Influence of Herbal Additives on the Physicochemical, Microbiological, Polyphenolic, and Sensory Profile of Green Tea-Based Kombucha
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Kombucha–Type Beverages
2.3. pH Measurement
2.4. Determination of Extract Content (Brix)
2.5. Determination of Polyphenol and Caffeine Contents
2.6. Sensory Evaluation
2.7. Analysis of Chemosensors
2.8. Microbiological Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analysis
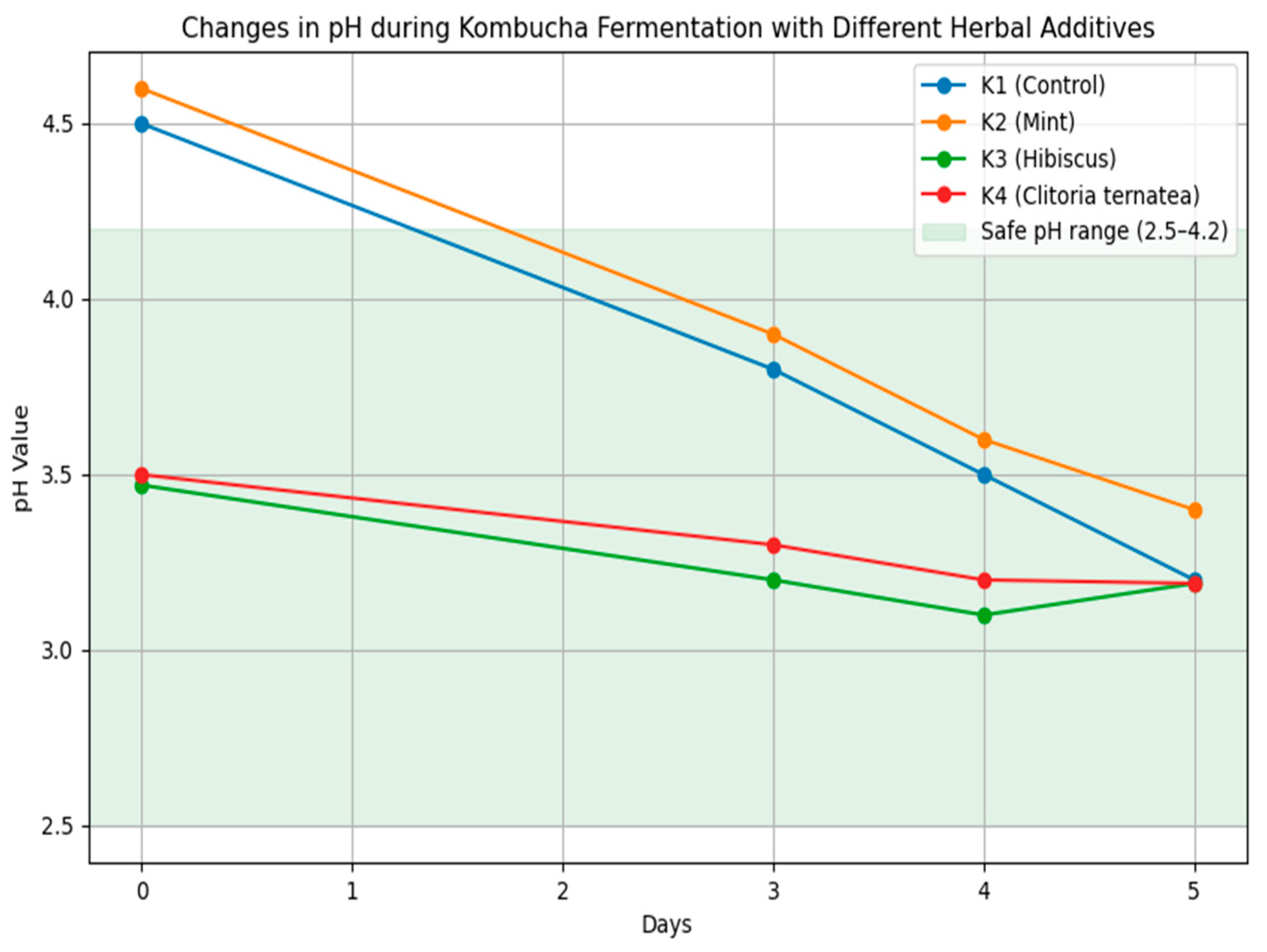
3.1.1. Changes in pH During Kombucha Fermentation
3.1.2. Extract Content
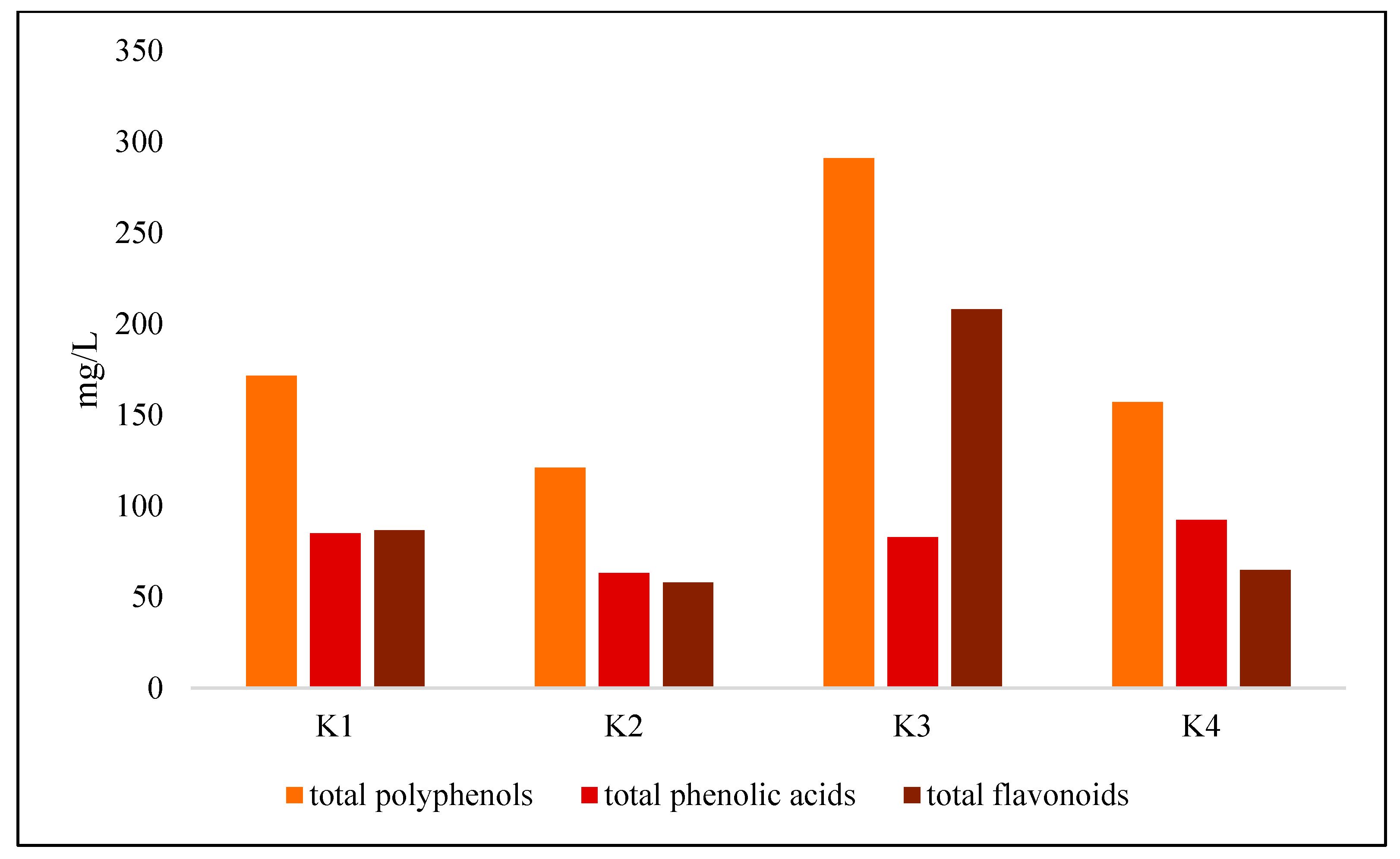
3.1.3. Polyphenol and Caffeine Content
Caffeine Content
3.2. Microbiological Analysis
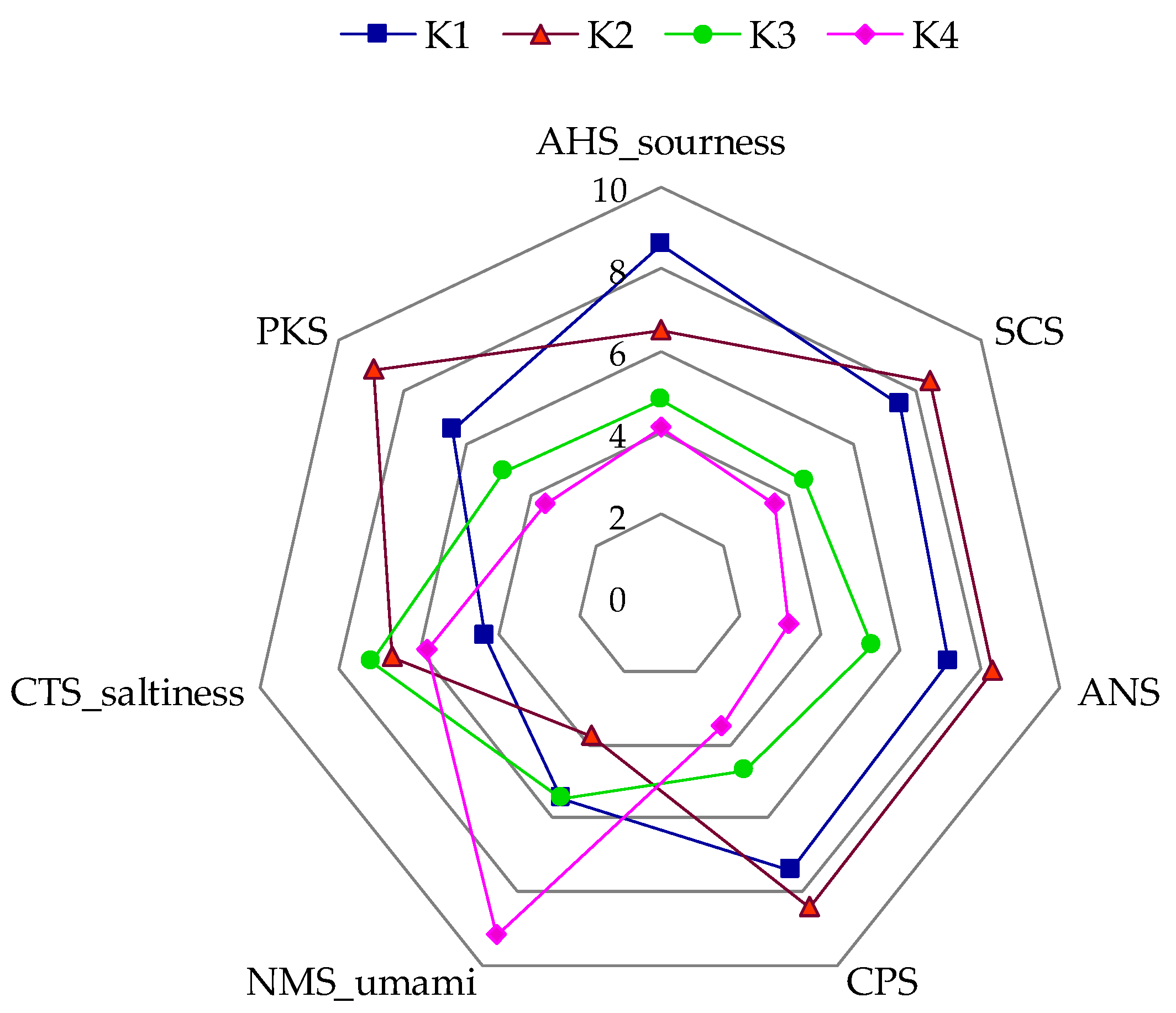
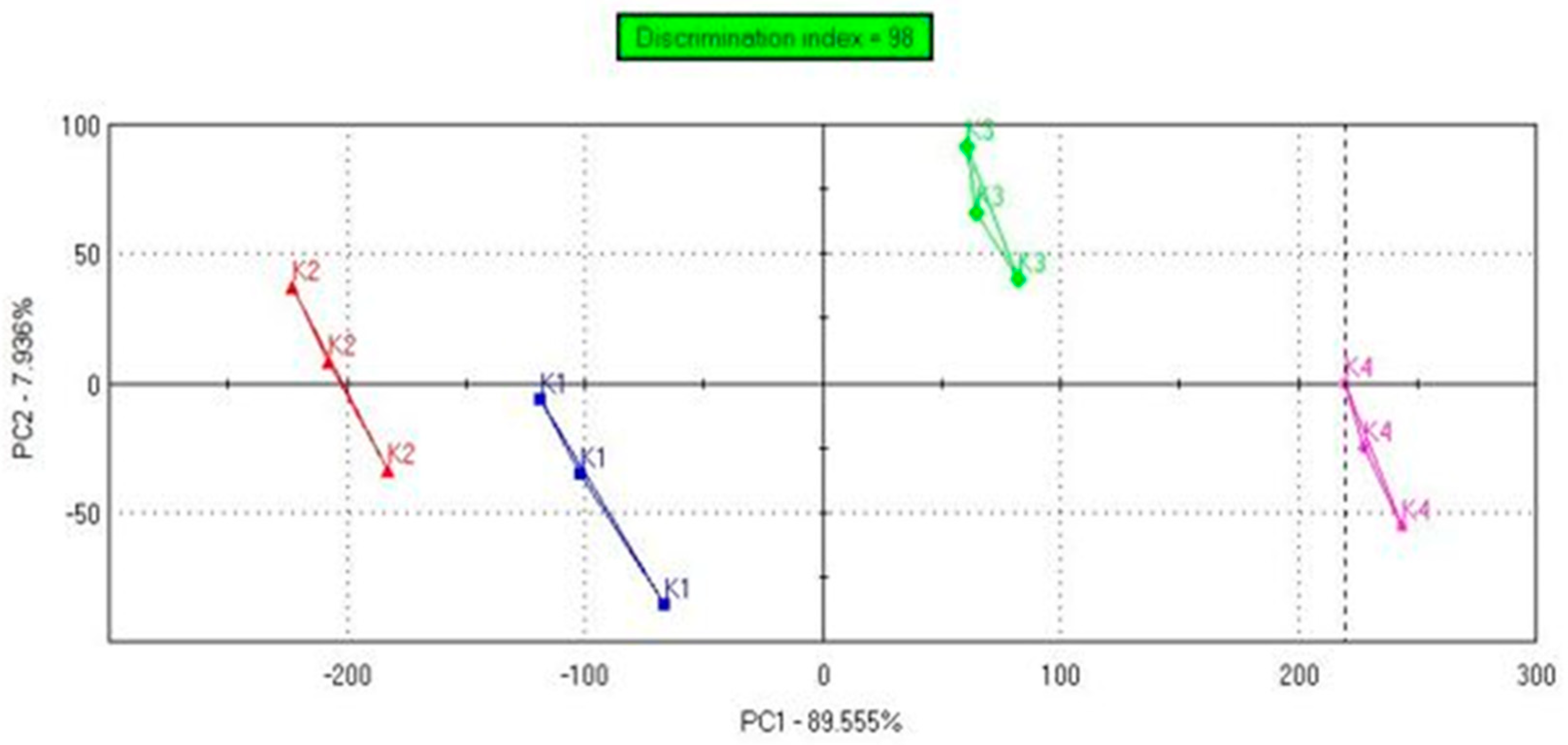
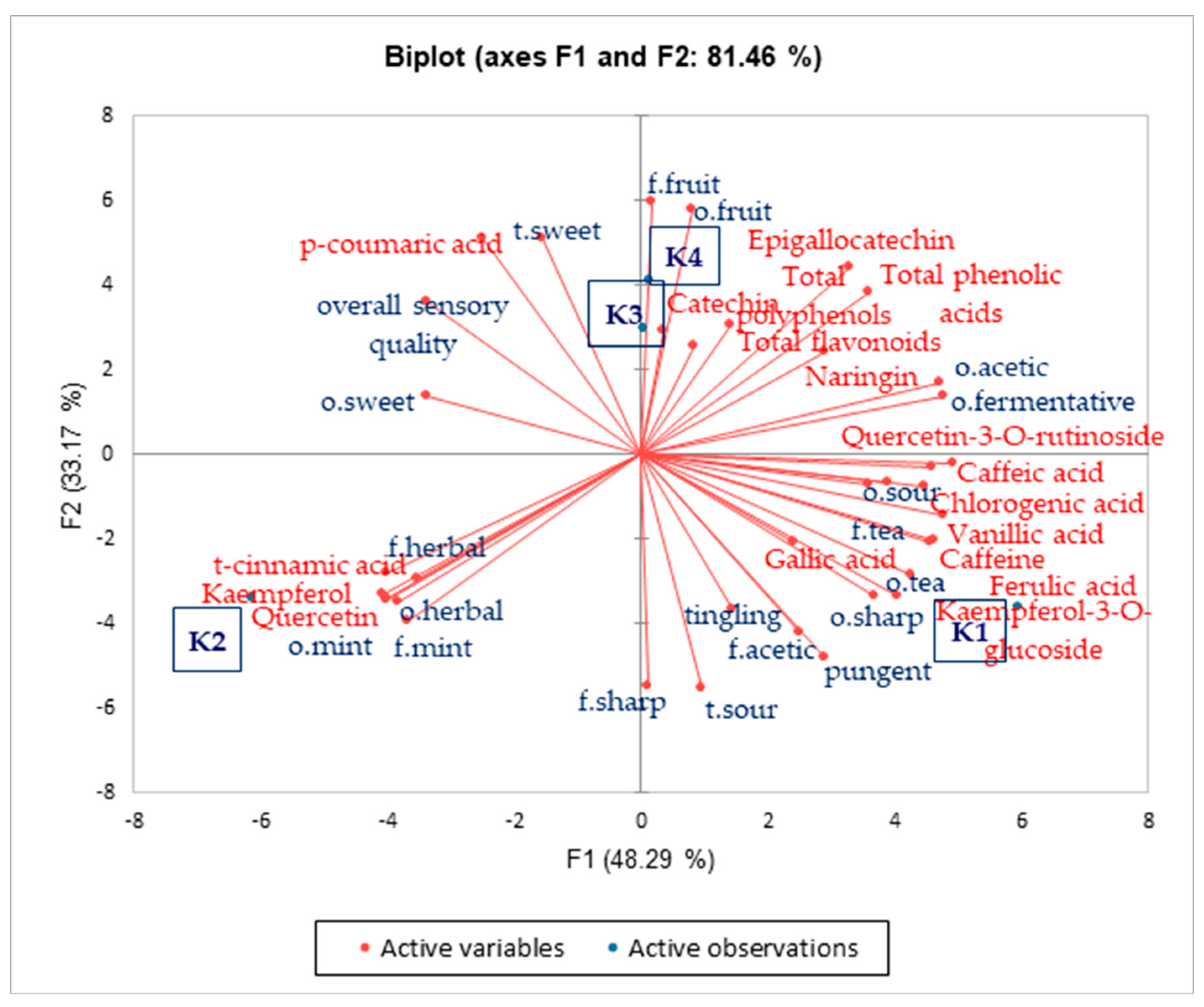
3.3. Sensory Evaluation and Analysis of Chemosensors
3.4. Limitations of This Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fekete, M.; Lehoczki, A.; Kryczyk-Poprawa, A.; Zábó, V.; Varga, J.T.; Bálint, M.; Fazekas-Pongor, V.; Csípő, T.; Rząsa-Duran, E.; Varga, P. Functional Foods in Modern Nutrition Science: Mechanisms, Evidence, and Public Health Implications. Nutrients 2025, 17, 2153. [Google Scholar] [CrossRef]
