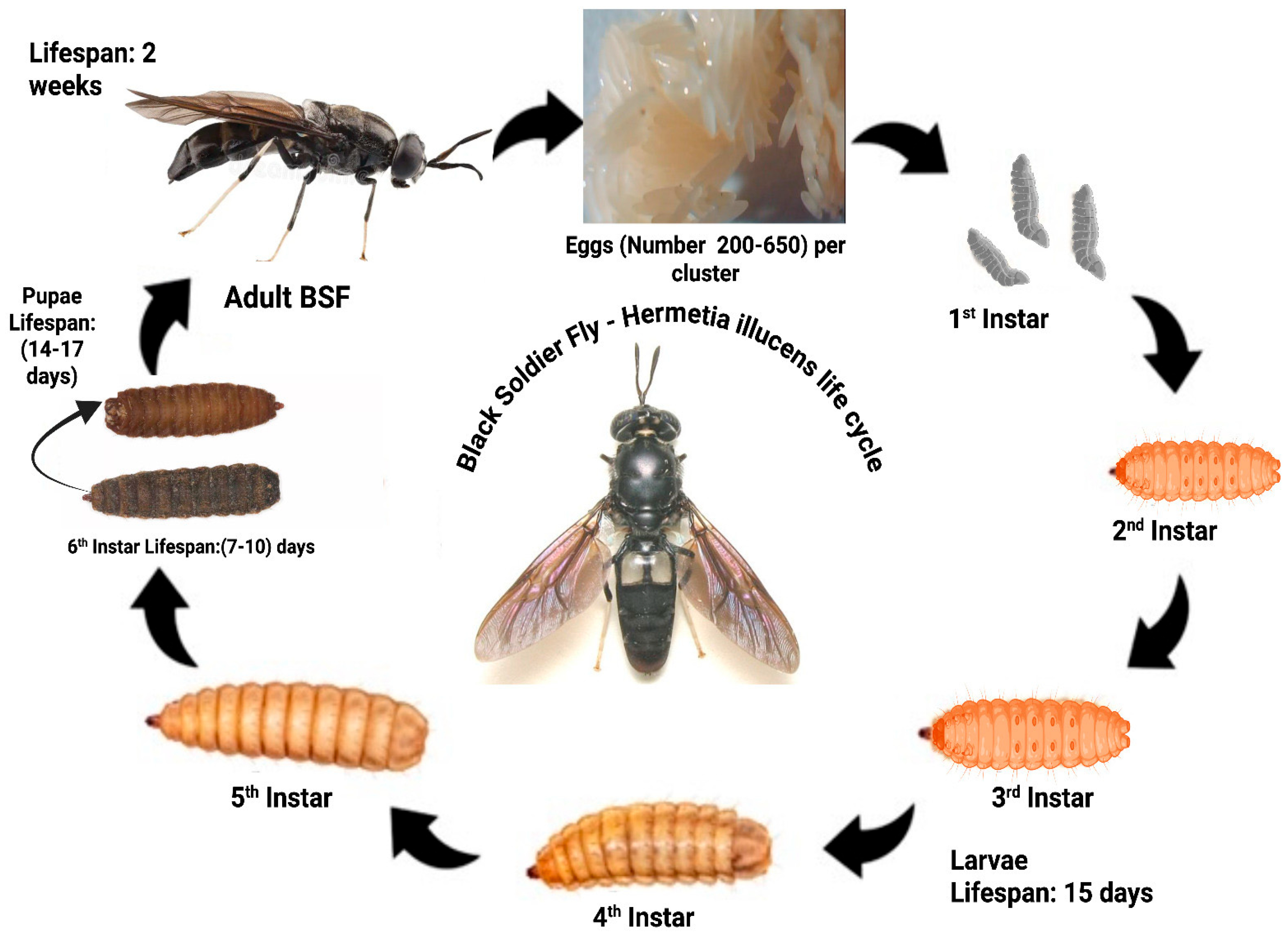
4.1. Life Cycle and Farming Practices
4.2. Safety Assessment for Human and Animal Consumption
4.3. Regulatory Landscape for Edible Insect Use
5. Bioactive Compounds in BSFL
5.1. Proteins and Amino Acid Profiles
5.2. Lipids and Fatty Acid Composition
5.3. Chitin and Chitosan: Chemical Structure and Biological Roles
5.4. Other Bioactives: Peptides, Antimicrobial Agents, Phenolics
6. Chitin-Derived Prebiotics: Mechanisms and Gut Health Benefits
6.1. Chemistry and Digestion of Chitin in the Gastrointestinal Tract
6.2. Role of Chitin and Derivatives as Dietary Fiber and Prebiotics
6.3. Impact on Gut Microbiota Diversity and Probiotic Growth
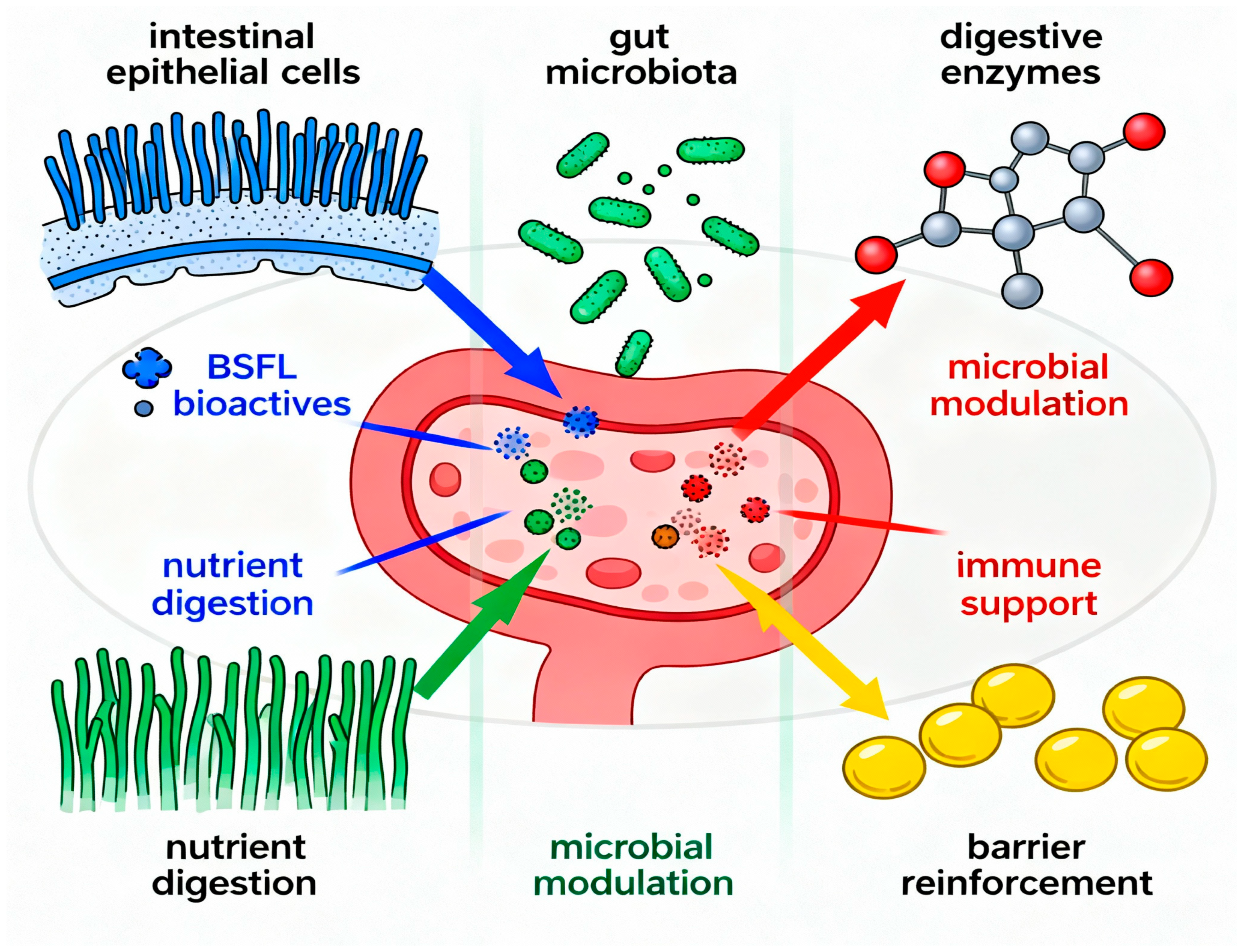
6.4. Effects on SCFAs Production, Gut Barrier Function, and Immune Modulation
6.5. Comparative Insights from Animal and Human Studies
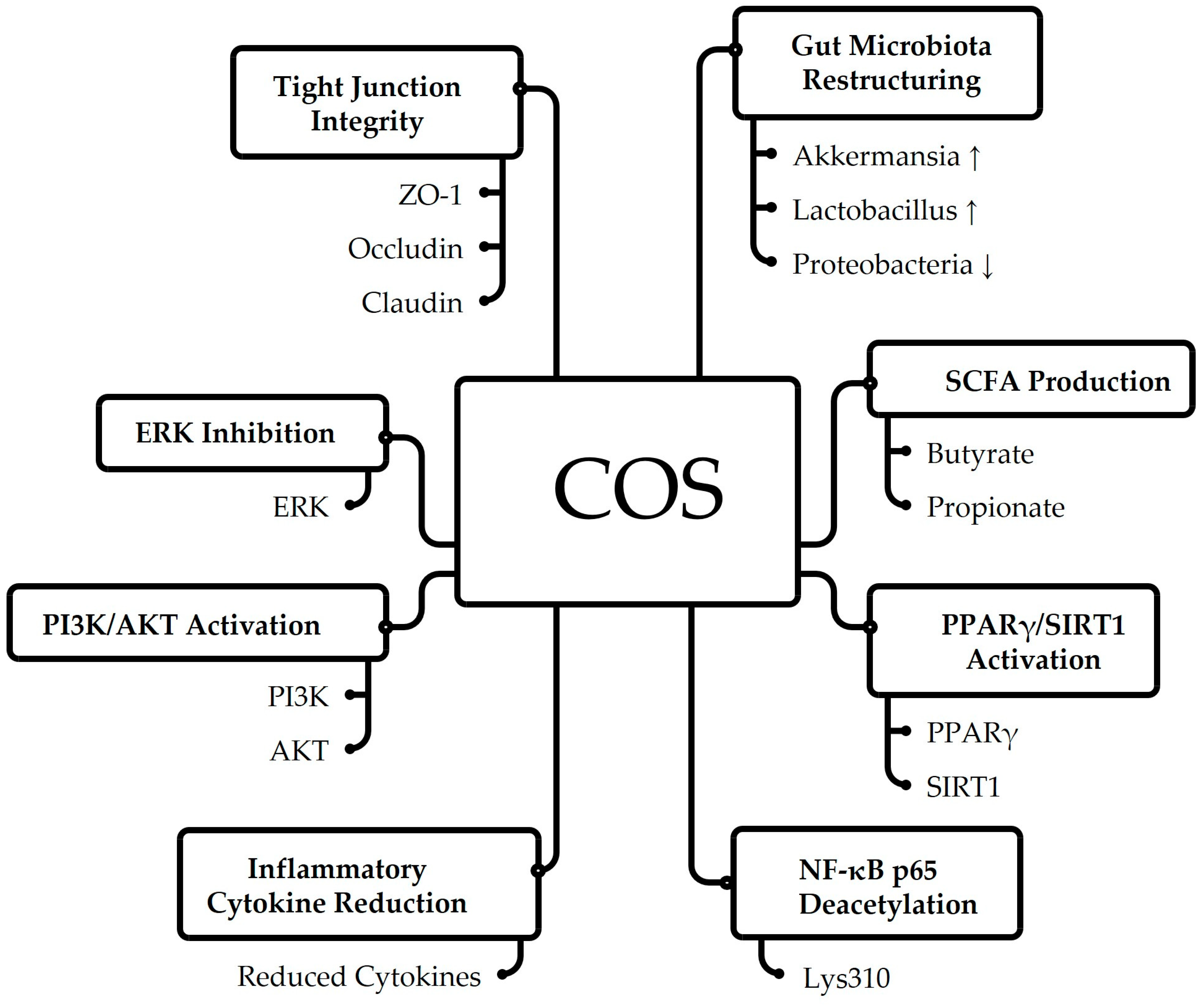
6.6. Mechanistic Pathways Linking BSFL-Derived Bioactives to Gut Health
7. Peptidomics of BSF Proteins
7.1. Overview of Peptidomics Technology and Its Application to Insect Proteins
7.2. Identification and Characterization of Bioactive Peptides from BSF
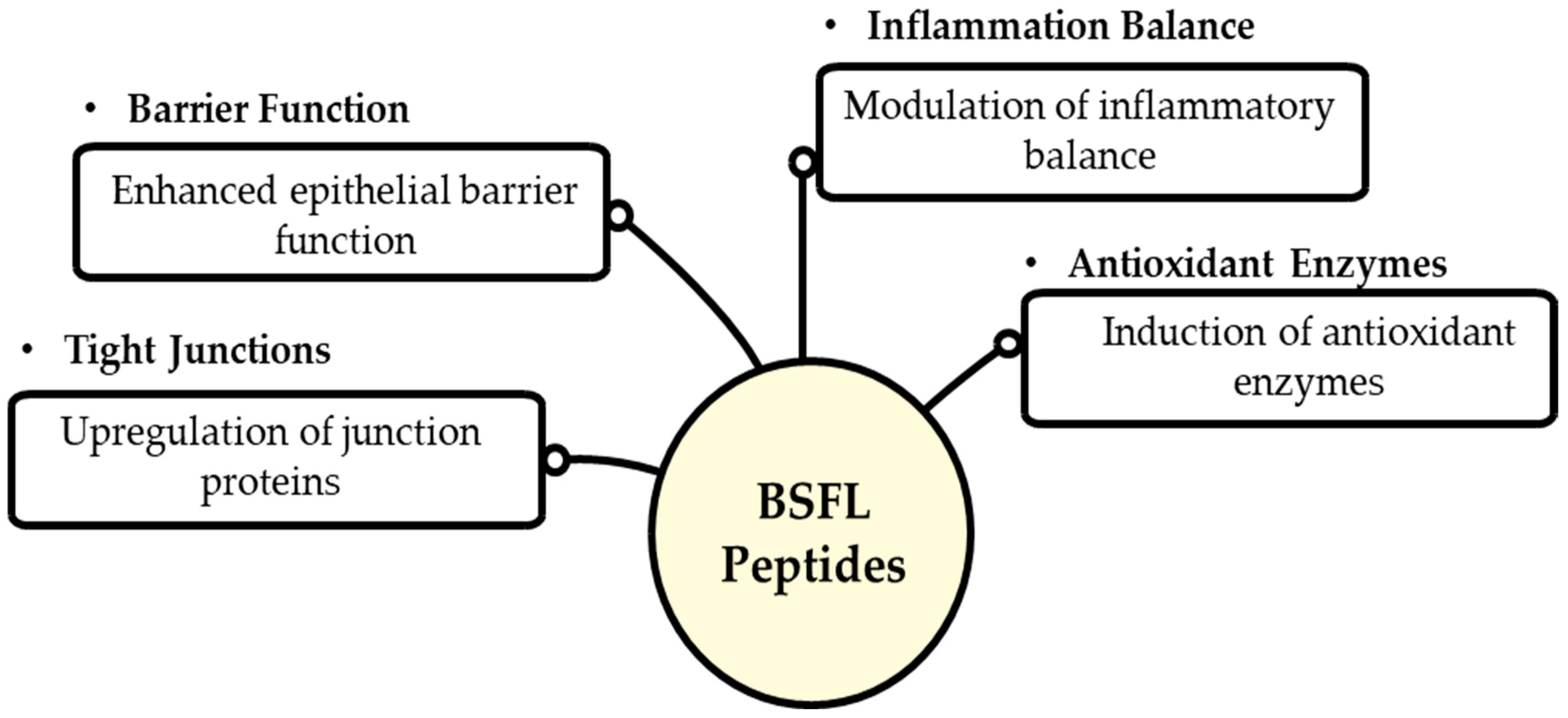
7.3. Biological Activities of Peptides Relevant to Gut Health
7.4. Potential Pathways Through Which Insect-Derived Peptides Influence Gut Microbiota and Host Health
8. Gut Microbiome Modulation by BSF-Derived Compounds
8.1. Interaction Between BSF Bioactives and Gut Microbial Communities
8.2. Effects on Microbial Diversity, Pathogen Inhibition, and Host Metabolism
8.3. Case Studies in Livestock, Aquaculture, and Experimental Human Models
9. Technological Considerations and Processing Influences
9.1. Methods of Harvesting, Drying, Defatting, and Extracting Bioactive Compounds
9.2. Impact of Processing on Stability and Bioavailability of Chitin and Peptides
9.3. Formulation Strategies for Incorporating BSF Bioactives into Functional Foods and Supplements
9.4. Economic Viability and Regional Industrialization Strategies
10. Safety, Allergenicity, and Regulatory Aspects
10.1. Microbial, Chemical, and Allergenic Risks in Edible Insect Consumption
10.2. Risk Mitigation Strategies and Quality Control Methods
10.3. Current and Evolving Regulatory Frameworks Globally for Edible Insect Products
11. Remarks and Future Perspectives
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazac, R.; Meinilä, J.; Korkalo, L.; Järviö, N.; Jalava, M.; Tuomisto, H.L. Incorporation of Novel Foods in European Diets Can Reduce Global Warming Potential, Water Use and Land Use by over 80%. Nat. Food. 2022, 3, 286–293. [Google Scholar] [CrossRef]
- Nirmal, N.; Anyimadu, C.F.; Khanashyam, A.C.; Bekhit, A.E.d.A.; Dhar, B.K. Alternative Protein Sources: Addressing Global Food Security and Environmental Sustainability. Sustain. Dev. 2025, 33, 3958–3969. [Google Scholar] [CrossRef]
- Lisboa, H.M.; Nascimento, A.; Arruda, A.; Sarinho, A.; Lima, J.; Batista, L.; Dantas, M.F.; Andrade, R. Unlocking the Potential of Insect-Based Proteins: Sustainable Solutions for Global Food Security and Nutrition. Foods 2024, 13, 1846. [Google Scholar] [CrossRef]
- Gil, M.; Rudy, M.; Duma-Kocan, P.; Stanisławczyk, R.; Krajewska, A.; Dziki, D.; Hassoon, W.H. Sustainability of Alternatives to Animal Protein Sources, a Comprehensive Review. Sustainability 2024, 16, 7701. [Google Scholar] [CrossRef]
- Graham, A.E.; Ledesma-Amaro, R. The Microbial Food Revolution. Nat. Commun. 2023, 14, 2231. [Google Scholar] [CrossRef]
- Malila, Y.; Owolabi, I.O.; Chotanaphuti, T.; Sakdibhornssup, N.; Elliott, C.T.; Visessanguan, W.; Karoonuthaisiri, N.; Petchkongkaew, A. Current Challenges of Alternative Proteins as Future Foods. npj Sci. Food 2024, 8, 53. [Google Scholar] [CrossRef]
- Wood, P.; Tavan, M. A Review of the Alternative Protein Industry. Curr. Opin. Food Sci. 2022, 47, 100869. [Google Scholar] [CrossRef]
- Barrett, M.; Chia, S.; Fischer, B.; Tomberlin, J. Welfare Considerations for Farming Black Soldier Flies, Hermetia illucens (Diptera: Stratiomyidae): A Model for the Insects as Food and Feed Industry. J. Insects Food Feed 2023, 9, 119–148. [Google Scholar] [CrossRef]
- FAO. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 978-92-5-107595-1. [Google Scholar]
- Rumpold, B.A.; Schlüter, O.K. Potential and Challenges of Insects as an Innovative Source for Food and Feed Production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, R.; Gaur, P.; Leal, É.; Lyu, X.; Ahmad, S.; Puri, P.; Chang, C.-M.; Raj, V.S.; Pandey, R.P. Unveiling Roles of Beneficial Gut Bacteria and Optimal Diets for Health. Front. Microbiol. 2025, 16, 1527755. [Google Scholar] [CrossRef] [PubMed]
- Safarchi, A.; Al-Qadami, G.; Tran, C.D.; Conlon, M. Understanding Dysbiosis and Resilience in the Human Gut Microbiome: Biomarkers, Interventions, and Challenges. Front. Microbiol. 2025, 16, 1559521. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Yang, X.; Li, Y.; Wang, Y.; Du, Y.; Wang, M.; Ye, R.; Wang, J.; Zhang, Y.; Chen, Y. Gut Microbiota Regulates Gut Homeostasis, Mucosal Immunity and Influences Immune-Related Diseases. Mol. Cell. Biochem. 2025, 480, 1969–1981. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Wu, C. Modulation of Gut Microbiota and Immune System by Probiotics, Pre-Biotics, and Post-Biotics. Front. Nutr. 2022, 8, 634897. [Google Scholar] [CrossRef]
- Yoo, S.; Jung, S.-C.; Kwak, K.; Kim, J.-S. The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health. Int. J. Mol. Sci. 2024, 25, 4834. [Google Scholar] [CrossRef]
- Wijesekara, T.; Abeyrathne, E.D.N.S.; Ahn, D.U. Effect of Bioactive Peptides on Gut Microbiota and Their Relations to Human Health. Foods 2024, 13, 1853. [Google Scholar] [CrossRef]
- Barathan, M.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. The Profound Influence of Gut Microbiome and Extracellular Vesicles on Animal Health and Disease. Int. J. Mol. Sci. 2024, 25, 4024. [Google Scholar] [CrossRef]
- Edo, G.I.; Mafe, A.N.; Ali, A.B.; Akpoghelie, P.O.; Yousif, E.; Apameio, J.I.; Isoje, E.F.; Igbuku, U.A.; Garba, Y.; Essaghah, A.E.A. Chitosan and Its Derivatives: A Novel Approach to Gut Microbiota Modulation and Immune System Enhancement. Int. J. Biol. Macromol. 2025, 289, 138633. [Google Scholar] [CrossRef] [PubMed]
- Stull, V.; Weir, T. Chitin and Omega-3 Fatty Acids in Edible Insects Have Underexplored Benefits for the Gut Microbiome and Human Health. Nat. Food. 2023, 4, 283–287. [Google Scholar] [CrossRef]
- Wijesekara, T.; Xu, B. New Insights into Sources, Bioavailability, Health-Promoting Effects, and Applications of Chitin and Chitosan. J. Agric. Food Chem. 2024, 72, 17138–17152. [Google Scholar] [CrossRef]
- Weththasinghe, P.; Rocha, S.D.; Øyås, O.; Lagos, L.; Hansen, J.Ø.; Mydland, L.T.; Øverland, M. Modulation of Atlantic Salmon (Salmo salar) Gut Microbiota Composition and Predicted Metabolic Capacity by Feeding Diets with Processed Black Soldier Fly (Hermetia illucens) Larvae Meals and Fractions. Anim. Microbiome 2022, 4, 9. [Google Scholar] [CrossRef]
- Quah, Y.; Tong, S.-R.; Bojarska, J.; Giller, K.; Tan, S.-A.; Ziora, Z.M.; Esatbeyoglu, T.; Chai, T.-T. Bioactive Peptide Discovery from Edible Insects for Potential Applications in Human Health and Agriculture. Molecules 2023, 28, 1233. [Google Scholar] [CrossRef]
- Rehman, K.u.; Hollah, C.; Wiesotzki, K.; Heinz, V.; Aganovic, K.; Rehman, R.u.; Petrusan, J.-I.; Zheng, L.; Zhang, J.; Sohail, S. Insect-Derived Chitin and Chitosan: A Still Unexploited Resource for the Edible Insect Sector. Sustainability 2023, 15, 4864. [Google Scholar] [CrossRef]
- Athanassiou, C.; Coudron, C.; Deruytter, D.; Rumbos, C.; Gasco, L.; Gai, F.; Sandrock, C.; De Smet, J.; Tettamanti, G.; Francis, A. A Decade of Advances in Black Soldier Fly Research: From Genetics to Sustainability. J. Insects Food Feed 2024, 11, 219–246. [Google Scholar] [CrossRef]
- Kirichenko-Babko, M.; Bieganowski, A. The Variety of Applications of Hermetia illucens in Industrial and Agricultural Areas-Review. Biology 2023, 12, 25. [Google Scholar]
