Development of Stored-Product Moths on Cricket Powder and Insect-Enriched Biscuits
Abstract
1. Introduction
2. Materials and Methods
2.1. Moth Stock Cultures
2.2. Description of the Rearing Substrate
2.3. Moth Development and Survival on Cricket Powder and Biscuits
2.4. Wingspan of Emerged Adults
2.5. Fertility of Females from Cricket Powder Test
2.6. Damage Assessment on Biscuits
2.7. Statistical Analysis
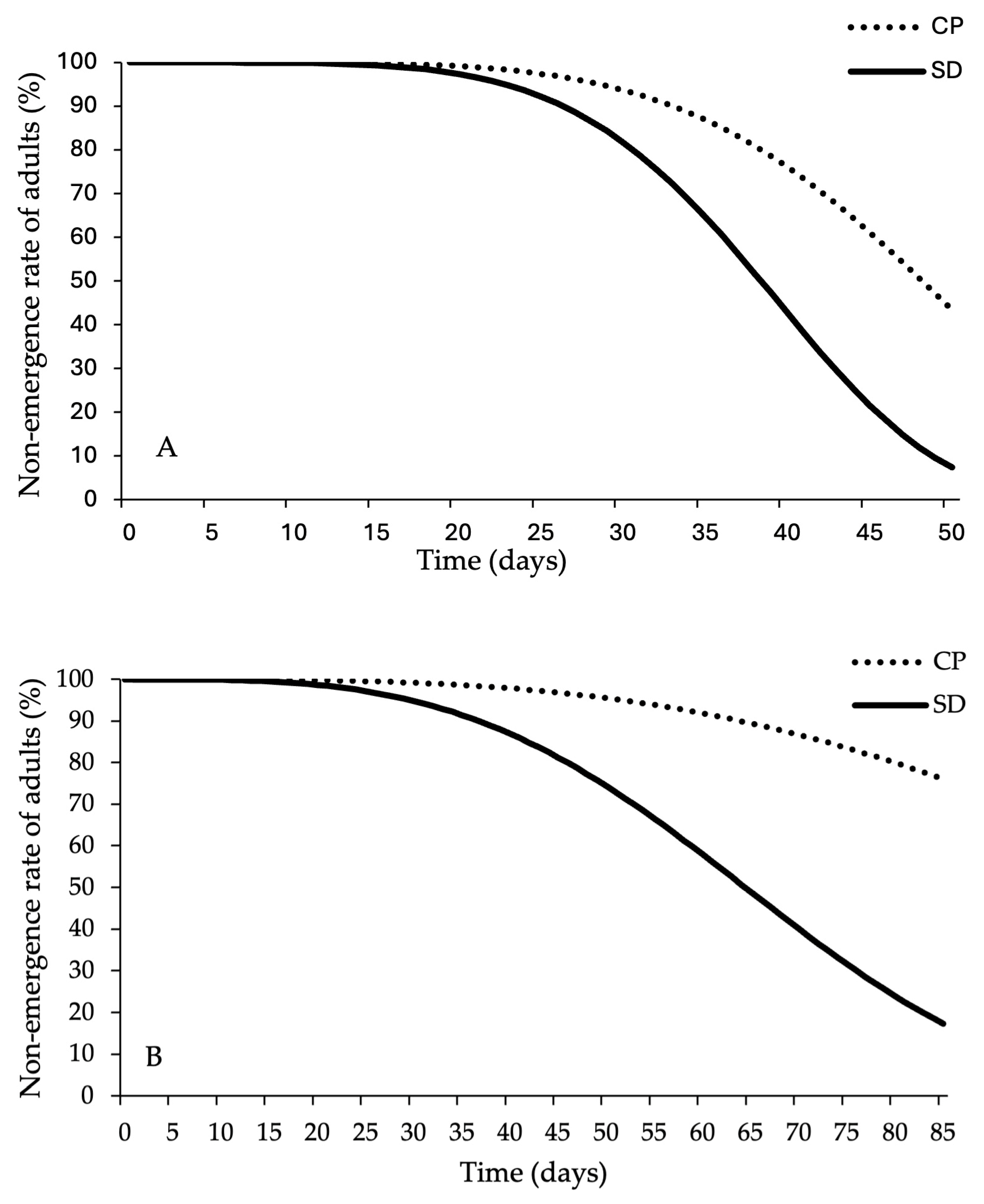
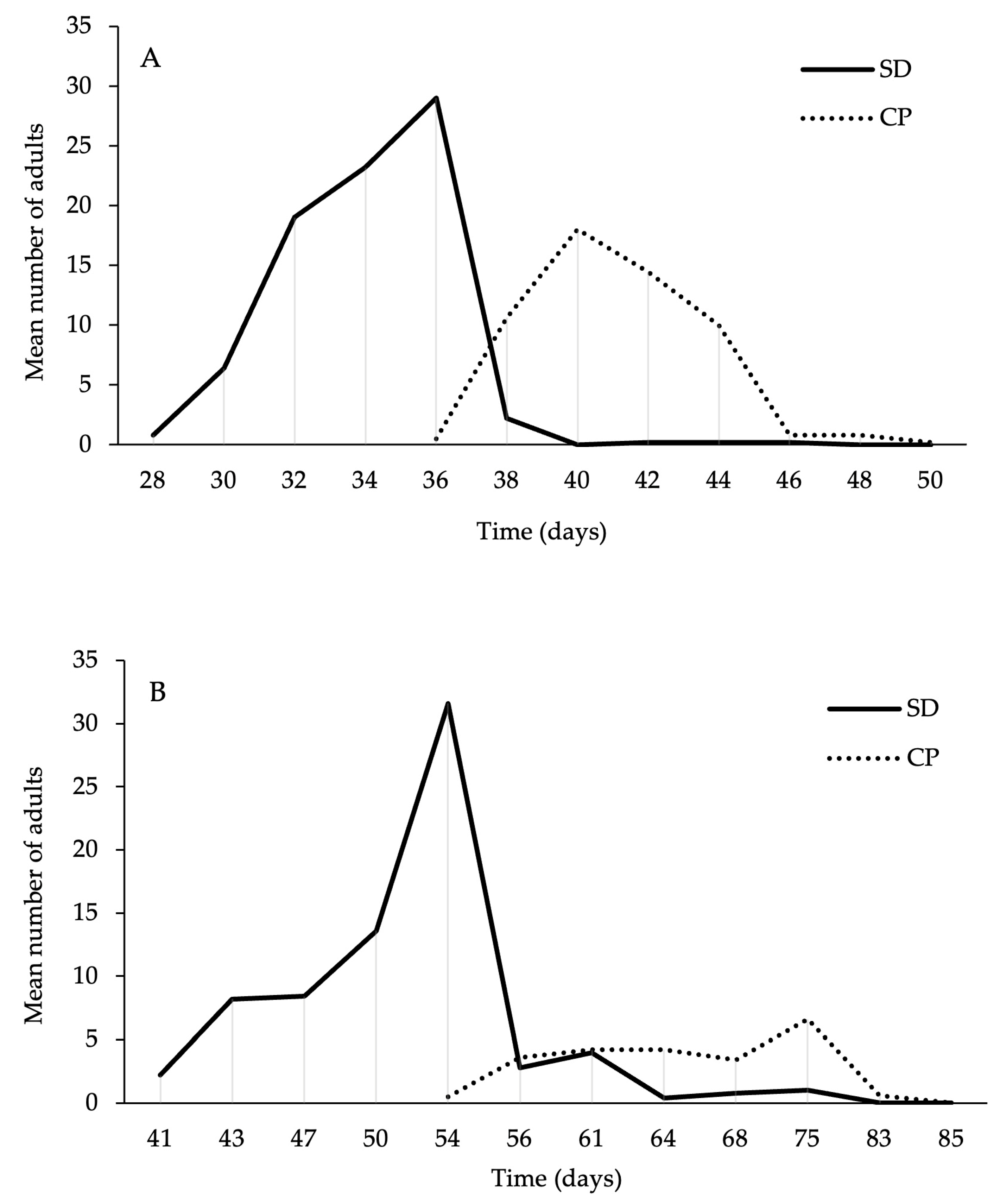
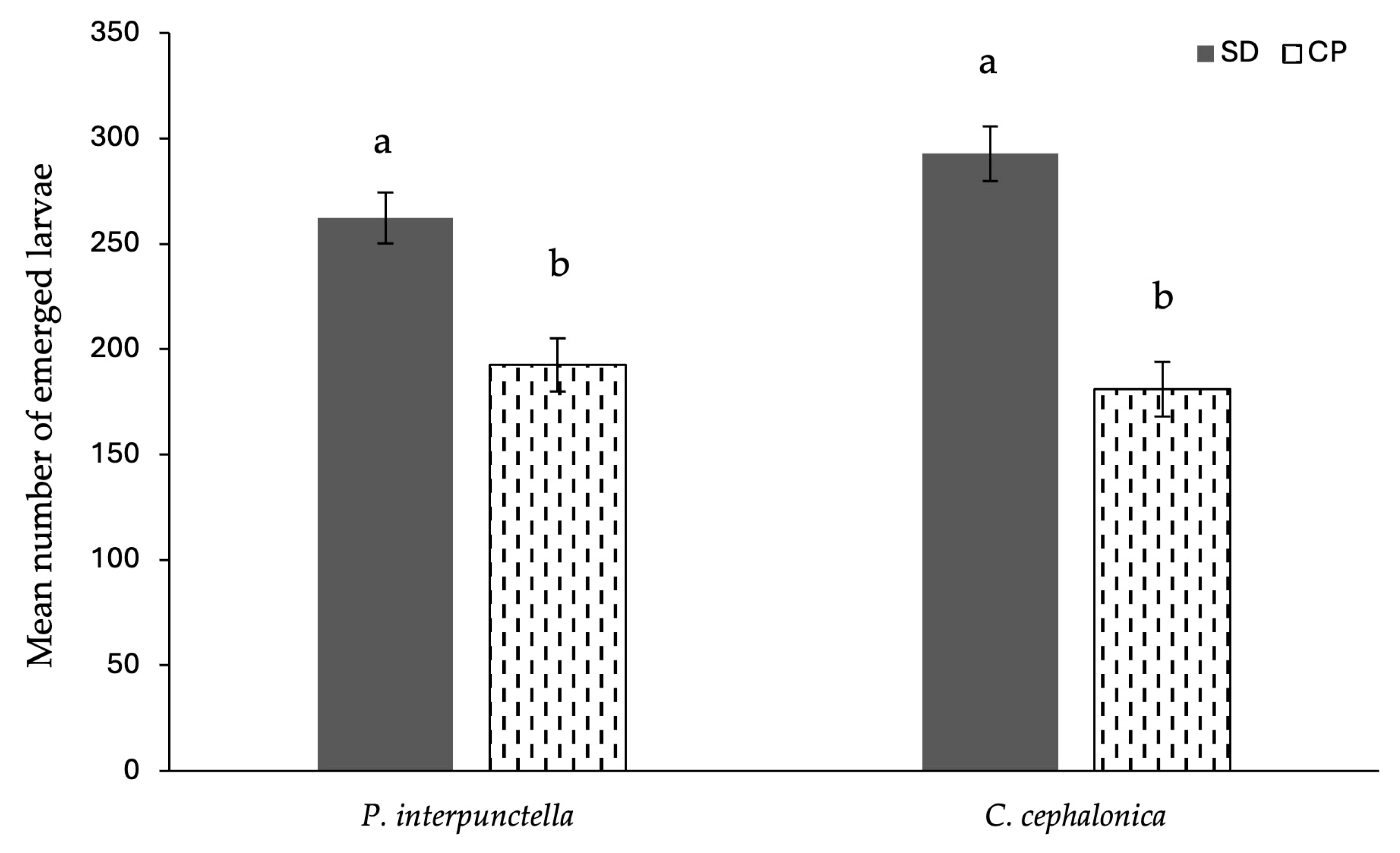
3. Results
3.1. Survival and Developmental Time Analysis
3.2. Wingspan Evaluation
3.3. Fertility of Females from Cricket Powder
3.4. Damage Evaluation in Biscuit Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belluco, S.; Losasso, C.; Maggioletti, M.; Alonzi, C.C.; Paoletti, M.G.; Ricci, A. Edible Insects in a Food Safety and Nutritional Perspective: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 12014. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). Edible Insects: Future Prospects for Food and Feed Security; FAO Forestry Paper 171; FAO: Rome, Italy, 2013. [Google Scholar]
- Fernandez-Cassi, X.; Supeanu, A.; Vaga, M.; Jansson, A.; Boqvist, S.; Vagsholm, I. The house cricket (Acheta domesticus) as a novel food: A risk profile. J. Insects Food Feed. 2019, 5, 137–158. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Mariod, A.A.; Saeed Mirghani, M.E.; Hussein, I. Chapter 48—Acheta domesticus House Cricket. In Unconventional Oilseeds and Oil Sources; Mariod, A.A., Saeed Mirghani, M.E., Hussein, I., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 323–325. [Google Scholar]