- Ali, A.; Rahut, D.B. Consumer demand for functional beverages. J. Consum. Res. 2019, 46, 123–134. [Google Scholar] [CrossRef]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Microbial composition of kombucha. J. Appl. Microbiol. 2021, 131, 1531–1542. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Wang, H.; Jiao, S.; Wu, J.; Hou, Y.; Sun, J.; Yuan, J. Chemical Profile and Antioxidant Capacity of Kombucha Tea by the Pure Cultured Kombucha. LWT 2022, 168, 113931. [Google Scholar] [CrossRef]
- Bortolomedi, V.; Silva, E.K.; Meireles, M.A.A. Health benefits of kombucha. J. Funct. Foods 2022, 89, 104938. [Google Scholar] [CrossRef]
- Bortolomedi, B.; Paglarini, C.; Brod, F. Bioactive compounds in kombucha: A review of substrate effect and fermentation conditions. Food Chem. 2022, 385, 132719. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Antioxidant properties of kombucha. J. Food Sci. 2020, 85, 2779–2787. [Google Scholar] [CrossRef]
- Zou, C.; Xu, Y.Q.; Huang, Y.B.; Yin, J.F. Kombucha Production and Its Bioactive Compounds Analysis. In Probiotic Foods and Beverages: Technologies and Protocols; Springer: New York, NY, USA, 2023; pp. 133–138. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef]
- Njieukam, J.A.; Ciccone, M.; Gottardi, D.; Ricci, A.; Parpinello, G.P.; Siroli, L.; Lanciotti, R.; Patrignani, F. Microbiological, Functional, and Chemico-Physical Characterization of Artisanal Kombucha: An Interesting Reservoir of Microbial Diversity. Foods 2024, 13, 1947. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Tan, Q.; Tang, Q.; Tong, Z.; Yang, M. Research Progress on Alternative Kombucha Substrate Transformation and the Resulting Active Components. Front. Microbiol. 2023, 14, 1254014. [Google Scholar] [CrossRef] [PubMed]
- Ivanišová, E.; Tokár, M.; Ivanišinová, O.; Kačániová, M. Chemical composition of kombucha. J. Food Sci. 2020, 85, 123–130. [Google Scholar] [CrossRef]
- Andrade, D.K.A.; Wang, B.; Lima, E.M.F.; Shebeko, S.K.; Ermakov, A.M.; Khramova, V.N.; Ivanova, I.V.; Rocha, R.d.S.; Vaz-Velho, M.; Mutukumira, A.N.; et al. Kombucha: An Old Tradition into a New Concept of a Beneficial, Health-Promoting Beverage. Foods 2025, 14, 1547. [Google Scholar] [CrossRef]
- Polanía, A.M.; Londoño, L.; Ramírez, C.; Bolívar, G. Influence of Ultrasound Application in Fermented Pineapple Peel on Total Phenolic Content and Antioxidant Activity. Fermentation 2022, 8, 314. [Google Scholar] [CrossRef]
- Alves, R.O.; de Oliveira, R.L.; de Moraes, M.M.; Santos, W.W.V.; Gomes da Câmara, C.A.; da Silva, S.P.; Porto, C.S.; Porto, T.S. Evaluation of the Impact of Fermentation Conditions, Scale Up and Stirring on Physicochemical Parameters, Antioxidant Capacity and Volatile Compounds of Green Tea Kombucha. Fermentation 2025, 11, 201. [Google Scholar] [CrossRef]
