Quantitative Analysis and Risk Assessment of Polycyclic Aromatic Hydrocarbons Using Gas Chromatography–Mass Spectrometry from Herbs and Spices Distributed in South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Sample Preparation for 4PAHs Evaluation
2.3. Extraction and Clean-Up for Preparation
2.4. Quantitative Analysis of 4PAHs by GC-MS
2.5. Validation of Analytical Method
2.6. Evaluation of TEQ in Herb and Spice
2.7. Exposure and Risk Assessment of PAHs in Herbs and Spices
2.8. Statistical Analysis
3. Results and Discussion
3.1. GC-MS Method Validation for Quantification of 4PAHs in Herbs and Spices
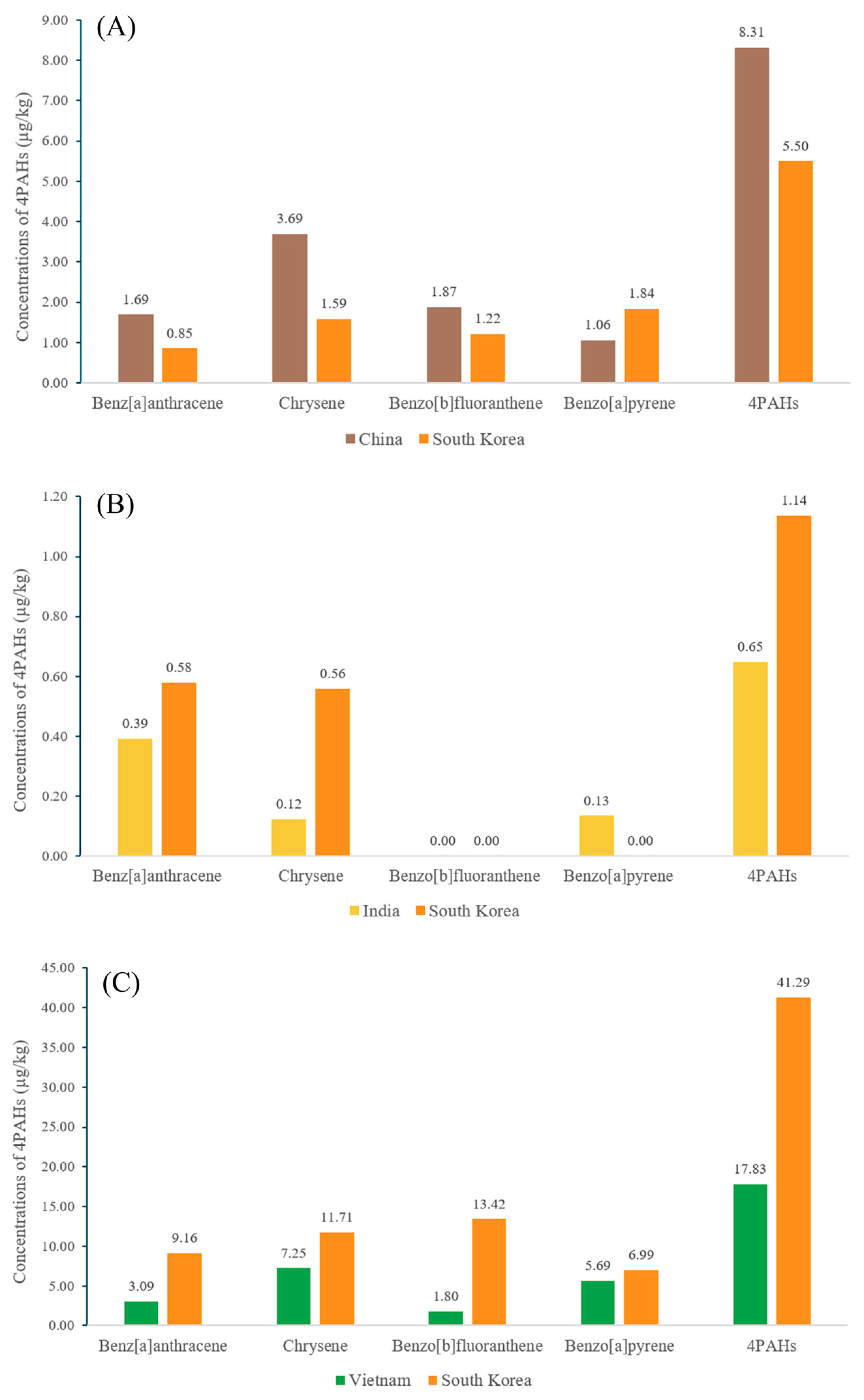
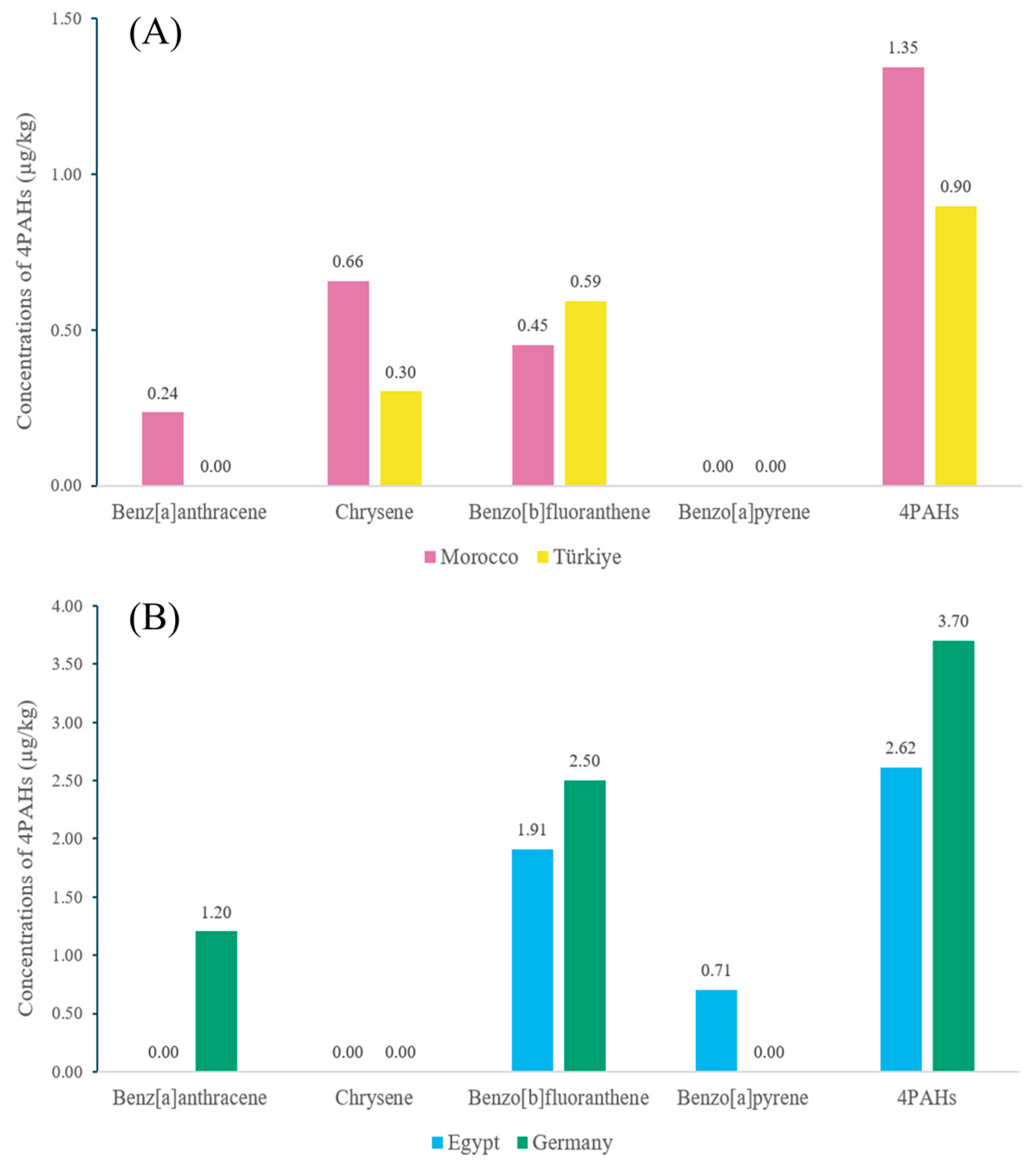
3.2. Evaluation of 4PAHs Content in Herbs and Spices
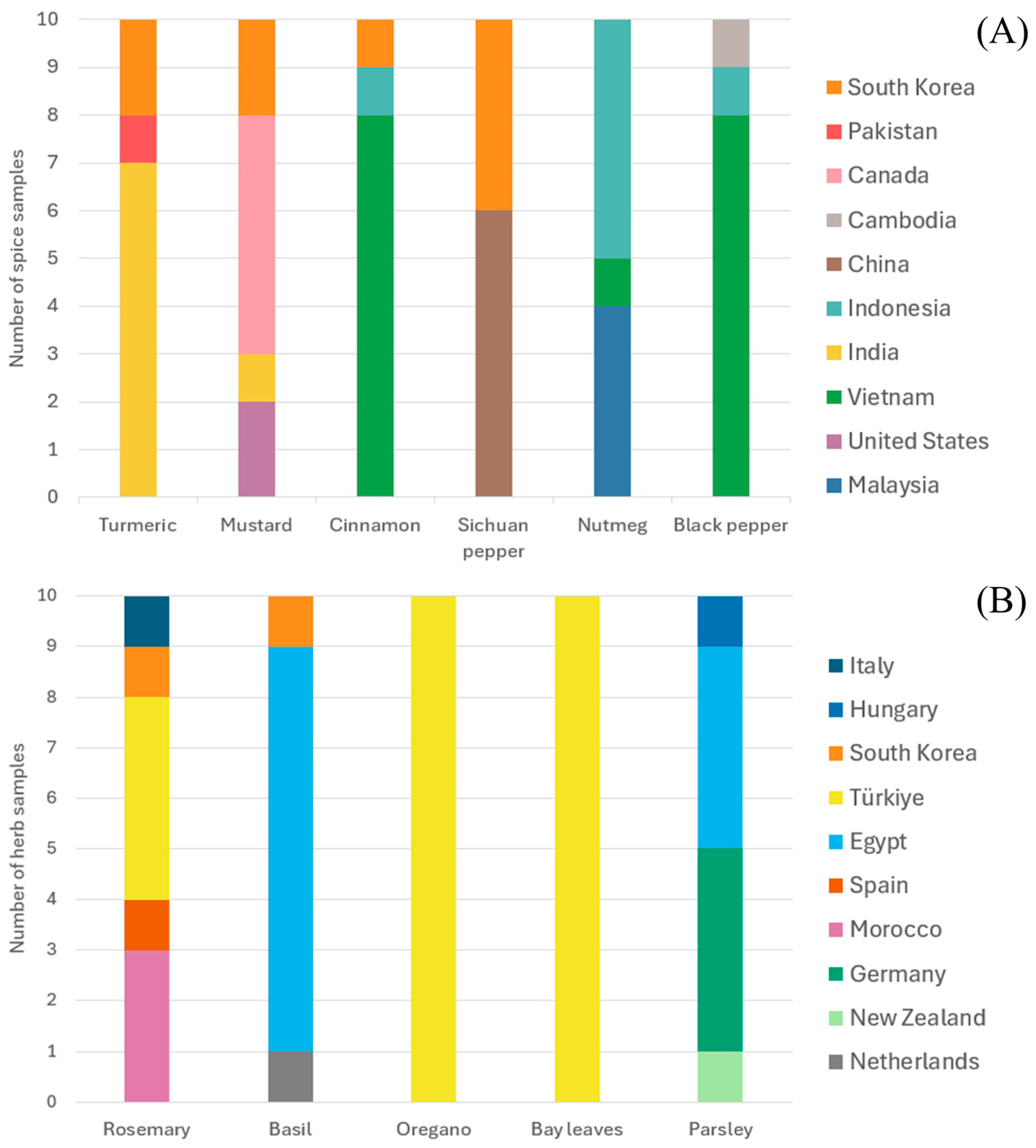
3.3. Interpretation of the Origins of PAH Contamination in Herbs and Spices
3.4. Risk Assessment of PAHs in Herbs and Spices
3.5. Dietary Risk Characterization of 4PAHs in Herbs and Spices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, M.; Song, X.; Ding, D.; Wang, Q.; Zhang, Z.; Tang, Z. Bioremediation of PAHs and heavy metals co-contaminated soils: Challenges and enhancement strategies. Environ. Pollut. 2022, 295, 118686. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Amirdivani, S.; Khorshidian, N.; Ghobadi Dana, M.; Mohammadi, R.; Mortazavian, A.M.; Quiterio de Souza, S.L.; Barbosa Rocha, H.; Raices, R. Polycyclic aromatic hydrocarbons in milk and dairy products. Int. J. Dairy Technol. 2019, 72, 120–131. [Google Scholar] [CrossRef]