- Lucas-González, R.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Effect of drying processes in the chemical, physico-chemical, techno-functional and antioxidant properties of flours obtained from house cricket (Acheta domesticus). Eur. Food Res. Technol. 2019, 245, 1451–1458. [Google Scholar] [CrossRef]
- Montowska, M.; Kowalczewski, P.Ł.; Rybicka, I.; Fornal, E. Nutritional value, protein and peptide composition of edible cricket powders. Food Chem. 2019, 289, 130–138. [Google Scholar] [CrossRef]
- Ho, I.; Peterson, A.; Madden, J.; Huang, E.; Amin, S.; Lammert, A. Will It Cricket? Product Development and Evaluation of Cricket (Acheta domesticus) Powder Replacement in Sausage, Pasta, and Brownies. Foods 2022, 11, 3128. [Google Scholar] [CrossRef]
- Mafu, A.; Ketnawa, S.; Phongthai, S.; Schönlechner, R.; Rawdkuen, S. Whole Wheat Bread Enriched with Cricket Powder as an Alternative Protein. Foods 2022, 11, 2142. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Gahukar, R.T.; Ghosh, S.; Jung, C. Chemical Composition, Nutrient Quality and Acceptability of Edible Insects Are Affected by Species, Developmental Stage, Gender, Diet, and Processing Method. Foods 2021, 10, 1036. [Google Scholar] [CrossRef]
- De la Luz Sánchez-Estrada, M.; Aguirre-Becerra, H.; Feregrino-Pérez, A.A. Bioactive compounds and biological activity in edible insects: A review. Heliyon 2024, 10, e24045. [Google Scholar] [CrossRef]
- Adeduntan, S.A. Nutritional and antinutritional characteristics of some insects foraging in Akure forest reserve Ondo state, Nigeria. J. Food Technol. 2005, 3, 563–567. [Google Scholar]
