1. Introduction
Dendrobium is among the first Orchidaceae plants listed in China’s Catalog of Substances with Both Medicinal and Edible Uses [
1]. Its dried stems have been included in the Pharmacopoeia of the People’s Republic of China (PRC Pharmacopoeia) since the 1977 edition, and the current 2020 edition still designates
Dendrobium nobile,
Dendrobium chrysotoxum,
Dendrobium fimbriatum, and their related species as official medicinal material sources.
Dendrobium denneanum Kerr., due to its high morphological similarity to medicinal
Dendrobium species and equivalent efficacy, is widely recognized in the industry as a broad-sense
Dendrobium resource with dual medicinal and edible properties. Currently, it is mainly produced in Leshan City, Ya’an City, and Meishan City of Sichuan Province [
2]; however, the differences in active ingredient contents and pharmacodynamic effects of
D. denneanum from different habitats remain unclear, which restricts the precise development and utilization of this resource.
The stems of
D. denneanum are rich in various components, including polysaccharides, alkaloids, sesquiterpenes, and flavonoids [
3]. Among these, polysaccharides—with a content ranging from 5.8% to 16.3%—are recognized as the core pharmacodynamic components [
4]. Considering the pathological characteristics of acute gastric ulcer (AGU), namely mucosal defects, oxidative stress imbalance, and excessive release of inflammatory factors [
5], existing studies have demonstrated that
Dendrobium polysaccharides exert gastroprotective effects through a “triple protective mechanism” (antioxidation-anti-inflammation-mucosal repair). Specifically: (1) They counteract oxidative damage in AGU by increasing superoxide dismutase (SOD) activity, reducing malondialdehyde (MDA) levels [
6,
7], or activating the Nrf2/HO-1 pathway to scavenge reactive oxygen species (ROS); (2) They alleviate inflammatory infiltration at ulcer sites by inhibiting the release of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) via the NF-κB/MAPK pathway [
8]; (3) They repair the tight junctions of gastric mucosal epithelium and enhance barrier function by upregulating the expression of occludin and claudin-1 [
9,
10].
Acute gastric ulcer is characterized by acute mucosal defects, bleeding, and inflammatory cell infiltration [
5], with a short disease course and numerous complications. Its pathogenesis is closely associated with the imbalance of gastric acid-pepsin, excessive ROS, and dysregulation of inflammatory factors; common inducing factors include ethanol, non-steroidal anti-inflammatory drugs (NSAIDs), and stress [
11]. Traditional chemical drugs (e.g., proton pump inhibitors, H
2 receptor antagonists) have obvious adverse reactions [
12], creating an urgent clinical need for safe and effective novel prevention and treatment strategies.
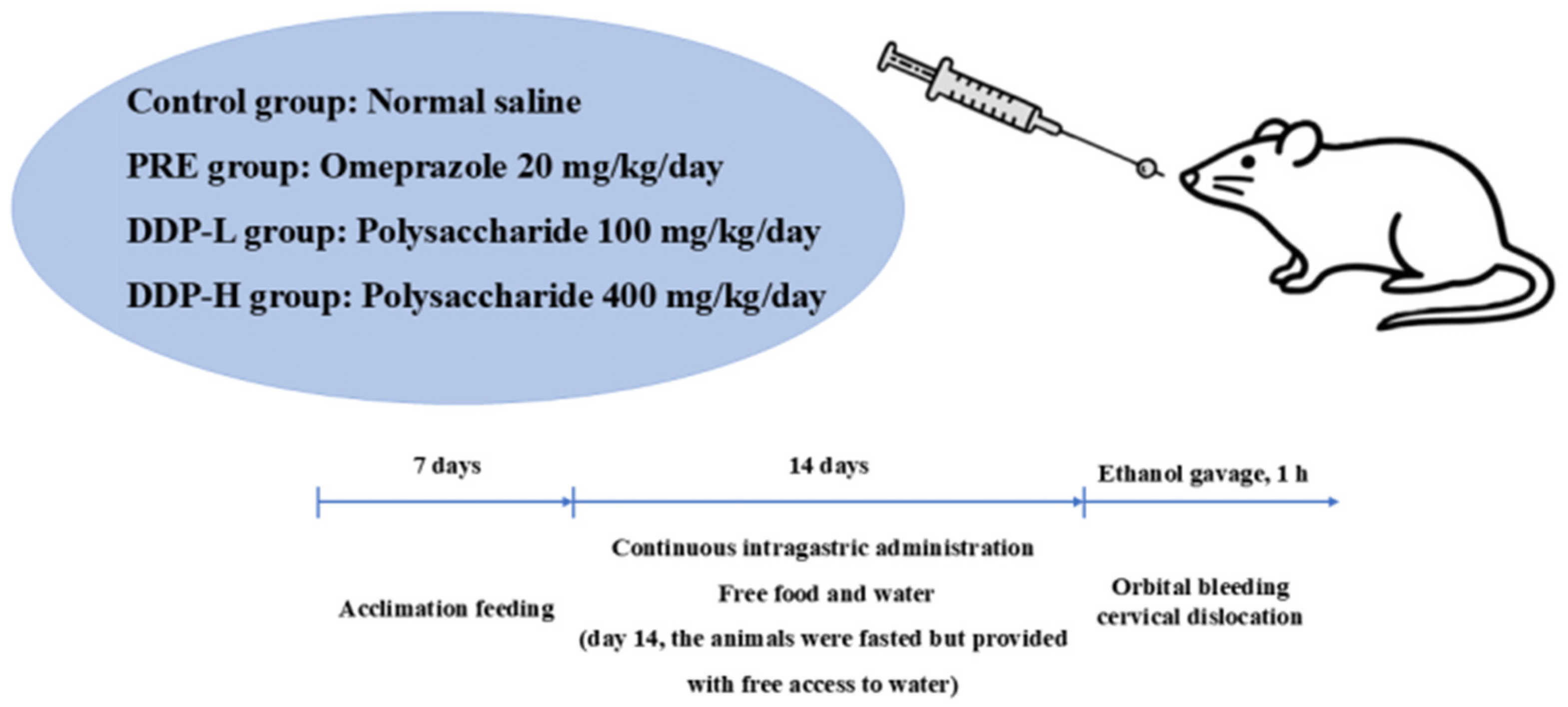
Given the significant gastroprotective activity of Dendrobium polysaccharides, the resource advantages of D. denneanum, and the research gap regarding the unclear pharmacodynamics of D. denneanum from different habitats, this study used ethanol-induced AGU rats as a model to systematically evaluate the gastric mucosal protective effect of D. denneanum polysaccharides and explore its underlying molecular mechanism. This work aims to provide a scientific basis for the development of anti-ulcer functional foods or candidate drugs derived from substances with both medicinal and edible properties.
