Comparative Analysis of Physicochemical Characteristics, Antioxidant Compound Contents, and Antioxidant Activities of Five Guava (Psidium guajava L.) Cultivars Harvested in Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Color
2.3. Soluble Solid Content (SSC), Titratable Acidity, pH, and Firmness
2.4. Extraction
2.5. Quantification of Individual Organic Acids
2.6. Quantification of Individual Sugars
2.7. Total Flavonoid Analysis
2.8. Total Phenolic Analysis
2.9. Polyphenol Analysis
2.10. Lycopene Analysis
2.11. Total Ascorbic Acid Analysis
2.12. DPPH Radical Scavenging Activity Analysis
2.13. ABTS Radical Scavenging Activity Analysis
2.14. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Qualities, Organic Acids, and Sugars of Guava Fruit
3.1.1. Color
3.1.2. Soluble Solid Content (SSC), Titratable Acidity, pH, and Firmness
3.1.3. Organic Acid Content
3.1.4. Sugar Content
3.2. Antioxidant Compound Contents and Antioxidant Activity Analysis of Guava Fruit
3.2.1. Total Flavonoid and Phenolic Contents
3.2.2. Polyphenol Content
3.2.3. Lycopene Content
3.2.4. Total Ascorbic Acid Content
3.2.5. DPPH and ABTS Radical Scavenging Activity
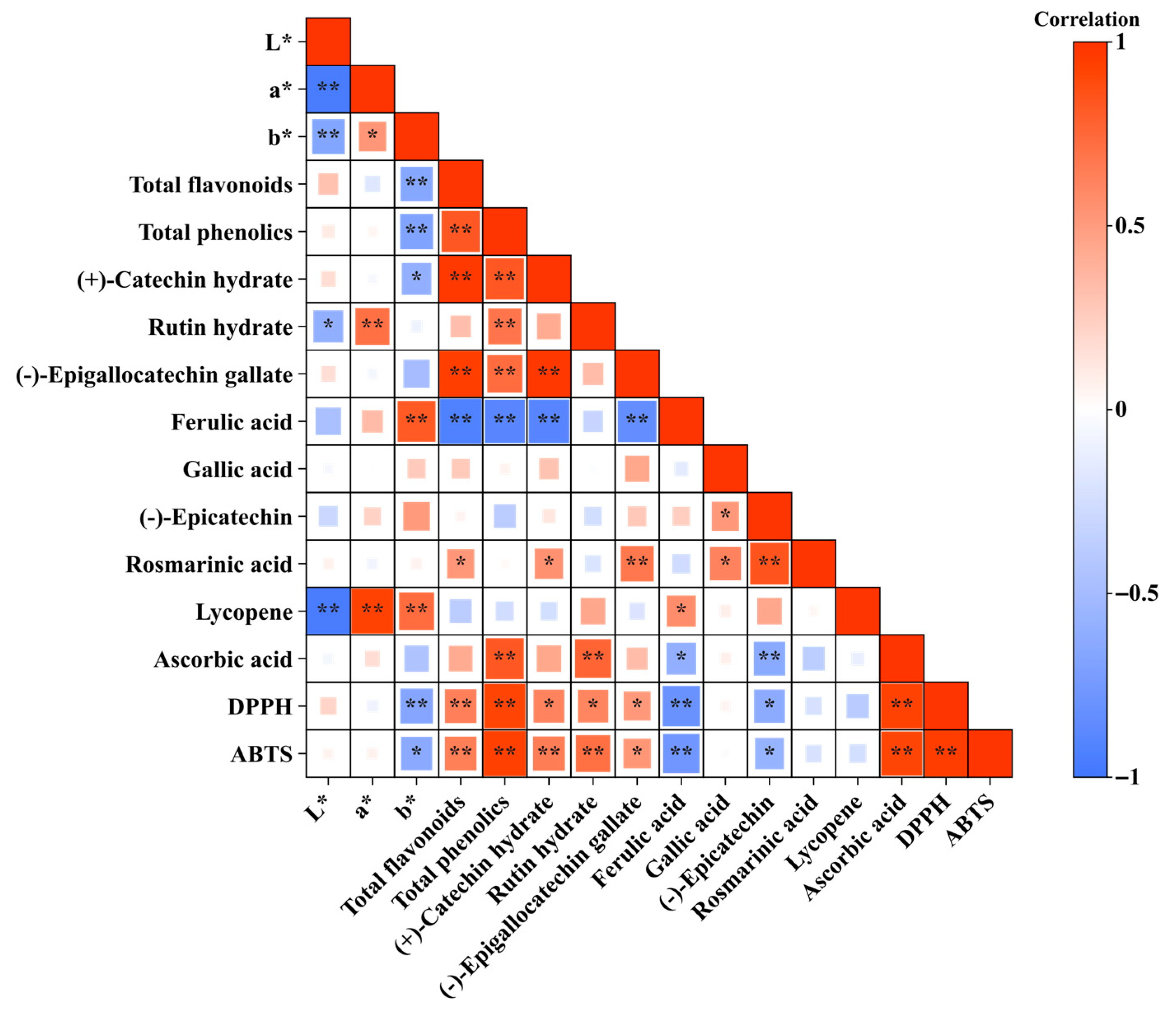
3.3. Pearson’s Correlation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vázquez-Manjarrez, N.; Ulaszewska, M.; Garcia-Aloy, M.; Mattivi, F.; Praticò, G.; Dragsted, L.O.; Manach, C. Biomarkers of intake for tropical fruits. Genes Nutr. 2020, 15, 11. [Google Scholar] [CrossRef]
- Carmona-Hernandez, J.C.; Taborda-Ocampo, G.; Valdez, J.C.; Bolling, B.W.; González-Correa, C.H. Polyphenol extracts from three Colombian Passifloras (passion fruits) prevent inflammation-induced barrier dysfunction of Caco-2 cells. Molecules 2019, 24, 4614. [Google Scholar] [CrossRef]
- de Albuquerque, M.A.C.; Levit, R.; Beres, C.; Bedani, R.; de LeBlanc, A.D.M.; Saad, S.M.I.; LeBlanc, J.G. Tropical fruit by-products water extracts as sources of soluble fibres and phenolic compounds with potential antioxidant, anti-inflammatory, and functional properties. J. Funct. Foods 2019, 52, 724–733. [Google Scholar] [CrossRef]