- Fernandez-Cassi, X.; Supeanu, A.; Jansson, A.; Boqvist, S.; Vagsholm, I. Novel foods: A risk profile for the house cricket (Acheta domesticus). EFSA J. 2018, 16, e16082. [Google Scholar] [CrossRef]
- Gachihi, A.; Tanga, C.; Nyambaka, H.; Kimiywe, J. Effect of processing methods on nutrient and anti-nutrient composition of grasshopper and termites. CYTA-J. Food 2023, 21, 745–750. [Google Scholar] [CrossRef]
- Pippinato, L.; Gasco, L.; Di Vita, G.; Mancuso, T. Current scenario in the European edible-insect industry: A preliminary study. J. Insects Food Feed 2020, 6, 371–382. [Google Scholar] [CrossRef]
- Bresciani, A.; Cardone, G.; Jucker, C.; Savoldelli, S.; Marti, A. Technological Performance of Cricket Powder (Acheta domesticus L.) in Wheat-Based Formulations. Insects 2022, 13, 546. [Google Scholar] [CrossRef]
- Sogari, G.; Menozzi, D.; Hartmann, C.; Mora, C. How to Measure Consumers Acceptance Towards Edible Insects?—A Scoping Review About Methodological Approaches. In Edible Insects in the Food Sector; Springer International Publishing: Cham, Switzerland, 2019; pp. 27–44. [Google Scholar] [CrossRef]
- Jensen, N.H.; Lieberoth, A. We Will Eat Disgusting Foods Together—Evidence of the Normative Basis of Western Entomophagy-Disgust from an Insect Tasting. Food Qual. Prefer. 2019, 72, 109–115. [Google Scholar] [CrossRef]
- Lammers, P.; Ullmann, L.M.; Fiebelkorn, F. Acceptance of Insects as Food in Germany: Is It about Sensation Seeking, Sustainability Consciousness, or Food Disgust? Food Qual. Prefer. 2019, 77, 78–88. [Google Scholar] [CrossRef]
- Woolf, E.; Zhu, Y.; Emory, K.; Zhao, J.; Liu, C. Willingness to Consume Insect-Containing Foods: A Survey in the United States. LWT 2019, 102, 100–105. [Google Scholar] [CrossRef]