- Macwan, S.; de Souza, T.S.P.; Dunshea, F.R.; DiGiacomo, K.; Suleria, H.A.R. Black Soldier Fly Larvae (Hermetica illucens) as a Sustainable Source of Nutritive and Bioactive Compounds, and Their Consumption Challenges. Anim. Prod. Sci. 2024, 64, AN23192. [Google Scholar] [CrossRef]
- Mshayisa, V.V.; Van Wyk, J.; Zozo, B. Nutritional, Techno-Functional and Structural Properties of Black Soldier Fly (Hermetia illucens) Larvae Flours and Protein Concentrates. Foods 2022, 11, 724. [Google Scholar] [CrossRef]
- Pedrazzani, C.; Righi, L.; Vescovi, F.; Maistrello, L.; Caligiani, A. Black Soldier Fly as a New Chitin Source: Extraction, Purification and Molecular/Structural Characterization. LWT 2024, 191, 115618. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B. Characterization of Chitin and Chitosan Derived from Hermetia illucens, a Further Step in a Circular Economy Process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef] [PubMed]
- Eke, M.; Tougeron, K.; Hamidovic, A.; Tinkeu, L.S.N.; Hance, T.; Renoz, F. Deciphering the Functional Diversity of the Gut Microbiota of the Black Soldier Fly (Hermetia illucens): Recent Advances and Future Challenges. Anim. Microbiome 2023, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Olivadese, M.; Dindo, M.L. Edible Insects: A Historical and Cultural Perspective on Entomophagy with a Focus on Western Societies. Insects 2023, 14, 690. [Google Scholar] [CrossRef]
- Granados-Echegoyen, C.; Vásquez-López, A.; Calderón-Cortés, N.; Gallego-Ocampo, H.L.; Gómez-Rodríguez, C.H.; Rodríguez-Vélez, J.M.; Sarmiento-Cordero, M.A.; Salamanca-Canizales, L.J.; Rodríguez-Vélez, B.; Arroyo-Balán, F. Brief Overview of Edible Insects: Exploring Consumption and Promising Sustainable Uses in Latin America. Front. Sustain. Food Syst. 2024, 8, 1385081. [Google Scholar] [CrossRef]
- Omuse, E.R.; Tonnang, H.E.Z.; Yusuf, A.A.; Machekano, H.; Egonyu, J.P.; Kimathi, E.; Mohamed, S.F.; Kassie, M.; Subramanian, S.; Onditi, J.; et al. The Global Atlas of Edible Insects: Analysis of Diversity and Commonality Contributing to Food Systems and Sustainability. Sci. Rep. 2024, 14, 5045. [Google Scholar] [CrossRef]
- Lange, K.W.; Nakamura, Y. Edible Insects as Future Food: Chances and Challenges. J. Future Foods 2021, 1, 38–46. [Google Scholar] [CrossRef]
- Matandirotya, N.R.; Filho, W.L.; Mahed, G.; Maseko, B.; Murandu, C.V. Edible Insects Consumption in Africa Towards Environmental Health and Sustainable Food Systems: A Bibliometric Study. Int. J. Environ. Res. Public Health 2022, 19, 14823. [Google Scholar] [CrossRef] [PubMed]
- Kipkoech, C.; Jaster-Keller, J.; Gottschalk, C.; Wesonga, J.; Maul, R. African Traditional Use of Edible Insects and Challenges Towards the Future Trends of Food and Feed. J. Insects Food Feed 2023, 9, 965–988. [Google Scholar] [CrossRef]
- Boukid, F.; Sogari, G.; Rosell, C.M. Edible Insects as Foods: Mapping Scientific Publications and Product Launches in the Global Market (1996–2021). J. Insects Food Feed 2022, 9, 353–368. [Google Scholar] [CrossRef]
- Florenca, S.G.; Guine, R.P.; Goncalves, F.J.; Barroca, M.J.; Ferreira, M.; Costa, C.A.; Correia, P.M.; Cardoso, A.P.; Campos, S.; Anjos, O. The Motivations for Consumption of Edible Insects: A Systematic Review. Foods 2022, 11, 3643. [Google Scholar] [CrossRef]
- Ros-Baro, M.; Casas-Agustench, P.; Díaz-Rizzolo, D.A.; Batlle-Bayer, L.; Adria-Acosta, F.; Aguilar-Martinez, A.; Medina, F.-X.; Pujola, M.; Bach-Faig, A. Edible Insect Consumption for Human and Planetary Health: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 11653. [Google Scholar] [CrossRef]
- Conway, A.; Jaiswal, S.; Jaiswal, A.K. The Potential of Edible Insects as a Safe, Palatable, and Sustainable Food Source in the European Union. Foods 2024, 13, 387. [Google Scholar] [CrossRef]
- Nachtigall, L.; Grune, T.; Weber, D. Proteins and Amino Acids from Edible Insects for the Human Diet—A Narrative Review Considering Environmental Sustainability and Regulatory Challenges. Nutrients 2025, 17, 1245. [Google Scholar] [CrossRef]
- Perez-Santaescolastica, C.; de Pril, I.; van de Voorde, I.; Fraeye, I. Fatty Acid and Amino Acid Profiles of Seven Edible Insects: Focus on Lipid Class Composition and Protein Conversion Factors. Foods 2023, 12, 4090. [Google Scholar] [CrossRef]
- Kolobe, S.D.; Manyelo, T.G.; Malematja, E.; Sebola, N.A.; Mabelebele, M. Fats and Major Fatty Acids Present in Edible Insects Utilised as Food and Livestock Feed. Vet. Anim. Sci. 2023, 22, 100312. [Google Scholar] [CrossRef]
- Bbosa, T.; Nakimbugwe, D.; Matthys, C.; Van Der Borght, M. A Systematic Review of Zinc, Iron and Vitamin B12 Content of Edible Insects and Comparison with Dietary Reference Values. Nutr. Res. Rev. 2025, 1–17. [Google Scholar] [CrossRef]
- Mwangi, M.N.; Oonincx, D.G.; Stouten, T.; Veenenbos, M.; Melse-Boonstra, A.; Dicke, M.; Van Loon, J.J. Insects as Sources of Iron and Zinc in Human Nutrition. Nutr. Res. Rev. 2018, 31, 248–255. [Google Scholar] [CrossRef]
- Kouřimská, L.; Adámková, A. Nutritional and Sensory Quality of Edible Insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Psarianos, M.; Aghababaei, F.; Schlüter, O.K. Bioactive Compounds in Edible Insects: Aspects of Cultivation, Processing and Nutrition. Food Res. Int. 2025, 203, 115802. [Google Scholar] [CrossRef]
- Ferri, I.; Canala, B.; Rossi, L. Unravelling the Role of Chitin and Chitosan in Prebiotic Activity and Correlation with Cancer: A Narrative Review. Nutr. Rev. 2024, 83, e2015–e2024. [Google Scholar] [CrossRef] [PubMed]
- Ushakova, N.; Dontsov, A.; Sakina, N.; Bastrakov, A.; Ostrovsky, M. Antioxidative Properties of Melanins and Ommochromes from Black Soldier Fly Hermetia illucens. Biomolecules 2019, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, P.; Dai, X.; Yao, X.; Zhou, S.; Ma, Q.; Liu, J.; Tian, S.; Zhu, J.; Zhang, J.; et al. Extraction, Physicochemical Properties, and Antioxidant Activity of Natural Melanin from Auricularia Heimuer Fermentation. Front. Nutr. 2023, 10, 1131542. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.S.S.; Villa, C.; Costa, J.; Ferreira, I.M.P.L.V.O.; Mafra, I. Edible Insects as a Novel Source of Bioactive Peptides: A Systematic Review. Foods 2023, 12, 2026. [Google Scholar] [CrossRef]
- Aiello, D.; Barbera, M.; Bongiorno, D.; Cammarata, M.; Censi, V.; Indelicato, S.; Mazzotti, F.; Napoli, A.; Piazzese, D.; Saiano, F. Edible Insects an Alternative Nutritional Source of Bioactive Compounds: A Review. Molecules 2023, 28, 699. [Google Scholar] [CrossRef]
- Sánchez-Estrada, M.; Aguirre-Becerra, H.; Feregrino-Pérez, A.A. Bioactive Compounds and Biological Activity in Edible Insects: A Review. Heliyon 2024, 10, e24045. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Chen, S.-C.; Xiao, J.-H.; Huang, D.-W. State-of-the-Art Review of Edible Insect: From Bioactives, Pretreatment to Enrichment. Food Biosci. 2024, 59, 103879. [Google Scholar] [CrossRef]
- Marra, A.; Hanson, M.A.; Kondo, S.; Erkosar, B.; Lemaitre, B. Drosophila Antimicrobial Peptides and Lysozymes Regulate Gut Microbiota Composition and Abundance. mBio 2021, 12, e0082421. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Swain, S.S.; Behera, A.; Sahoo, G.; Mahapatra, P.K.; Panda, S.K. Antimicrobial Peptides Derived from Insects Offer a Novel Therapeutic Option to Combat Biofilm: A Review. Front. Microbiol. 2021, 12, 661195. [Google Scholar] [CrossRef]
- Torres-Castillo, J.A.; Olazarán-Santibáñez, F.E. Insects as Source of Phenolic and Antioxidant Entomochemicals in the Food Industry. Front. Nutr. 2023, 10, 1133342. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, L.; Varriale, L.; Dipineto, L.; Pace, A.; Menna, L.F.; Fioretti, A. Insect Derived Lauric Acid as Promising Alternative Strategy to Antibiotics in the Antimicrobial Resistance Scenario. Front. Microbiol. 2021, 12, 620798. [Google Scholar] [CrossRef]
- Alejandro Ruiz, F.E.; Ortega Jácome, J.F.; Tejera, E.; Alvarez-Suarez, J.M. Edible Insects as Functional Foods: Bioactive Compounds, Health Benefits, Safety Concerns, Allergenicity, and Regulatory Considerations. Front. Nutr. 2025, 12, 1571084. [Google Scholar] [CrossRef] [PubMed]