4. Discussion
This study systematically analyzed the content, structural characteristics, in vitro antioxidant activity, and gastric mucosal protective effect of Dendrobium denneanum polysaccharides (DDP) from different habitats. The core finding is that there are significant differences in total sugar content, molecular weight distribution, and monosaccharide composition of DDP from different habitats, and these structural characteristics are directly associated with their experimental activities. This provides a scientific basis for the precise development and utilization of DDP.
This study found that the total sugar content (20–51.49%) and yield (0.29–1.76%) of DDP from 22 habitats varied significantly, which may be closely related to local cultivation methods and environmental conditions [
18]. In the field of Chinese medicinal materials, the regulatory effect of environmental factors on the accumulation of bioactive substances has been widely confirmed: for example, The results showed that the optimal conditions for the growth of
P. purpureum were salinity 34 ppt, N:P ratio 169:1, and pH 8; for polysaccharide acquisition, they were salinity 17 ppt, N:P ratio 14:1, and pH 8; for phycoerythrin acquisition, salinity 17 ppt, N:P ratio 68:1, and pH 8; and for lipid acquisition, salinity 34 ppt, N:P ratio 1:1, and pH 8 [
19]; Ecological factors such as annual mean precipitation and average temperature significantly affect the content of bioactive components in
Sinopodophyllum hexandrum (Royle) T.S. Ying, with annual mean precipitation being the key determinant and showing a significant negative correlation with bioactive components. Notably, climatic factors contribute more to the variation than edaphic factors, and habitats like Jingyuan (Ningxia) are favorable for the accumulation of specific bioactive components [
20]. Light intensity regulates the biosynthesis of terpenoid bioactive components in
Schizonepeta tenuifolia Briq. via m
6A RNA methylation: dark conditions increase menthone content by approximately 40-fold, while high light intensity promotes limonene accumulation [
21]. In contrast, The microbial effects on monoterpene accumulation in citrus from the core region were further verified via synthetic community (SynCom) experiments. Rhizosphere microorganisms activated terpene synthesis and promoted monoterpene accumulation by interacting with the host immune system, while soil-derived endophytes with terpene synthesis potential might enhance citrus monoterpene accumulation by providing monoterpene precursors [
22]. It is speculated that factors such as light intensity, precipitation distribution, soil fertility, and biotic interference in different habitats may regulate the activity of polysaccharide synthesis-related enzymes (e.g., UDP-glucosyltransferase) and metabolic pathways, ultimately leading to differences in DDP content. However, this study has not identified specific key environmental factors, which means the conclusions cannot yet directly guide the directional cultivation of DDP raw materials. In the future, it is necessary to combine detailed environmental data of each habitat (e.g., annual sunshine hours, soil pH) and use correlation analysis and controlled experiments (e.g., artificial regulation of light gradients) to further identify the core environmental factors affecting DDP accumulation, thereby achieving precise “environment-content” matching.