- Dotto, J.M.; Abihudi, S.A. Nutraceutical value of Carica papaya: A review. Sci. Afr. 2021, 13, e00933. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Wee, Y.J.; Ye, W.; Korivi, M. Nutritional composition and bioactive compounds in three different parts of mango fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef]
- Angulo-López, J.E.; Flores-Gallegos, A.C.; Torres-León, C.; Ramírez-Guzmán, K.N.; Martínez, G.A.; Aguilar, C.N. Guava (Psidium guajava L.) Fruit and Valorization of Industrialization By-Products. Processes 2021, 9, 1075. [Google Scholar] [CrossRef]
- Arévalo-Marín, E.; Casas, A.; Landrum, L.; Shock, M.P.; Alvarado-Sizzo, H.; Ruiz-Sanchez, E.; Clement, C.R. The Taming of Psidium guajava: Natural and Cultural History of a Neotropical Fruit. Front. Plant Sci. 2021, 12, 714763. [Google Scholar] [CrossRef]
- Kamath, J.; Rahul, N.; Ashok Kumar, C.; Lakshmi, S. Psidium guajava L: A review. Int. J. Green Pharm. 2008, 2, 9–12. [Google Scholar] [CrossRef]
- Ngbolua, K.; Lufuluabo, L.G.; Moke, L.E.; Bongo, G.; Liyongo, C.I.; Ashande, C.M.; Sapo, B.S.; Zoawe, B.G.; Mpiana, P.T. A review on the Phytochemistry and Pharmacology of Psidium guajava L. (Myrtaceae) and Future direction. Discov. Phytomedicine 2018, 5, 7–13. [Google Scholar] [CrossRef]
- Cheon, W.; Seo, D.; Kim, Y. Antioxidative and hepatocyte protective effects of guava (Psidium guajava L.) leaves cultivated in Korea. Korean J. Food Nutr. 2019, 32, 33–40. [Google Scholar]
- Tousif, M.I.; Nazir, M.; Saleem, M.; Tauseef, S.; Shafiq, N.; Hassan, L.; Hussian, H.; Montesano, D.; Naviglio, D.; Zengin, G.; et al. Psidium guajava L. An Incalculable but Underexplored Food Crop: Its Phytochemistry, Ethnopharmacology, and Industrial Applications. Molecules 2022, 27, 7016. [Google Scholar] [CrossRef]
- USDA. Guavas, Common, Raw (SR Legacy, 173044) U.S. Department of Agriculture. 2019. Available online: https://fdc.nal.usda.gov/food-details/173044/nutrients (accessed on 9 October 2025).