- Marangon, A.; Paul, G.; Zaghi, R.; Marchese, L.; Gatti, G. Chitin Extracted from Black Soldier Fly Larvae at Different Growth Stages. Polymers 2024, 16, 2861. [Google Scholar] [CrossRef]
- Eggink, K.M.; Dalsgaard, J. Chitin Contents in Different Black Soldier Fly (Hermetia illucens) Life Stages. J. Insects Food Feed 2023, 9, 855–864. [Google Scholar] [CrossRef]
- Querejeta, M.; Hervé, V.; Perdereau, E.; Marchal, L.; Herniou, E.A.; Boyer, S.; Giron, D. Changes in Bacterial Community Structure across the Different Life Stages of Black Soldier Fly (Hermetia illucens). Microb. Syst. 2023, 86, 1254–1267. [Google Scholar] [CrossRef] [PubMed]
- Laursen, S.F.; Flint, C.A.; Bahrndorff, S.; Tomberlin, J.K.; Kristensen, T.N. Reproductive Output and Other Adult Life-History Traits of Black Soldier Flies Grown on Different Organic Waste and by-Products. Waste Manag. 2024, 181, 136–144. [Google Scholar] [CrossRef]
- Klüber, P.; Arous, E.; Zorn, H.; Rühl, M. Protein- and Carbohydrate-Rich Supplements in Feeding Adult Black Soldier Flies (Hermetia illucens) Affect Life History Traits and Egg Productivity. Life 2023, 13, 355. [Google Scholar] [CrossRef]
- Muraro, T.; Lalanne, L.; Pelozuelo, L.; Calas-List, D. Mating and Oviposition of a Breeding Strain of Black Soldier Fly Hermetia illucens (Diptera: Stratiomyidae): Polygynandry and Multiple Egg-Laying. J. Insects Food Feed 2024, 10, 1423–1435. [Google Scholar] [CrossRef]
- Auger, L.; Deschamps, M.-H.; Vandenberg, G.; Derome, N. Microbiota Is Structured by Gut Regions, Life Stage, and Diet in the Black Soldier Fly (Hermetia illucens). Front. Microbiol. 2023, 14, 1221728. [Google Scholar] [CrossRef]
- Elkadaoui, S.; Azzi, M.; Desbrieres, J.; Zim, J.; El Hachimi, Y.; Tolaimate, A. Valorization of Hermetia illucens Breeding Rejects by Chitins and Chitosans Production. Influence of Processes and Life Cycle on Their Physicochemical Characteristics. Int. J. Biol. Macromol. 2024, 266, 131314. [Google Scholar] [CrossRef]
- Kotsou, K.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Lalas, S.I. Exploiting Agri-Food Waste as Feed for Tenebrio molitor Larvae Rearing: A Review. Foods 2024, 13, 1027. [Google Scholar] [CrossRef] [PubMed]
- Muurmann, A.T.; Eriksen, N.T.; Rasmussen, J.A.; Limborg, M.T.; Tomberlin, J.K.; Gilbert, M.T.P.; Bahrndorff, S. Growth and Metabolic Performance of House Fly and Black Soldier Fly Larvae Differ across Densities and Waste-Based Growth Substrates. Waste Manag. 2025, 193, 529–538. [Google Scholar] [CrossRef]
- Nayak, A.; Klüber, P. The Hidden Drivers: Unraveling the Impact of Density, Moisture, and Scale on Hermetia illucens Rearing. PLoS ONE 2025, 20, e0317049. [Google Scholar] [CrossRef]
- Zhang, Q.-H.; Puniamoorthy, N. Impact of Rearing Substrates on Black Soldier Fly Growth and Fertility: A Semi-Industrial Scale Study to Optimize Egg Collection. Insects 2025, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, G.; Franco, A.; De Smet, J.; Scieuzo, C.; Salvia, R.; Falabella, P. Larval Frass of Hermetia illucens as Organic Fertilizer: Composition and Beneficial Effects on Different Crops. Insects 2024, 15, 293. [Google Scholar] [CrossRef]
- Alagappan, S.; Dong, A.; Hoffman, L.; Cozzolino, D.; Mantilla, S.O.; James, P.; Yarger, O.; Mikkelsen, D. Microbial Safety of Black Soldier Fly Larvae (Hermetia illucens) Reared on Food Waste Streams. Waste Manag. 2025, 194, 221–227. [Google Scholar] [CrossRef]
- Brulé, L.; Misery, B.; Baudouin, G.; Yan, X.; Guidou, C.; Trespeuch, C.; Foltyn, C.; Anthoine, V.; Moriceau, N.; Federighi, M.; et al. Evaluation of the Microbial Quality of Hermetia illucens Larvae for Animal Feed and Human Consumption: Study of Different Types of Rearing Substrates. Foods 2024, 13, 1587. [Google Scholar] [CrossRef]
- Hoek-van den Hil, E.F.; Meijer, N.P.; Van Rozen, K.; Elissen, H.; van Wikselaar, P.G.; Brust, H.; Te Loeke, N.A.J.M.; de Rijk, T.; Tienstra, M.; van de Schans, M.G.M.; et al. Safety of Black Soldier Fly (Hermetia illucens) Larvae Reared on Waste Streams of Animal and Vegetal Origin and Manure. J. Insects Food Feed 2023, 10, 771–783. [Google Scholar] [CrossRef]
- Heuel, M.; Kreuzer, M.; Gangnat, I.D.M.; Frossard, E.; Zurbrügg, C.; Egger, J.; Dortmans, B.; Gold, M.; Mathys, A.; Jaster-Keller, J.; et al. Low Transfer of Cadmium, Lead and Aflatoxin B1 to Eggs and Meat of Laying Hens Receiving Diets with Black Soldier Fly Larvae Reared on Contaminated Substrates. Anim. Feed Sci. Technol. 2023, 304, 115733. [Google Scholar] [CrossRef]
- Zulkifli, N.F.N.M.; Seok-Kian, A.Y.; Seng, L.L.; Mustafa, S.; Kim, Y.-S.; Shapawi, R. Nutritional Value of Black Soldier Fly (Hermetia illucens) Larvae Processed by Different Methods. PLoS ONE 2022, 17, e0263924. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; Thongpea, S.; Paengkoum, S.; Purba, R.A.P.; et al. Nutritional Composition of Black Soldier Fly Larvae (Hermetia illucens L.) and Its Potential Uses as Alternative Protein Sources in Animal Diets: A Review. Insects 2022, 13, 831. [Google Scholar] [CrossRef] [PubMed]
- Mufungwe, J.; Namukonde, N.; Mwaanga, P.; Johnson, T.; Siamujompa, M.; Mwango, N.C.; Ngoma, J.; Hang’ombe, B.M. Critical Safety Concerns in the Production of Black Soldier Fly (Hermetia illucens) Larvae in Africa. Discov. Food 2025, 5, 74. [Google Scholar] [CrossRef]
- Delgado, L.; Garino, C.; Moreno, F.J.; Zagon, J.; Broll, H. Sustainable Food Systems: Eu Regulatory Framework and Contribution of Insects to the Farm-to-Fork Strategy. Food Res. Int. 2022, 39, 6955–6976. [Google Scholar] [CrossRef]
- Vale-Hagan, W.; Singhal, S.; Grigoletto, I.; Totaro-Fila, C.; Theodoridou, K.; Koidis, A. Edible Insects in Mixed-Sourced Protein Meals for Animal Feed and Food: An Eu Focus. Food Humanit. 2023, 1, 1180–1187. [Google Scholar] [CrossRef]
- Grabowski, N.T.; Tchibozo, S.; Abdulmawjood, A.; Acheuk, F.; M’Saad Guerfali, M.; Sayed, W.A.; Plötz, M. Edible Insects in Africa in Terms of Food, Wildlife Resource, and Pest Management Legislation. Foods 2020, 9, 502. [Google Scholar] [CrossRef]
- Bekele, M.; Grace, D.; Knight-Jones, T.J.D.; Mutua, F.; Lindahl, J.F.; Daivadanam, M. Implementation Gaps in Food Safety Interventions: Evidence from a Multi-Vocal Review Focusing on Animal-Source Foods in Ethiopia. Front. Sustain. Food Syst. 2025, 9, 1546347. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Marimuthu, S.B.; Meijer, N. Regulations on Insects as Food and Feed: A Global Comparison. J. Insects Food Feed 2021, 7, 849–856. [Google Scholar] [CrossRef]
- Alagappan, S.; Rowland, D.; Barwell, R.; Cozzolino, D.; Mikkelsen, D.; Olarte Mantilla, S.M.; James, P.; Yarger, O.; Hoffman, L. Organic Side Streams (Bioproducts) as Substrate for Black Soldier Fly. Anim. Prod. Sci. 2022, 62, 1639–1651. [Google Scholar] [CrossRef]
- Sogari, G.; Amato, M.; Palmieri, R.; Hadj Saadoun, J.; Formici, G.; Verneau, F.; Mancini, S. The Future Is Crawling: Evaluating the Potential of Insects for Food and Feed Security. Curr. Res. Food Sci. 2023, 6, 100504. [Google Scholar] [CrossRef]
- Fuso, A.; Barbi, S.; Macavei, L.I.; Luparelli, A.V.; Maistrello, L.; Montorsi, M.; Sforza, S.; Caligiani, A. Effect of the Rearing Substrate on Total Protein and Amino Acid Composition in Black Soldier Fly. Foods 2021, 10, 1773. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.C.R.; Lehnen, C.R.; Marcato, S.M. Black Soldier Fly (Hermetia illucens) as a Protein Ingredient in Poultry Feed. World’s Poult. Sci. J. 2024, 80, 1123–1154. [Google Scholar] [CrossRef]
- Cheng, V.; Shoveller, A.K.; Huber, L.-A.; Kiarie, E.G. Comparative Protein Quality in Black Soldier Fly Larvae Meal Vs. Soybean Meal and Fish Meal Using Classical Protein Efficiency Ratio (Per) Chick Growth Assay Model. Poult. Sci. 2023, 102, 102255. [Google Scholar] [CrossRef]
- Fukuda, E.P.; Cox, J.R.; Wickersham, T.A.; Drewery, M.L. Evaluation of Black Soldier Fly Larvae (Hermetia illucens) as a Protein Supplement for Beef Steers Consuming Low-Quality Forage. Transl. Anim. Sci. 2022, 6, txac018. [Google Scholar] [CrossRef]