- Prajapati, K.; Prajapati, J.; Patel, D.; Patel, R.; Varshnei, A.; Saraf, M.; Goswami, D. Multidisciplinary Advances in Kombucha Fermentation, Health Efficacy, and Market Evolution. Arch. Microbiol. 2024, 206, 366. [Google Scholar] [CrossRef]
- Edo, R.; Kowalska, A.; Nowak, M. Organic Acids in Hibiscus Infusions and Their Impact on pH. Herbs 2023, 9, 101–112. [Google Scholar]
- Galus, S.; Podolska, G. The Influence of Hibiscus sabdariffa L. Flower Concentration on the Physicochemical Properties of Infusions. Żywność. Nauka Technol. Jakość 2021, 28, 45–58. [Google Scholar]
- Padmanabhan, V.; Kumar, S.S.; Giridhar, P. Phytochemicals and UHPLC-QTOF-HRMS Characterisation of Bioactives of Butterfly Pea (Clitoria ternatea L.) Seeds and Their Antioxidant Potentials. Food Chem. 2024, 433, 137373. [Google Scholar] [CrossRef]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in Free-Radical Scavenging Ability of Kombucha Tea During Fermentation. Food Chem. 2008, 109, 227–234. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Dybka-Ślepień, K.; Antolak, H. Wpływ warunków fermentacji na jakość sensoryczną kombuchy. Żywność Nauka Technol. Jakość 2019, 24, 45–54. [Google Scholar]
- Górecki, M.; Hallmann, E. The Antioxidant Content of Coffee and Its In Vitro Activity as an Effect of Its Production Method and Roasting and Brewing Time. Antioxidants 2020, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 13299:2016; Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. ISO: Geneva, Switzerland, 2016.