- Schöller, M.E.; Flinn, P.W.; Grieshop, M.J.; Zd’árková, E. Biological control of stored product pests. In Insect Management for Food Storage and Processing; Heaps, J.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 67–87. [Google Scholar]
- Karunarathne, E.V.U.P.; Dissanayaka, D.M.S.K.; Wijayaratne, L.K.W. Development and evaluation of diets for rearing Cadra cautella (Walker) (Lepidoptera: Pyralidae) and Corcyra cephalonica (Stainton) (Lepidoptera: Pyralidae) using readily- available materials. RUSL 2020, 5, 79–85. [Google Scholar]
- Rumbos, C.I.; Rigopoulou, M.; Athanassiou, C.G. Are insect meals prone to insect infestation during storage? Development of major storage insects on substrates based on Tenebrio molitor larvae meal. J. Pest Sci. 2020, 93, 1359–1367. [Google Scholar] [CrossRef]
- Rigopoulou, M.; Rumbos, C.; Athanassiou, C. Evaluation of the susceptibility of Alphitobius diaperinus meal to infestations by major stored-product beetle species. ESPR 2023, 30, 73628–73635. [Google Scholar] [CrossRef]
- Trematerra, P.; Colacci, M.; Messia, M.C.; Trivisonno, M.C.; Reale, A.; Boscaino, F.; Germinara, G.S. Olfactory response of Sitophilus zeamais adults to odours of semolina pasta and semolina pasta enriched with different amounts of Acheta domesticus powder. Insects 2024, 15, 634. [Google Scholar] [CrossRef]
- Soderstrom, E.; Hinsch, R.; Bongers, A.; Brandl, D.; Hoogendorn, H. Detecting adult Phycitinae (Lepidoptera: Pyralidae) infestations in a raisin-marketing channel. J. Econ. Entomol. 1987, 80, 1229–1232. [Google Scholar] [CrossRef]