- Rani, D.J.; Vijayanchali, S.S. Phytochemical, Antioxidant Activity and Lycopene Analysis of Red Guava Fruits. J. Res. Ext. Dev. 2017, 6, 25–30. [Google Scholar] [CrossRef]
- Lok, B.; Babu, D.; Tabana, Y.; Dahham, S.S.; Adam, M.A.A.; Barakat, K.; Sandai, D. The Anticancer Potential of Psidium guajava (Guava) Extracts. Life 2023, 13, 346. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Shin, Y. Antioxidant profile, antioxidant activity, and physicochemical characteristics of strawberries from different cultivars and harvest locations. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 587–595. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- Yang, H.; Kim, Y.-J.; Shin, Y. Influence of Ripening Stage and Cultivar on Physicochemical Properties and Antioxidant Compositions of Aronia Grown in South Korea. Foods 2019, 8, 598. [Google Scholar] [CrossRef]
- Bicanic, D.; Fogliano, V.; Luterotti, S.; Swarts, J.; Piani, G.; Graziani, G. Quantification of lycopene in tomato products: Comparing the performances of a newly proposed direct photothermal method and high-performance liquid chromatography. J. Sci. Food Agric. 2005, 85, 1149–1153. [Google Scholar] [CrossRef]
- Park, H.; Kim, Y.-J.; Shin, Y. Estimation of Daily Intake of Lycopene, Antioxidant Contents and Activities from Tomatoes, Watermelons, and Their Processed Products in Korea. Appl. Biol. Chem. 2020, 63, 50. [Google Scholar] [CrossRef]
- Joung, M.; Kim, Y.J.; Shin, Y. Assessment of lycopene, polyphenols, antioxidant compounds, and activities in colored cherry tomato cultivars harvested in Korea. Food Sci. Biotechnol. 2025, 34, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Aghel, N.; Ramezani, Z.; Amirfakhrian, S. Isolation and quantification of lycopene from tomato cultivated in dezfoul, Iran. Jundishapur J. Nat. Pharm. Prod. 2011, 6, 9–15. [Google Scholar]
- Terada, M.; Watanabe, Y.; Kunitomo, M.; Hayashi, E. Differential rapid analysis of ascorbic acid and ascorbic acid 2-sulfate by dinitrophenylhydrazine method. Anal. Biochem. 1978, 84, 604–608. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, Y.-J.; Shin, Y. Assessment of Physicochemical Quality, Antioxidant Content and Activity, and Inhibition of Cholinesterase between Unripe and Ripe Blueberry Fruit. Foods 2020, 9, 690. [Google Scholar] [CrossRef]
- Soares, F.D.; Pereira, T.; Marcia, O.; Marques, M.; Monteiro, A.R. Volatile and non-volatile chemical composition of the white guava fruit (Psidium guajava) at different stages of maturity. Food Chem. 2007, 100, 15–21. [Google Scholar] [CrossRef]
- Pasupuleti, V.; Kulkarni, S.G. Lycopene fortification on the quality characteristics of beverage formulations developed from pink flesh guava (Psidium guajava L.). J. Food Sci. Technol. 2014, 51, 4126–4131. [Google Scholar] [CrossRef]