- Alomirah, H.; Al-Zenki, S.; Al-Hooti, S.; Zaghloul, S.; Sawaya, W.; Ahmed, N.; Kannan, K. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control 2011, 22, 2028–2035. [Google Scholar] [CrossRef]
- Pérez-Cadahía, B.; Laffon, B.; Pásaro, E.; Méndez, J. Evaluation of PAH bioaccumulation and DNA damage in mussels (Mytilus galloprovincialis) exposed to spilled Prestige crude oil. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 138, 453–460. [Google Scholar] [CrossRef]
- Guillén, M.D.; Sopelana, P. Polycyclic aromatic hydrocarbons in diverse foods. Food Saf. 2003, 1, 175–198. [Google Scholar]
- Rose, M.; Holland, J.; Dowding, A.; Petch, S.R.; White, S.; Fernandes, A.; Mortimer, D. Investigation into the formation of PAHs in foods prepared in the home to determine the effects of frying, grilling, barbecuing, toasting and roasting. Food Chem. Toxicol 2015, 78, 1–9. [Google Scholar] [CrossRef]
- Iwegbue, C.M.; Onyonyewoma, U.A.; Bassey, F.I.; Nwajei, G.E.; Martincigh, B.S. Concentrations and health risk of polycyclic aromatic hydrocarbons in some brands of biscuits in the Nigerian market. Hum. Ecol. Risk Assess. 2015, 21, 338–357. [Google Scholar] [CrossRef]
- Ishizaki, A.; Saito, K.; Hanioka, N.; Narimatsu, S.; Kataoka, H. Determination of polycyclic aromatic hydrocarbons in food samples by automated on-line in-tube solid-phase microextraction coupled with high-performance liquid chromatography-fluorescence detection. J. Chromatogr. A 2010, 1217, 5555–5563. [Google Scholar] [CrossRef] [PubMed]
- The Government of the Hong Kong Special Administrative Region. Available online: https://www.cfs.gov.hk/english/programme/programme_rafs/programme_rafs_fc_01_49.html (accessed on 21 October 2025).