- PN-EN ISO 8589:2010/A1:2014-07; Sensory Analysis—General Guidelines for the Design of a Sensory Analysis Laboratory. ISO: Geneva, Switzerland, 2016.
- PN-EN ISO 8586:2014-03; Sensory Analysis—General Guidelines for the Selection, Training, and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO: Geneva, Switzerland, 2016.
- PN-EN ISO 7218:2024-12; Microbiology of Food and Animal Feed—General Requirements and Principles of Microbiological Testing. ISO: Geneva, Switzerland, 2024. Available online: https://sklep.pkn.pl/pn-en-iso-7218-2024-12e.html (accessed on 12 June 2025).
- PN-ISO 15214:2002; Microbiology of Food and Feed. Horizontal Method for Enumeration of Mesophilic Lactic Acid Bacteria. ISO: Geneva, Switzerland, 2002. Available online: https://www.iso.org/standard/26853.html (accessed on 12 June 2025).
- PN-ISO 21527-1:2009; Microbiology of Food and Animal Feed—Horizontal Method for the Enumeration of Yeasts and Molds—Part 1: Colony Count Method in Products with Water Activity Greater Than 0.95. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/38275.html (accessed on 12 June 2025).
- PN-EN ISO 7932:2005; Microbiology of Food and Animal Feed—Horizontal Method for the Enumeration of Presumptive Bacillus cereus. ISO: Geneva, Switzerland, 2005. Available online: https://www.iso.org/standard/38219.html (accessed on 12 June 2025).
