Bioactive Potential of Tocosh Supplemented with Selenium-Enriched Saccharomyces Cerevisiae Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Selenium-Enriched Yeast Biomass
2.2. ICP-OES Selenium Quantification
2.3. Selenium-Enriched Tocosh
2.4. In Vitro Glycemic Index
2.5. Simulated In Vitro Gastrointestinal Digestion
2.6. DPPH Radical Scavenging Activity
2.7. In Vitro Nitric Oxide Inhibition
2.8. In Vitro Cytotoxicity Assay
2.9. Statistical Analysis
3. Results and Discussion
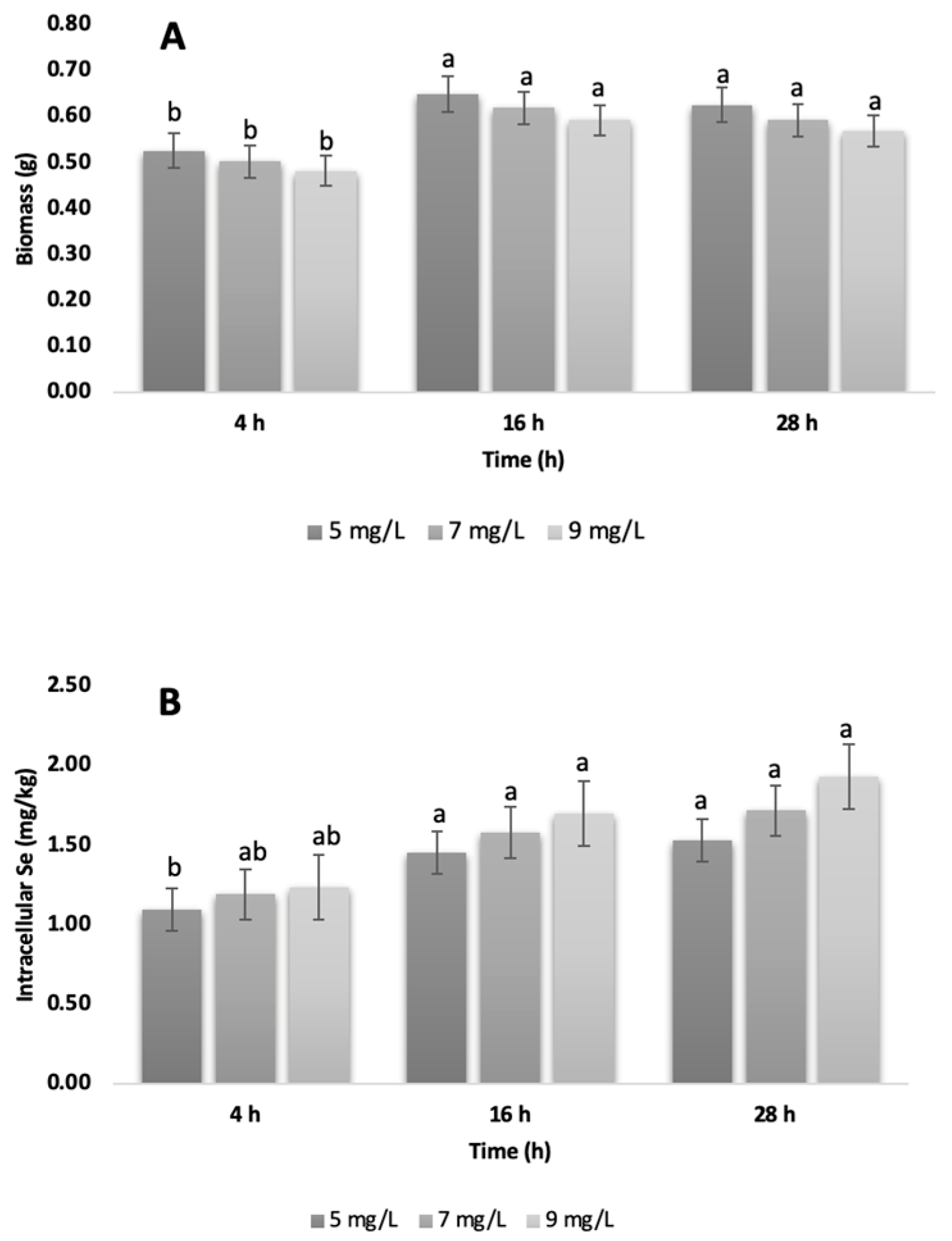
3.1. Obtaining Selenized Biomass
3.2. ICP-OES Selenium Quantification
3.3. In Vitro Glycemic Index
3.4. Antioxidant Activity
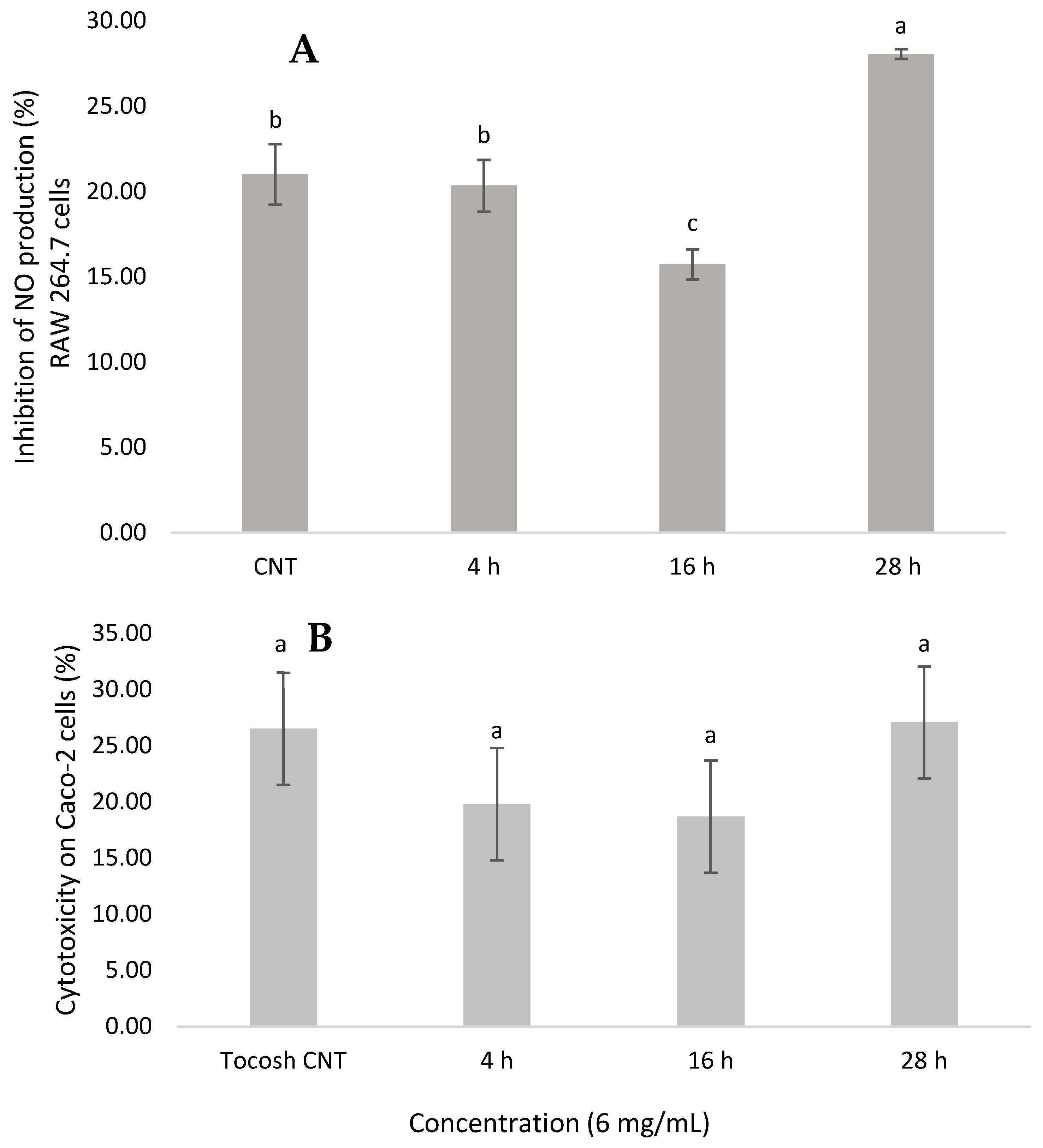
3.5. Anti-Inflammatory Effects of Se-Enriched Tocosh
3.6. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Se | Selenium |
| NO | Nitric oxide |
| YEPD | Yeast extract peptone dextrose |
| GPx | Glutathione peroxidases |
| ROS | Reactive oxygen species |
| CFUs | Colony-forming units |
| ICP-OES | Inductively coupled plasma optical emission spectrometry |
| RSD | Standard deviations |
| eGI | Estimated glycemic index |
| PBS | Phosphate-buffered saline |
| UV–Vis | UV–visible spectrum |
| AUC | Area under the curve |
| SGID | In vitro simulated gastrointestinal digestion |
| DPPH | Radical scavenging activity |
References
- Escandón, K.; Gonzalez-Rojas, J.C.; Carrera Flores, M.J.; Felix, D.G.; Lazo-Vélez, M.A. In Vitro Digestibility and Physicochemical Properties of Potato (Solanum tuberosum) Fermented by Traditional and Alternative Processes under Water Currents. ACS Food Sci. Technol. 2023, 3, 465–469. [Google Scholar] [CrossRef]
- Velasco-Chong, J.R.; Herrera-Calderón, O.; Rojas-Armas, J.P.; Hañari-Quispe, R.D.; Figueroa-Salvador, L.; Peña-Rojas, G.; Andía-Ayme, V.; Yuli-Posadas, R.Á.; Yepes-Perez, A.F.; Aguilar, C. TOCOSH FLOUR (Solanum tuberosum L.): A toxicological assessment of traditional peruvian fermented potatoes. Foods 2020, 9, 719. [Google Scholar] [CrossRef]