- Kumari, P.; Mankar, A.; Karuna, K.; Homa, F.; Meiramkulova, K.; Siddiqui, M.W. Mineral Composition, Pigments, and Postharvest Quality of Guava Cultivars Commercially Grown in India. J. Agric. Food Res. 2020, 2, 100061. [Google Scholar] [CrossRef]
- Moon, P.; Fu, Y.; Bai, J.; Plotto, A.; Crane, J.; Chambers, A. Assessment of fruit aroma for twenty-seven guava (Psidium guajava) accessions through three fruit developmental stages. Sci. Hortic. 2018, 238, 375–383. [Google Scholar] [CrossRef]
- Shalu Ran, S.R.; Sharma, J.R.; Jakhar, M.S. Assessment of genetic diversity and diversity relationship in different varieties of guava using morphological characterization. Plant Arch. 2017, 17, 307–311. [Google Scholar]
- Mehta, S.K.; Singh, K.K.; Jat, D.K.; Rana, D.K. Comparative studies of physico-chemical characteristics of various cultivars of guava (Psidium guajava L.) under sub tropical valley condition of Garhwal Himalaya (UttaraKhand), India. Plant Arch. 2016, 16, 361–364. [Google Scholar]
- Chen, H.C.; Sheu, M.J.; Lin, L.Y.; Wu, C.M. Nutritional composition and volatile compounds in guava. Fresh Proced. 2007, 1, 132–139. [Google Scholar]
- Setiasih, I.S.; Rialita, T.; Sumanti, D.M.; Hanidah, I. Characteristics of guava (Psidium guajava L.) treated with ozonation during ambient storage. KnE Life Sci. 2017, 2, 448–458. [Google Scholar] [CrossRef]
- Lee, S.; Choi, H.K.; Cho, S.K.; Kim, Y.S. Metabolic analysis of guava (Psidium guajava L.) fruits at different ripening stages using different data-processing approaches. J. Chromatogr. B 2010, 878, 2983–2988. [Google Scholar] [CrossRef]
- Muñoz-Arrieta, R.; Esquivel-Alvarado, D.; Alfaro-Viquez, E.; Rodriguez-Salazar, M.; Alvarez-Valverde, V.; Rodriguez, G.; Reed, J.D. Nutritional and chemical composition of the Costa Rican guava (Psidium friedrichsthalianum [O. Berg] Nied): An underexploited edible fruit with nutritional and industrial potential. ACS Food Sci. Technol. 2021, 1, 1970–1978. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Winter, J.; Montero, M.L.; Zimmermann, B.F.; Schieber, A. Characterization of Phytochemicals in Costa Rican Guava (Psidium Friedrichsthalianum-Nied.) Fruit and Stability of Main Compounds during Juice Processing—(U)HPLC-DAD-ESI-TQD-MSn. J. Food Compos. Anal. 2019, 75, 26–42. [Google Scholar] [CrossRef]
- Srivastava, K.K.; Soni, S.K.; Kumar, D.; Dwivedi, S.K. Effect of different bagging materials on guava fruit physiology and its quality attributes. Plant Physiol. Rep. 2023, 28, 238–246. [Google Scholar] [CrossRef]
- Yousaf, A.A.; Abbasi, K.S.; Ahmad, A.; Hassan, I.; Sohail, A.; Qayyum, A.; Akram, M.A. Physico-chemical and Nutraceutical Characterization of Selected Indigenous Guava (Psidium guajava L.) Cultivars. Food Sci. Technol. 2021, 41, 47–58. [Google Scholar] [CrossRef]