The biological activity of polysaccharides is determined by their structural characteristics. In this study, the structural data obtained via gel permeation chromatography (GPC), high-performance liquid chromatography (HPLC), and Fourier transform infrared spectroscopy (FT-IR) directly correspond to the in vitro antioxidant activity and gastric mucosal protective activity, clarifying the core mechanism of the “structure-function” relationship of DDP.
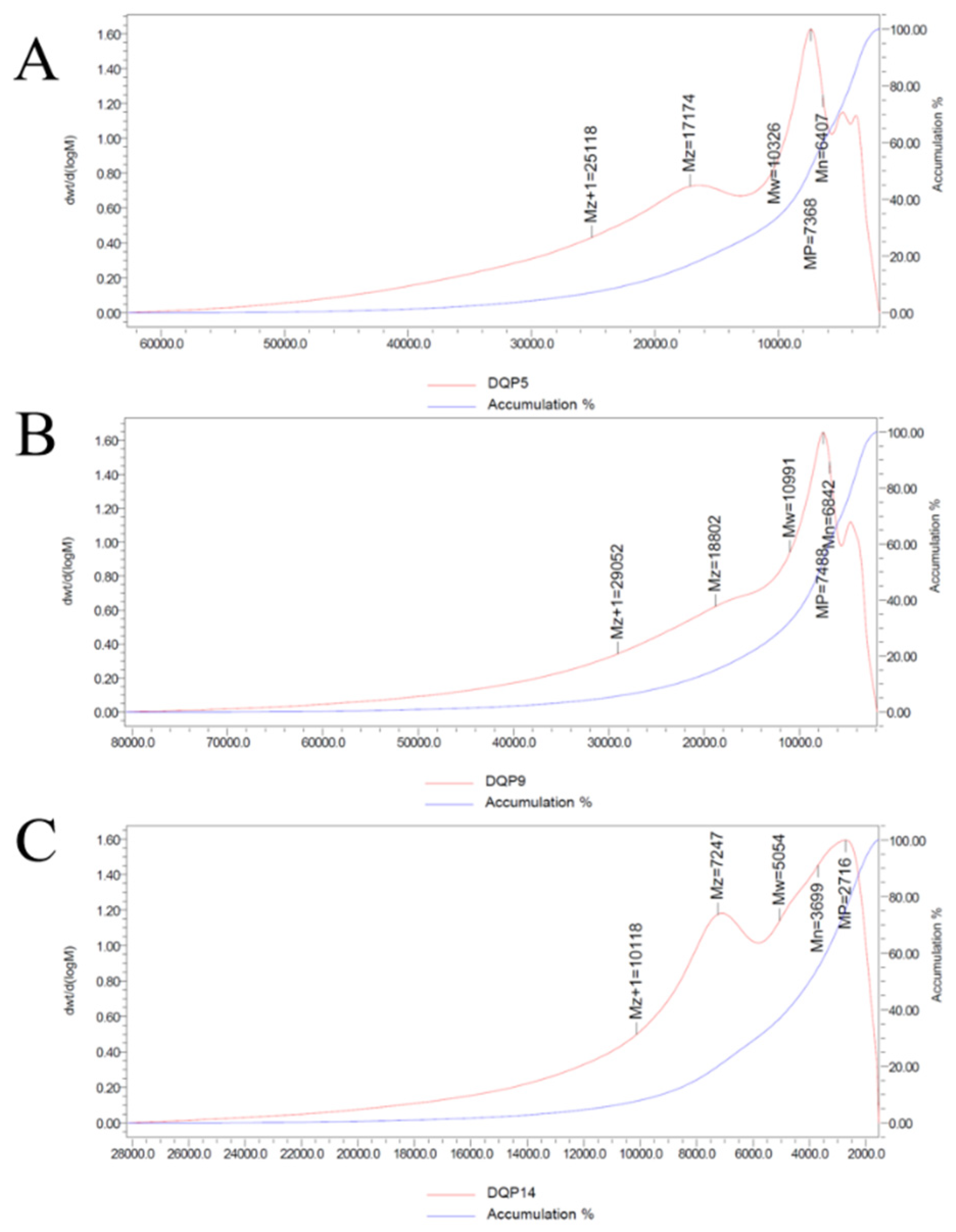
GPC results showed that the weight-average molecular weight (Mw) of DDP from 3 representative habitats was all less than 20 kDa. Among them, DDP5 (10.326 kDa) and DDP9 (10.991 kDa) were concentrated in the range of 10–11 kDa, while DDP14 (5.05 kDa) was a low-molecular-weight component. This is consistent with the research conclusion that low-molecular-weight polysaccharides have activity advantages: for instance, a comparison between two
Tremella fuciformis acidic polysaccharides with different molecular weights, namely TFP (2238 kDa) and TFLP (molecular weight data to be supplemented), revealed that TFLP exhibited superior immunostimulatory and antioxidant activities compared to TFP [