- Enciso, S.; Medina, J.; Mauricio, F.; Mauricio-Vilchez, C.; Alvitez-Temoche, D.; Vilchez, L.; Mayta-Tovalino, F. Antibacterial effectiveness of four concentrations of the hydroalcoholic extract of Solanum tuberosum (Tocosh) against Streptococcus mutans ATCC 25175TM: A Comparative In Vitro Study. Int. J. Dent. 2020, 2020, 8856382. [Google Scholar] [CrossRef]
- Villanueva-Flores, F.; Garcia-Atutxa, I. From forgotten to frontier: Vindicating Latin America’s indigenous biotechnology. J. Ethnobiol. Ethnomed. 2025, 21, 52. [Google Scholar] [CrossRef]
- Dávila-Vega, J.P.; Gastelum-Hernández, A.C.; Serrano-Sandoval, S.N.; Serna-Saldívar, S.O.; Guitiérrez-Uribe, J.A.; Milán-Carrillo, J.; Martínez-Cuesta, M.C.; Guardado-Félix, D. Metabolism and anticancer mechanisms of selocompounds: Comprehensive review. Biol. Trace Elem. Res. 2023, 201, 3626–3644. [Google Scholar] [CrossRef]
- Serna-Saldivar, S.O.; Lazo-Vélez, M.A. Production of Selenium-enriched Breads and Their Nutritional and Nutraceutical Properties. In Bread and Its Fortification for Nutrition and Health Benefits; CRC Press: London, UK, 2015; pp. 122–131. [Google Scholar]
- Lazo-Vélez, M.A.; Gutiérrez-Díaz, V.A.; Ramírez-Medrano, A.; Serna-Saldívar, S.O. Effect of sodium selenite addition and sponge dough fermentation on selenomethionine generation during production of yeast-leavened breads. J. Cereal Sci. 2013, 58, 164–169. [Google Scholar] [CrossRef]
- Lazo-Vélez, M.A.; Chávez-Santoscoy, A.; Serna-Saldivar, S.O. Selenium-enriched breads and their benefits in human nutrition and health as affected by agronomic, milling, and baking factors. Cereal Chem. 2015, 92, 134–144. [Google Scholar] [CrossRef]
- Guardado-Félix, D.; Lazo-Vélez, M.A.; Pérez-Carrillo, E.; Panata-Saquicili, D.E.; Serna-Saldívar, S.O. Effect of partial replacement of wheat flour with sprouted chickpea flours with or without selenium on physicochemical, sensory, antioxidant and protein quality of yeast-leavened breads. LWT 2020, 129, 109517. [Google Scholar] [CrossRef]
- Guardado-Félix, D.; Lazo-Vélez, M.A.; Serna-Saldivar, S.O. Protein-Selenized enriched breads. In Flour and Breads and Their Fortification in Health and Disease Prevention; Academic Press: San Diego, CA, USA, 2019; pp. 307–317. [Google Scholar]