- Prigioniero, A.; Postiglione, A.; Zuzolo, D.; Niinemets, Ü.; Tartaglia, M.; Scarano, P.; Mercurio, M.; Germinario, C.; Izzo, F.; Trifuoggi, M. Leaf surface functional traits influence particulate matter and polycyclic aromatic hydrocarbons air pollution mitigation: Insights from Mediterranean urban forests. J. Clean. Prod. 2023, 418, 138158. [Google Scholar] [CrossRef]
- Du, W.; Jiang, S.; Lei, Y.; Wang, J.; Cui, Z.; Xiang, P.; Chang0, Z.; Duan, W.; Shen, G.; Qin, Y. Occurrence, formation mechanism, and health risk of polycyclic aromatic hydrocarbons in barbecued food. Ecotoxicol. Environ. Saf. 2025, 293, 118046. [Google Scholar] [CrossRef]
- Holland, J.; Rose, M.; Petch, S. Occurrence of polycyclic aromatic hydrocarbons in herbs, spices, supplements and tea. In Report to the Food Standards Agency; Food Standards Agency (FSA): London, UK, 2015; pp. 2–73. [Google Scholar]
- Szternfeld, P.; Marakis, A.; Scippo, M.-L.; Van Hoeck, E.; Joly, L. Polycyclic aromatic hydrocarbons in spices and dried herbs and associated risk for the Belgian population. Food Addit. Contam. Part B 2022, 15, 292–300. [Google Scholar] [CrossRef]
- Guo, X.; Chen, F.; Pan, S. Pollution, traceability, and health risk of polycyclic aromatic hydrocarbons in traditional Chinese spices. J. Food Compos. Anal. 2025, 142, 107390. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (MFDS). General Test Method for Benzo[a]pyrene in Food. Available online: https://www.mfds.go.kr (accessed on 18 August 2025).
- Nisbet, I.C.; Lagoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Korea Disease Control and Prevention Agency. Korea National Health and Nutrition Examination Survey (KNHANES), 2023 Dataset. Available online: https://www.khidi.or.kr/kps/dhraStat/result2?menuId=MENU01653&year=2023 (accessed on 18 August 2025).
- National Health Insurance Service. 2023 Health Checkup Statistical Yearbook. Available online: https://www.nhis.or.kr/nhis/together/wbhaec07000m01.do?mode=view&articleNo=10848529&article.offset=0&articleLimit=10 (accessed on 11 August 2025).
- EFSA. Polycyclic aromatic hydrocarbons in food-scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008, 6, 724. [Google Scholar] [CrossRef]
- AOAC International. Draft SMPR 2021.XXX: Quantitative Mycotoxins in Cannabis; Available online: https://www.aoac.orgAOAC International: Rockville, MD, USA, 2021; (accessed on 6 October 2025).
- European Commission. Commission Regulation (EU) No 836/2011 of 19 August 2011 amending Regulation (EC) No 333/2007 laying down the methods of sampling and analysis for the official control of the levels of lead, cadmium, mercury, inorganic tin, 3-MCPD and benzo (a) pyrene in foodstuffs. Off. J. Eur. Union 2011, 215, 9–16. [Google Scholar]
- Di Bella, G.; Potortì, A.G.; Ben Tekaya, A.; Beltifa, A.; Ben Mansour, H.; Sajia, E.; Bartolomeo, G.; Naccari, C.; Dugo, G.; Lo Turco, V. Organic contamination of Italian and Tunisian culinary herbs and spices. J. Environ. Sci. Health B 2019, 54, 345–356. [Google Scholar] [CrossRef]
- Szternfeld, P.; Marchi, J.; Malysheva, S.V.; Joly, L. Modular method for the determination of polycyclic aromatic hydrocarbons in spices and dried herbs by gas chromatography–tandem mass spectrometry. Food Anal. Methods 2019, 12, 2383–2391. [Google Scholar] [CrossRef]
- Rozentale, I.; Lun, A.Y.; Zacs, D.; Bartkevics, V. The occurrence of polycyclic aromatic hydrocarbons in dried herbs and spices. Food Control 2018, 83, 45–53. [Google Scholar] [CrossRef]
- Rahman, M.M.; Abd El-Aty, A.; Shim, J.-H. Matrix enhancement effect: A blessing or a curse for gas chromatography?—A review. Anal. Chim. Acta 2013, 801, 14–21. [Google Scholar] [CrossRef]
- Bogdanović, T.; Petričević, S.; Listeš, E.; Pleadin, J. Polycyclic aromatic hydrocarbons in selected food items coming from the Croatian market. Ital. J. Food Sci. 2019, 31, 661–680. [Google Scholar]
- Tomaniová, M.; Kocourek, V.; Hajšlová, J. Polycyklické aromatické uhlovodíky v potravinách. Chem. Listy 1997, 91, 357–366. [Google Scholar]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Shahina, Z.; Molaeitabari, A.; Sultana, T.; Dahms, T.E.S. Cinnamon leaf and clove essential oils are potent inhibitors of Candida albicans virulence traits. Microorganisms 2022, 10, 1989. [Google Scholar] [CrossRef]
- Schmidt, E.; Jirovetz, L.; Buchbauer, G.; Eller, G.A.; Stoilova, I.; Krastanov, A.; Stoyanova, A.; Geissler, M. Composition and antioxidant activities of the essential oil of cinnamon (Cinnamomum zeylanicum Blume) leaves from Sri Lanka. J. Essent. Oil-Bear. Plants 2006, 9, 170–182. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Falah, F.; Lavi Arab, F.; Vasiee, M.; Tabatabaee Yazdi, F. Chemical composition and antioxidant, antimicrobial, and antiproliferative activities of Cinnamomum zeylanicum bark essential oil. Evid.-Based Complement. Altern. Med. 2020, 2020, 5190603. [Google Scholar] [CrossRef]
- Parthasarathy, V.A.; Chempakam, B.; Zachariah, T.J. Chemistry of Spices; CABI Head Office: Wallingford, UK, 2008; pp. 124–145. [Google Scholar]
- Francis, G.W.; Christy, A.A.; Øygarden, J. Pyrolytic formation of polycyclic aromatic hydrocarbons from sesquiterpenes. Food Chem. 2012, 135, 1316–1322. [Google Scholar] [CrossRef][Green Version]
- Rodrigues Sampaio, G.; Guizellini, G.M.; Alves da Silva, S.; Palma de Almeida, A.; Pinaffi-Langley, A.C.; Macedo Rogero, M.; Costa De Camargo, A.; Torres, E. Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, analytical methods, and strategies to reduce their formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef]
- da Silva, S.A.; de Rossi, G.Z.; de Almeida, A.P.; Guizellini, G.M.; Torres, E.A.F.d.S.; Rogero, M.M.; Sampaio, G.R. Occurrence and exposure to polycyclic aromatic hydrocarbons (PAHs) in traditional dry-cured or smoked meat products from Brazil. Food Prod. Process. Nutr. 2024, 6, 82. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Mevzuat Bilgi Sistemi. Available online: https://www.mevzuat.gov.tr/mevzuat?MevzuatNo=40394&MevzuatTur=7&MevzuatTertip=5 (accessed on 5 October 2025).