23]. Small-molecular-weight polysaccharide (DANP-II) was extracted from
Taraxacum mongolicum via ultrasound-assisted enzymatic extraction (UAEE). Compared with other high-molecular-weight polysaccharides from
Taraxacum mongolicum, DANP-II exhibited a more potent ability to alleviate H
2O
2-induced cell damage, reduce the cell apoptosis rate, and demonstrated strong antioxidant activity [
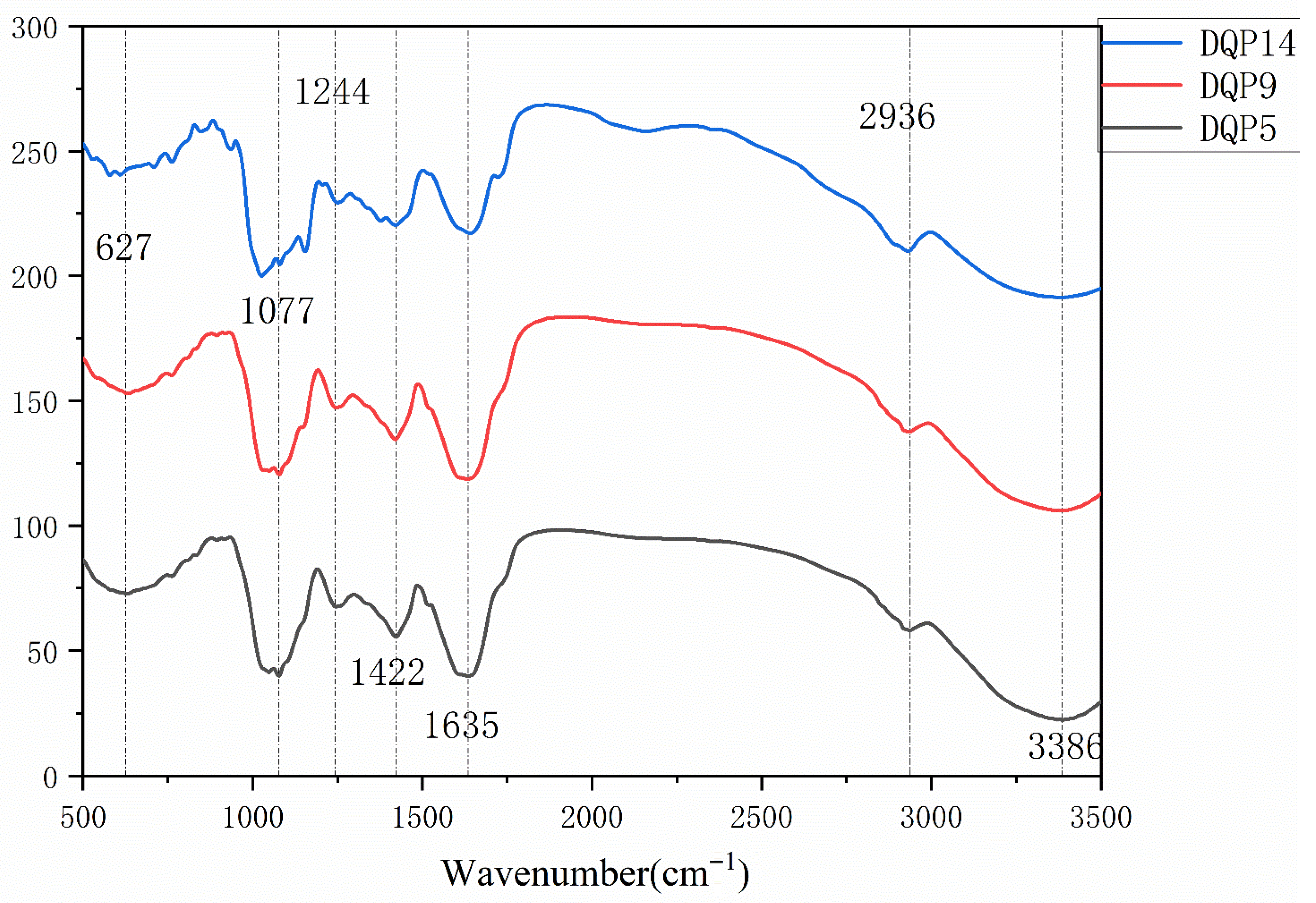
24]. From the perspective of structural basis, the broad peak at 3386.55 cm
−1 in the FT-IR spectrum confirmed that DDP is rich in hydroxyl groups (O-H). Low-molecular-weight DDP has smaller steric hindrance, making it easier to expose active hydroxyl groups. Therefore, although the in vitro antioxidant activity of DDP14 (with a low-molecular-weight characteristic of 5.05 kDa) was significantly lower than that of DDP5 and DDP9, its ulcer inhibition rate at low doses was higher than that of DDP5 and DDP9. It is speculated that DDP14 may penetrate gastric epithelial cell membranes to directly scavenge intracellular reactive oxygen species (ROS), which also explains why low-molecular-weight DDP exhibits better in vivo protective effects.