- Kaiser, C.; Michaelis, S.; Mitchell, A. Methods in Yeast Genetics; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1994; Volume 234. [Google Scholar]
- Rodríguez, G.; Altamirano, C.; Ramírez, C. Evaluación de la viabilidad celular y contenido de selenio en levaduras cultivadas con selenito de sodio. Rev. Peru. Biotecnol. 2019, 5, 22–29. [Google Scholar]
- Kieliszek, M.; Waśko, A.; Michalak, K.; Kot, A.M.; Piwowarek, K.; Winiarczyk, S. Effect of selenium and methods of protein extraction on the proteomic profile of Saccharomyces yeast. Open Life Sci. 2022, 17, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Eyinla, T.E.; Sanusi, R.A.; Maziya-Dixon, B. Evaluation of in vitro and in vivo Glycemic Index of common staples made from varieties of White Yam (Dioscorea rotundata). Front. Nutr. 2022, 9, 983212. [Google Scholar] [CrossRef]
- Álvarez-Quinteros, J.F.; Lazo-Vélez, M.A.; Cordero-Clavijo, L.M.; Antunes-Ricardo, M.; Avilés-González, J.; Villacrés, E.; Serna-Saldívar, S.O. Protein Concentrates from Andean Plants (Plukenetia volubilis and Lupinus mutabilis): Impact on Protein Quality and In Vitro Biological Activity in Bread. ACS Food Sci. Technol. 2025, 5, 2156–2166. [Google Scholar] [CrossRef]
- Ru, W.; Pang, Y.; Gan, Y.; Liu, Q.; Bao, J. Phenolic Compounds and Antioxidant Activities of Potato Cultivars with White, Yellow, Red and Purple Flesh. Antioxidants 2019, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Clavijo, L.M.; Mejía-Valdez, D.; Antunes-Ricardo, M.; Lazo-Vélez, M.A.; Guajardo-Flores, D. Evaluating sacha inchi (Plukenetia volubilis) oil stability and physicochemical properties: A comparison between conventional extraction and supercritical fluids. Food Chem. 2025, 463, 141132. [Google Scholar] [CrossRef]
- Mejía-Valdez, D.; Antunes-Ricardo, M.; Martínez-Ávila, M.; Ortega-Hernandez, E.; Guajardo-Flores, D. Liposomal encapsulation of Chenopodium berlandieri extracts rich in oleanolic acid: Improved bioactivities targeting metabolic syndrome prevention. Food Funct. 2025, 16, 4994–5007. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hong, L.; Shi, M. Production of Methylselenocysteine in Saccharomyces cerevisiae LG6 by continuous fermentation. Bioresour. Technol. Rep. 2021, 13, 100627. [Google Scholar] [CrossRef]