- Legislation.gov.uk. Available online: https://www.legislation.gov.uk/eur/2015/1933/annex (accessed on 5 October 2025).
- United States Department of Agriculture Foreign Agricultural Service (USDA FAS). China Releases Standard on Maximum Levels of Contaminants in Foods. Available online: https://www.fas.usda.gov/data/china-china-releases-standard-maximum-levels-contaminants-foods-0 (accessed on 17 October 2025).
- Hwang, H.-J.; Lee, S.-H.; Kim, Y.-Y.; Shin, H.-S. Polycyclic aromatic hydrocarbon risk assessment and analytical methods using QuEchERS pretreatment for the evaluation of herbal medicine ingredients in Korea. Foods 2021, 10, 2200. [Google Scholar] [CrossRef]
- Yu, L.; Cao, Y.; Zhang, J.; Cui, Z.; Sun, H. Isotope dilution-GC-MS/MS analysis of 16 polycyclic aromatic hydrocarbons in selected medicinal herbs used as health food additives. Food Addit. Contam. Part A 2012, 29, 1800–1809. [Google Scholar] [CrossRef]
- Coleto, J.M.; Martín, A.; Horrillo, A.; Mesías, F.J.; Velázquez, R. An approach to the consumption of smoked paprika in spain and its impact on the intake of polycyclic aromatic hydrocarbons. Foods 2021, 10, 973. [Google Scholar] [CrossRef]
- Lodovici, M.; Akpan, V.; Casalini, C.; Zappa, C.; Dolara, P. Polycyclic aromatic hydrocarbons in Laurus nobilis leaves as a measure of air pollution in urban and rural sites of Tuscany. Chemosphere 1998, 36, 1703–1712. [Google Scholar] [CrossRef]
- Zhan, X.-H.; Ma, H.-L.; Zhou, L.-X.; Liang, J.-R.; Jiang, T.-H.; Xu, G.-H. Accumulation of phenanthrene by roots of intact wheat (Triticum acstivnm L.) seedlings: Passive or active uptake? BMC Plant Biol. 2010, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.E.; Sánchez-Palencia, Y.; Gallego, J.L.; Borrego, Á.G.; Baragano, D.; Torres, T. Deposition of atmospheric polycyclic aromatic hydrocarbons in rural areas: Current data and historical record from an ombrotrophic peatland. Int. J. Coal Geol. 2023, 268, 104199. [Google Scholar] [CrossRef]
- Wang, J.; Bao, H.; Zhang, H.; Li, J.; Hong, H.; Wu, F. Effects of cuticular wax content and specific leaf area on accumulation and partition of PAHs in different tissues of wheat leaf. Environ. Sci. Pollut. Res. 2020, 27, 18793–18802. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, Q.; Tian, S.; Yang, Z.; Yang, Y.; Shao, P.; Liu, Y. Occurrences of Deposited Polycyclic Aromatic Hydrocarbons in Wax of Plant Leaves Using Laser Scanning Microscopy and Gas Chromatography–Mass Spectrometry. Atmosphere 2024, 15, 1165. [Google Scholar] [CrossRef]
- Xi, D.; Li, J.; Kuang, Y.W.; Xu, Y.M.; Zhu, X.M. Influence of traffic exhausts on elements and polycyclic aromatic hydrocarbons in leaves of medicinal plant Broussonetia papyrifera. Atmos. Pollut. Res. 2013, 4, 370–376. [Google Scholar] [CrossRef][Green Version]
- Borgulat, J.; Staszewski, T. Fate of PAHs in the vicinity of aluminum smelter. Environ. Sci. Pollut. Res. 2018, 25, 26103–26113. [Google Scholar] [CrossRef]
- Klingberg, J.; Strandberg, B.; Grundström, M.; Sjöman, H.; Wallin, G.; Pleijel, H. Variation in Polycyclic Aromatic Compound (PAC) concentrations in a Norway spruce stand close to a major traffic route—Influence of distance and season. Water Air Soil Pollut. 2023, 234, 563. [Google Scholar] [CrossRef]
- Kakareka, S.V.; Kukharchyk, T.I. PAH emission from the open burning of agricultural debris. Sci. Total Environ. 2003, 308, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Silvergren, S.; Spinicci, S.; Mashayekhy Rad, F.; Nilsson, U.; Westerholm, R.; Johansson, C. Contribution of wood burning to exposures of PAHs and oxy-PAHs in Eastern Sweden. Atmos. Chem. Phys. 2022, 22, 11359–11379. [Google Scholar] [CrossRef]
- Li, Y.; Duan, X. Wet deposition of polycyclic aromatic hydrocarbons in a remote area of Central South China from 2014 to 2017. Atmos. Sci. Lett. 2024, 25, e1201. [Google Scholar] [CrossRef]
- Pratt, G.; Herbrandson, C.; Krause, M.; Schmitt, C.; Lippert, C.; McMahon, C.; Ellickson, K. Measurements of gas and particle polycyclic aromatic hydrocarbons (PAHs) in air at urban, rural and near-roadway sites. Atmos. Environ. 2018, 179, 268–278. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Cabezudo, M.D. Effect of drying method on the volatiles in bay leaf (Laurus nobilis L.). J. Agric. Food Chem. 2002, 50, 4520–4524. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. Code of practice for the reduction of contamination of food with polycyclic aromatic hydrocarbons (PAH) from smoking and direct drying processes. CAC/RCP 2009, 68, 1–16. [Google Scholar]