- Gautam, A.; Gyawali, I.; Poudel, S.; Devkota, S.; Acharya, R.; Kandel, M.; Subedi, D. Insects as Food and Feed Source: A Comprehensive Review on Nutritional Value, Food Safety Concern, Environmental Benefits, Economic Potential, Technological Innovations, Challenges, and Future Prospects. Food Front. 2025. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Y.; He, X.; Tan, B.; Liao, Z.; Chen, A.; Gu, X.; Li, X.; Chen, X.; Chen, B.; et al. Comprehensive Utilization of Black Soldier Fly (Hermetia illucens) Larvae: Extraction, Recovery and Characterization of Peptide, Chitin and Melanin and Scaling-up Trial. Sep. Purif. Technol. 2025, 361, 131262. [Google Scholar] [CrossRef]
- Van Etten, C.H.; Hubbard, J.E.; Mallan, J.M.; Smith, A.K.; Blessin, C.W. Amino Acids in Soybeans, Amino Acid Composition of Soybean Protein Fractions. J. Agric. Food Chem. 1959, 7, 129–131. [Google Scholar] [CrossRef]
- Suryati, T.; Julaeha, E.; Farabi, K.; Ambarsari, H.; Hidayat, A.T. Lauric Acid from the Black Soldier Fly (Hermetia illucens) and Its Potential Applications. Sustainability 2023, 15, 10383. [Google Scholar] [CrossRef]
- Almeida, C.; Murta, D.; Nunes, R.; Baby, A.R.; Fernandes, Â.; Barros, L.; Rijo, P.; Rosado, C. Characterization of Lipid Extracts from the Hermetia illucens Larvae a Nd Their Bioactivities for Potential Use as Pharmaceutical and Cosmetic Ingredients. Heliyon 2022, 8, e09455. [Google Scholar] [CrossRef] [PubMed]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty Acid Composition of Black Soldier Fly Larvae (Hermetia illucens)—Possibilities and Limitations for Modification through Diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef]
- Opatovsky, I.; Vitenberg, T.; Jonas-Levi, A.; Gutman, R. Does Consumption of Baker’s Yeast (Saccharomyces cerevisiae) by Black Soldier Fly (Diptera: Stratiomyidae) Larvae Affect Their Fatty Acid Composition? J. Insect Sci. 2021, 21, 5. [Google Scholar] [CrossRef]
- Cattaneo, A.; Meneguz, M.; Dabbou, S. The Fatty Acid Composition of Black Soldier Fly Larvae: The Influence of Feed Substrate and Applications in the Feed Industry. J. Insects Food Feed 2023, 10, 533–558. [Google Scholar] [CrossRef]
- Richter, H.; Gover, O.; Schwartz, B. Anti-Inflammatory Activity of Black Soldier Fly Oil Associated with Modulation of Tlr Signaling: A Metabolomic Approach. Int. J. Mol. Sci. 2023, 24, 10634. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Montevecchi, G.; Zanasi, L.; Bortolini, S.; Macavei, L.I.; Masino, F.; Maistrello, L.; Antonelli, A. Lipid Profile and Growth of Black Soldier Flies (Hermetia illucens, Stratiomyidae) Reared on by-Products from Different Food Chains. J. Sci. Food Agric. 2020, 100, 3648–3657. [Google Scholar] [CrossRef] [PubMed]
- Heuel, M.; Kreuzer, M.; Sandrock, C.; Leiber, F.; Mathys, A.; Gold, M.; Zurbrügg, C.; Gangnat, I.D.M.; Terranova, M. Transfer of Lauric and Myristic Acid from Black Soldier Fly Larval Lipids to Egg Yolk Lipids of Hens Is Low. Lipids 2021, 56, 423–435. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural Modification, Biological Activity and Application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Soetemans, L.; Uyttebroek, M.; Bastiaens, L. Characteristics of Chitin Extracted from Black Soldier Fly in Different Life Stages. Int. J. Biol. Macromol. 2020, 165, 3206–3214. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.N.; Chin, Y.L.; Chen, W.N. Comparison of Sustainable Lipid and Protein Removal Methods for the Isolation of Insect Chitin from Black Soldier Fly Exoskeleton. ACS Food Sci. Technol. 2021, 1, 698–706. [Google Scholar] [CrossRef]
- Xiong, A.; Ruan, L.; Ye, K.; Huang, Z.; Yu, C. Extraction of Chitin from Black Soldier Fly (Hermetia illucens) and Its Puparium by Using Biological Treatment. Life 2023, 13, 1424. [Google Scholar] [CrossRef]
- Witono, J.R.; Setyadi, F.F.; Deandra, P.P.; Wanta, K.C.; Miryanti, A.; Santoso, H.; Astuti, D.A.; Bulin, C.D.Q. A Comprehensive Analysis of Chitin Extraction from the Black Soldier Fly for Chitosan Production. Period. Polytech. Chem. Eng. 2024, 68, 507–522. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Liang, S.-H.; Lai, W.-L.; Lee, J.-X.; Wang, Y.-P.; Liu, Y.-T.; Wang, S.-H.; Lee, M.-H. Sustainable Extraction of Chitin from Spent Pupal Shell of Black Soldier Fly. Processes 2021, 9, 976. [Google Scholar] [CrossRef]
- Guan, Z.; Feng, Q. Chitosan and Chitooligosaccharide: The Promising Non-Plant-Derived Pre Biotics with Multiple Biological Activities. Int. J. Mol. Sci. 2022, 23, 6761. [Google Scholar] [CrossRef]
- Kipkoech, C.; Kinyuru, J.N.; Imathiu, S.; Meyer-Rochow, V.B.; Roos, N. In Vitro Study of Cricket Chitosan’s Potential as a Prebiotic and a Promoter of Probiotic Microorganisms to Control Pathogenic Bacteria in the Human Gut. Foods 2021, 10, 2310. [Google Scholar] [CrossRef]
- Teo, H.P.; Law, K.W.; Eric Chan, W.C.; Michelle Soo, O.Y. Antibacterial Properties of Chitosan Isolated from the Black Soldier Fly, Hermetia illucens. Sains Malays. 2022, 51, 3923–3935. [Google Scholar] [CrossRef]
- Liu, S.; Raheel Tariq, M.; Zhang, Q.; Wang, H.; Wang, F.; Zheng, C.; Li, K.; Zhuang, Z.; Wang, L. Dietary Influence on Growth, Physicochemical Stability, and Antimicrobial Mechanisms of Antimicrobial Peptides in Black Soldier Fly Larvae. Insects 2024, 15, 872. [Google Scholar] [CrossRef] [PubMed]
- Praseatsook, K.; Vachiraarunwong, A.; Taya, S.; Setthaya, P.; Sato, K.; Wanibuchi, H.; Wongpoomchai, R.; Dejkriengkraikul, P.; Gi, M.; Yodkeree, S. Anticancer and Antioxidant Effects of Bioactive Peptides from Black Soldier Fly Larvae (Hermetia illucens). Nutrients 2025, 17, 645. [Google Scholar] [CrossRef]
- Lu, J.; Guo, Y.; Muhmood, A.; Zeng, B.; Qiu, Y.; Wang, P.; Ren, L. Probing the Antioxidant Activity of Functional Proteins and Bioactive Peptides in Hermetia illucens Larvae Fed with Food Wastes. Sci. Rep. 2022, 12, 2799. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.-C.; Lee, Y.-H.; Ong, J.-H.; Manan, F.A.; Sabri, M.Z.; Chai, T.-T. Exploring the Potential of Black Soldier Fly Larval Proteins as Bioact Ive Peptide Sources through in Silico Gastrointestinal Proteolysis: A Cheminformatic Investigation. Catalysts 2023, 13, 605. [Google Scholar] [CrossRef]
- Riolo, K.; Rotondo, A.; La Torre, G.L.; Marino, Y.; Franco, G.A.; Crupi, R.; Fusco, R.; Di Paola, R.; Oliva, S.; De Marco, G.; et al. Cytoprotective and Antioxidant Effects of Hydrolysates from Black Soldier Fly (Hermetia illucens). Antioxidants 2023, 12, 519. [Google Scholar] [CrossRef]
- Leni, G.; Del Vecchio, L.; Dellapina, C.; Moliterni, V.M.C.; Caligiani, A.; Cirlini, M. Black Soldier Fly Larvae Grown on Hemp Fiber: Nutritional Composition and Production of Potential Bioactive Peptides. Macromol 2024, 4, 135–149. [Google Scholar] [CrossRef]
- Moretta, A.; Salvia, R.; Scieuzo, C.; Di Somma, A.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A Bioinformatic Study of Antimicrobial Peptides Identified in the Black Soldier Fly (Bsf) Hermetia illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 16875. [Google Scholar] [CrossRef]
- Gasco, L.; Biancarosa, I.; Liland, N.S. From Waste to Feed: A Review of Recent Knowledge on Insects as Producers of Protein and Fat for Animal Feeds. Curr. Opin. Green Sustain. Chem. 2020, 23, 67–79. [Google Scholar] [CrossRef]
- Anil, S. Potential Medical Applications of Chitooligosaccharides. Polymers 2022, 14, 3558. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Zhao, Z.; Pi, X.; Meng, Y.; Fei, D.; Liu, D.; Wang, X. Effect of Chitooligosaccharides on Human Gut Microbiota and Antiglycation. Carbohydr. Polym. 2020, 242, 116413. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, Y.; Luo, X.; Wang, C.; Wang, N.; He, H.; Zhang, T.; Chen, L. Chitooligosaccharides Modulate Glucose-Lipid Metabolism by Suppressing Smyd3 Pathways and Regulating Gut Microflora. Mar. Drugs 2020, 18, 69. [Google Scholar] [CrossRef]
- Refael, G.; Riess, H.T.; Levi, C.S.; Magzal, F.; Tamir, S.; Koren, O.; Lesmes, U. Responses of the Human Gut Microbiota to Physiologically Digested Insect Powders or Isolated Chitin Thereof. Future Foods 2022, 6, 100197. [Google Scholar] [CrossRef]