- Naryal, A.; Acharya, S.; Bhardwaj, A.K.; Kant, A.; Chaurasia, O.P.; Stobdan, T. Altitudinal effect on sugar contents and sugar profiles in dried apricot (Prunus armeniaca L.) fruit. J. Food Compos. Anal. 2019, 76, 27–32. [Google Scholar] [CrossRef]
- Chiveu, J.; Naumann, M.; Kehlenbeck, K.; Pawelzik, E. Variation in fruit chemical and mineral composition of Kenyan guava (Psidium guajava L.): Inferences from climatic conditions, and fruit morphological traits. J. Appl. Bot. Food Qual. 2019, 92, 151–159. [Google Scholar]
- Sviech, F.; Cardoso, P.; Oliveira, R.A.; Ubbink, J.; Prata, A.S. State diagrams and water sorption isotherms of pitanga, ciriguela araza, mango, and guava. J. Food Process Eng. 2023, 46, e14370. [Google Scholar] [CrossRef]
- Patel, P.; Sunkara, R.; Walker, L.T.; Verghese, M. Effect of Drying Techniques on Antioxidant Capacity of Guava Fruit. Food Nutr. Sci. 2016, 7, 544–554. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Singh, S.; Singh, R.P.; Kumar, A. Determination of antioxidant capacity, total phenolics and antimicrobial properties of spray-dried guava extract for value-added processing. J. Food Process. Technol. 2014, 5, 1000368. [Google Scholar] [CrossRef]
- Sanguansil, S.; Boonprakob, U.; Thaipong, K. Quantification of antioxidant content in fruit of guava germplasm. Acta. Hortic. 2014, 1024, 385–390. [Google Scholar] [CrossRef]
- Omayio, D.G.; Abong’, G.O.; Okoth, M.W.; Gachuiri, C.K.; Mwangombe, A.W. Physicochemical and processing qualities of guava varieties in Kenya. Int. J. Fruit Sci. 2022, 22, 329–345. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of guava fruit: Comparison with some local fruits. Sunway Acad. J. 2006, 3, 9–20. [Google Scholar]
- Stevenson, D.E.; Hurst, R.D. Polyphenolic phytochemicals—Just antioxidants or much more? Cell Mol. Life. Sci. 2007, 64, 2900–2916. [Google Scholar] [CrossRef]
- Fu, L.; Lu, W.; Zhou, X. Phenolic compounds and in vitro antibacterial and antioxidant activities of three tropic fruits: Persimmon, guava, and sweetsop. BioMed Res. Int. 2016, 2016, 4287461. [Google Scholar] [CrossRef]
- dos Santos, W.N.L.; da Silva Sauthier, M.C.; dos Santos, A.M.P.; de Andrade Santana, D.; Azevedo, R.S.A.; da Cruz Caldas, J. Simultaneous determination of 13 phenolic bioactive compounds in guava (Psidium guajava L.) by HPLC-PAD with evaluation using PCA and Neural Network Analysis (NNA). Microchem. J. 2017, 133, 583–592. [Google Scholar] [CrossRef]