- Zhang, L.; Wu, H.-T.; Yang, F.-X.; Zhang, J.-H. Evaluation of Soxhlet extractor for one-step biodiesel production from Zanthoxylum bungeanum seeds. Fuel Process. Technol. 2015, 131, 452–457. [Google Scholar] [CrossRef]
- Li, X.-Q.; Kang, R.; Huo, J.-C.; Xie, Y.-H.; Wang, S.-W.; Cao, W. Wound-healing activity of Zanthoxylum bungeanum maxim seed oil on experimentally burned rats. Pharmacogn. Mag. 2017, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-S.; Han, H.-J. Chemical constituents of Korean chopi (Zanthoxylum piperitum) and sancho (Zanthoxylum schinifolium). Korean J. Food Sci. Technol. 1996, 28, 19–27. [Google Scholar]
- Kubatova, A.; Št’ávová, J.; Seames, W.S.; Luo, Y.; Sadrameli, S.M.; Linnen, M.J.; Baglayeva, G.V.; Smoliakova, I.P.; Kozliak, E.I. Triacylglyceride thermal cracking: Pathways to cyclic hydrocarbons. Energy Fuels 2012, 26, 672–685. [Google Scholar] [CrossRef]
- Sinha, S.; Rahman, R.K.; Raj, A. On the role of resonantly stabilized radicals in polycyclic aromatic hydrocarbon (PAH) formation: Pyrene and fluoranthene formation from benzyl–indenyl addition. Phys. Chem. Chem. Phys. 2017, 19, 19262–19278. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Wang, S.; Zou, Y.; Zhang, J.; Liang, L.; Wen, C.; Li, Y.; Xu, X.; He, X. Relationship between PAH4 formation and thermal reaction products in model lipids and possible pathways of PAHs formation. J. Hazard. Mater. 2024, 465, 133374. [Google Scholar] [CrossRef]
- Lai, Y.-W.; Stephen Inbaraj, B.; Chen, B.-H. Effects of Oil and Processing conditions on formation of heterocyclic amines and polycyclic aromatic hydrocarbons in pork fiber. Foods 2023, 12, 3504. [Google Scholar] [CrossRef] [PubMed]
- Llamas, A.; Al-Lal, A.-M.; García-Martínez, M.-J.; Ortega, M.F.; Llamas, J.F.; Lapuerta, M.; Canoira, L. Polycyclic Aromatic Hydrocarbons (PAHs) produced in the combustion of fatty acid alkyl esters from different feedstocks: Quantification, statistical analysis and mechanisms of formation. Sci. Total Environ. 2017, 586, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Li, J.; Yao, Z. Changes in PAHs levels in edible oils during deep-frying process. Food Control 2016, 66, 233–240. [Google Scholar] [CrossRef]
- Reizer, E.; Csizmadia, I.G.; Nehez, K.; Viskolcz, B.; Fiser, B. Theoretical investigation of benzo (a) pyrene formation. Chem. Phys. Lett. 2021, 772, 138564. [Google Scholar] [CrossRef]
- Hwang, K.-W.; Son, D.; Jo, H.-W.; Kim, C.H.; Seong, K.C.; Moon, J.-K. Levels of curcuminoid and essential oil compositions in turmerics (Curcuma longa L.) grown in Korea. Appl. Biol. Chem. 2016, 59, 209–215. [Google Scholar] [CrossRef]
- Awasthi, P.; Dixit, S. Chemical composition of Curcuma Longa leaves and rhizome oil from the plains of Northern India. J. Young Pharm. 2009, 1, 322. [Google Scholar]
- Raina, V.; Srivastava, S.; Jain, N.; Ahmad, A.; Syamasundar, K.; Aggarwal, K. Essential oil composition of Curcuma longa L. cv. Roma from the plains of northern India. Flavour Fragr. J. 2002, 17, 99–102. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Liu, D. Effects of oxygenated biofuel additives on soot formation: A comprehensive review of laboratory-scale studies. Fuel 2022, 313, 122635. [Google Scholar] [CrossRef]
- McEnally, C.S.; Pfefferle, L.D. Sooting tendencies of oxygenated hydrocarbons in laboratory-scale flames. Environ. Sci. Technol. 2011, 45, 2498–2503. [Google Scholar] [CrossRef]
- Bierkandt, T.; Gaiser, N.; Bachmann, J.; Oßwald, P.; Köhler, M. Terpene speciation: Analytical insights into the oxidation and pyrolysis of limonene and 1,8-cineole via molecular-beam mass spectrometry. Combust. Flame 2025, 272, 113854. [Google Scholar] [CrossRef]
- Hoang, S.L.; Le, T.N.; Nguyen, H.T. Comparison of Chemical Profile and Antibacterial Activity of Cinnamomum cassia Bark Essential Oil from Three different Vietnamese Provinces. Indian J. Agric. Res. 2025, 59, 1060. [Google Scholar] [CrossRef]
- Vu, D.P.U.; Ho, D.M. Chemical composition of cinnamomum Cassia oil in Vietnam and its Spatial repellent effects against the Aedes aegypti. J. Tech. Educ. Sci. 2022, 17, 130–134. [Google Scholar] [CrossRef]
- Cha, J.; Kim, C.-T.; Kim, T.-E.; Cho, Y.-J. Optimization of subcritical extraction process for cinnamon (Cinnamomum Cassia Blume) using response surface methodology. Food Sci. Biotechnol. 2019, 28, 1703–1711. [Google Scholar] [CrossRef]
- Kim, L.; Jeon, H.-J.; Kim, Y.-C.; Yang, S.-H.; Choi, H.; Kim, T.-O.; Lee, S.-E. Monitoring polycyclic aromatic hydrocarbon concentrations and distributions in rice paddy soils from Gyeonggi-do, Ulsan, and Pohang. Appl. Biol. Chem. 2019, 62, 18. [Google Scholar] [CrossRef]
- Shao, X.; Xu, Y.; Zhang, W.; Lv, J. Polycyclic aromatic hydrocarbons (PAHs) pollution in agricultural soil in Tianjin, China. Soil Sediment Contam. Int. J. 2015, 24, 343–351. [Google Scholar] [CrossRef]
- Kwon, H.-O.; Choi, S.-D. Polycyclic aromatic hydrocarbons (PAHs) in soils from a multi-industrial city, South Korea. Sci. Total Environ. 2014, 470, 1494–1501. [Google Scholar] [CrossRef]
- Ma, W.-L.; Liu, L.-Y.; Tian, C.-G.; Qi, H.; Jia, H.-L.; Song, W.-W.; Li, Y.-F. Polycyclic aromatic hydrocarbons in Chinese surface soil: Occurrence and distribution. Environ. Sci. Pollut. Res. Int. 2015, 22, 4190–4200. [Google Scholar] [CrossRef]
- Duc, T.V.; Lan, C.D.T.; Tra, M.N. Residue of selected persistent organic pollutants (POPs) in soil of some areas in Vietnam. In Biochemical Toxicology-Heavy Metals and Nanomaterials; IntechOpen: London, UK, 2019; pp. 87–100. [Google Scholar]
- Liu, F.; Xu, Y.; Liu, J.; Liu, D.; Li, J.; Zhang, G.; Li, X.; Zou, S.; Lai, S. Atmospheric deposition of polycyclic aromatic hydrocarbons (PAHs) to a coastal site of Hong Kong, South China. Atmos. Environ. 2013, 69, 265–272. [Google Scholar] [CrossRef]
- Nacar, S. Trends of high and low values of annual and seasonal precipitation in Turkey. Sustainability 2023, 15, 16523. [Google Scholar] [CrossRef]
- Hssaisoune, M.; Bouchaou, L.; Sifeddine, A.; Bouimetarhan, I.; Chehbouni, A. Moroccan groundwater resources and evolution with global climate changes. Geosciences 2020, 10, 81. [Google Scholar] [CrossRef]
- Hanedar, A.; Alp, K.; Kaynak, B.; Avşar, E. Toxicity evaluation and source apportionment of polycyclic aromatic hydrocarbons (PAHs) at three stations in Istanbul, Turkey. Sci. Total Environ. 2014, 488, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Deabji, N.; Fomba, K.W.; dos Santos Souza, E.J.; Mellouki, A.; Herrmann, H. Influence of anthropogenic activities on metals, sugars and PAHs in PM10 in the city of Fez, Morocco: Implications on air quality. Environ. Sci. Pollut. Res. 2024, 31, 25238–25257. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, J.; Liu, Y.; Peng, J.; Wu, L.; Mao, H. PM2.5-Bound Polycyclic Aromatic Hydrocarbons (PAHs), Nitrated PAHs (NPAHs) and Oxygenated PAHs (OPAHs) in Typical Traffic-Related Receptor Environments. J. Geophys. Res. Atmos. 2022, 127, e2021JD035951. [Google Scholar] [CrossRef]
- Lohwasser, U.; Declercq, M.; Börner, A.; Struckmeyer, T.; Budahn, H.; Krüger, H.; Ulrich, D.; Marthe, F. The German parsley germplasm collection-interaction of morphological, molecular and phytochemical characters. In Proceedings of the IV International Symposium on Breeding Research on Medicinal and Aromatic Plants-ISBMAP2009 860, Ljubljana, Slovenia, 17–21 June 2009; pp. 235–240. [Google Scholar]
- Farouk, A.; Ali, H.; Al-Khalifa, A.R.; Mohsen, M.; Fikry, R. Aroma volatile compounds of parsley cultivated in the Kingdom of Saudi Arabia and Egypt extracted by hydrodistillation and headspace solid-phase microextraction. Int. J. Food Prop. 2017, 20, S2868–S2877. [Google Scholar] [CrossRef]
- Moustafa, Y.; Abdelwahab, M. Evaluation of four novel imported and Egyptian curly and non-curly leafed parsley genotypes for yield and essential oil composition under the Egyptian sandy soil growing conditions. J. Basic Appl. Res. Biomed. 2025, 2, 345–352. [Google Scholar]
- Bierkandt, T.; Hoener, M.; Gaiser, N.; Hansen, N.; Köhler, M.; Kasper, T. Experimental flat flame study of monoterpenes: Insights into the combustion kinetics of α-pinene, β-pinene, and myrcene. Proc. Combust. Inst. 2021, 38, 2431–2440. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeong, T.C.; Kim, J.H. In vitro and in vivo metabolism of myristicin in the rat. J. Chromatogr. B Biomed. Sci. Appl. 1998, 705, 367–372. [Google Scholar] [CrossRef]
- Wornat, M.J.; Ledesma, E.B.; Marsh, N.D. Polycyclic aromatic hydrocarbons from the pyrolysis of catechol (ortho-dihydroxybenzene), a model fuel representative of entities in tobacco, coal, and lignin. Fuel 2001, 80, 1711–1726. [Google Scholar] [CrossRef]
- Lomnicki, S.; Truong, H.; Dellinger, B. Mechanisms of product formation from the pyrolytic thermal degradation of catechol. Chemosphere 2008, 73, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Tsiodra, I.; Grivas, G.; Tavernaraki, K.; Bougiatioti, A.; Apostolaki, M.; Paraskevopoulou, D.; Gogou, A.; Parinos, C.; Oikonomou, K.; Tsagkaraki, M. Annual exposure to polycyclic aromatic hydrocarbons in urban environments linked to wintertime wood-burning episodes. Atmos. Chem. Phys. 2021, 21, 17865–17883. [Google Scholar] [CrossRef]
- Wang, P.; Jin, B.; Lian, C.; Guo, K.; Ma, C. Comparative analysis of polycyclic aromatic hydrocarbons and halogenated polycyclic aromatic hydrocarbons in different parts of Perilla frutescens (L.) Britt. Molecules 2022, 27, 3133. [Google Scholar] [CrossRef]
- Li, S.-Q.; Ni, H.-G.; Zeng, H. PAHs in polystyrene food contact materials: An unintended consequence. Sci. Total Environ. 2017, 609, 1126–1131. [Google Scholar] [CrossRef] [PubMed]


| Sample Type | Compounds | Target Ion | Qualitative Ions | Retention Time | Equation (y = ax + b) | Linearity (R2) | LOD (1) (μg/kg) | LOQ (2) (μg/kg) |
|---|---|---|---|---|---|---|---|---|
| Spice (mustard) | Benz[a]anthracene | 228 | 226, 229 | 22.793 | y = 0.0085x − 0.0013 | 1.000 | 0.08 | 0.24 |
| Chrysene | 23.246 | y = 0.0115x + 0.0010 | 0.998 | 0.15 | 0.46 | |||
| Benzo[b]fluoranthene | 252 | 250, 253 | 30.462 | y = 0.0158x + 0.0037 | 0.999 | 0.18 | 0.55 | |
| Benzo[a]pyrene | 32.161 | y = 0.0109x + 0.0016 | 0.999 | 0.12 | 0.37 | |||
| Herb (parsley) | Benz[a]anthracene | 228 | 226, 229 | 22.793 | y = 0.0082x − 0.0014 | 0.999 | 0.17 | 0.51 |
| Chrysene | 23.246 | y = 0.0113x + 0.0010 | 1.000 | 0.11 | 0.32 | |||
| Benzo[b]fluoranthene | 252 | 250, 253 | 30.462 | y = 0.0150x + 0.0021 | 1.000 | 0.13 | 0.41 | |
| Benzo[a]pyrene | 32.161 | y = 0.0097x + 0.0018 | 0.999 | 0.15 | 0.46 |
| Sample Type | Compounds | Recovery (%) | ||||
|---|---|---|---|---|---|---|
| 1 μg/kg | 2 μg/kg | 5 μg/kg | 10 μg/kg | 20 μg/kg | ||
| Spice (mustard) | Benz[a]anthracene | 88.6 ± 1.2 | 98.4 ± 1.1 | 101.0 ± 1.2 | 116.2 ± 0.9 | 116.5 ± 0.2 |
| Chrysene | 102.1 ± 1.3 | 98.6 ± 0.4 | 107.6 ± 1.3 | 103.3 ± 1.0 | 98.1 ± 1.3 | |
| Benzo[b]fluoranthene | 96.3 ± 0.5 | 99.9 ± 0.6 | 93.0 ± 1.2 | 97.7 ± 0.7 | 93.5 ± 1.4 | |
| Benzo[a]pyrene | 95.6 ± 1.0 | 96.1 ± 0.6 | 100.0 ± 1.5 | 112.2 ± 0.4 | 109.0 ± 1.2 | |
| Herb (parsley) | Benz[a]anthracene | 109.3 ± 0.7 | 97.2 ± 0.8 | 94.2 ± 0.3 | 104.1 ± 1.4 | 112.9 ± 1.3 |
| Chrysene | 108.3 ± 1.4 | 101.1 ± 1.1 | 97.7 ± 1.1 | 97.6 ± 0.9 | 96.5 ± 1.1 | |
| Benzo[b]fluoranthene | 94.5 ± 0.3 | 90.7 ± 0.5 | 85.0 ± 0.0 | 89.4 ± 0.6 | 89.0 ± 0.9 | |
| Benzo[a]pyrene | 91.4 ± 1.2 | 97.7 ± 0.9 | 95.0 ± 0.3 | 96.4 ± 0.8 | 99.8 ± 1.2 | |
| Sample Type | Compounds | Intra-Day (n = 3) | Inter-Day (n = 3) | |||
|---|---|---|---|---|---|---|
| μg/kg | Accuracy (%) (1) | RSD (%) (2) | Accuracy (%) | RSD (%) | ||
| Spice | Benz[a]anthracene | 1 | 88.6 | 1.7 | 88.1 | 1.3 |
| 2 | 98.6 | 1.5 | 98.2 | 0.5 | ||
| 5 | 101.2 | 1.5 | 100.7 | 0.5 | ||
| 10 | 116.2 | 0.9 | 115.8 | 0.8 | ||
| 20 | 116.6 | 0.3 | 116.6 | 0.1 | ||
| Chrysene | 1 | 102.1 | 1.6 | 102.6 | 1.2 | |
| 2 | 98.6 | 0.5 | 98.5 | 0.5 | ||
| 5 | 107.4 | 1.7 | 107.4 | 0.9 | ||
| 10 | 103.4 | 1.3 | 103.7 | 1.1 | ||
| 20 | 98.1 | 1.7 | 97.5 | 1.5 | ||
| Benzo[b]fluoranthene | 1 | 96.3 | 0.7 | 96.1 | 0.5 | |
| 2 | 99.9 | 0.8 | 99.7 | 0.4 | ||
| 5 | 92.9 | 1.7 | 93.4 | 1.0 | ||
| 10 | 97.8 | 1.0 | 97.9 | 0.6 | ||
| 20 | 93.4 | 2.0 | 93.6 | 0.6 | ||
| Benzo[a]pyrene | 1 | 95.5 | 1.4 | 95.1 | 1.2 | |
| 2 | 96.1 | 0.8 | 96.3 | 0.4 | ||
| 5 | 99.8 | 2.0 | 99.5 | 1.5 | ||
| 10 | 112.3 | 0.5 | 112.4 | 0.4 | ||
| 20 | 109.0 | 1.4 | 108.5 | 1.1 | ||
| Herb | Benz[a]anthracene | 1 | 109.4 | 0.8 | 109.6 | 0.6 |
| 2 | 97.2 | 1.1 | 96.9 | 0.9 | ||
| 5 | 94.2 | 0.5 | 94.3 | 0.1 | ||
| 10 | 104.4 | 1.7 | 104.3 | 1.2 | ||
| 20 | 112.6 | 1.5 | 112.6 | 1.1 | ||
| Chrysene | 1 | 108.1 | 1.8 | 107.8 | 1.0 | |
| 2 | 101.1 | 1.3 | 100.7 | 1.0 | ||
| 5 | 97.9 | 1.4 | 97.5 | 0.3 | ||
| 10 | 97.8 | 1.2 | 97.6 | 0.6 | ||
| 20 | 96.8 | 1.5 | 96.7 | 0.9 | ||
| Benzo[b]fluoranthene | 1 | 94.5 | 0.5 | 94.4 | 0.3 | |
| 2 | 90.7 | 0.8 | 90.7 | 0.3 | ||
| 5 | 85.0 | 0.0 | 85.0 | 0.0 | ||
| 10 | 89.3 | 0.9 | 89.2 | 0.7 | ||
| 20 | 89.1 | 1.3 | 88.7 | 0.5 | ||
| Benzo[a]pyrene | 1 | 91.3 | 1.7 | 91.8 | 1.1 | |
| 2 | 97.7 | 1.2 | 98.1 | 1.0 | ||
| 5 | 95.0 | 0.4 | 95.2 | 0.3 | ||
| 10 | 96.5 | 1.1 | 96.1 | 0.5 | ||
| 20 | 99.6 | 1.7 | 100.2 | 0.4 | ||
| Sample Type | Sample | N (1) | Mean ± SD (μg/kg) | ||||
|---|---|---|---|---|---|---|---|
| Benz[a]anthracene | Chrysene | Benzo[b]fluoranthene | Benzo[a]pyrene | Σ4PAHs | |||
| Spice | Mustard | 10 | 0.18 ± 0.45 | 0.42 ± 1.12 | 0.15 ± 0.49 | 0.29 ± 0.92 | 0.26 ± 0.77 |
| Nutmeg | 10 | 1.05 ± 0.81 | 1.09 ± 0.95 | 0.00 ±0.00 | 0.12 ± 0.28 | 0.56 ± 0.80 | |
| Black pepper | 10 | 1.86 ± 1.98 | 3.13 ± 2.70 | 0.65 ± 1.86 | 0.93 ± 1.56 | 1.64 ± 2.22 | |
| Sichuan pepper | 10 | 1.36 ± 1.87 | 2.85 ± 4.66 | 1.61 ± 2.11 | 1.37 ± 2.44 | 1.80 ± 2.93 | |
| Cinnamon | 10 | 3.39 ± 4.86 | 6.98 ± 5.27 | 2.78 ± 4.30 | 6.18 ± 3.46 | 4.83 ± 4.71 | |
| Turmeric | 10 | 0.39 ± 0.44 | 0.20 ± 0.42 | 0.00 ±0.00 | 0.09 ± 0.20 | 0.17 ± 0.34 | |
| Herb | Basil | 10 | 0.83 ± 0.58 | 0.93 ± 0.83 | 1.30 ± 2.46 | 1.75 ± 2.84 | 1.23 ± 1.90 |