- Rodriguez-Best, C.A. Cuantificación Cromatográfica y Electrométrica de Selenometionina y Selenocisteína Intracelular de Levadura Saccharomyces cerevisiae en Función de la Concentración de Selenito en el Medio de Cultivo. Ph.D. Thesis, Universidad Nacional Mayor de San Marcos, Lima, Peru, 2019. [Google Scholar]
- Ponce de León, C.A.; Montes-Bayón, M.; Blanco-González, E.; Sanz-Medel, A.; Encinar, J.R. Selenium incorporation into Saccharomyces cerevisiae cells: A study of different incorporation methods. J. Appl. Microbiol. 2002, 92, 602–610. [Google Scholar] [CrossRef]
- Rodríguez Best, C.A.; Ceroni Galloso, M.; Villegas Silva, E.F.; Rebaza Cárdenas, T.D. Selenio total y viabilidad celular en la obtención de selenio-levadura. Rev. Soc. Química Perú 2019, 85, 518–526. [Google Scholar] [CrossRef]
- Kieliszek, M.; Blazejak, S.; Gientka, I.; Bzducha-Wróbel, A. Accumulation and metabolism of selenium by yeast cells. Appl. Microbiol. Biotechnol. 2016, 99, 5373–5382. [Google Scholar] [CrossRef]
- Zare, H.; Vahidi, H.; Owlia, P.; Khujin, M.H.; Khamisabadi, A. Yeast enriched with selenium: A promising source of selenomethionine and seleno-proteins. Trends Pept. Protein Sci. 2017, 1, 130–134. [Google Scholar]
- Herrero, E.; Wellinger, R.E. Yeast as a model system to study metabolic impact of selenium compounds. Microb. Cell 2015, 2, 139. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Bzducha-Wróbel, A.; Kot, A.M. Effect of selenium on growth and antioxidative system of yeast cells. Mol. Biol. Rep. 2019, 46, 1797–1808. [Google Scholar] [CrossRef]
- Oraby, M.M.; Allababidy, T.; Ramadan, E.M. The bioavailability of selenium in Saccharomyces cerevisiae. Ann. Agric. Sci. 2015, 60, 307–315. [Google Scholar] [CrossRef]
- Nie, X.; Yang, X.; He, J.; Liu, P.; Shi, H.; Wang, T.; Zhang, D. Bioconversion of inorganic selenium to less toxic selenium forms by microbes: A review. Front. Bioeng. Biotechnol. 2023, 11, 1167123. [Google Scholar] [CrossRef] [PubMed]