- PN-EN ISO 21528-2:2017-08; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae. Part 2: Colony-Count Technique. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/63504.html (accessed on 12 June 2025).
- .Sreeramulu, G.; Zhu, Y.; Knol, W. Kombucha Fermentation and Its Antimicrobial Activity. J. Agric. Food Chem. 2000, 48, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Almeida, R.; Silva, M. pH Dynamics in Green Tea Kombucha Fermentation. J. Food Ferment. 2022, 8, 201–210. [Google Scholar]
- Majid, M.; Khan, M.I.; Ali, A.; Hussain, A.; Imran, M.; Xu, X.; Aadil, R.M. Comparative Study of Kombucha Fermentation with Different Herbal Additives. Foods 2023, 12, 789–798. [Google Scholar] [CrossRef]
- Kluz, M.I.; Pietrzyk, K.; Pastuszczak, M.; Kacaniova, M.; Kita, A.; Kapusta, I.; Puchalski, C. Microbiological and Physicochemical Composition of Various Types of Homemade Kombucha Beverages Using Alternative Kinds of Sugars. Foods 2022, 11, 1523. [Google Scholar] [CrossRef]
- Pawluś, P.; Kolniak-Ostek, J. Innovative Analogs of Unpasteurized Kombucha Beverages: Comparative Analysis of Mint/Nettle Kombuchas, Considering Their Health-Promoting Effect, Polyphenolic Compounds and Chemical Composition. Int. J. Mol. Sci. 2024, 25, 7572. [Google Scholar] [CrossRef]
- Aliki, T.; Archountoula, C.; Evaggelos, Z.; Panagiotis, H.; Dimitra, H. Antioxidant activity of mint (Mentha piperita L.) of Greek flora and identification of its bioactive compounds. Org. Med. Chem. 2021, 11, 555814. [Google Scholar]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha Tea—A Double Power of Bioactive Compounds from Tea and Symbiotic Culture of Bacteria and Yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef]
- Saimaiti, A.; Huang, S.-Y.; Xiong, R.-G.; Wu, S.-X.; Zhou, D.-D.; Yang, Z.-J.; Luo, M.; Gan, R.-Y.; Li, H.-B. Antioxidant Capacities and Polyphenol Contents of Kombucha Beverages Produced from Vine Tea and Sweet Tea. Antioxidants 2022, 11, 1655. [Google Scholar] [CrossRef]
- Barros, V.C.; Botelho, V.A.; Chisté, R.C. Alternative Substrates for the Development of Fermented Beverages Analogous to Kombucha: An Integrative Review. Foods 2024, 13, 1768. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2022, 24, 340. [Google Scholar] [CrossRef]
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K.A. Chemical Composition of Kombucha. Beverages 2022, 8, 45. [Google Scholar] [CrossRef]
- Jarosz, M.; Wierzejska, R.; Mojska, H.; Świderska, K.; Siuba, M. Zawartość kofeiny w produktach spożywczych. Bromatol. Chem. Toksykol. 2009, 42, 776–781. [Google Scholar]
- Kolla, H.B.; Makam, S.S.; Reddy, P.N. Mapping of conserved immunodominant epitope peptides in the outer membrane porin (Omp) L of prominent Enterobacteriaceae pathogens associated with gastrointestinal infections. J. Genet. Eng. Biotechnol. 2023, 21, 146. [Google Scholar] [CrossRef]
- Kitwetcharoen, H.; Phung, L.T.; Klanrit, P.; Thanonkeo, S.; Tippayawat, P.; Yamada, M.; Thanonkeo, P. Kombucha Healthy Drink—Recent Advances in Production, Chemical Composition and Health Benefits. Fermentation 2023, 9, 48. [Google Scholar] [CrossRef]
- Pătrînjan, R.T.; Morar, A.; Ban-Cucerzan, A.; Popa, S.A.; Imre, M.; Morar, D.; Imre, K. Systematic Review of the Occurrence and Antimicrobial Resistance Profile of Foodborne Pathogens from Enterobacteriaceae in Wild Ungulates Within the European Countries. Pathogens 2024, 13, 1046. [Google Scholar] [CrossRef]
- Fabricio, M.F.; Mann, M.B.; Kothe, C.I.; Frazzon, J.; Tischer, B.; Flôres, S.H.; Ayub, M.A.Z. Effect of freeze-dried kombucha culture on microbial composition and assessment of metabolic dynamics during fermentation. Food Microbiol. 2022, 101, 103889. [Google Scholar] [CrossRef]
- Ramesh, M.M.; Venkatappa, A.H.; Bhat, R. Natural sources, mechanisms, and sensory evaluation of umami and kokumi flavour compounds in food. Eur. Food Res. Technol. 2025, 251, 1467–1488. [Google Scholar] [CrossRef]
- Kumar, S.R.; Sherief, S.H.; Tawale, H.; Chavan, A.B.; Mohammed, J.S.; Latha, C.M.; Jayalekshmi, R.; Biju, C.; Borse, G.N.; Gupta, V.L. A comprehensive evaluation of Clitoria ternatea—Its botanical description, phytochemistry, pharmacological activities, and traditional uses. Asian J. Biol. Biomed. Sci. 2024, 6, 5979–5997. [Google Scholar]
- Wang, Q.J.; Mielby, L.A.; Junge, J.Y.; Bertelsen, A.S.; Kidmose, U.; Spence, C.; Byrne, D.V. The role of intrinsic and extrinsic sensory factors in sweetness perception of food and beverages: A review. Foods 2019, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, H.; Hashemi Gahruie, H.; Golmakani, M.T.; Eskandari, M.H.; Movahedi, M. Effect of medicinal plant type and concentration on physicochemical, antioxidant, antimicrobial, and sensorial properties of kombucha. Food Sci. Nutr. 2018, 6, 2568–2577. [Google Scholar] [CrossRef] [PubMed]
- Neffe-Skocińska, K.; Sionek, B.; Ścibisz, I.; Kołożyn-Krajewska, D. Acid contents and the effect of fermentation condition of Kombucha tea beverages on physicochemical, microbiological and sensory properties. CyTA—J. Food 2017, 15, 601–607. [Google Scholar] [CrossRef]
- Dartora, B.; Hickert, L.R.; Fabricio, M.F.; Ayub, M.A.Z.; Furlan, J.M.; Wagner, R.; Sant’Anna, V. Understanding the effect of fermentation time on physicochemical characteristics, sensory attributes, and volatile compounds in green tea kombucha. Food Res. Int. 2023, 174, 113569. [Google Scholar] [CrossRef]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Harangozo, Ľ.; Kántor, A.; Kačániová, M. The evaluation of chemical, antioxidant, antimicrobial and sensory properties of kombucha tea beverage. J. Food Sci. Technol. 2020, 57, 1840–1846. [Google Scholar] [CrossRef]
- Oliveira de, I.; Santos-Buelga, C.; Aquino, Y.; Barros, L.; Heleno, S.A. New frontiers in the exploration of phenolic com-pounds and other bioactives as natural preservatives. Food Biosci. 2025, 68, 106571. [Google Scholar] [CrossRef]
- Paissoni, M.A.; Waffo-Teguo, P.; Ma, W.; Jourdes, M.; Rolle, L.; Teissedre, P.-L. Chemical and sensorial investigation of in-mouth sensory properties of grape anthocyanins. Sci. Rep. 2018, 8, 17098. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Wang, Z.; Peng, H.; Jiang, L.; Yang, P.; Li, W. Analysis of Taste Quality Differences Between High and Low Grades of Ninghong Tea: From the Perspective of Sensory, Metabolite, and Taste Activity Values. Foods 2024, 13, 3957. [Google Scholar] [CrossRef] [PubMed]
| Day | pH | |||
|---|---|---|---|---|
| K1 | K2 | K3 | K4 | |
| 0 | 4.15 ± 0.01 b | 4.40 ± 0.00 a | 3.47 ± 0.02 d | 3.99 ± 0.04 c |
| 3 | 3.27 ± 0.01 c | 3.64 ± 0.01 a | 3.23 ± 0.02 c | 3.32 ± 0.01 b |
| 4 | 3.22 ± 0.02 b | 3.40 ± 0.01 a | 3.17 ± 0.01 c | 3.19 ± 0.01 b |
| 5 | 3.21 ± 0.01 b | 3.40 ± 0.01 a | 3.19 ± 0.02 b | 3.19 ± 0.01 b |
| Sample | Extract Content [%] |
|---|---|
| K1 | 6.50 ± 0.16 c |
| K2 | 7.00 ± 0.22 b |
| K3 | 7.83 ± 0.05 a |
| K4 | 7.83 ± 0.05 a |
| Bioactive Compound [mg/L] | K1 | K2 | K3 | K4 |
|---|---|---|---|---|
| Total polyphenols | 171.57 ± 2.72 b | 121.10 ± 4.07 c | 291.00 ± 3.95 a | 157.17 ± 3.07 b |
| Total phenolic acids | 84.92 ± 1.09 b | 63.09 ± 1.86 c | 82.87 ± 2.73 a | 92.35 ± 1.95 b |
| Gallic acid | 1.76 ± 0.10 a | 1.42 ± 0.10 b | 1.75 ± 0.01 a | 1.19 ± 0.05 c |
| Chlorogenic acid | 17.92 ± 0.35 a | 10.61 ± 0.25 b | 9.60 ± 1.99 b | 16.33 ± 0.19 a |
| Caffeic acid | 39.81 ± 0.44 a | 17.57 ± 0.44 c | 27.15 ± 0.35 b | 29.26 ± 0.72 b |
| p-coumaric acid | 3.05 ± 0.07 c | 19.04 ± 0.66 b | 28.95 ± 0.35 a | 29.90 ± 0.90 a |
| Ferulic acid | 11.31 ± 0.21 a | 6.57 ± 0.26 c | 8.23 ± 0.18 b | 7.37 ± 0.13 b |