- Dhianawaty, D.; Atik, N.; Dwiwina, R.G.; Muda, I. Preliminary Identification and Quantification of Four Secondary Metabolites, Total Tannin and Total Flavonoid Contents in Guava Fruit Ethanol Extract. Pharmacogn. J. 2022, 14, 350–357. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental factors regulate plant secondary metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- González, I.A.; Osorio, C.; Meléndez-Martínez, A.J.; González-Miret, M.L.; Heredia, F.J. Application of tristimulus colorimetry to evaluate colour changes during the ripening of Colombian guava (Psidium guajava L.) varieties with different carotenoid pattern. Int. J. Food Sci. Technol. 2011, 46, 840–848. [Google Scholar] [CrossRef]
- Musaa, K.H.; Abdullah, A.; Subramaniam, V. Flavonoid profile and antioxidant activity of pink guava. ScienceAsia 2015, 41, 149–154. [Google Scholar] [CrossRef]
- Nwaichi, E.O.; Chuku, L.C.; Oyibo, N.J. Profile of Ascorbic Acid, Beta-Carotene and Lycopene in Guava, Tomatoes, Honey and Red Wine. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 39–43. [Google Scholar]
- Amorim, A.G.N.; Souza, J.M.T.; Santos, R.C.; Gullón, B.; Oliveira, A.; Santos, L.F.A.; Virgino, A.L.E.; Mafud, A.C.; Petrilli, H.M.; Mascarenhas, Y.P.; et al. HPLC-DAD, ESI–MS/MS, and NMR of Lycopene Isolated From P. Guajava L. and Its Biotechnological Applications. Eur. J. Lipid Sci. Technol. 2018, 120, 1700330. [Google Scholar] [CrossRef]
- Nanda, B.; Sailaja, M.; Mohapatra, P.; Pradhan, R.K.; Nanda, B.B. Green solvents: A suitable alternative for sustainable chemistry. Mater. Today Proc. 2021, 47, 1234–1240. [Google Scholar] [CrossRef]
- Morón-Ortiz, Á.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J. Sustainable green extraction of carotenoid pigments: Innovative technologies and bio-based solvents. Antioxidants 2024, 13, 239. [Google Scholar] [CrossRef]
- Calvo, F.G.; María, F.; Monteagudo, J. Green and Bio-Based Solvents. Top. Curr. Chem. 2018, 376, 18. [Google Scholar] [CrossRef] [PubMed]
- Colucci Cante, R.; Gallo, M.; Varriale, L.; Garella, I.; Nigro, R. Recovery of carotenoids from tomato pomace using a hydrofluorocarbon solvent in sub-critical conditions. Appl. Sci. 2022, 12, 2822. [Google Scholar] [CrossRef]
- Jawaheer, B.; Goburdhun, D.; Ruggoo, A. Effect of processing and storage of guava into jam and juice on the ascorbic acid content. Plant Foods Hum. Nutr. 2003, 58, 1–12. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Valente, A.; Albuquerque, T.G.; Sanches-Silva, A.; Costa, H.S. Ascorbic acid content in exotic fruits: A contribution to produce quality data for food composition databases. Food Res. Int. 2011, 44, 2237–2242. [Google Scholar] [CrossRef]
- Leong, L.P.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Arabia, A.; Munné-Bosch, S.; Munoz, P. Ascorbic acid as a master redox regulator of fruit ripening. Postharvest Biol. Technol. 2024, 207, 112614. [Google Scholar] [CrossRef]