HPLC analysis revealed that DDP from the 3 habitats was mainly composed of glucose (Glc, 49.67–84.73%), mannose (Man, 8.29–12.25%), and galactose (Gal, 0.96–16.41%), but differences in their molar ratios directly affected activity. DDP5 had the highest Gal content (16.41%), with a Man:Glc:Gal molar ratio of 10.39:49.67:16.41. The characteristic peak of β-glycosidic bonds at 890 cm
−1 in the FT-IR spectrum suggested that DDP5 may form a highly branched “comb-like” structure. This topological structure can delay free radical chain reactions through steric hindrance, which directly corresponds to the strongest hydroxyl radical scavenging activity of this sample (IC
50 = 0.89 mg/mL). This result is consistent with the high antioxidant activity of the galactose-rich exopolysaccharide produced by
Limosilactobacillus fermentum YL-11 [
25].
The Man content of DDP9 (18.29%) was significantly higher than that of other samples. It is speculated that DDP9 may bind to immunoglobulin-like receptors (e.g., CD206) via the C-2 hydroxyl groups of Man residues [
26], thereby assisting in enhancing anti-inflammatory activity. In the experiment, the TNF-α level in the high-dose DDP9 group (23.5 ± 2.1 pg/mL) was significantly lower than that in the DDP14 group (28.7 ± 3.4 pg/mL), which confirms this structural speculation. In contrast, DDP14 had a Gal content of only 0.96% and was mainly composed of linear glucans. Its in vitro antioxidant activity and in vivo anti-inflammatory activity were weaker than those of DDP5 and DDP9, which is consistent with the research conclusion that linear highland barley β-glucans have lower activity than branched structures [
27]. In addition, the weak absorption peak near 670 cm
−1 in the FT-IR spectrum suggested that DDP5 and DDP9 may contain small amounts of fucose and rhamnose. These special monosaccharides may further optimize the spatial conformation of polysaccharides, but their specific contributions need to be verified by subsequent purification of single polysaccharide components.
FT-IR analysis showed that the strong absorption peak of DDP at 3420 cm
−1 confirmed the high abundance of hydroxyl groups, which is the basis for its antioxidant activity; the C-H stretching vibration peak at 2936.93 cm
−1 indicated a stable pyranose ring structure; the weak absorption peak at 1240 cm
−1 implied the possible presence of sulfate groups (S=O stretching vibration), while the absence of an obvious peak at 1730 cm
−1 indicated low acetylation of DDP. Sulfate groups may promote electron transfer by enhancing the negative charge of polysaccharides, thereby improving activity. For example, The polysaccharide from
Sargassum carpophyllum with the highest sulfated polysaccharide content exhibited the most potent effects in vitro: it inhibited nitric oxide (NO) production, reduced the release of cellular NO and reactive oxygen species (ROS), and suppressed the secretion of proinflammatory mediators in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages [
28]. The weak sulfation characteristic of DDP in this study may be an important supplement to its activity, but the specific effect needs to be further verified through sulfation modification experiments.
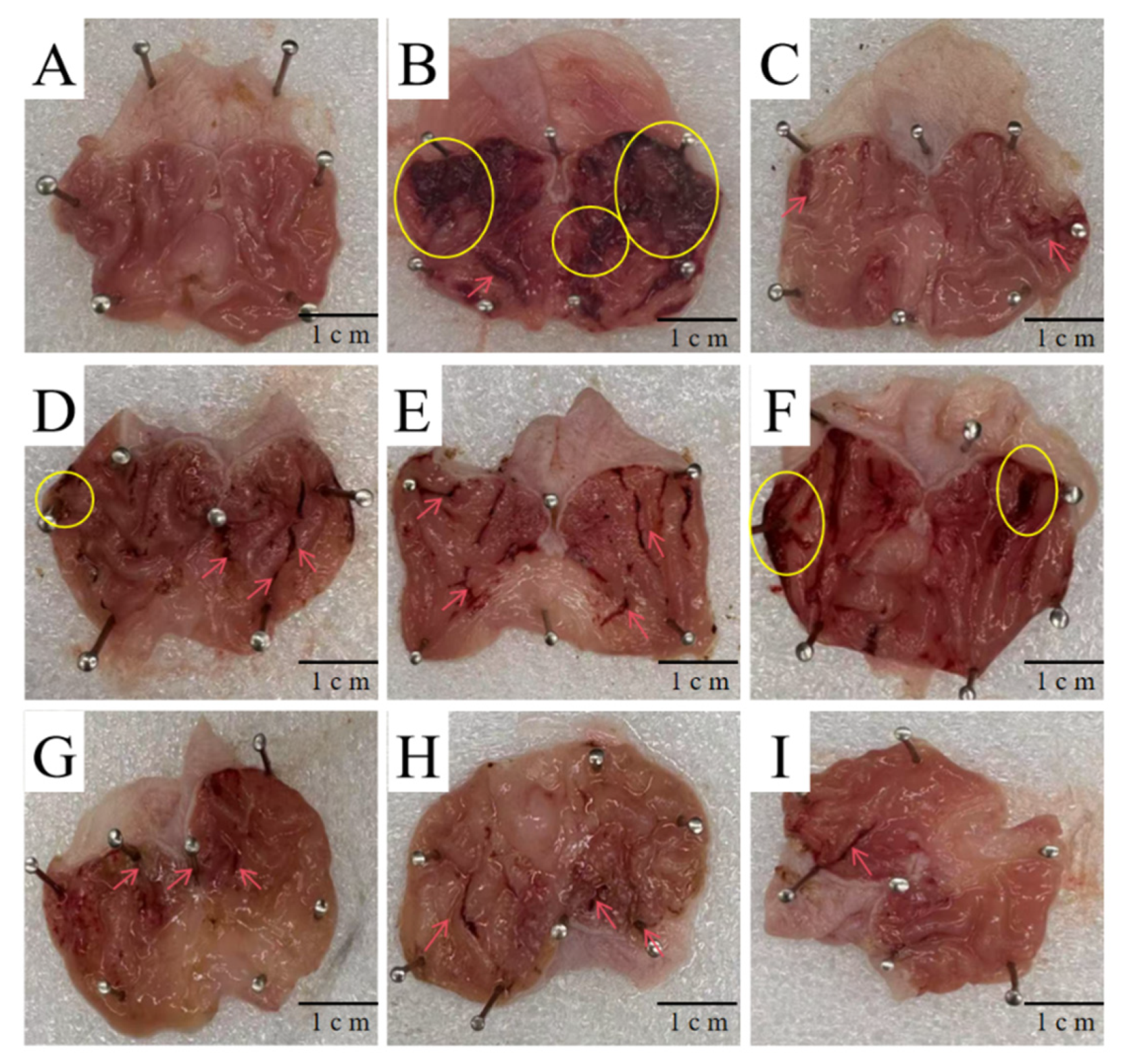
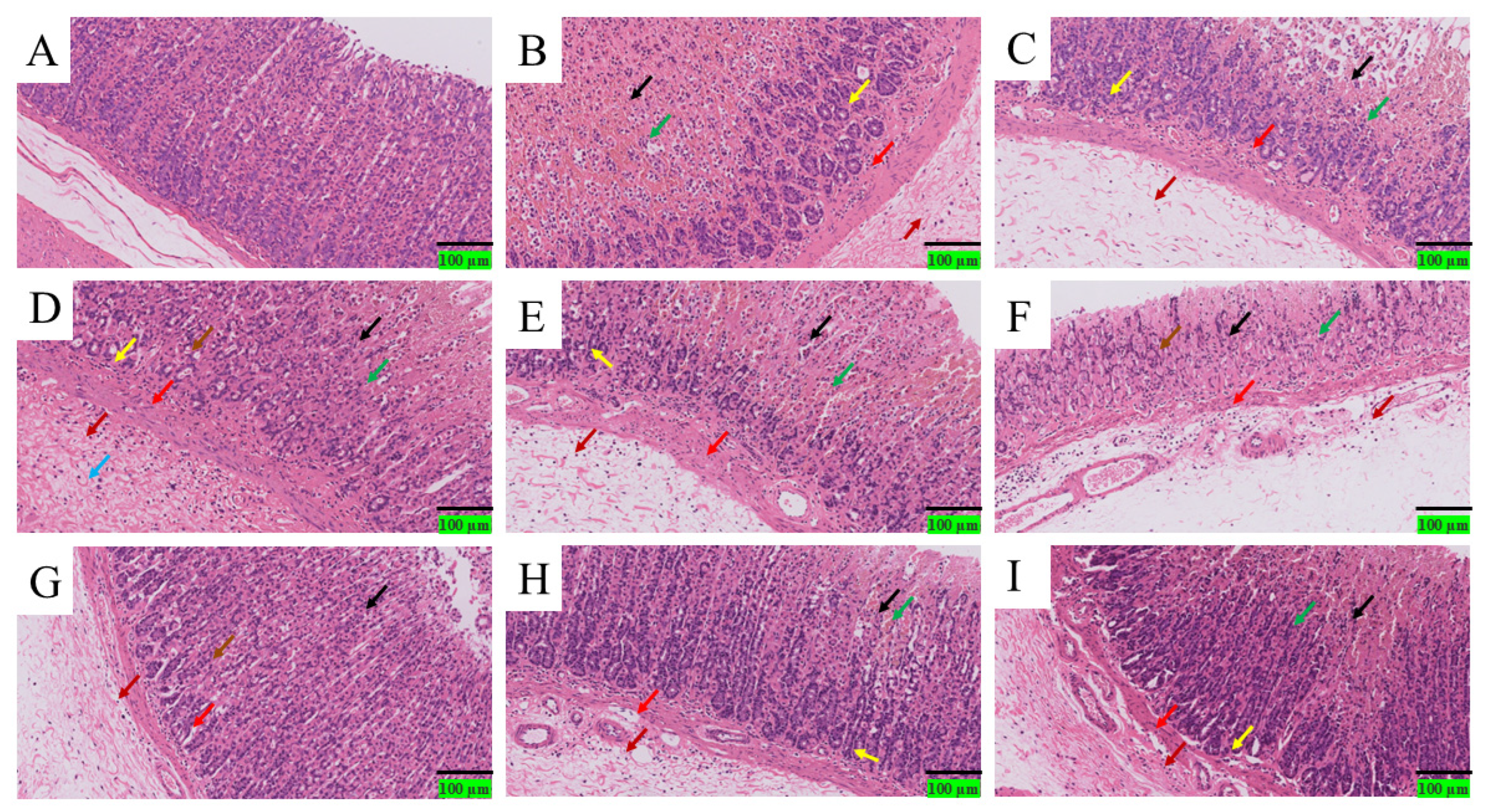
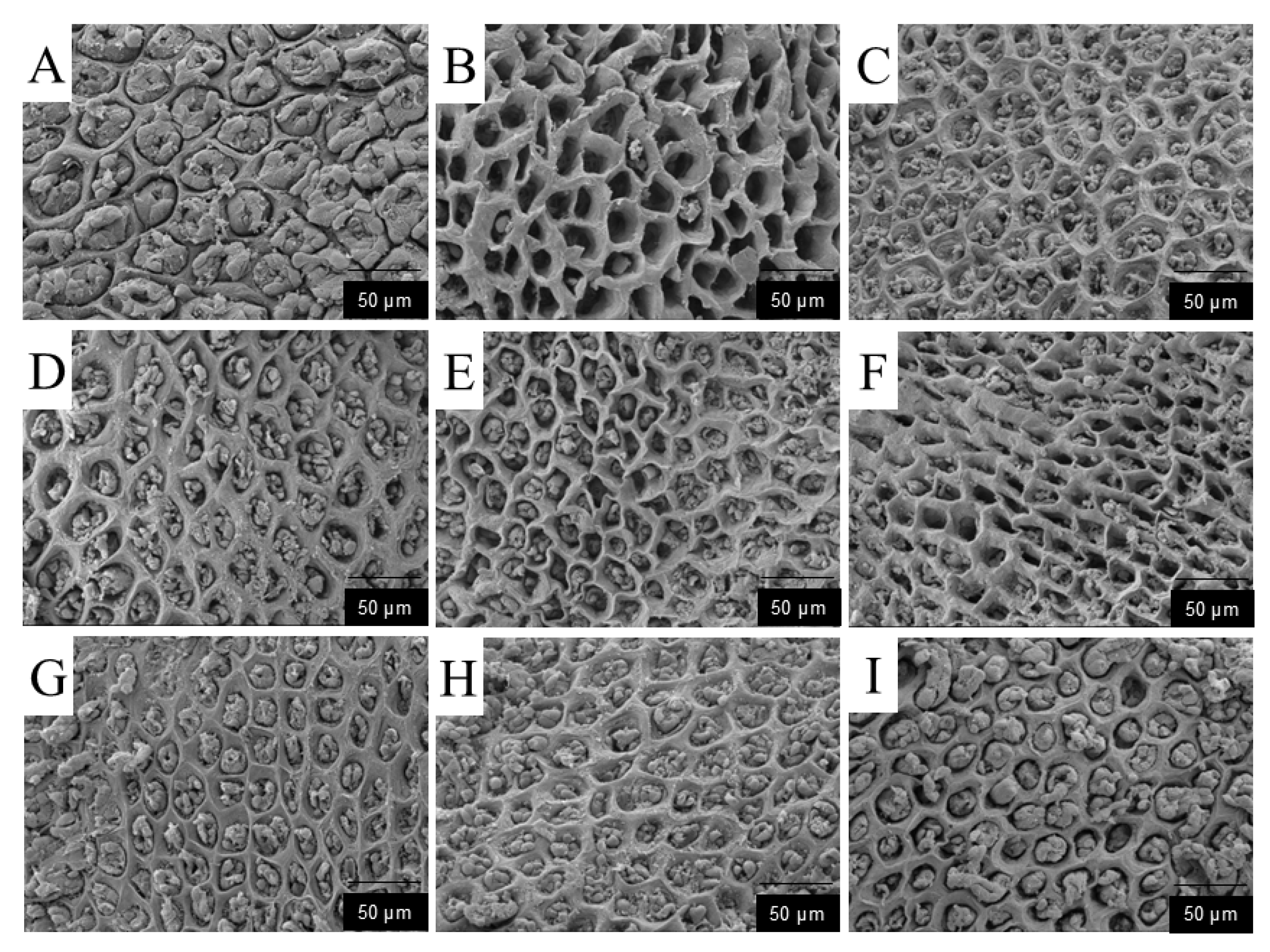
Based on experimental data, the protective effect of DDP against ethanol-induced acute gastric ulcers can be summarized as a “triple synergistic mechanism,” which is directly associated with structural characteristics: Antioxidant damage: Low-molecular-weight DDP (e.g., DDP14) can penetrate cell membranes, significantly increasing the activities of SOD (2.3 ± 0.2 U/mg prot) and CAT (1.8 ± 0.1 U/mg prot) in gastric tissue, while decreasing MDA content (1.5 ± 0.1 nmol/mg prot). This is consistent with its structural characteristic of sufficient hydroxyl group exposure. Anti-inflammatory regulation: DDP9 with high Man content can more effectively reduce the levels of TNF-α (23.5 ± 2.1 pg/mL) and IL-6 (35.2 ± 2.8 pg/mL), while increasing PGE2 content (120.3 ± 5.2 pg/mL). It is speculated that DDP9 may inhibit inflammatory pathways by regulating immune receptor signals. Mucosal repair: Scanning electron microscopy showed that the diameter of ulcerative concave holes in gastric pits was reduced to less than 5 μm in the high-dose DDP group, and the tight junctions of epithelial cells were restored. Combined with the β-glycosidic bond characteristic indicated by FT-IR, it is speculated that DDP may enhance barrier function by upregulating the expression of tight junction proteins (occludin, ZO-1), but this speculation has not been verified by molecular detection.