- Ji, X.; Zhu, L.; Chang, K.; Zhang, R.; Chen, Y.; Yin, H.; Jin, J.; Zhao, L. Chitooligosaccahrides: Digestion Characterization and Effect of the Degree of Polymerization on Gut Microorganisms to Manage the Metabolome Functional Diversity in Vitro. Carbohydr. Polym. 2022, 275, 118716. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Mondragon, A.d.C.; Lamas, A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Animal-Origin Prebiotics Based on Chitin: An Alternative for the Future? A Critical Review. Foods 2020, 9, 782. [Google Scholar] [CrossRef]
- Benchamas, G.; Huang, G.; Huang, S.; Huang, H. Preparation and Biological Activities of Chitosan Oligosaccharides. Trends Food Sci. Technol. 2021, 107, 38–44. [Google Scholar] [CrossRef]
- Zhai, X.; Li, C.; Ren, D.; Wang, J.; Ma, C.; Abd El-Aty, A.M. The Impact of Chitooligosaccharides and Their Derivatives on the in Vitro and in Vivo Antitumor Activity: A Comprehensive Review. Carbohydr. Polym. 2021, 266, 118132. [Google Scholar] [CrossRef]
- Zhou, D.-Y.; Wu, Z.-X.; Yin, F.-W.; Song, S.; Li, A.; Zhu, B.-W.; Yu, L.-L. Chitosan and Derivatives: Bioactivities and Application in Foods. Annu. Rev. Food Sci. Technol. 2021, 12, 407–432. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, L.; Qu, H.; Zhou, H.; Yang, H.; Chen, H. Physicochemical Characterization, Adsorption Function and Prebiotic Effect of Chitin-Glucan Complex from Mushroom Coprinus Comatus. Int. J. Biol. Macromol. 2022, 206, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Rajan, D.K.; Ganesan, A.R.; Divya, D.; Johansen, J.; Zhang, S. Chitin, Chitosan and Chitooligosaccharides as Potential Growth Promoters and Immunostimulants in Aquaculture: A Comprehensive Review. Int. J. Biol. Macromol. 2023, 251, 126285. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, M.; Van den Abbeele, P.; Ghyselinck, J.; Marzorati, M.; Rohs, E.; Birkett, A. Comparative Effect of 22 Dietary Sources of Fiber on Gut Microbiota of Healthy Humans in Vitro. Front. Nutr. 2021, 8, 700571. [Google Scholar] [CrossRef]
- Rezende, E.S.V.; Lima, G.C.; Naves, M.M.V. Dietary Fibers as Beneficial Microbiota Modulators: A Proposed Classification by Prebiotic Categories. Nutrition 2021, 89, 111217. [Google Scholar] [CrossRef]
- Abdi, R.; Joye, I.J. Prebiotic Potential of Cereal Components. Foods 2021, 10, 2338. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Li, H.; Ren, Y.; Geng, Y.; Lu, Z.; Shi, J.; Xu, Z. Similarities and Differences of Oligo/Poly-Saccharides’ Impact on Human Fecal Microbiota Identified by in Vitro Fermentation. Appl. Microbiol. Biotechnol. 2021, 105, 7475–7486. [Google Scholar] [CrossRef]
- Bai, J.; Li, Y.; Li, T.; Zhang, W.; Fan, M.; Zhang, K.; Qian, H.; Zhang, H.; Qi, X.; Wang, L. Comparison of Different Soluble Dietary Fibers During the in Vitro Fermentation Process. J. Agric. Food Chem. 2021, 69, 7446–7457. [Google Scholar] [CrossRef] [PubMed]
- Vinelli, V.; Biscotti, P.; Martini, D.; Del Bo’, C.; Marino, M.; Meroño, T.; Nikoloudaki, O.; Calabrese, F.M.; Turroni, S.; Taverniti, V.; et al. Effects of Dietary Fibers on Short-Chain Fatty Acids and Gut Microbiot a Composition in Healthy Adults: A Systematic Review. Nutrients 2022, 14, 2559. [Google Scholar] [CrossRef]
- Zhong, C.; Ukowitz, C.; Domig, K.J.; Nidetzky, B. Short-Chain Cello-Oligosaccharides: Intensification and Scale-up of Their Enzymatic Production and Selective Growth Promotion among Probiotic Bacteria. J. Agric. Food Chem. 2020, 68, 8557–8567. [Google Scholar] [CrossRef]
- Na, K.; Wei, J.; Zhang, L.; Fang, Y.; Li, X.; Lu, S.; Guo, X. Effects of Chitosan Oligosaccharides (Cos) and Fmt from Cos-Dosed Mice on Intestinal Barrier Function and Cell Apoptosis. Carbohydr. Polym. 2022, 297, 120043. [Google Scholar] [CrossRef]
- Young, W.; Arojju, S.K.; McNeill, M.R.; Rettedal, E.; Gathercole, J.; Bell, N.; Payne, P. Feeding Bugs to Bugs: Edible Insects Modify the Human Gut Microbiome in an in Vitro Fermentation Model. Front. Microbiol. 2020, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Anshory, M.; Effendi, R.M.R.A.; Kalim, H.; Dwiyana, R.F.; Suwarsa, O.; Nijsten, T.E.C.; Nouwen, J.L.; Thio, H.B. Butyrate Properties in Immune-Related Diseases: Friend or Foe? Fermentation 2023, 9, 205. [Google Scholar] [CrossRef]
- Xie, L.; Alam, M.J.; Marques, F.Z.; Mackay, C.R. A Major Mechanism for Immunomodulation: Dietary Fibres and Acid Metabo Lites. Semin. Immunol. 2023, 66, 101737. [Google Scholar] [CrossRef]
- Korsten, S.G.P.J.; Vromans, H.; Garssen, J.; Willemsen, L.E.M. Butyrate Protects Barrier Integrity and Suppresses Immune Activation in a Caco-2/Pbmc Co-Culture Model While Hdac Inhibition Mimics Butyrate in Restoring Cytokine-Induced Barrier Disruption. Nutrients 2023, 15, 2760. [Google Scholar] [CrossRef]
- Li, Y.; Wu, L.; Yong, Y.; Niu, X.; Gao, Y.; Zhou, Q.; Xie, H.; Liu, X.; Li, Y.; Yu, Z.; et al. Enhancing Gut Barrier Integrity: Upregulation of Tight Junction Proteins by Chitosan Oligosaccharide through the Erk1/2 Signaling Pathway. Nutrition 2024, 124, 112428. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.-H. Butyrate Producers, “the Sentinel of Gut”: Their Intestinal Significance with and Beyond Butyrate, and Prospective Use as Microbial Therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The Interplay between Gut Microbiota, Short-Chain Fatty Acids, and Implications for Host Health and Disease. Gut Microbes 2024, 16, 2393270. [Google Scholar] [CrossRef]
- Liu, H.; Lu, H.; Wang, Y.; Yu, C.; He, Z.; Dong, H. Unlocking the Power of Short-Chain Fatty Acids in Ameliorating Intesti Nal Mucosal Immunity: A New Porcine Nutritional Approach. Front. Cell. Infect. Microbiol. 2024, 14, 1449030. [Google Scholar] [CrossRef]
- Ayman, U.; Akter, L.; Islam, R.; Bhakta, S.; Rahman, M.A.; Islam, M.R.; Sultana, N.; Sharif, A.; Jahan, M.R.; Rahman, M.S.; et al. Dietary Chitosan Oligosaccharides Improves Health Status in Broilers for Safe Poultry Meat Production. Ann. Agric. Sci. 2022, 67, 90–98. [Google Scholar] [CrossRef]
- Lan, R.; Wu, F.; Wang, Y.; Lin, Z.; Wang, H.; Zhang, J.; Zhao, Z. Chitosan Oligosaccharide Improves Intestinal Function by Promoting Intestinal Development, Alleviating Intestinal Inflammatory Response, and Enhancing Antioxidant Capacity in Broilers Aged D 1 to 14. Poult. Sci. 2024, 103, 103381. [Google Scholar] [CrossRef]
- Gu, Y.F.; Chen, Y.P.; Jin, R.; Wang, C.; Wen, C.; Zhou, Y.M. Dietary Chitooligosaccharide Supplementation Alleviates Intestinal Barrier Damage, and Oxidative and Immunological Stress in Lipopolysaccharide-Challenged Laying Hens. Poult. Sci. 2022, 101, 101701. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Tang, D.; Dong, R.; Feng, Q. Chitosan Oligosaccharide Ameliorates Metabolic Syndrome Induced by Overnutrition Via Altering Intestinal Microbiota. Front. Nutr. 2021, 8, 743492. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jin, J.; Hou, F.; Song, B.; Li, Z.; Zhao, Y. Effects of Black Soldier Fly Larvae Oil on Growth Performance, Immunit Y and Antioxidant Capacity, and Intestinal Function and Microbiota of Broilers. J. Appl. Poult. Res. 2022, 31, 100292. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Y.; Chen, Y.; Qu, H.; Zhao, Y.; Wen, C.; Zhou, Y. Dietary Chitooligosaccharide Inclusion as an Alternative to Antibiotics Improves Intestinal Morphology, Barrier Function, Antioxidant Capacity, and Immunity of Broilers at Early Age. Animals 2019, 9, 493. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzmanr, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly Via Activation of Amp-Activated Protein Kinase in Caco-2 Cell Monolayers12. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, L.; Yu, J.; Shao, S. Advances in the Application and Mechanism of Bioactive Peptides in the Treatment of Inflammation. Front. Immunol. 2024, 15, 1413179. [Google Scholar] [CrossRef]