- Sinha, R.N.; Watters, F.L. Insect Pests of Flour Mills, Grain Elevators, and Feed Mills and Their Control; Agriculture Canada Publication: Ottawa, ON, Canada, 1985; pp. 1–290. [Google Scholar]
- Cox, P.D. The suitability of dried fruits, almonds and carobs for the development of Ephestia figulilella Gregson, E. calidella (Guenee) and E. cautella (Walker) (Lepidoptera: Phycitidae). J. Stored Prod. Res. 1975, 11, 229–233. [Google Scholar] [CrossRef]
- Na, J.H.; Ryoo, M.I. The influence of temperature on development of Plodia interpunctella (Lepidoptera: Pyralidae) on dried vegetable commodities. J. Stored Prod. Res. 2000, 36, 125–129. [Google Scholar] [CrossRef]
- Burks, C.S.; Johnson, J.A. Biology, behavior, and ecology of stored fruit and nut insects. In Stored Product Protection; Hagstrum, D.W., Phillips, T.W., Cuperus, G., Eds.; Kansas State University: Manhattan, KA, USA, 2012; pp. 21–32. [Google Scholar]
- Limonta, L.; Locatelli, D.P.; Broglia, T.; Baumgartner, J. Cohort development models for integrated Corcyra cephalonica population management (Stainton). Boll. Zool. Agrar. Bachic. 2009, 41, 215–226. [Google Scholar]
- Jucker, C.; Belluco, S.; Oddon, S.B.; Ricci, A.; Bonizzi, L.; Lupi, D.; Savoldelli, S.; Biasato, I.; Caimi, C.; Mascaretti, A.; et al. Impact of some local organic by-products on Acheta domesticus growth and meal production. J. Insects Food Feed 2022, 8, 631–640. [Google Scholar] [CrossRef]
- Pierce, F.N.; Metcalfe, J.W. The Genitalia of the British Pyrales with the Deltoids and Plumes; FN Pierce: Oundle, UK, 1938. [Google Scholar]
- Abdi, M.K.; Lupi, D.; Jucker, C.; Hardy, I.C. