Antimicrobial and Biofilm Inhibiting Potential of Two Romanian Linden Honeys
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Palynologic Assessment of the Honey Samples
2.3. Determination of Impurities
2.4. Determination of Humidity
2.5. Determination of Acidity
2.6. Determination of pH
2.7. Determination of Reducing Sugar
2.8. Determination of Total Phenolic Content (TPC)
2.9. Determination of Flavonoid Content (FC)
2.10. Determination of Antioxidant Capacity (DPPH)
2.11. Determination of Mineral Substance Content (Ash)
2.12. Antimicrobial Activity
2.12.1. Bacterial Strains
2.12.2. Bacterial Cultures
2.12.3. Bacterial Cell Viability Testing
2.12.4. Inhibition Rate of Biofilm Formation
2.13. Statistical Analyses
3. Results
3.1. Palynological Analysis
| Species | Asteraceae | Tiliaceae | Lamiaceae | Fabaceae | Rosaceae | Boraginaceae | Others | |
|---|---|---|---|---|---|---|---|---|
| Honey Sample | ||||||||
| Sample A | 12.15% | 47.85% | 10.35% | 8.75% | 7.50% | 6.60% | 6.80% | |
| Sample B | 11.62% | 51.75% | 3.25% | 9.25% | 8.25% | 7.5% | 8.38% | |
3.2. Physico-Chemical Characterization
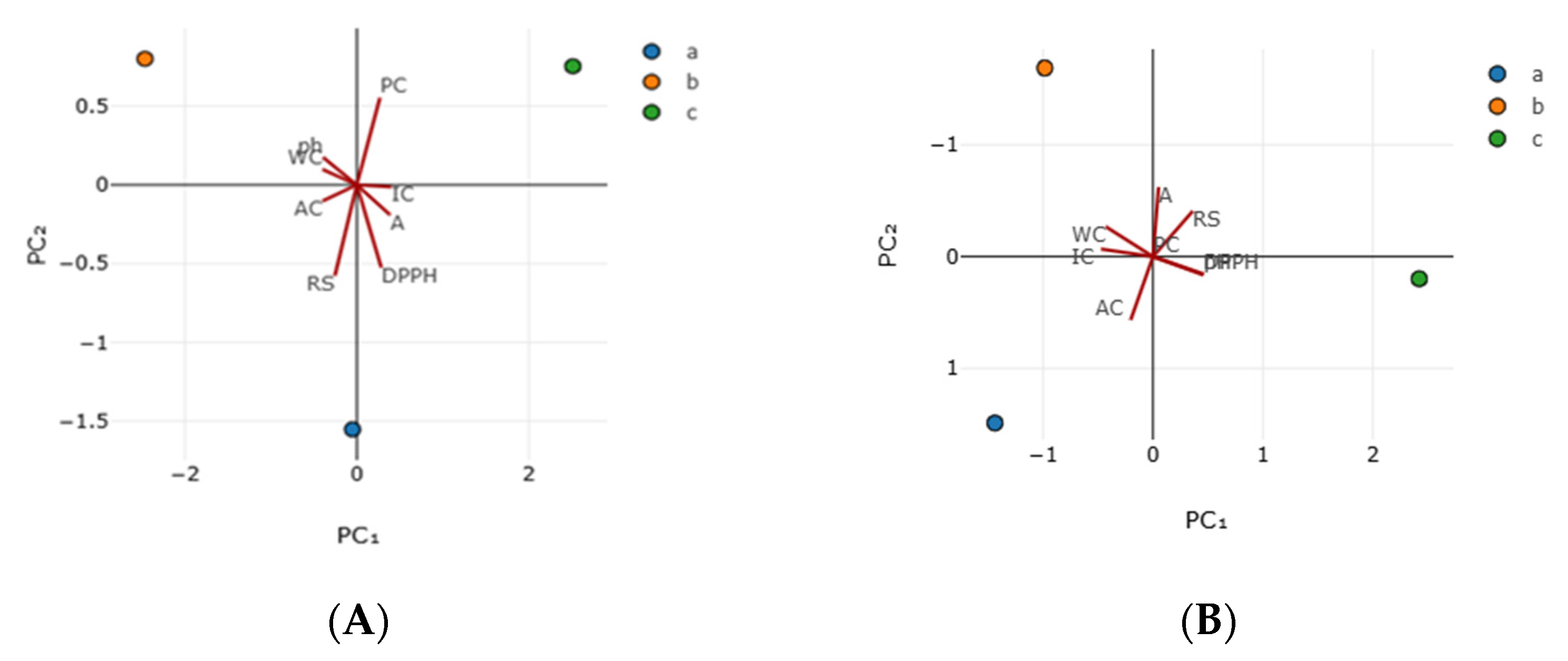
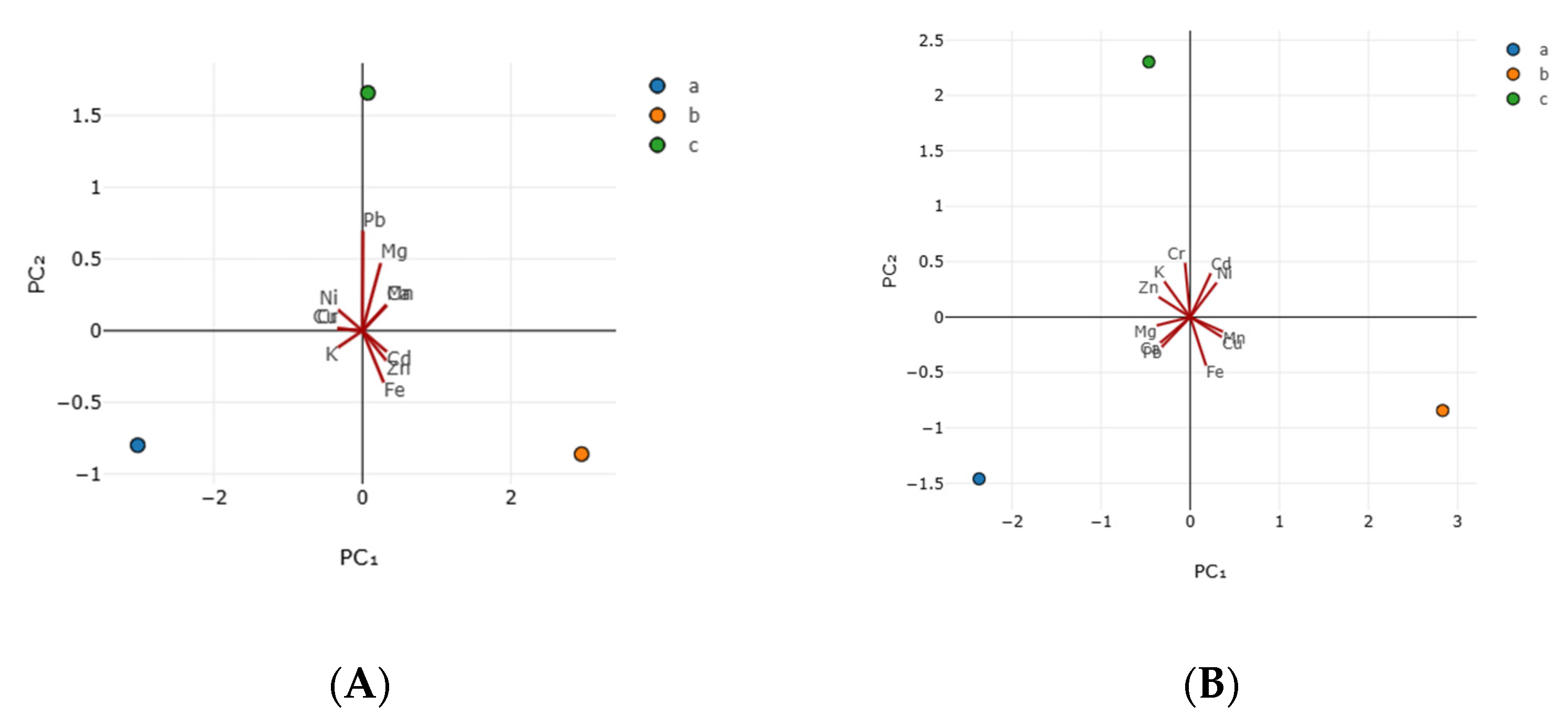
3.3. Antioxidant Activity
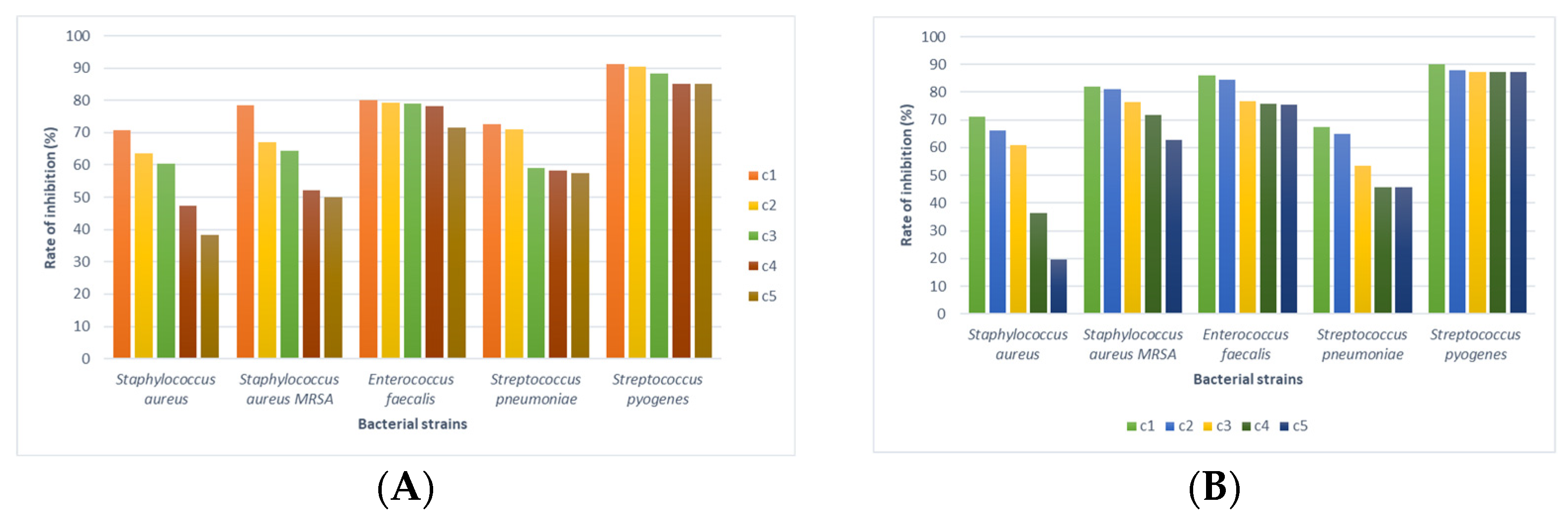
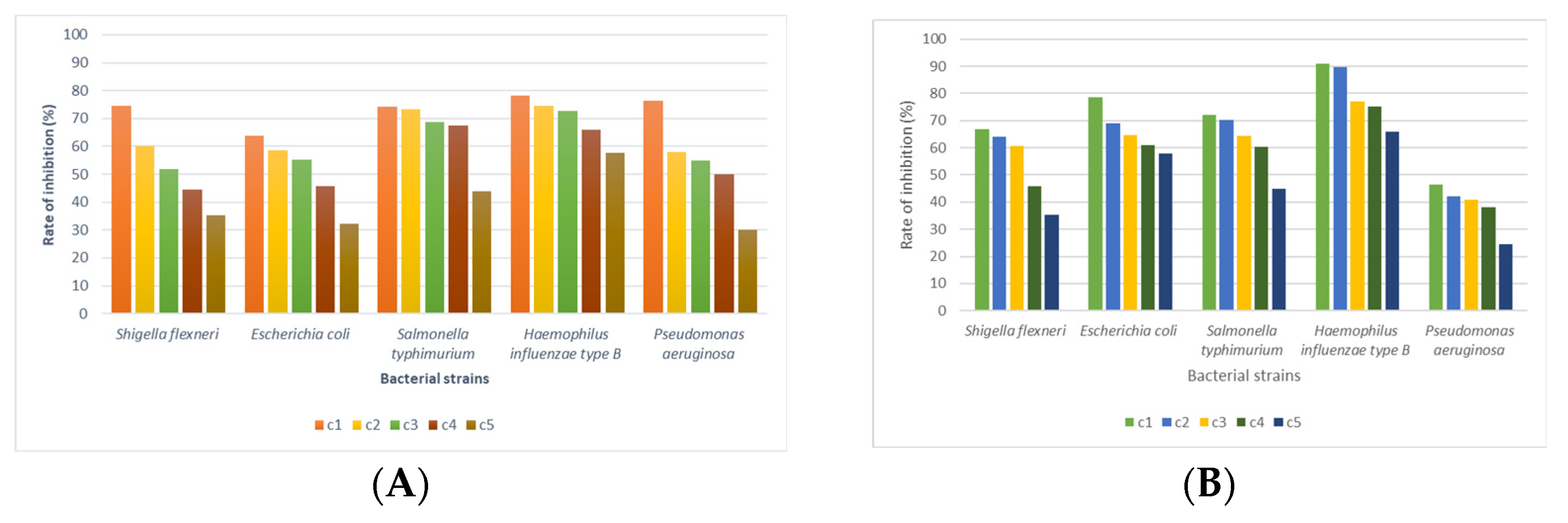
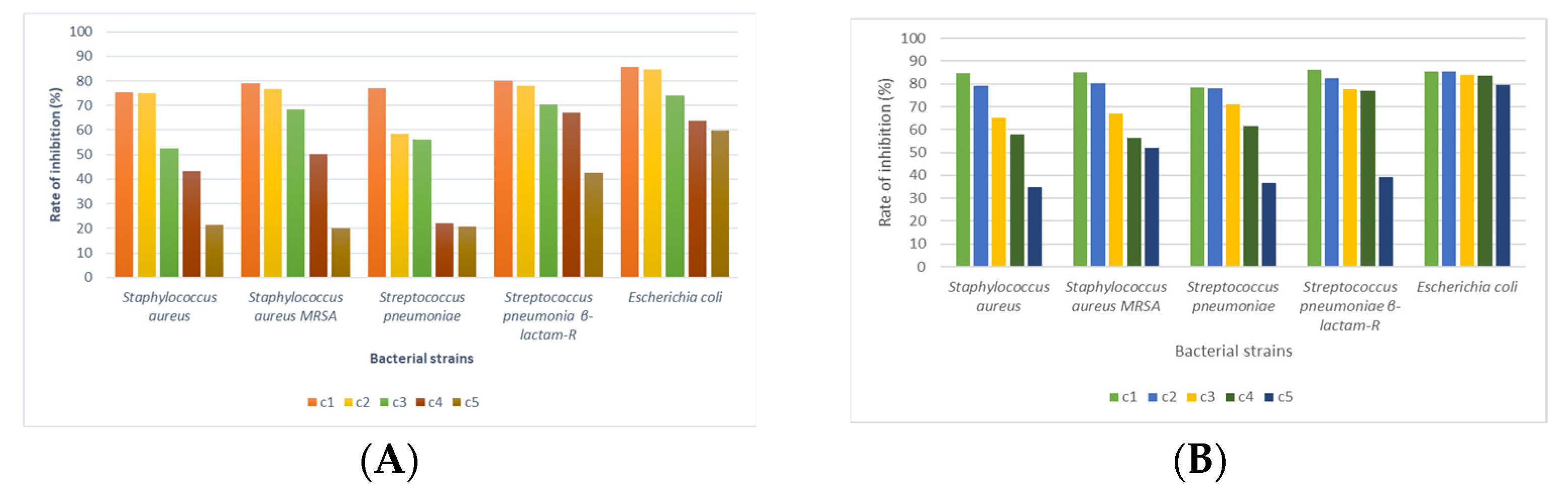
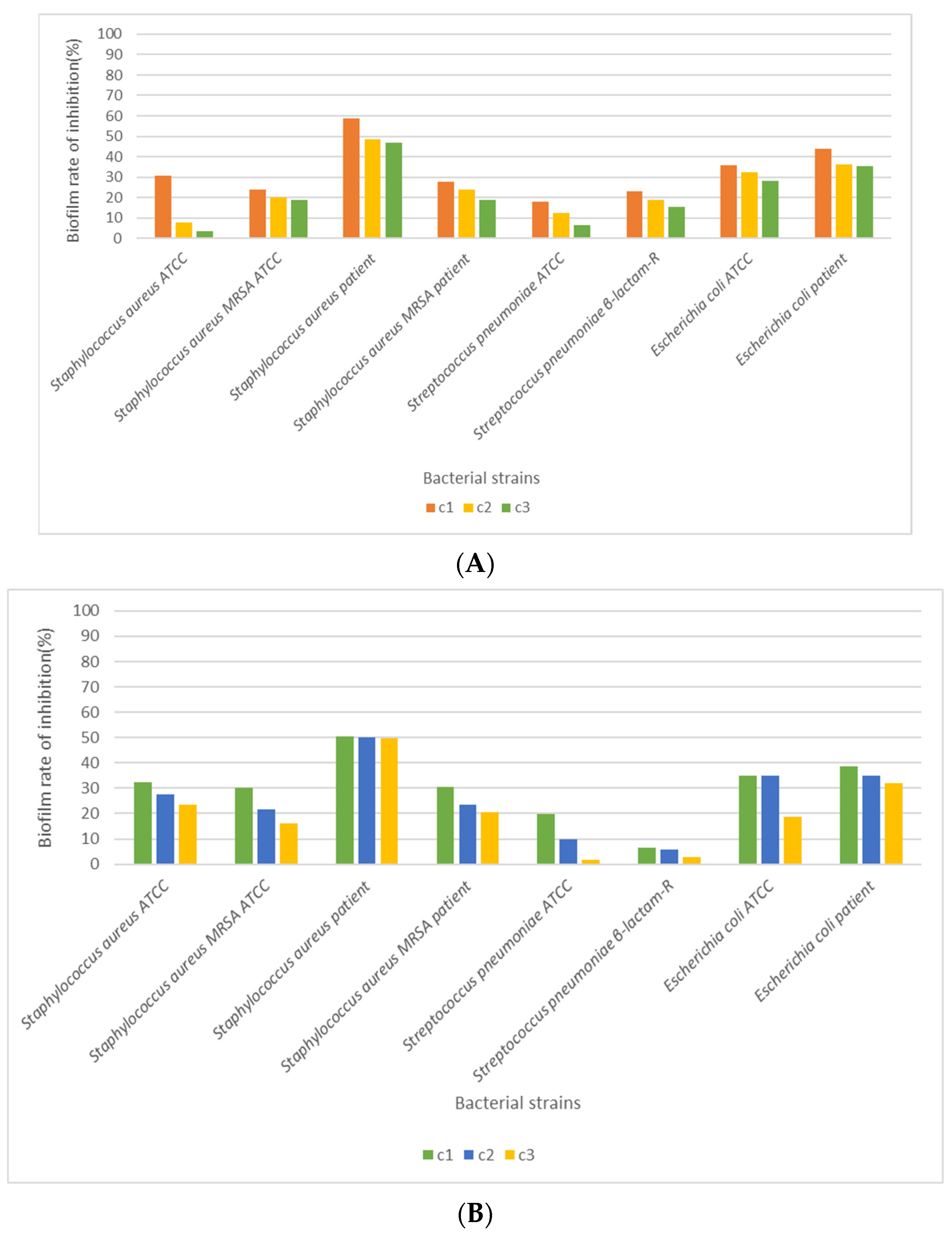
3.4. Antimicrobial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WC | Water content |
| IC | Impurities |
| AC | Ash |
| A | Acidity |
| RS | Reducing sugars |
| TPC | Phenolic compounds |
| PCA | Principal component analysis |
| Cu | Copper |
| Ni | Nickel |
| Cr | Chromium |
| Pb | Lead |
| Fe | Iron |
| Zn | Zinc |
| Mn | Manganese |
| Ca | Calcium |
| Mg | Magnesium |
| K | Potassium |
References
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Rao, P.V.; Krishnan, K.T.; Salleh, N.; Gan, S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Rev. Bras. Farmacogn. 2016, 26, 657–664. [Google Scholar] [CrossRef]
- Escuredo, O.; Míguez, M.; Fernández-González, M.; Seijo, M.C. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013, 138, 851–856. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Gonz´alez-Param´as, A.M. Chemical composition of honey. In Bee Products-Chemical and Biological Properties; Springer: Heidelberg, Germany, 2017; pp. 43–82. [Google Scholar]
- Gela, A.; Gebresilassie, A.; Atikem, A.; Teferi Damto, T.; Woldehawariat, Y. Physicochemical Properties and Botanical Sources of Honey from Different Areas of Ethiopia: An Implication for Quality Control. J. Food Qual. 2023, 2023, 9051595. [Google Scholar] [CrossRef]
- Tornuk, F.; Karaman, S.; Ozturk, I.; Toker, O.S.; Tastemur, B.; Sagdic, O.; Dogan, M.; Kayacier, A. Quality characterization of artisanal and retail Turkish blossom honeys: Determination of physicochemical, microbiological, bioactive properties and aroma profile. Ind. Crops Prod. 2013, 46, 124–131. [Google Scholar] [CrossRef]
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Felsner, M.L.; Cano, C.B.; Bruns, R.E.; Watanabe, H.M.; Almeida-Muradian, L.B.; Matos, J.R. Characterization of monofloral honeys by ash contents through a hierarchical design. J. Food Compos. Anal. 2004, 17, 737–747. [Google Scholar] [CrossRef]
- Sabatini, A.G. Il miele: Origine, composizione e proprieta. In Conscere il Miele; Sabatini, A.G., Botolotti, L., Marcazzan, G.L., Eds.; Avenue Media: Bologna, Italy, 2007; pp. 3–37. [Google Scholar]
- Karabagias, I.K.; Louppis, A.P.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and geographical discrimination of Greek pine and thyme honeys based on their mineral content, using chemometrics. Eur. Food Res. Technol. 2017, 243, 101–113. [Google Scholar] [CrossRef]
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant activities and total phenolics of different types of honey. Nutr. Res. 2002, 22, 1041–1047. [Google Scholar] [CrossRef]
- Gheldof, N.; Engeseth, N.J. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J. Agric. Food Chem. 2002, 50, 3050–3055. [Google Scholar] [CrossRef]
- Andersen, M.; Markham, K.R. Flavonoids chemistry, biochemistry and applications. In Separation and Quantification of Flavonoids; Taylor & Francis: New York, NY, USA, 2006. [Google Scholar]
- Stanek, N.; Jasicka-Misiak, I. HPTLC Phenolic Profiles as Useful Tools for the Authentication of Honey. Food Anal. Methods 2018, 11, 2979–2989. [Google Scholar] [CrossRef]
- Interstate Standard 19792-2017; Natural Honey. Specifications. Standartinform: Moscow, Russia, 2017.
- Kędzierska-Matysek, M.; Florek, M.; Wolanciuk, A.; Skałecki, P. Effect of freezing and room temperatures storage for 18 months on quality of raw rapeseed honey (Brassica napus). J. Food Sci. Technol. 2016, 53, 3349–3355. [Google Scholar] [CrossRef]
- Salhi, I.; Samet, Y.; Trabelsi, M. Direct electrochemical determination of very low levels of 5-hydroxymethyl furfural in natural honey by cyclic and square wave voltammetric techniques. J. Electroanal. Chem. 2020, 837, 114326. [Google Scholar] [CrossRef]
- Besir, A.; Yazici, F.; Mortas, M.; Gul, O. A novel spectrophotometric method based on Seliwanoff test to determine 5-(Hydroxymethyl) furfural (HMF) in honey: Development, in house validation and application. LWT—Food Sci. Technol. 2021, 139, 110602. [Google Scholar] [CrossRef]
- Laolue, P.; Lerdsri, J. Development of square wave voltammetry method using working electrodes modified with nickel oxide and carbon black for determination of 5-hydroxymethylfurfural in honey. J. Food Compos. Anal. 2023, 124, 105699. [Google Scholar] [CrossRef]
- Gheldof, N.; Wang, X.H.; Engeseth, N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef] [PubMed]
- Tonks, A.J.; Dudley, E.; Porter, N.; Parton, J.; Brazier, J.; Smith, E.; Tonks, A. A 5.8-kDa component of manuka honey stimulates immune cells via TLR4. J. Leukocyte Biol. 2007, 82, 1147–1155. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 2464507. [Google Scholar] [CrossRef]
- Patruica, S.; Alexa, E.; Obistioiu, D.; Cocan, I.; Radulov, I.; Berbecea, A.; Lazar, R.N.; Simiz, E.; Vicar, N.M.; Hulea, A.; et al. Chemical Composition, Antioxidant and Antimicrobial Activity of Some Types of Honey from Banat Region, Romania. Molecules 2022, 27, 4179. [Google Scholar] [CrossRef]
- Hulea, A.; Obistioiu, D.; Cocan, I.; Alexa, E.; Negrea, M.; Neacșu, A.-G.; Hulea, C.; Pascu, C.; Costinar, L.; Iancu, I.; et al. Diversity of Monofloral Honey Based on the Antimicrobial and Antioxidant Potential. Antibiotics 2022, 11, 595. [Google Scholar] [CrossRef]
- Mathew, C.; Tesfaye, W.; Rasmussen, P.; Peterson, G.M.; Bartholomaeus, A.; Sharma, M.; Thomas, J. Manuka Oil—A Review of Antimicrobial and Other Medicinal Properties. Pharmaceuticals 2020, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Drain, J.; Fleming, M.O. Palliative management of malodorous squamous cell carcinoma of the oral cavity with Manuka honey. J. Wound Ostomy Cont. 2015, 42, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Al-Rahbi, B.; Zakaria, R.; Othman, Z.; Hassan, A.; Ismail, Z.I.M.; Muthuraju, S. Tualang honey supplement improves memory performance and hippocampal morphology in stressed ovariectomized rats. Acta Histochem. 2014, 116, 79–88. [Google Scholar] [CrossRef]
- Mosavat, M.; Ooi, F.K.; Mohamed, M. Effects of honey supplementation combined with different jumping exercise intensities on bone mass, serum bone metabolism markers and gonadotropins in female rats. BMC Complement. Altern. Med. 2014, 14, 126. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Honey-a novel antidiabetic agent. Int. J. Biol. Sci. 2012, 8, 913. [Google Scholar] [CrossRef]
- Yaghoobi, N.; Al-Waili, N.; Ghayour-Mobarhan, M.; Parizadeh, S.; Abasalti, Z.; Yaghoobi, Z.; Yaghoobi, F.; Esmaeili, H.; Kazemi-Bajestani, S.; Aghasizadeh, R. Natural honey and cardiovascular risk factors; effects on blood glucose, cholesterol, triacylglycerole, CRP, and body weight compared with sucrose. Sci. World J. 2008, 8, 463–469. [Google Scholar] [CrossRef]
- Bodó, A.; Radványi, L.; Koszegi, T.; Csepregi, R.; Nagy, D.U.; Farkas, Á.; Kocsis, M. Melissopalynology, antioxidant activity and multielement analysis of two types of early spring honeys from Hungary. Food Biosci. 2020, 35, 100587. [Google Scholar] [CrossRef]
- Bueno-Costa, F.M.; Zambiazi, R.C.; Bohmer, B.W.; Chaves, F.C.; Da Silva, W.P.; Zanusso, J.T.; Dutra, I. Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul, Brazil. LWT-Food Sci. Technol. 2016, 65, 333–340. [Google Scholar] [CrossRef]
- Sousa, J.M.; de Souza, E.L.; Marques, G.; Meireles, B.; de Cordeiro, Â.T.M.; Gullón, B.; Pintado, M.M.; Magnani, M. Polyphenolic profile and antioxidant and antibacterial activities of monofloral honeys produced by Meliponini in the Brazilian semiarid region. Food Res. Int. 2016, 84, 61–68. [Google Scholar] [CrossRef]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef]
- Balázs, V.L.; Nagy Radványi, L.; Filep, R.; Kerekes, E.; Kocsis, B.; Kocsis, M.; Farkas, Á. In Vitro Antibacterial and Antibiofilm Activity of Hungarian Honeys against Respiratory Tract Bacteria. Foods 2021, 10, 1632. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef]
- Brudzynski, K.; Abubaker, K.; St-Martin, L.; Castle, A. Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front. Microbiol. 2011, 2, 213. [Google Scholar] [CrossRef]
- Kuś, P.M.; Szweda, P.; Jerković, I.; Tuberoso, C.I. Activity of Polish unifloral honeys against pathogenic bacteria and its correlation with colour, phenolic content, antioxidant capacity and other parameters. Lett. Appl. Microbiol. 2016, 62, 269–276. [Google Scholar] [CrossRef]
- Kwakman, P.H.; Zaat, S.A. Antibacterial components of honey. IUBMB Life 2012, 64, 48–55. [Google Scholar] [CrossRef]
- Mundo, M.A.; Padilla-Zakour, O.I.; Worobo, R.W. Growth inhibition of foodborne pathogens and food spoilage organisms by select raw honeys. Int. J. Food Microbiol. 2004, 97, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barbarić, A.; Saftić Martinović, L.; Marijanović, Z.; Juretić, L.; Jurič, A.; Petrović, D.; Šoljić, V.; Gobin, I. Integrated Chemical and Biological Evaluation of Linden Honeydew Honey from Bosnia and Herzegovina: Composition and Cellular Effects. Foods 2025, 14, 1668. [Google Scholar] [CrossRef] [PubMed]
- Von Der Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized Methods of Melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar] [CrossRef]
- Lazar, R.N.; Alexa, E.; Obistioiu, D.; Cocan, I.; Patruica, S. The effect of the use of essential oil in the feed of bee families on honey chemical composition and antimicrobial activity. Appl. Sci. 2022, 12, 1094. [Google Scholar] [CrossRef]
- Gergen, I. Agri-Food Products Analysis; EUROSTAMPA Publishing House: Timișoara, Romania, 2004; 316p, ISBN 973-687-271-8. [Google Scholar]
- Horwitz, W. Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Vlad, C.S.; Vlaia, L.; Vlaia, V.; Dumitraşcu, D.; Filimon, M.N.; Popescu, R.; Cimporescu, A.; Dehelean, C.; Onwubiko, C.E.; Vlad, D.C. Chromatographic analysis and antibacterial potential of extracts of Gnetum africanum. Farmacia 2019, 67, 1083–1090. [Google Scholar] [CrossRef]
- Filimon, M.N.; Popescu, R.; Sinitean, A.; Maniu, P.; Dumitrescu, G.; Verdes, D.; Vlad, C.S. The Assessment of Chitosan Solutions Effects on Bacterial Strains. Rev. Chim. 2018, 69, 1485–1488. [Google Scholar] [CrossRef]
- Popescu, R.; Filimon, M.N.; Vlad, D.C.; Verdes, D.; Moatar, A.; Moisei, G.; Gurani, K.; Caraba, I.V.; Petculescu Ciochina, L.; Pinzaru, I.; et al. Antiproliferative and antibacterial potential of tetrahexylammonium bromide-based ionic liquids. Exp. Ther. Med. 2021, 22, 672. [Google Scholar] [CrossRef] [PubMed]
- Danilescu, O.; Bourosh, P.; Bulhac, I.; Shova, S.; Kravtsov, V.C.; Caraba, M.N.; Caraba, I.V.; Popescu, R.; Crisan, M.; Haidu, D.; et al. Laminated dihydrazone Zn(II) coordination polymer with prospects for sensory and multifunctional biomedical applications. Polyhedron 2024, 258, 117039. [Google Scholar] [CrossRef]
- PCA—Principal Component Analysis Essentials—Articles—STHDA. 2017. Available online: https://www.sthda.com/english/articles/31-principal-component-methods-in-r-practical-guide/112-pca-principal-component-analysis-essentials/?utm_source=chatgpt.com (accessed on 7 January 2025).
- Interpreting Feature Scores in Principal Component Analysis (PCA)|by Andrea Grianti|The Startup|Medium. 2020. Available online: https://medium.com/swlh/interpreting-principal-components-fifa20-players-use-case-639fde373bac (accessed on 7 January 2025).
- Kaskoniene, V.; Venskutonis, P.R.; Ceksteryte, V. Carbohydrate composition and electrical conductivity of different origin honeys from Lithuania. Food Sci. Technol. 2010, 43, 801–807. [Google Scholar]
- Machado De-Melo, A.A.; Almeida-Muradian, L.B.D.; Sancho, M.T.; Pascual-Maté, A. Composition and properties of Apis mellifera honey: A review. J. Apic. Res. 2018, 57, 5–37. [Google Scholar] [CrossRef]
- Pascual-Maté, A.; Osés, S.M.; Marcazzan, G.L.; Gardini, S.; Muiño, M.A.F.; Sancho, M.T. Sugar composition and sugar-related parameters of honeys from the northern Iberian Plateau. J. Food Compos. Anal. 2018, 74, 34–43. [Google Scholar] [CrossRef]
- Taha, A.A.; Balabel, N.M.; Elshishtawy, H.M. Physicochemical Characterization and Antimicrobial Activity of Sidr Honey Produced by Dwarf Honey Bees (Apis florea F.). J. Plant Prot. Pathol. 2019, 10, 621–628. [Google Scholar] [CrossRef]
- Baloš, M.Ž.; Jakšić, S.; Popov, N.; Mihaljev, Ž.; Pelić, D.L. Comparative study of water content in honey produced in different years. Arch. Vet. Med. 2019, 12, 43–53. [Google Scholar] [CrossRef]
- Gallina, A.; Stocco, N.; Mutinelli, F. Karl Fischer Titration to determine moisture in honey: A new simplified approach. Food Control 2010, 21, 942–944. [Google Scholar] [CrossRef]
- Olalekan-Adeniran, M.A.; Ogunwolu, S.O. Comparative Quality Evaluation of Oven-Roasted and Honey-Coated Cashew (Anarcadium occidentale, L.) Nut produced using Locally Fabricated Cashew Nut Processing Machine in Nigeria. Int. J. Environ. Agric. Biotechnol. 2018, 3, 266194. [Google Scholar]
- Council of the European Communities. 2001. Council Directive 2001/110/EC Relating to Honey. Available online: https://eur-lex.europa.eu/eli/dir/2001/110/oj/eng (accessed on 11 April 2025).
- Mato, I.; Huidobro, J.F.; Simal-Lozano, J.; Sancho, M.T. Significance of nonaromatic organic acids in honey. J. Food Prot. 2003, 66, 2371–2376. [Google Scholar] [CrossRef]
- Bogdanov, S. Honey Composition. HoneyBook, San Francisco. Volume 9, pp. 27–36. Available online: https://www.researchgate.net/publication/304011775_Honey_Composition (accessed on 11 April 2025).
- Terrab, A.; Díez, M.J.; Heredia, F.J. Characterisation of Moroccan unifloral honeys by their physicochemical characteristics. Food Chem. 2002, 79, 373–379. [Google Scholar] [CrossRef]
- Campone, L.; Piccinelli, A.L.; Pagano, I.; Carabetta, S.; Di Sanzo, R.; Russo, M.; Rastrelli, L. Determination of phenolic compounds in honey using dispersiveliquid–liquid microextraction. J. Chromatogr. A 2014, 1334, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant Activity, Total Phenolic Content, Individual Phenolics and Physicochemical Parameters Suitability for Romanian Honey Authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Chirsanova, A.; Capcanari, T.; Boistean, A.; Siminiuc, R. Physico-Chemical Profile of Four Types of Honey from the South of the Republic of Moldova. Food Nutr. Sci. 2021, 12, 874–888. [Google Scholar] [CrossRef]
- Shantal Rodríguez Flores, M.; Escuredo, O.; Carmen Seijo, M. Assessment of physicochemical and antioxidant characteristics ofQuercus pyrenaica honeydew honeys. Food Chem. 2015, 166, 101–106. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Karabournioti, S.; Karabagias, V.K.; Badeka, A.V. Palynological, physico-chemical and bioactivity parameters determination, of a less common Greek honeydew honey: “dryomelo”. Food Control 2020, 109, 106940. [Google Scholar] [CrossRef]
- Vasic, V.; Gašic, U.; Stankovic, D.; Lušic, D.; Vukic-Lušic, D.; Milojkovic-Opsenica, D.; Tešic, Ž.; Trifkovic, J. Towards better quality criteria of European honeydew honey: Phenolic profile and antioxidant capacity. Food Chem. 2019, 274, 629–641. [Google Scholar] [CrossRef]
- Camina, M.J.; G Pellerano, R.; J Marchevsky, E. Geographical and botanicalclassification of honeys and apicultural products by chemometric methods. A review. Curr. Anal. Chem. 2012, 8, 408–425. [Google Scholar] [CrossRef]
- Alves, A.; Ramos, A.; Gonçalves, M.M.; Bernardo, M.; Mendes, B. Antioxidantactivity, quality parameters and mineral content of Portuguese monofloral honeys. J Food Compos. Anal. 2013, 30, 130–138. [Google Scholar] [CrossRef]
- Alqarni, A.S.; Owayss, A.A.; Mahmoud, A.A.; Hannan, M.A. Mineral contentand physical properties of local and imported honeys in Saudi Arabia. J. Saudi Chem. Soc. 2014, 18, 618–625. [Google Scholar] [CrossRef]
- Nguyen, H.T.L.; Panyoyai, N.; Kasapis, S.; Pang, E.; Mantri, N. Honey and itsrole in relieving multiple facets of atherosclerosis. Nutrients 2019, 11, 167. [Google Scholar] [CrossRef]
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical properties, minerals, trace elements, and heavy metals in honey of different origins: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 219–233. [Google Scholar] [CrossRef]
- El-Haskoury, R.; Al-Waili, N.; Kamoun, Z.; Makni, M.; Al-Waili, H.; Lyoussi, B. Antioxidant activity and protective effect of carob honey in CCl4-induced kidneyand liver injury. Arch. Med. Res. 2018, 49, 306–313. [Google Scholar] [CrossRef]
- Mracevic, S.D.; Krstic, M.; Lolic, A.; Ražic, S. Comparative study of the chemical composition and biological potential of honeyfrom different regions of Serbia. Microchem. J. 2020, 152, 104420. [Google Scholar] [CrossRef]
- Conti, M.; Botre, F. Honeybees and their products as potential bioindicators of heavymetals contamination. Environ. Monit. Assess. 2000, 69, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S. Contaminants of bee products. Apidologie 2006, 37, 1–18. [Google Scholar] [CrossRef]
- Zugravu, C.; Parvu, M.; Patrascu, D.; Stoian, A. Correlations between Lead and Cadmium pollution of Honey and Environmental Heavy Metal Presence in Two Romanian Counties. Bull. UASVM Agric. 2009, 66, 230–233. [Google Scholar] [CrossRef]
- Rusin, M.; Domagalska, J.; Rogala, D.; Razzaghi, M.; Szymala, I. The concentration of cadmium and lead in vegetables and fruits. Sci. Rep. 2021, 11, 11913. [Google Scholar] [CrossRef]
- Martiniaková, M.; Omelka, R.; Jančová, A.; Stawarz, R.; Formicki, G. Heavy metal content in the femora of a yellow-necked mouse (Apodemus flavicollis) and wood mouse (Apodemus sylvaticus) from different types of polluted environments in Slovakia. Environ. Monit. Assess. 2010, 171, 651–660. [Google Scholar] [CrossRef]
- Adamchuk, L. Improvement of the method of botanical identification of honey. Food Sci. Technol. 2021, 14, 31–42. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Kosanović, M.; Bilandžić, N.; Sedak, M.; Čalopek, B. Variations in lead, cadmium, arsenic, and mercury concentrations during honeybee wax processing using casting technology. Arh. Hig. Rada Toksikol. 2016, 67, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Lambert, O.; Piroux, M.; Puyo, S.; Thorin, C.; Larhantec, M.; Delbac, F.; Pouliquen, H. Bees, honey, and pollen as sentinels for lead environmental contamination. Environ. Pollut. 2012, 170, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Zheplinska, M.; Mushtruk, M.; Vasyliv, V.; Kuts, A.; Slobodyanyuk, N.; Bal-Prylypko, L.; Nikolaenko, M.; Kokhan, O.; Reznichenko, Y.; Salavor, O. The micronutrient profile of medicinal plant extracts. Potravin. Slovak J. Food Sci. 2021, 15, 528–535. [Google Scholar] [CrossRef]
- Aghamirlou, H.M.; Khadem, M.; Rahmani, A.; Sadeghian, M.; Mahvi, A.H.; Akbarzadeh, A.; Nazmara, S. Heavy metals determination in honey samples using inductively coupled plasma-optical emission spectrometry. J. Environ. Health Sci. Eng. 2015, 13, 39. [Google Scholar] [CrossRef]
- Colabucci, A.; Turco, A.C.; Ciprotti, M.; Di Gregorio, M.; Sorbo, A.; Ciaralli, L. Analysis of Cadmium and Lead in honey: Direct-determination by Graphite Furnace Atomic Absorption Spectrometry. Acta Imeko 2022, 5, 10–14. [Google Scholar] [CrossRef][Green Version]
- Suhag, Y.; Nanda, V. Optimization for spray drying process parameters of nutritionally rich honey powder using response surface methodology. Cogent Food Agric. 2016, 2, 1176631. [Google Scholar] [CrossRef]
- Scripcă, L.A. Cercetări Privind Procesul de Cristalizare a Mierii și Metode Inovative de Prevenire a Acestuia. Doctoral Thesis, Universitatea Stefan cel Mare, Suceava, Romania, 2021. [Google Scholar]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef]
- Majtan, J.; Bucekova, M.; Kafantaris, I.; Szweda, P.; Hammer, K.; Mossialos, D. Honey antibacterial activity: A neglected aspect of honey quality assurance as functional food. Trends Food Sci. Technol. 2021, 118, 870–886. [Google Scholar] [CrossRef]
- Farkasovska, J.; Bugarova, V.; Godocikova, J.; Majtan, V.; Majtan, J. The role of hydrogen peroxide in the antibacterial activity of different floral honeys. Eur. Food Res. Technol. 2019, 245, 2739–2744. [Google Scholar] [CrossRef]
- Brudzynski, K. A current perspective on hydrogen peroxide production in honey. A review. Food Chem. 2020, 332, 127229. [Google Scholar] [CrossRef]
- Tsavea, E.; Vardaka, F.-P.; Savvidaki, E.; Kellil, A.; Kanelis, D.; Bucekova, M.; Grigorakis, S.; Godocikova, J.; Gotsiou, P.; Dimou, M.; et al. Physicochemical Characterization and Biological Properties of Pine Honey Produced across Greece. Foods 2022, 11, 943. [Google Scholar] [CrossRef]
- Núñez-Gómez, V.; San Mateo, M.; Sánchez-Martínez, L.; Periago, M.J. Antibacterial Effect of Spanish Honeys of Different Botanical Origins against Staphylococcus epidermidis. Int. J. Mol. Sci. 2024, 25, 6590. [Google Scholar] [CrossRef]
- Ianovici, N.; Ionuti, A.; Zbircea, S.; Crasovan, G. Preliminary contribution to the characterization of commercial unifloral honey samples by melissopalynology analysis. Ann. West Univ. Timişoara Ser. Biol. 2008, XI, 85–89. [Google Scholar]

| Sample A | Sample B | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Mean ± Standard Deviation (x ± SD) | Min | Max | Mean ± Standard Deviation (x ± SD) | Min | Max | p | |
| Water content (WC)(%) | 15.23 ± 0.03 | 15.21 | 15.26 | 19.21 ± 0.02 | 19.19 | 19.22 | <0.0001 | |
| Impurities (IC) (mg/100 g) | 71 ± 0.01 | 71 | 72 | 68 ± 0.01 | 67 | 68 | 0.0015 | |
| Ash (AC) (g/100 g) | 0.48 ± 0.06 | 0.42 | 0.53 | 0.47 ± 0.03 | 0.45 | 0.50 | 0.7504 | |
| Acidity (A) (VNaOH) (mL) | 4.84 ± 0.11 | 4.87 | 4.93 | 6.10 ± 0.05 | 6.05 | 6.15 | <0.0001 | |
| pH | 3.51 ± 0.07 | 3.45 | 3.58 | 3.53 ± 0.07 | 3.48 | 3.48 | 0.6634 | |
| Reducing sugars (RS) (%) | 66.83 ± 0.04 | 66.79 | 66.87 | 71.74 ± 3.21 | 69.38 | 73.41 | 0.0268 | |
| DPPH (%) | 80.39 ± 0.02 | 80.37 | 80.41 | 77.51 ± 3.21 | 75.17 | 81.17 | 0.1153 | |
| Phenolic compounds (TPC) (mg/kg) | 862.32 ± 0.88 | 861.56 | 863.28 | 871.33 ± 0.00 | 871.33 | 871.33 | <0.0001 | |
| Flavonoid content (FC) (mg QE/100 g) | 242.34 ± 0.59 | 241.75 | 242.93 | 268.22 ± 0.94 | 268.89 | 267.56 | <0.0001 | |
| Principal Components | Sample A | Sample B | ||
|---|---|---|---|---|
| PC1 | PC2 | PC1 | PC2 | |
| Eigen value | 6.192 | 1.808 | 4.445 | 2.552 |
| % of variance | 77.399 | 22.601 | 63.540 | 36.459 |
| Parameters | Vector1 | Vector2 | Vector1 | Vector2 |
| WC | −0.398 | 0.098 | −0.429 | −0.267 |
| IC | 0.402 | −0.013 | −0.471 | −0.067 |
| AC | −0.398 | −0.103 | −0.203 | 0.566 |
| A | 0.388 | −0.191 | 0.051 | −0.622 |
| pH | −0.391 | 0.174 | 0.459 | 0.156 |
| RS | −0.254 | −0.576 | 0.361 | −0.407 |
| DPPH | 0.285 | −0.525 | 0.457 | 0.164 |
| TPC | 0.269 | 0.556 | 0 | 0 |
| Principal Components | Sample A | Sample B | ||
|---|---|---|---|---|
| PC1 | PC2 | PC1 | PC2 | |
| Eigen value | 8.935 | 2.066 | 6.925 | 4.075 |
| % of variance | 81.223 | 18.777 | 62.957 | 37.043 |
| Parameters | Eigen-Vector1 | Eigen-Vector2 | Vector1 | Vector2 |
| Cd | 0.327 | −0.146 | 0.230 | 0.394 |
| Cu | −0.335 | 0.015 | 0.354 | −0.179 |
| Ni | −0.327 | 0.146 | 0.296 | 0.310 |
| Cr | −0.335 | 0.015 | −0.058 | 0.489 |
| Pb | 0.007 | 0.696 | −0.319 | −0.269 |
| Zn | 0.319 | −0.207 | −0.354 | 0.181 |
| Fe | 0.286 | −0.361 | 0.178 | −0.438 |
| Mn | 0.323 | 0.179 | 0.366 | −0.132 |
| Ca | 0.323 | 0.179 | −0.336 | −0.232 |
| Mg | 0.246 | 0.472 | −0.376 | −0.076 |
| K | −0.329 | −0.117 | −0.289 | 0.321 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nan, A.; Mituletu, M.; Dumitrescu, G.; Caraba, I.V.; Pet, I.; Sinitean, A.; Matica, M.A.; Liliana, P.C.; Pet, E.; Popescu, R.; et al. Antimicrobial and Biofilm Inhibiting Potential of Two Romanian Linden Honeys. Foods 2025, 14, 3594. https://doi.org/10.3390/foods14213594
Nan A, Mituletu M, Dumitrescu G, Caraba IV, Pet I, Sinitean A, Matica MA, Liliana PC, Pet E, Popescu R, et al. Antimicrobial and Biofilm Inhibiting Potential of Two Romanian Linden Honeys. Foods. 2025; 14(21):3594. https://doi.org/10.3390/foods14213594
Chicago/Turabian StyleNan, Alexandru, Mihai Mituletu, Gabi Dumitrescu, Ion Valeriu Caraba, Ioan Pet, Adrian Sinitean, Mariana Adina Matica, Petculescu Chiochina Liliana, Elena Pet, Roxana Popescu, and et al. 2025. "Antimicrobial and Biofilm Inhibiting Potential of Two Romanian Linden Honeys" Foods 14, no. 21: 3594. https://doi.org/10.3390/foods14213594
APA StyleNan, A., Mituletu, M., Dumitrescu, G., Caraba, I. V., Pet, I., Sinitean, A., Matica, M. A., Liliana, P. C., Pet, E., Popescu, R., & Caraba, M. N. (2025). Antimicrobial and Biofilm Inhibiting Potential of Two Romanian Linden Honeys. Foods, 14(21), 3594. https://doi.org/10.3390/foods14213594