- Vajdi, M.; Hassanizadeh, S.; Gholami, Z.; Bagherniya, M. Selenium supplementation effect on glycemic control: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Pharmacol. Res. 2023, 195, 106888. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzynska, K. Catechins and Selenium Species—How They React with Each Other. Molecules 2023, 28, 5897. [Google Scholar] [CrossRef]
- Martínez, F.G.; Moreno-Martin, G.; Pescuma, M.; Madrid-Albarrán, Y.; Mozzi, F. Biotransformation of selenium by lactic acid bacteria: Formation of seleno-nanoparticles and seleno-amino acids. Front. Bioeng. Biotechnol. 2020, 8, 506. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Bordoni, A.; Aureli, F.; Cubadda, F.; Gianotti, A. Sourdough fermentation favorably influences selenium biotransformation and the biological effects of flatbread. Nutrients 2018, 10, 1898. [Google Scholar] [CrossRef]
- Simsek, S.; El, S.N. In vitro starch digestibility, estimated glycemic index and antioxidant potential of taro (Colocasia esculenta L. Schott) corm. Food Chem. 2015, 168, 257–261. [Google Scholar] [CrossRef]
- Ukom, A.N.; Nwanagba, L.N.; Oguzie, C.O. Antioxidant activity, glycemic index and glycemic load of boiled and roasted cocoyam (Xanthosoma mafaffa Schott) tuber. J. Adv. Food Sci. Technol. 2022, 9, 21–30. [Google Scholar] [CrossRef]
- Peres, M.; Costa, H.S.; Silva, M.A.; Albuquerque, T.G. The health effects of low glycemic index and low glycemic load interventions on prediabetes and type 2 diabetes mellitus: A literature review of RCTs. Nutrients 2023, 15, 5060. [Google Scholar] [CrossRef]
- Jiménez, E.; Yépez, A.; Pérez-Cataluña, A.; Vásquez, E.R.; Dávila, D.Z.; Vignolo, G.; Aznar, R. Exploring diversity and biotechnological potential of lactic acid bacteria from tocosh-traditional Peruvian fermented potatoes-by high throughput sequencing (HTS) and culturing. LWT 2018, 87, 567–574. [Google Scholar] [CrossRef]