- Hu, Z.; Xia, M.; Wang, G.; Jia, L.; Ji, H.; Sun, J.; Yu, H. A Superior Chitin Product: Black Soldier Fly Larvae Chitin, Beneficial to Growth Performance, Muscle Quality and Health Status of Largemouth Bass Micropterus Salmoides, in Comparison to Shrimp Chitin. Aquaculture 2025, 595, 741667. [Google Scholar] [CrossRef]
- Smets, R.; Verbinnen, B.; Van De Voorde, I.; Aerts, G.; Claes, J.; Van Der Borght, M. Sequential Extraction and Characterisation of Lipids, Proteins, and Chitin from Black Soldier Fly (Hermetia illucens) Larvae, Prepupae, and Pupae. Waste Biomass Valorization 2020, 11, 6455–6466. [Google Scholar] [CrossRef]
- Bose, U.; Broadbent, J.A.; Juhász, A.; Karnaneedi, S.; Johnston, E.B.; Stockwell, S.; Byrne, K.; Limviphuvadh, V.; Maurer-Stroh, S.; Lopata, A.L.; et al. Comparison of Protein Extraction Protocols and Allergen Mapping from Black Soldier Fly Hermetia illucens. J. Proteom. 2022, 269, 104724. [Google Scholar] [CrossRef]
- Bose, U.; Juhasz, A.; Stockwell, S.; Escobar-Correas, S.; Marcora, A.; Paull, C.; Broadbent, J.A.; Wijffels, G. Unpacking the Proteome and Metaproteome of the Black Soldier Fly Larvae: Efficacy and Complementarity of Multiple Protein Extraction Protocols. ACS Omega 2023, 8, 7319–7330. [Google Scholar] [CrossRef]
- Meng, L.; Yu, D.; Lin, J.; Hu, Y.; Peng, N.; Zhao, S. Structural Characterization, Hepg2 Cell Cytoprotective Ability, and an Antioxidant Mechanism of Novel Antioxidant Peptides Identified from Black Soldier Fly Larvae (Hermetia illucens L.). Food Chem. 2025, 463, 141462. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Batista, P.; Gomes, J.E.G.; da Silva, R.; Pintado, M.M. Screening of Novel Bioactive Peptides from Goat Casein: In Silico to in Vitro Validation. Int. J. Mol. Sci. 2022, 23, 2439. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wu, J. Impact of Food-Derived Bioactive Peptides on Gut Function and Health. Food Res. Int. 2021, 147, 110485. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Holman, D.R.; Puntasecca, C.J.; Polevoi, D.; Rubin, S.J.S.; Rogalla, S. Antimicrobial Peptides and the Gut Microbiome in Inflammatory Bowel Disease. World J. Gastroenterol. 2021, 27, 7402–7422. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Zong, X.; Wang, C.; Shi, C.; Wang, F.; Wang, Y.; Lu, Z. Peptides Derived from Fermented Soybean Meal Suppresses Intestinal Inf Lammation and Enhances Epithelial Barrier Function in Piglets. Food Agric. Immunol. 2019, 31, 120–135. [Google Scholar] [CrossRef]
- Li, M.; Ge, Q.; Du, H.; Jiang, P.; Bao, Z.; Chen, D.; Lin, S. Potential Mechanisms Mediating the Protective Effects of Tricholoma Matsutake Derived Peptides in Mitigating Dss-Induced Colitis. J. Agric. Food Chem. 2021, 69, 5536–5546. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Oh, H.; Oh, N.S.; Seo, Y.; Kang, J.; Park, M.H.; Kim, K.S.; Kang, S.H.; Yoon, Y. Anti-Inflammatory Effect of a Peptide Derived from the Synbiotics, Fermented Cudrania tricuspidata with Lactobacillus gasseri on Inflammatory Bowel Disease. Mediat. Inflamm. 2020, 2020, 3572809. [Google Scholar] [CrossRef]
- Zhu, W.; Ren, L.; Zhang, L.; Qiao, Q.; Farooq, M.Z.; Xu, Q. The Potential of Food Protein-Derived Bioactive Peptides against Chronic Intestinal Inflammation. Mediat. Inflamm. 2020, 2020, 6817156. [Google Scholar] [CrossRef]
- Koutsos, E.; Modica, B.; Freel, T. Immunomodulatory Potential of Black Soldier Fly Larvae: Applications B Eyond Nutrition in Animal Feeding Programs. Transl. Anim. Sci. 2022, 6, txac084. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; D’Ambrosio, F.; Caruso, S.; Gatto, R.; Caruso, S. Bioactive Peptides Derived from Edible Insects: Effects on Human Health and Possible Applications in Dentistry. Nutrients 2023, 15, 4611. [Google Scholar] [CrossRef]
- Tarahi, M.; Aghababaei, F.; McClements, D.J.; Pignitter, M.; Hadidi, M. Bioactive Peptides Derived from Insect Proteins: Preparation, Biologic Al Activities, Potential Applications, and Safety Issues. Food Chem. 2025, 465, 142113. [Google Scholar] [CrossRef] [PubMed]
- Lachat, J.; Lextrait, G.; Jouan, R.; Boukherissa, A.; Yokota, A.; Jang, S.; Ishigami, K.; Futahashi, R.; Cossard, R.; Naquin, D.; et al. Hundreds of Antimicrobial Peptides Create a Selective Barrier for Insect Gut Symbionts. Proc. Natl. Acad. Sci. USA 2024, 121, e2401802121. [Google Scholar] [CrossRef]
- Cardoso, M.H.; Meneguetti, B.T.; Oliveira-Júnior, N.G.; Macedo, M.L.R.; Franco, O.L. Antimicrobial Peptide Production in Response to Gut Microbiota Imbalance. Peptides 2022, 157, 170865. [Google Scholar] [CrossRef]
- Mu, J.; Lin, Q.; Liang, Y. An Update on the Effects of Food-Derived Active Peptides on the Intestinal Microecology. Crit. Rev. Food Sci. Nutr. 2022, 63, 11625–11639. [Google Scholar] [CrossRef]
- Yang, Q.; Lyu, S.; Xu, M.; Li, S.; Du, Z.; Liu, X.; Shang, X.; Yu, Z.; Liu, J.; Zhang, T. Potential Benefits of Egg White Proteins and Their Derived Peptides in the Regulation of the Intestinal Barrier and Gut Microbiota: A Compre Hensive Review. J. Agric. Food Chem. 2023, 71, 13168–13180. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Jaffar, S.; Xu, Y.; Qi, Y. The Intestinal Immune Defense System in Insects. Int. J. Mol. Sci. 2022, 23, 15132. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, L.; Generalovic, T.; Ali, Y.M.; Seilly, D.; Sivanesan, K.; Kalmar, L.; Pipan, M.; Christie, G.; Grant, A.J. A Novel Family of Defensin-Like Peptides from Hermetia illucens with Antibacterial Properties. BMC Microbiol. 2024, 24, 167. [Google Scholar] [CrossRef]
- Pimchan, T.; Hamzeh, A.; Siringan, P.; Thumanu, K.; Hanboonsong, Y.; Yongsawatdigul, J. Antibacterial Peptides from Black Soldier Fly (Hermetia illucens) Larvae: Mode of Action and Characterization. Sci. Rep. 2024, 14, 26469. [Google Scholar] [CrossRef]
- Bonomini, M.G.; Verstringe, S.; Bruggeman, G.; Vandercruyssen, R.; Carmans, H.; Caligiani, A. Characterisation, Antibacterial Activity, and Prebiotic Potential of Dried Hermetia illucens L. Larvae and of Their Fractions. J. Insects Food Feed 2024, 11, 273–288. [Google Scholar] [CrossRef]
- Salam, M.; Bolletta, V.; Meng, Y.; Yakti, W.; Grossule, V.; Shi, D.; Hayat, F. Exploring the Role of the Microbiome of the H. Illucens (Black Soldier Fly) for Microbial Synergy in Optimizing Black Soldier Fly Rearing and Subsequent Applications. Environ. Pollut. 2024, 363, 125055. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, J.; Cheng, X.; Li, C.; Yuan, Z. Relationship of Black Soldier Fly Larvae (Bsfl) Gut Microbiota and Bioconversion Efficiency with Properties of Substrates. Waste Manag. 2024, 180, 106–114. [Google Scholar] [CrossRef]
- Spragge, F.; Bakkeren, E.; Jahn, M.T.; Araujo, E.B.N.; Pearson, C.F.; Wang, X.; Pankhurst, L.; Cunrath, O.; Foster, K.R. Microbiome Diversity Protects against Pathogens by Nutrient Blocking. Science 2023, 382, eadj3502. [Google Scholar] [CrossRef]
- Junaid, M.; Lu, H.; Li, Y.; Liu, Y.; Din, A.U.; Qi, Z.; Xiong, Y.; Yan, J. Novel Synergistic Probiotic Intervention: Transcriptomic and Metabolomic Analysis Reveals Ameliorative Effects on Immunity, Gut Barrier, and Metabolism of Mice During Salmonella typhimurium Infection. Genes 2024, 15, 435. [Google Scholar] [CrossRef]
- Heumel, S.; de Rezende Rodovalho, V.; Urien, C.; Specque, F.; Brito Rodrigues, P.; Robil, C.; Delval, L.; Sencio, V.; Descat, A.; Deruyter, L.; et al. Shotgun Metagenomics and Systemic Targeted Metabolomics Highlight Indo Le-3-Propionic Acid as a Protective Gut Microbial Metabolite against Influenza Infection. Gut Microbes 2024, 16, 2325067. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, H.; Yang, Y.; Yang, X.; Li, X.; Zhong, W.; Wen, B.; He, F.; Li, J. Reinventing Gut Health: Leveraging Dietary Bioactive Compounds for the Prevention and Treatment of Diseases. Front. Nutr. 2024, 11, 1491821. [Google Scholar] [CrossRef]
- Fu, Y.; Lyu, J.; Wang, S. The Role of Intestinal Microbes on Intestinal Barrier Function and Host Immunity from a Metabolite Perspective. Front. Immunol. 2023, 14, 1277102. [Google Scholar] [CrossRef] [PubMed]