- Samee, W.; Engkalohakul, M.; Nebbua, N.; Direkrojanavuti, P.; Sornchaithawatwong, C.; Kamkaen, N. Correlation Analysis between Total Acid, Total Phenolic and Ascorbic Acid Contents in Fruit Extracts and Their Antioxidant Activities. Thai. Pharm. Health Sci. J. 2006, 1, 196–203. [Google Scholar]
- Du, G.; Li, M.; Ma, F.; Liang, D. Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Suwanwong, Y.; Boonpangrak, S. Phytochemical contents, antioxidant activity, and anticancer activity of three common guava cultivars in Thailand. Eur. J. Integr. Med. 2021, 42, 101290. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant Capacity and Phenolic Content of Selected Tropical Fruits from Malaysia, Extracted with Different Solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Comp. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]


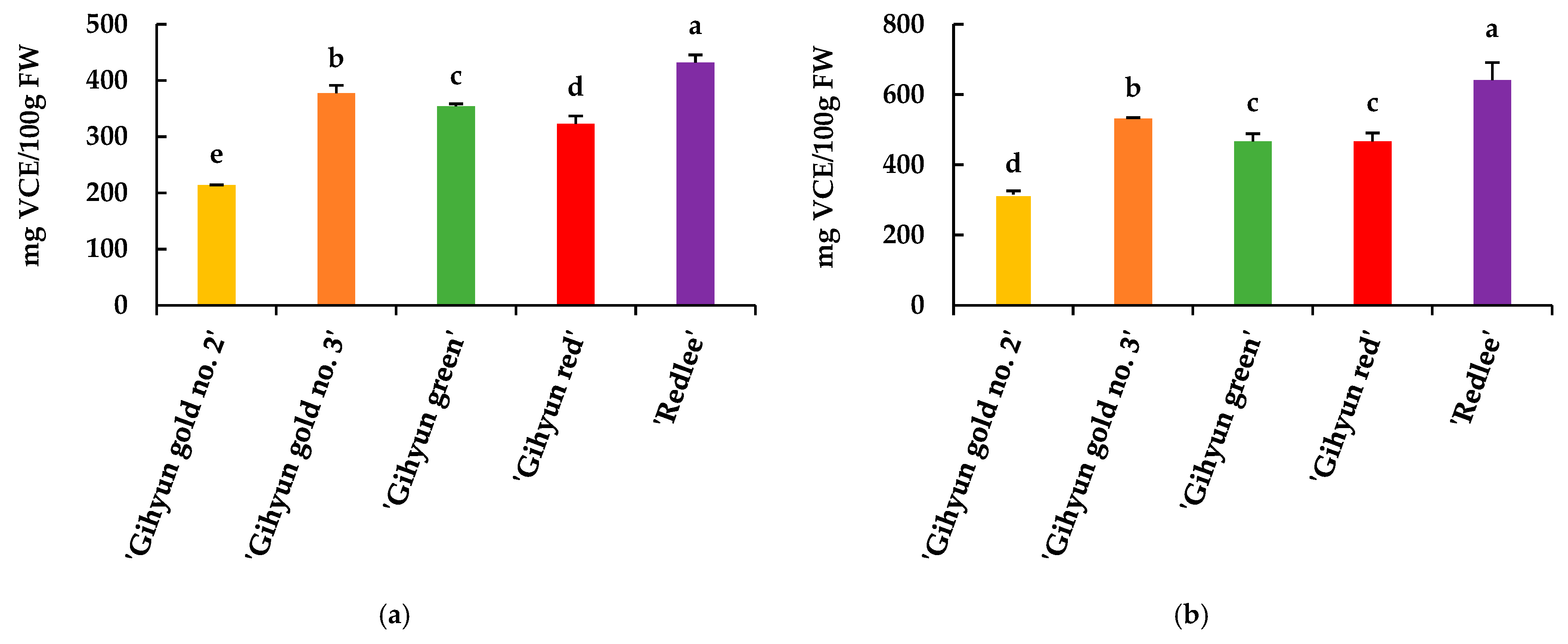
| Cultivar | Part | L* (Lightness) | a* (Redness) | b* (Yellowness) |
|---|---|---|---|---|
| ‘Gihyun gold no. 2’ | Skin | 68.28 ± 1.36 b | −2.94 ± 2.89 d | 40.73 ± 1.21 a |
| Flesh | 63.40 ± 1.30 bc | 21.75 ± 1.23 b | 30.07 ± 1.69 d | |
| ‘Gihyun gold no. 3’ | Skin | 60.31 ± 0.64 c | −16.30 ± 0.70 e | 35.36 ± 0.47 c |
| Flesh | 83.06 ± 1.70 a | −0.37 ± 0.41 d | 14.27 ± 2.32 f | |
| ‘Gihyun green’ | Skin | 66.64 ± 5.80 b | −16.23 ± 1.46 e | 38.26 ± 3.08 abc |
| Flesh | 82.32 ± 2.40 a | −2.62 ± 1.38 d | 20.44 ± 3.84 e | |
| ‘Gihyun red’ | Skin | 65.83 ± 5.12 b | 4.59 ± 4.87 c | 36.05 ± 3.17 bc |
| Flesh | 82.35 ± 0.97 a | −0.13 ± 0.34 d | 14.50 ± 2.41 f | |
| ‘Redlee’ | Skin | 67.95 ± 0.52 b | −2.91 ± 3.34 d | 40.05 ± 1.15 ab |
| Flesh | 64.36 ± 2.63 bc | 25.82 ± 1.66 a | 18.42 ± 0.85 e |
| Cultivar | SSC (°Brix) | Titratable Acidity (%) | pH | Firmness (N) |
|---|---|---|---|---|
| ‘Gihyun gold no. 2’ | 11.77 ± 0.12 c | 1.57 ± 0.02 b | 3.98 ± 0.01 a | 7.65 ± 0.34 c |
| ‘Gihyun gold no. 3’ | 10.67 ± 0.12 d | 1.76 ± 0.04 a | 3.82 ± 0.01 c | 9.28 ± 0.11 a |
| ‘Gihyun green’ | 10.50 ± 0.17 d | 1.33 ± 0.01 d | 3.89 ± 0.02 b | 6.83 ± 0.61 d |
| ‘Gihyun red’ | 13.90 ± 0.00 a | 1.54 ± 0.03 b | 3.77 ± 0.01 d | 8.50 ± 0.58 ab |
| ‘Redlee’ | 12.70 ± 0.10 b | 1.39 ± 0.02 c | 3.99 ± 0.01 a | 8.39 ± 0.33 bc |
| Cultivar | Citric Acid | Malic Acid | Oxalic Acid | Acetic Acid | Fumaric Acid | Total Sum |
|---|---|---|---|---|---|---|
| ‘Gihyun gold no. 2’ | 439.08 ± 0.90 b | 178.56 ± 0.16 a | 13.20 ± 0.14 b | N.D. | 0.19 ± 0.00 a | 631.03 |
| ‘Gihyun gold no. 3’ | 620.87 ± 2.45 a | 36.54 ± 2.06 e | 17.15 ± 0.13 a | N.D. | 0.11 ± 0.00 c | 674.67 |
| ‘Gihyun green’ | 381.59 ± 1.03 d | 92.64 ± 0.71 d | 7.01 ± 0.00 c | N.D. | 0.07 ± 0.00 d | 481.31 |
| ‘Gihyun red’ | 413.90 ± 1.78 c | 141.48 ± 0.75 c | 2.14 ± 0.02 e | N.D. | 0.07 ± 0.00 e | 557.59 |