| Vanillic acid | 8.90 ± 0.05 a | 4.04 ± 0.10 b | 5.64 ± 0.05 c | 5.71 ± 0.04 c |
| t-cinnamic acid | 2.17 ± 0.13 b | 3.85 ± 0.23 a | 1.55 ± 0.08 c | 2.58 ± 0.15 b |
| Total flavonoids | 86.65 ± 2.04 b | 58.01 ± 2.36 c | 208.13 ± 1.54 a | 64.83 ± 1.31 c |
| Epigallocatechin | 1.41 ± 0.20 a | 0.94 ± 0.05 b | 1.49 ± 0.02 a | 1.62 ± 0.11 a |
| Catechin | 24.18 ± 1.24 b | 11.77 ± 0.47 c | 165.82 ± 0.51 a | 19.44 ± 1.20 b |
| Quercetin-3-O-rutinoside | 24.08 ± 0.30 a | 12.59 ± 0.26 b | 15.40 ± 0.16 b | 20.00 ± 0.32 a |
| Naringin | 3.33 ± 0.09 b | 1.91 ± 0.13 c | 2.29 ± 0.09 c | 4.13 ± 0.04 a |
| Kaempferol-3-O-glucoside | 28.98 ± 0.37 | 15.03 ± 0.60 c | 18.48 ± 0.40 b | 15.48 ± 0.19 c |
| Quercetin | 2.34 ± 0.15 b | 11.16 ± 0.75 a | 1.92 ± 0.13 c | 2.33 ± 0.12 b |
| Kaempferol | 2.33 ± 0.01 b | 4.60 ± 0.43 a | 2.72 ± 0.32 b | 1.82 ± 0.03 c |
| Caffeine | 321.91 ± 3.50 a | 114.34 ± 5.47 c | 162.60 ± 1.02 b | 172.08 ± 1.18 b |
| Kombucha Beverages | TVC | AAB | LAB | Yeast and Mould | ENT | BC |
|---|---|---|---|---|---|---|
| K1 | 7.08 ± 0.09 a | 3.03 ± 0.05 a | <1.00 | 6.17 ± 0.04 a | <1.00 | <1.00 |
| K2 | 7.04 ± 0.05 a | 2.26 ± 0.41 b | <1.00 | 6.09 ± 0.08 a | <1.00 | <1.00 |
| K3 | 6.38 ± 0.21 b | 2.06 ± 0.10 b | 2.24 ± 1.97 a | 6.11 ± 0.03 a | <1.00 | <1.00 |
| K4 | 6.35 ± 0.17 b | 2.39 ± 0.46 b | 3.61 ± 3.16 b | 6.32 ± 0.22 a | <1.00 | <1.00 |
| Attributes | Sample Code | |||
|---|---|---|---|---|
| K1 | K2 | K3 | K4 | |
| Visual attribute | ||||
| clarity | 4.74 b | 7.60 a | 7.78 a | 7.11 a |
| Odor attributes | ||||
| acetic | 4.87 a | 3.09 b | 4.46 a | 4.34 a |
| sharp | 3.76 | 3.10 | 2.94 | 3.20 |
| sour | 3.49 | 2.90 | 3.30 | 3.03 |
| fermentative | 4.22 a | 2.41 b | 3.51 a | 3.75 a |
| sweet | 2.50 | 2.85 | 2.95 | 2.61 |
| fruit | 2.67 | 2.53 | 3.30 | 3.25 |
| tea | 2.18 | 1.98 | 2.01 | 1.97 |
| mint | 0.41 b | 7.54 a | 0.13 b | 0.26 b |
| herbal | 2.58 ab | 3.22 a | 2.55 ab | 2.42 b |
| Taste/flavor attributes | ||||
| acetic | 5.93 a | 5.09 b | 5.23 b | 4.33 c |
| sharp | 4.91 a | 4.83 a | 4.17 b | 3.24 c |
| tingling | 4.49 a | 3.79 b | 4.20 ab | 2.41 c |
| sour | 5.31 a | 4.98 a | 4.49 b | 3.88 c |
| sweet | 2.63 c | 3.35 b | 4.70 a | 4.02 ab |
| fruit | 2.91 b | 2.89 b | 4.13 a | 4.35 a |
| tea | 2.41 | 2.08 | 2.07 | 2.32 |
| mint | 0.27 b | 7.35 a | 0.20 b | 0.27 b |
| herbal | 1.95 | 2.46 | 1.88 | 2.06 |
| pungent | 3.69 a | 3.03 ab | 2.83 b | 2.68 b |
| overall sensory quality | 5.08 d | 6.73 b | 6.24 c | 7.37 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gantner, M.; Piotrowska, A.; Kostyra, E.; Hallmann, E.; Ponder, A.; Sionek, B.; Neffe-Skocińska, K. Influence of Herbal Additives on the Physicochemical, Microbiological, Polyphenolic, and Sensory Profile of Green Tea-Based Kombucha. Foods 2025, 14, 3497. https://doi.org/10.3390/foods14203497
Gantner M, Piotrowska A, Kostyra E, Hallmann E, Ponder A, Sionek B, Neffe-Skocińska K. Influence of Herbal Additives on the Physicochemical, Microbiological, Polyphenolic, and Sensory Profile of Green Tea-Based Kombucha. Foods. 2025; 14(20):3497. https://doi.org/10.3390/foods14203497
Chicago/Turabian StyleGantner, Magdalena, Anna Piotrowska, Eliza Kostyra, Ewelina Hallmann, Alicja Ponder, Barbara Sionek, and Katarzyna Neffe-Skocińska. 2025. "Influence of Herbal Additives on the Physicochemical, Microbiological, Polyphenolic, and Sensory Profile of Green Tea-Based Kombucha" Foods 14, no. 20: 3497. https://doi.org/10.3390/foods14203497
APA StyleGantner, M., Piotrowska, A., Kostyra, E., Hallmann, E., Ponder, A., Sionek, B., & Neffe-Skocińska, K. (2025). Influence of Herbal Additives on the Physicochemical, Microbiological, Polyphenolic, and Sensory Profile of Green Tea-Based Kombucha. Foods, 14(20), 3497. https://doi.org/10.3390/foods14203497