- de Souza Vilela, J.; Kheravii, S.K.; Sharma Bajagai, Y.; Kolakshyapati, M.; Zimazile Sibanda, T.; Wu, S.-B.; Andrew, N.R.; Ruhnke, I. Inclusion of up to 20% Black Soldier Fly Larvae Meal in Broiler Chicken Diet Has a Minor Effect on Caecal Microbiota. PeerJ 2023, 11, e15857. [Google Scholar] [CrossRef] [PubMed]
- Ndotono, E.W.; Khamis, F.M.; Bargul, J.L.; Tanga, C.M. Insights into the Gut Microbial Communities of Broiler Chicken Fed Black Soldier Fly Larvae-Desmodium-Based Meal as a Dietary Protein Source. Microorganisms 2022, 10, 1351. [Google Scholar] [CrossRef]
- Leeper, A.; Benhaïm, D.; Smárason, B.Ö.; Knobloch, S.; Òmarsson, K.L.; Bonnafoux, T.; Pipan, M.; Koppe, W.; Björnsdóttir, R.; Øverland, M. Feeding Black Soldier Fly Larvae (Hermetia illucens) Reared on Organic Rest Streams Alters Gut Characteristics of Atlantic Salmon (Salmo salar). J. Insects Food Feed 2022, 8, 1355–1372. [Google Scholar] [CrossRef]
- Elhag, O.; Zhang, Y.; Xiao, X.; Cai, M.; Zheng, L.; Jordan, H.R.; Tomberlin, J.K.; Huang, F.; Yu, Z.; Zhang, J. Inhibition of Zoonotic Pathogens Naturally Found in Pig Manure by Black Soldier Fly Larvae and Their Intestine Bacteria. Insects 2022, 13, 66. [Google Scholar] [CrossRef]
- Huang, C.; Hernandez, C.E.; Wall, H.; Tahamtani, F.M.; Ivarsson, E.; Sun, L. Live Black Soldier Fly (Hermetia illucens) Larvae in Feed for Laying Hens: Effects on Hen Gut Microbiota and Behavior. Poult. Sci. 2024, 103, 103429. [Google Scholar] [CrossRef]
- Xia, J.; Ge, C.; Yao, H. Antimicrobial Peptides from Black Soldier Fly (Hermetia illucens) as Potential Antimicrobial Factors Representing an Alternative to Antibiotics in Livestock Farming. Animals 2021, 11, 1937. [Google Scholar] [CrossRef]
- Hurtado-Ribeira, R.; Villanueva-Bermejo, D.; García-Risco, M.R.; Hernández, M.D.; Sánchez-Muros, M.J.; Fornari, T.; Vázquez, L.; Martin, D. Evaluation of the Interrelated Effects of Slaughtering, Drying, and De-Fatting Methods on the Composition and Properties of Black Soldier Fly (Hermetia illucens) Larvae Fat. Curr. Res. Food Sci. 2023, 7, 100633. [Google Scholar] [CrossRef]
- Lehmad, M.; Nomeir, B.; Hidra, N.; El Hachimi, Y.; Abdenouri, N. Impact of Hybrid Drying on the Drying Kinetics, Nutritional, Physicochemical, Functional, Structural, and Thermal Properties of Black Soldier Fly Larvae. J. Insects Food Feed 2025, 1, 1–26. [Google Scholar] [CrossRef]
- Cruz, V.A.; Ferreira, N.J.; Le Roux, E.; Destandau, E.; de Oliveira, A.L. Intensification of the Sfe Using Ethanol as a Cosolvent and Integration of the Sfe Process with Sc-Co2 Followed by Ple Using Pressurized Ethanol of Black Soldier Fly (Hermetia illucens L.) Larvae Meal-Extract Yield and Characterization. Foods 2024, 13, 1620. [Google Scholar] [CrossRef]
- Pan, J.; Xu, H.; Dabbour, M.; Kumah Mintah, B.; Chen, W.; Yang, F.; Zhang, Z.; Cheng, Y.; Dai, C.; He, R.; et al. Effect of Alkaline Ph-Shifting Process on Extraction Rate, Structural, and Functional Properties of Black Soldier Fly (Hermetia illucens) Larvae Protein. LWT 2023, 185, 115180. [Google Scholar] [CrossRef]
- Bogusz, R.; Bryś, J.; Onopiuk, A.; Pobiega, K.; Tomczak, A.; Kowalczewski, P.Ł.; Rybak, K.; Nowacka, M. The Impact of Drying Methods on the Quality of Blanched Yellow Mealworm (Tenebrio molitor L.). Larvae. Mol. 2024, 29, 3679. [Google Scholar] [CrossRef]
- Hurtado-Ribeira, R.; Hernández, D.M.; Villanueva-Bermejo, D.; García-Risco, M.R.; Hernández, M.D.; Vázquez, L.; Fornari, T.; Martin, D. The Interaction of Slaughtering, Drying, and Defatting Methods Differently Affects Oxidative Quality of the Fat from Black Soldier Fly (Hermetia illucens) Larvae. Insects 2023, 14, 368. [Google Scholar] [CrossRef]
- Keil, C.; Grebenteuch, S.; Kröncke, N.; Kulow, F.; Pfeif, S.; Kanzler, C.; Rohn, S.; Boeck, G.; Benning, R.; Haase, H. Systematic Studies on the Antioxidant Capacity and Volatile Compound Profile of Yellow Mealworm Larvae (T. Molit. L.) Under Different Drying Regimes. Insects 2022, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zaini, N.S.; Lim, E.J.; Ahmad, N.H.; Gengatharan, A.; Wan-Mohtar, W.A.A.Q.I.; Abd Rahim, M.H. The Review of Cooking, Drying, and Green Extraction Methods on General Nutritional Properties of Mealworms and Locusts. Food Bioprocess Technol. 2023, 16, 1904–1918. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Le, T.-D.; Suttikhana, I. Stability and Bioactivity of Peptides in Food Matrices Based on Processing Conditions. Food Res. Int. 2023, 168, 112786. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Pino, F. Bioactive Food-Derived Peptides for Functional Nutrition: Effect of Fortification, Processing and Storage on Peptide Stability and Bioactivity within Food Matrices. Food Chem. 2023, 406, 135046. [Google Scholar] [CrossRef]
- Queiroz, L.S.; Casanova, F.; Feyissa, A.H.; Jessen, F.; Ajalloueian, F.; Perrone, I.T.; de Carvalho, A.F.; Mohammadifar, M.A.; Jacobsen, C.; Yesiltas, B. Physical and Oxidative Stability of Low-Fat Fish Oil-in-Water Emulsions Stabilized with Black Soldier Fly (Hermetia illucens) Larvae Protein Concentrate. Foods 2021, 10, 2977. [Google Scholar] [CrossRef]
- Wang, J.; Jousse, M.; Jayakumar, J.; Fernández-Arteaga, A.; de Lamo-Castellví, S.; Ferrando, M.; Güell, C. Black Soldier Fly (Hermetia illucens) Protein Concentrates as a Sustainable Source to Stabilize O/W Emulsions Produced by a Low-Energy High-Throughput Emulsification Technology. Foods 2021, 10, 1048. [Google Scholar] [CrossRef]
- Bessa, L.W.; Pieterse, E.; Marais, J.; Hoffman, L.C. Techno-Functional Properties of Black Soldier Fly (Hermetia illucens) Larvae. J. Insects Food Feed 2022, 8, 1047–1060. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Quintanar-Guerrero, D.; Liceaga, A.M.; Zambrano-Zaragoza, M.L. Encapsulation of Bioactive Peptides: A Strategy to Improve the Stability, Protect the Nutraceutical Bioactivity and Support Their Food Applications. RSC Adv. 2022, 12, 6449–6458. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Gómez, B.; Munekata, P.E.S.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of Promising Bioactive Compounds to Improve Their Absorption, Stability, Functionality and the Appearance of the Final Food Products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.W.; Pieterse, E.; Marais, J.; Hoffman, L.C. Why for Feed and Not for Human Consumption? The Black Soldier Fly Larvae. Comp. Rev. Food Sci. Food Safe 2020, 19, 2747–2763. [Google Scholar] [CrossRef] [PubMed]
- Muslykhah, U.; Phupaboon, S.; Suriyapha, C.; Matra, M.; Wanapat, M. Encapsulation of Protein-Based Bioactive from Black Soldier Fly for Ruminant Feeding. J. Agric. Food Res. 2024, 18, 101325. [Google Scholar] [CrossRef]
- Lehmad, M.; Nomeir, B.; Hidra, N.; Lhomme, P.; El Hachimi, Y.; Abdenouri, N. Comparative Analysis of Drying Technologies for Black Soldier Fly (Hermetia illucens) Larvae: Multidimensional Assessment. J. Insects Food Feed 2025, 1, 1–21. [Google Scholar] [CrossRef]
- Lehmad, M.; Hidra, N.; Lhomme, P.; Mghazli, S.; Hachimi, Y.E.; Abdenouri, N. Environmental, Economic and Quality Assessment of Hybrid Solar-Electric Drying of Black Soldier Fly (Hermetia illucens) Larvae. Renew. Energy 2024, 226, 120401. [Google Scholar] [CrossRef]
- Chakawa, D.; Goosen, N. Protein Recovery from Black Soldier Fly Larvae Using Enzymatic Hydrolysis and Alkaline Extraction. J. Insects Food Feed 2025, 1, 1–22. [Google Scholar] [CrossRef]
- Niyonsaba, H.; Höhler, J.; Kooistra, J.; Van der Fels-Klerx, H.; Meuwissen, M. Profitability of Insect Farms. J. Insects Food Feed 2021, 7, 923–934. [Google Scholar] [CrossRef]
- Leipertz, M.; Hogeveen, H.; Saatkamp, H. Economic Supply Chain Modelling of Industrial Insect Production in the Netherlands. J. Insects Food Feed 2024, 10, 1361–1385. [Google Scholar] [CrossRef]
- Bessa, L.W.; Pieterse, E.; Marais, J.; Dhanani, K.; Hoffman, L.C. Food Safety of Consuming Black Soldier Fly (Hermetia illucens) Larvae: Microbial, Heavy Metal and Cross-Reactive Allergen Risks. Foods 2021, 10, 1934. [Google Scholar] [CrossRef]