| ‘Redlee’ | 345.93 ± 0.09 e | 162.42 ± 0.68 b | 6.17 ± 0.01 d | 3.46 ± 0.05 | 0.17 ± 0.00 b | 518.15 |
| Cultivar | Fructose | Glucose | Sucrose | Total Sum |
|---|---|---|---|---|
| ‘Gihyun gold no. 2’ | 4.46 ± 0.02 b | 3.48 ± 0.03 a | 0.41 ± 0.03 d | 8.35 |
| ‘Gihyun gold no. 3’ | 2.53 ± 0.05 c | 1.33 ± 0.01 c | 2.44 ± 0.06 b | 6.30 |
| ‘Gihyun green’ | 4.35 ± 0.19 b | 2.58 ± 0.09 b | 0.15 ± 0.01 e | 7.08 |
| ‘Gihyun red’ | 5.41 ± 0.16 a | 3.44 ± 0.10 a | 1.35 ± 0.12 c | 10.20 |
| ‘Redlee’ | 2.35 ± 0.01 c | 1.43 ± 0.04 c | 4.84 ± 0.05 a | 8.62 |
| Cultivar | (+)-Catechin Hydrate | Rutin Hydrate | Epigallocatechin Gallate | Ferulic Acid | Gallic Acid | Epicatechin | Rosmarinic Acid |
|---|---|---|---|---|---|---|---|
| ‘Gihyun gold no. 2’ | 10.07 ± 0.18 d | 11.34 ± 0.07 c | 0.81 ± 0.02 c | 0.95 ± 0.01 a | 0.38 ± 0.01 c | 0.53 ± 0.02 a | 0.07 ± 0.00 b |
| ‘Gihyun gold no. 3’ | 55.55 ± 0.53 a | 15.93 ± 0.12 b | 7.48 ± 0.33 a | 0.09 ± 0.00 e | 0.44 ± 0.01 a | 0.39 ± 0.03 b | 0.12 ± 0.00 a |
| ‘Gihyun green’ | 10.07 ± 0.15 d | 3.62 ± 0.01 e | 0.34 ± 0.01 d | 0.59 ± 0.01 b | 0.40 ± 0.02 b | N.D. | N.D. |
| ‘Gihyun red’ | 19.66 ± 0.05 c | 6.13 ± 0.05 d | 1.06 ± 0.02 c | 0.50 ± 0.01 c | 0.21 ± 0.01 e | N.D. | N.D. |
| ‘Redlee’ | 36.81 ± 1.15 b | 64.81 ± 0.73 a | 3.69 ± 0.06 b | 0.36 ± 0.01 d | 0.34 ± 0.01 d | N.D. | N.D. |
| Cultivar | Lycopene | Total Ascorbic Acid |
|---|---|---|
| ‘Gihyun gold no. 2’ | 5.21 ± 0.20 a | 114.43 ± 1.48 e |
| ‘Gihyun gold no. 3’ | N.D. | 198.27 ± 3.91 c |
| ‘Gihyun green’ | N.D. | 214.50 ± 6.10 b |
| ‘Gihyun red’ | N.D. | 164.96 ± 4.27 d |
| ‘Redlee’ | 3.60 ± 0.77 b | 292.38 ± 4.40 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, B.; Shin, Y. Comparative Analysis of Physicochemical Characteristics, Antioxidant Compound Contents, and Antioxidant Activities of Five Guava (Psidium guajava L.) Cultivars Harvested in Korea. Foods 2025, 14, 3645. https://doi.org/10.3390/foods14213645
Choi B, Shin Y. Comparative Analysis of Physicochemical Characteristics, Antioxidant Compound Contents, and Antioxidant Activities of Five Guava (Psidium guajava L.) Cultivars Harvested in Korea. Foods. 2025; 14(21):3645. https://doi.org/10.3390/foods14213645
Chicago/Turabian StyleChoi, Bohee, and Youngjae Shin. 2025. "Comparative Analysis of Physicochemical Characteristics, Antioxidant Compound Contents, and Antioxidant Activities of Five Guava (Psidium guajava L.) Cultivars Harvested in Korea" Foods 14, no. 21: 3645. https://doi.org/10.3390/foods14213645
APA StyleChoi, B., & Shin, Y. (2025). Comparative Analysis of Physicochemical Characteristics, Antioxidant Compound Contents, and Antioxidant Activities of Five Guava (Psidium guajava L.) Cultivars Harvested in Korea. Foods, 14(21), 3645. https://doi.org/10.3390/foods14213645