In addition, this study found that the approximately 3% total phenols in DDP may have a synergistic effect with polysaccharides: the phenolic hydroxyl groups of phenolic substances can form hydrogen bonds with the hydroxyl groups of polysaccharides, further enhancing free radical scavenging ability [
29]; at the same time, phenols can inhibit myeloperoxidase activity and assist in reducing inflammatory infiltration [
30]. This “polysaccharide-phenol” synergistic effect may be an important reason why the activity of DDP is superior to that of a single component, but the specific synergistic ratio and mode of action still need to be verified after separation and purification.
This study speculates that signaling pathways such as Nrf2/ARE and NF-κB may be involved in the protective effect of DDP, but direct experimental evidence is currently lacking. This is only a reasonable hypothesis based on existing results, not a definitive conclusion. In the future, it will be necessary to detect the expression levels of Nrf2, HO-1 (Nrf2 downstream target protein), and p65 (NF-κB subunit) via Western blot to verify whether these pathways are involved.
Based on the above limitations, future research can be carried out in three directions: Directional screening of high-quality raw materials: Combine environmental data (e.g., light, soil nutrients) of different habitats with DDP activity, use multiple regression analysis to identify key environmental factors, and establish a “habitat-structure-activity” prediction model to guide the standardized cultivation of high-quality DDP raw materials. Elucidation of specific mechanisms of action: Use Western blot and RT-PCR to detect the expression of Nrf2/ARE pathway-related genes (e.g., SOD1, CAT) and tight junction proteins (occludin, ZO-1), so as to clarify the molecular targets of DDP in regulating the gastric mucosal barrier. Advancement of preclinical translational research: Conduct in vivo pharmacokinetic studies of DDP (e.g., determination of half-life and bioavailability), and evaluate its safety through long-term administration experiments (e.g., 3-month gavage), so as to provide complete data support for the development of functional foods or candidate drugs.
In conclusion, this study is the first to clarify the structure-activity relationship of DDP from different habitats, confirming that DDP protects gastric mucosa through a “antioxidant-anti-inflammatory-mucosal repair” synergistic effect. Moreover, DDP with low molecular weight and high Gal/Man ratio exhibits better activity, which provides a core basis for the precise development of DDP.