- Viltres-Portales, M.; Sánchez-Martín, M.J.; Llugany, M.; Boada, R.; Valiente, M. Selenium biofortification of microgreens: Influence on phytochemicals, pigments and nutrients. Plant Physiol. Biochem. 2024, 206, 108283. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Huang, Y.; Li, L.; Liu, Y.; Liu, L.; Wang, L.; Tong, L.; Wang, F.; Fan, B. A review of plant selenium-enriched proteins/peptides: Extraction, detection, bioavailability, and effects of processing. Molecules 2023, 28, 1223. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Wang, X.; Xiong, H.; Qiu, T.; Zhang, H.; Guo, F.; Jiang, L.; Sun, Y. Anti-inflammatory effects of three selenium-enriched brown rice protein hydrolysates in LPS-induced RAW264. 7 macrophages via NF-κB/MAPKs signaling pathways. J. Funct. Foods 2021, 76, 104320. [Google Scholar] [CrossRef]
- Wallenberg, M.; Misra, S.; Wasik, A.M.; Marzano, C.; Björnstedt, M.; Gandin, V.; Fernandes, A.P. Selenium induces a multi-targeted cell death process in addition to ROS formation. J. Cell. Mol. Med. 2014, 18, 671–684. [Google Scholar] [CrossRef]


| IC50 | ||
|---|---|---|
| Sample | IC50 (mg/mL) | |
| Tocosh CNT | >200 | |
| Se-enriched tocosh | 4 h | >200 |
| 16 h | >200 | |
| 28 h | 22.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Rojas, G.; Escriba-Gutierrez, E.; Andía-Ayme, V.; Cordero-Clavijo, L.M.; Lazo-Vélez, M.A. Bioactive Potential of Tocosh Supplemented with Selenium-Enriched Saccharomyces Cerevisiae Biomass. Foods 2025, 14, 3153. https://doi.org/10.3390/foods14183153
Peña-Rojas G, Escriba-Gutierrez E, Andía-Ayme V, Cordero-Clavijo LM, Lazo-Vélez MA. Bioactive Potential of Tocosh Supplemented with Selenium-Enriched Saccharomyces Cerevisiae Biomass. Foods. 2025; 14(18):3153. https://doi.org/10.3390/foods14183153
Chicago/Turabian StylePeña-Rojas, Gilmar, Edgar Escriba-Gutierrez, Vidalina Andía-Ayme, L. Mateo Cordero-Clavijo, and Marco A. Lazo-Vélez. 2025. "Bioactive Potential of Tocosh Supplemented with Selenium-Enriched Saccharomyces Cerevisiae Biomass" Foods 14, no. 18: 3153. https://doi.org/10.3390/foods14183153
APA StylePeña-Rojas, G., Escriba-Gutierrez, E., Andía-Ayme, V., Cordero-Clavijo, L. M., & Lazo-Vélez, M. A. (2025). Bioactive Potential of Tocosh Supplemented with Selenium-Enriched Saccharomyces Cerevisiae Biomass. Foods, 14(18), 3153. https://doi.org/10.3390/foods14183153