- Kolakowski, B.M.; Johaniuk, K.; Zhang, H.; Yamamoto, E. Analysis of Microbiological and Chemical Hazards in Edible Insects Available to Canadian Consumers. J. Food Prot. 2021, 84, 1575–1581. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Sousa-Pinto, B.; Fonseca, J.; Fonseca, S.C.; Cunha, L.M. Edible Insects and Food Safety: Allergy. J. Insects Food Feed 2021, 7, 833–847. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, S.; Kuang, H.; Tang, C.; Song, J. Edible Insects as Ingredients in Food Products: Nutrition, Functional Properties, Allergenicity of Insect Proteins, and Processing Modifications. Crit. Rev. Food Sci. Nutr. 2023, 64, 10361–10383. [Google Scholar] [CrossRef]
- De Marchi, L.; Wangorsch, A.; Zoccatelli, G. Allergens from Edible Insects: Cross-Reactivity and Effects of Processing. Curr. Allergy Asthma Rep. 2021, 21, 35. [Google Scholar] [CrossRef]
- Cappelli, A.; Cini, E.; Lorini, C.; Oliva, N.; Bonaccorsi, G. Insects as Food: A Review on Risks Assessments of Tenebrionidae and Gryllidae in Relation to a First Machines and Plants Development. Food Control 2020, 108, 106877. [Google Scholar] [CrossRef]
- Kooh, P.; Jury, V.; Laurent, S.; Audiat-Perrin, F.; Sanaa, M.; Tesson, V.; Federighi, M.; Boué, G. Control of Biological Hazards in Insect Processing: Application of Hac. Foods 2020, 9, 1528. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.M.; Meijer, N.; Hoek-van den Hil, E.F.; van der Fels-Klerx, H.J. Chemical Food Safety Hazards of Insects Reared for Food and Feed. J. Insects Food Feed 2021, 7, 823–831. [Google Scholar] [CrossRef]
- Ojha, S.; Bußler, S.; Psarianos, M.; Rossi, G.; Schlüter, O.K. Edible Insect Processing Pathways and Implementation of Emerging Technologies. J. Insects Food Feed 2021, 7, 877–900. [Google Scholar] [CrossRef]
- Yan, X.; Laurent, S.; Federighi, M.; Boué, G.; Jury, V. Processing Edible Insects into Powders: A Review of Available Processes and Potential Microbial Inactivation Methods. J. Insects Food Feed 2022, 9, 325–338. [Google Scholar] [CrossRef]
- Vandeweyer, D.; De Smet, J.; Van Looveren, N.; Van Campenhout, L. Biological Contaminants in Insects as Food and Feed. J. Insects Food Feed 2021, 7, 807–822. [Google Scholar] [CrossRef]
- IPIFF. Insects as Feed Eu Legislation—Aquaculture, Poultry & Pig Species; IPIFF: Brussels, Belgium, 2025. [Google Scholar]
- Imathiu, S. Benefits and Food Safety Concerns Associated with Consumption of Edibl E Insects. NFS J. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Żuk-Gołaszewska, K.; Gałęcki, R.; Obremski, K.; Smetana, S.; Figiel, S.; Gołaszewski, J. Edible Insect Farming in the Context of the Eu Regulations and Marketing-an Overview. Insects 2022, 13, 446. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Tettey, E.; Yunusa, B.M.; Ngah, N.; Debrah, S.K.; Yang, X.; Fernando, I.; Povetkin, S.N.; Shah, M.A. Legal Situation and Consumer Acceptance of Insects Being Eaten as Human Food in Different Nations across the World–a Comprehensive Review. Comp. Rev. Food Sci. Food Safe 2023, 22, 4786–4830. [Google Scholar] [CrossRef] [PubMed]
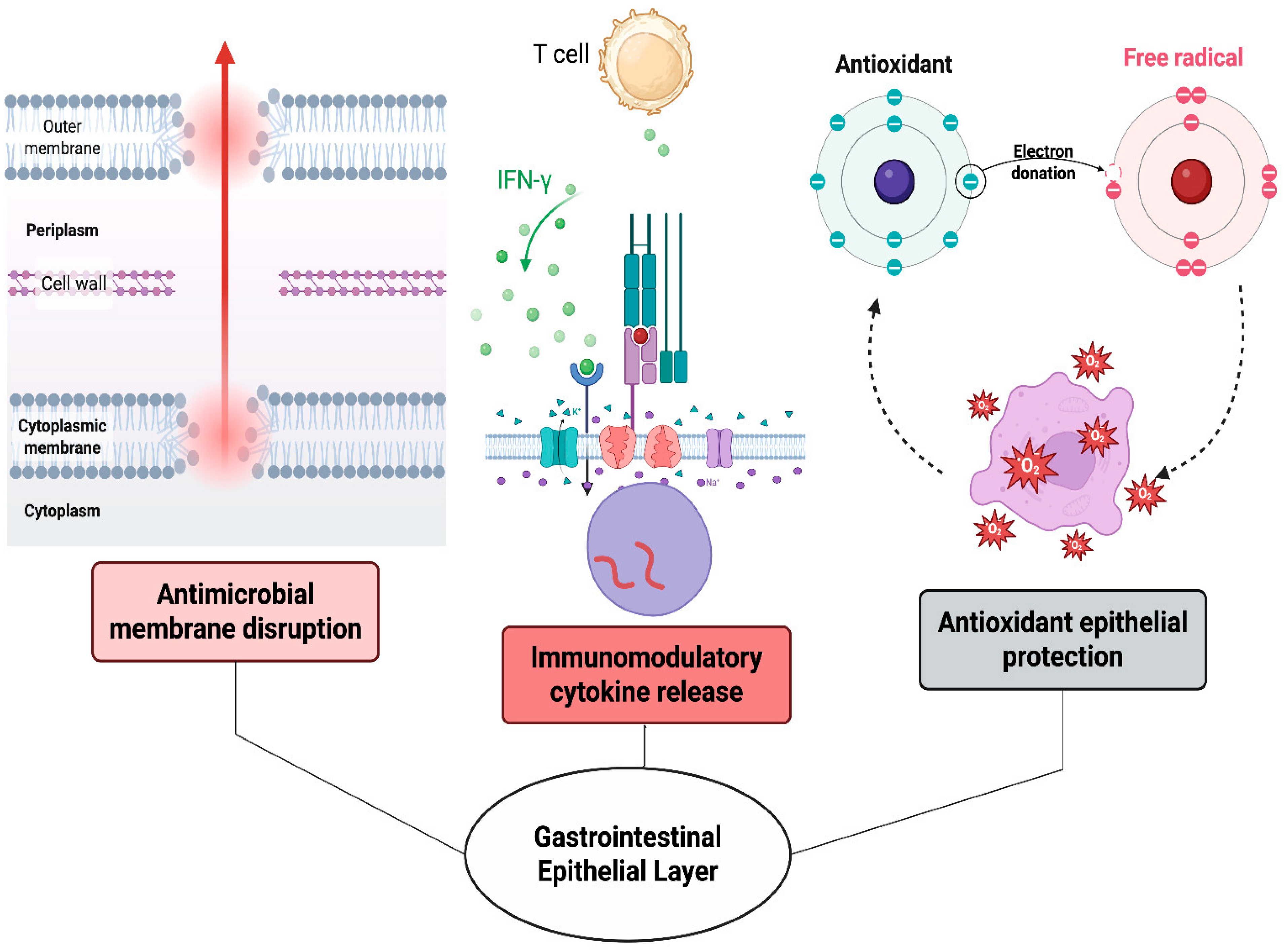
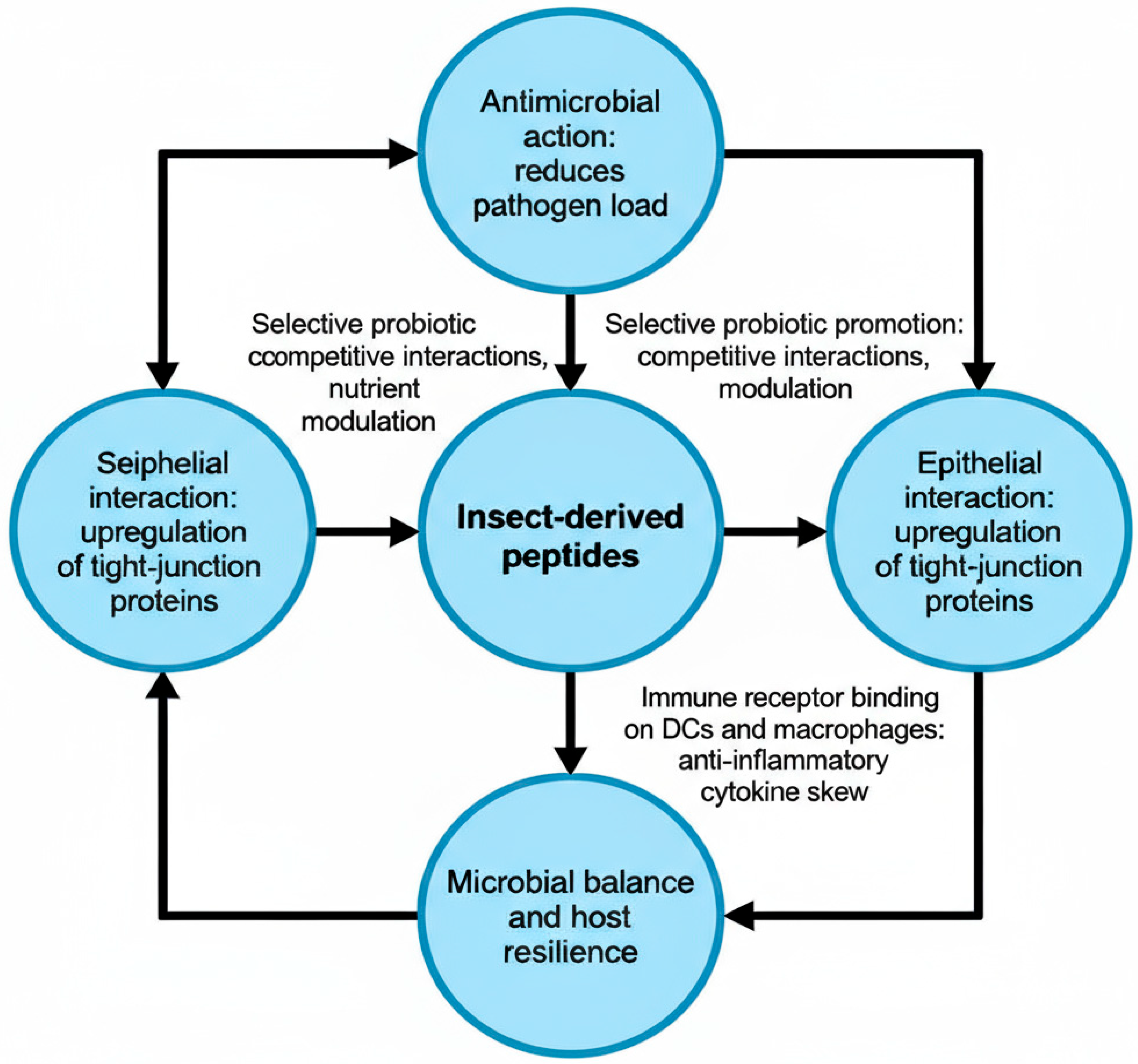
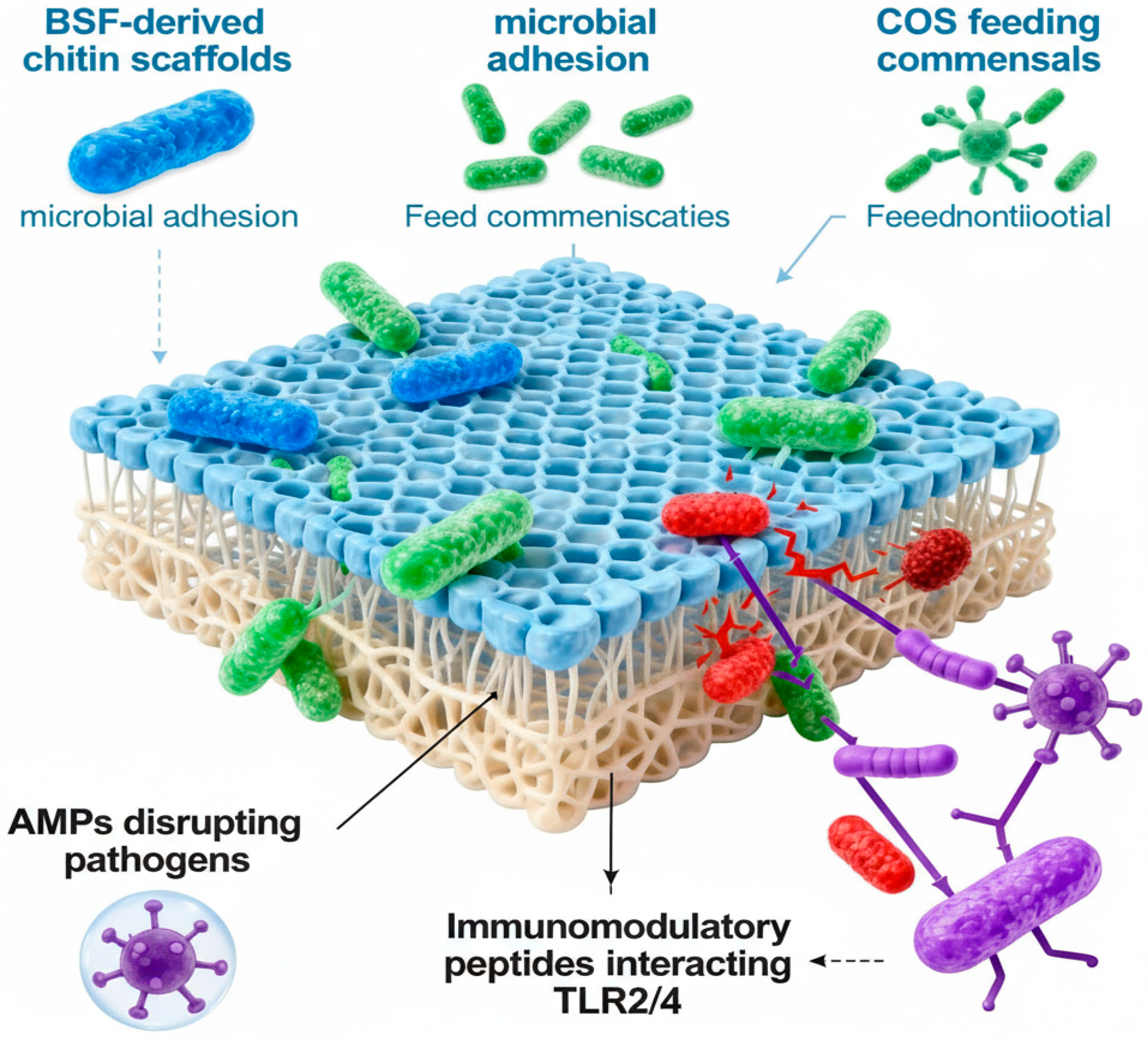
| Amino Acids | BSFL | Beef | Soy |
|---|---|---|---|
| Lysine | 6.2 | 6.0 | 5.7 |
| Leucine | 8.5 | 7.9 | 7.0 |
| Valine | 5.7 | 5.4 | 4.8 |
| Methionine | 1.9 | 1.6 | 1.3 |
| Cysteine | 1.1 | 0.4 | 0.1 |
| Threonine | 4.3 | 4.1 | 3.8 |
| Phenylalanine | 4.7 | 4.6 | 4.5 |
| Tryptophan | 1.3 | 1.2 | 1.1 |
| Fatty Acid | Abundance (%) | Health Implications |
|---|---|---|
| Lauric acid | 36 | Antimicrobial, supports gut barrier |
| Palmitic acid | 12 | Energy source, structural lipid component |
| Oleic acid | 28 | Anti-inflammatory, cardioprotective |
| Linoleic acid | 18 | Essential omega-6, supports cell signaling |
| Stearic acid | 6 | Neutral effect on serum cholesterol |
| Property | Chitin | Chitosan | Gut Health Role |
|---|---|---|---|
| Degree of acetylation | ~90% | ~50% | Determines solubility and fermentability |
| Molecular weight (kDa) | 100–200 | 50–100 | Influences prebiotic efficacy |
| Solubility | Insoluble in water | Soluble in acidic solutions | Enables selective microbial fermentation |
| Biological activity | Structural support | Antimicrobial, prebiotic | Modulates microbiota and strengthens the mucosal barrier |
| BSFL Compounds | Microbial Target | Metabolites | Host Receptor | Effect | Ref. |
|---|---|---|---|---|---|
| BSF-COS | F. prausnitzii | Butrate | CPR109A | Enhanced barrier | [105] |
| BSF-AMPI | Lactobacillus | Lactate | TLR2 | Reduced TNF-α | [154] |
| BSF-CPP | B. lungum | Acetate | GPR43 | Anti-inflammatory | [155] |
| Model | BSF-Derived Compound | Key Outcomes |
|---|---|---|
| Broiler chickens | 5% COS | 20% increase in villus-to-crypt ratio; significant reduction in cecal Enterobacteriaceae counts |
| Shrimp aquaculture | AMP-enriched BSF protein hydrolysate | 1.5 log reduction in Vibrio spp. loads; 15% increase in survival rate |
| Pilot human trial | 4 g day−1 COS for six weeks | 30% increase in stool Bifidobacterium abundance; improved bowel regularity |
| Processing Method | Energy Consumption (kWh/kg) | Processing Time | Capital Investment | Bioactivity Retention | Operating Costs | Industrial Suitability | Regional Considerations |
|---|---|---|---|---|---|---|---|
| Hot Air Drying (60–70 °C) | 1.5–2.5 | 18–24 h | Low ($50–100 K for medium scale) | Moderate (60–70% peptide retention) | Low (labor, energy) | Highly established technology | Suitable for all regions; limited climate control needed |
| Freeze-Drying | 5–10 (4–10x—higher than HAD) | 24–48 h | Very High ($500 K–2 M for industrial units) | Excellent (>90% peptide/chitin retention) | Very High (energy, maintenance) | Limited—niche high-value applications | Most suitable for temperate/cold climates; prohibitive in energy-scarce regions |
| Enzymatic Extraction (Chitin/Peptides) | 0.5–1.2 (moderate heating) | 4–12 h | Moderate–High ($200–500 K) | Excellent (85–95% targeted bioactives) | Moderate–High (enzyme costs, pH control) | Moderate—requires technical expertise | Viable in regions with enzyme availability and trained personnel |
| Supercritical CO2 Extraction (Lipids) | 2–4 | 2–6 h | Very High ($800 K–3 M) | Excellent (>95% lipid quality) | High (CO2 pressure maintenance) | Moderate—for high-value lipid products | Requires stable infrastructure; suitable for developed markets |
| Combined Hot Air + Enzymatic Processing | 2–3 | 12–20 h | Moderate ($150–350 K) | Good (75–85% overall retention) | Moderate | Highly balanced cost–benefit | Optimal for developing regions; combines affordability with quality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhasyani, T.; Ebeid, T.; Ghonimy, M.; Alharbi, S.; Hassan, M.F.Y.; Jarallah, A.; Alkhurayji, M.; Abdellatif, A.A.H.; Barakat, H. Harnessing Edible Insect Bioactives for Gut Health: A Comprehensive Review on Chitin-Derived Prebiotics and Peptidomic Insights from the Black Soldier Fly. Foods 2025, 14, 3654. https://doi.org/10.3390/foods14213654
Alhasyani T, Ebeid T, Ghonimy M, Alharbi S, Hassan MFY, Jarallah A, Alkhurayji M, Abdellatif AAH, Barakat H. Harnessing Edible Insect Bioactives for Gut Health: A Comprehensive Review on Chitin-Derived Prebiotics and Peptidomic Insights from the Black Soldier Fly. Foods. 2025; 14(21):3654. https://doi.org/10.3390/foods14213654
Chicago/Turabian StyleAlhasyani, Thamer, Tarek Ebeid, Mohamed Ghonimy, Saif Alharbi, Mohamed F. Y. Hassan, Abdullah Jarallah, Mohammed Alkhurayji, Ahmed A. H. Abdellatif, and Hassan Barakat. 2025. "Harnessing Edible Insect Bioactives for Gut Health: A Comprehensive Review on Chitin-Derived Prebiotics and Peptidomic Insights from the Black Soldier Fly" Foods 14, no. 21: 3654. https://doi.org/10.3390/foods14213654
APA StyleAlhasyani, T., Ebeid, T., Ghonimy, M., Alharbi, S., Hassan, M. F. Y., Jarallah, A., Alkhurayji, M., Abdellatif, A. A. H., & Barakat, H. (2025). Harnessing Edible Insect Bioactives for Gut Health: A Comprehensive Review on Chitin-Derived Prebiotics and Peptidomic Insights from the Black Soldier Fly. Foods, 14(21), 3654. https://doi.org/10.3390/foods14213654