| Oregano | 10 | 0.32 ± 0.50 | 1.10 ± 1.31 | 3.18 ± 3.26 | 7.13 ± 6.68 | 2.94 ± 4.51 | |
| Parsley | 10 | 0.35 ± 0.50 | 1.76 ± 1.77 | 0.00 ±0.00 | 0.48 ± 1.52 | 0.65 ± 1.33 | |
| Rosemary | 10 | 0.07 ± 0.22 | 0.36 ± 0.69 | 0.61 ± 1.03 | 0.77 ± 2.43 | 0.45 ± 1.34 | |
| Bay leaves | 10 | 5.12 ± 5.33 | 7.61 ± 6.50 | 0.88 ± 1.07 | 0.79 ± 1.05 | 3.60 ± 5.04 | |
| Sample Type | Sample | Benz[a]anthracene | Chrysene | Benzo[b]fluoranthene | Benzo[a]pyrene | Σ4PAHs | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Detection Rate (%) | Median (Q1–Q3) (μg/kg) | Detection Rate (%) | Median (Q1–Q3) (μg/kg) | Detection Rate (%) | Median (Q1–Q3) (μg/kg) | Detection Rate (%) | Median (Q1–Q3) (μg/kg) | Detection Rate (%) | Median (Q1–Q3) (μg/kg) | ||
| Spice | Mustard | 20.0 | 0.00 (0.00–0.00) | 20.0 | 0.00 (0.00–0.00) | 10.0 | 0.00 (0.00–0.00) | 10.0 | 0.00 (0.00–0.00) | 15.0 | 0.00 (0.00–0.00) |
| Nutmeg | 80.0 | 1.01 (0.41–1.62) | 70.0 | 1.08 (0.16–1.66) | 0.0 | 0.00 (0.00–0.00) | 20.0 | 0.00 (0.00–0.00) | 42.5 | 0.00 (0.00–0.98) | |
| Black pepper | 80.0 | 1.66 (0.85–1.82) | 90.0 | 2.93 (1.88–3.27) | 20.0 | 0.00 (0.00–0.00) | 60.0 | 0.47 (0.00–0.56) | 62.5 | 0.63 (0.00–2.51) | |
| Sichuan pepper | 60.0 | 0.71 (0.00–1.31) | 60.0 | 1.03 (0.00–2.69) | 40.0 | 0.00 (0.00–3.63) | 40.0 | 0.00 (0.00–1.48) | 50.0 | 0.31 (0.00–3.03) | |
| Cinnamon | 80.0 | 1.62 (1.20–1.95) | 90.0 | 5.88 (4.16–7.59) | 70.0 | 0.88 (0.14–3.94) | 90.0 | 7.02 (5.26–8.74) | 82.5 | 4.29 (0.98–7.15) | |
| Turmeric | 50.0 | 0.25 (0.00–0.75) | 20.0 | 0.00 (0.00–0.00) | 0.0 | 0.00 (0.00–0.00) | 20.0 | 0.00 (0.00–0.00) | 22.5 | 0.00 (0.00–0.00) | |
| Herb | Basil | 80.0 | 0.75 (0.63–1.24) | 70.0 | 1.22 (0.16–1.47) | 40.0 | 0.00 (0.00–1.02) | 40.0 | 0.00 (0.00–2.26) | 57.5 | 0.69 (0.00–1.42) |
| Oregano | 40.0 | 0.00 (0.00–0.66) | 50.0 | 0.48 (0.00–1.96) | 70.0 | 2.25 (0.39–4.76) | 70.0 | 6.95 (0.64–10.84) | 23.0 | 0.91 (0.00–3.50) | |
| Parsley | 40.0 | 0.00 (0.00–0.67) | 70.0 | 1.14 (0.22–2.80) | 0.0 | 0.00 (0.00–0.00) | 10.0 | 0.00 (0.00–0.00) | 12.0 | 0.00 (0.00–0.76) | |
| Rosemary | 10.0 | 0.00 (0.00–0.00) | 30.0 | 0.00 (0.00–0.33) | 30.0 | 0.00 (0.00–1.02) | 10.0 | 0.00 (0.00–0.00) | 20.0 | 0.00 (0.00–0.00) | |
| Bay leaves | 90.0 | 3.00 (2.10–6.06) | 100.0 | 5.78 (4.73–8.88) | 60.0 | 0.60 (0.00–1.34) | 40.0 | 0.00 (0.00–1.79) | 29.0 | 2.03 (0.00–4.73) | |
| Sample Type | Sample | TEQBaP (μg/kg) | TEQΣ4PAHs (μg/kg) | MOE—Average Dietary Exposure | MOE—95th Percentile Dietary Exposure | ||
|---|---|---|---|---|---|---|---|
| BaP | Σ4PAHs | BaP | Σ4PAHs | BaP | Σ4PAHs | ||
| Spice | Mustard | 0.29 | 0.33 | 4.97 × 109 | 2.14 × 1010 | 1.17 × 108 | 5.04 × 108 |
| Nutmeg | 0.12 | 0.24 | 1.96 × 1011 | 4.90 × 1011 | 6.49 × 107 | 1.62 × 108 | |
| Black pepper | 0.93 | 1.22 | 4.67 × 107 | 1.74 × 108 | 4.57 × 107 | 1.70 × 108 | |
| Sichuan pepper | 1.37 | 1.70 | 1.23 × 109 | 4.82 × 109 | 8.57 × 107 | 3.37 × 108 | |
| Cinnamon | 6.18 | 6.87 | 1.00 × 109 | 4.38 × 109 | 1.39 × 106 | 6.07 × 106 | |
| Turmeric | 0.09 | 0.14 | 1.42 × 108 | 4.82 × 108 | 4.15 × 106 | 1.41 × 107 | |
| Herb | Basil | 1.75 | 1.98 | 1.62 × 109 | 6.98 × 109 | 2.02 × 107 | 8.70 × 107 |
| Oregano | 7.13 | 7.50 | 7.67 × 109 | 3.55 × 1010 | 6.56 × 108 | 3.03 × 109 | |
| Parsley | 0.48 | 0.54 | 3.06 × 109 | 1.34 × 1010 | 7.89 × 107 | 3.45 × 108 | |
| Rosemary | 0.77 | 0.84 | 3.74 × 109 | 1.66 × 1010 | 2.33 × 108 | 1.03 × 109 | |
| Bay leaves | 0.79 | 1.46 | 2.66 × 109 | 6.94 × 109 | 2.11 × 107 | 5.51 × 107 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sa, S.-H.; Lim, K.-J.; Shin, H.-S. Quantitative Analysis and Risk Assessment of Polycyclic Aromatic Hydrocarbons Using Gas Chromatography–Mass Spectrometry from Herbs and Spices Distributed in South Korea. Foods 2025, 14, 3595. https://doi.org/10.3390/foods14213595
Sa S-H, Lim K-J, Shin H-S. Quantitative Analysis and Risk Assessment of Polycyclic Aromatic Hydrocarbons Using Gas Chromatography–Mass Spectrometry from Herbs and Spices Distributed in South Korea. Foods. 2025; 14(21):3595. https://doi.org/10.3390/foods14213595
Chicago/Turabian StyleSa, Seung-Hyun, Kyung-Jik Lim, and Han-Seung Shin. 2025. "Quantitative Analysis and Risk Assessment of Polycyclic Aromatic Hydrocarbons Using Gas Chromatography–Mass Spectrometry from Herbs and Spices Distributed in South Korea" Foods 14, no. 21: 3595. https://doi.org/10.3390/foods14213595
APA StyleSa, S.-H., Lim, K.-J., & Shin, H.-S. (2025). Quantitative Analysis and Risk Assessment of Polycyclic Aromatic Hydrocarbons Using Gas Chromatography–Mass Spectrometry from Herbs and Spices Distributed in South Korea. Foods, 14(21), 3595. https://doi.org/10.3390/foods14213595







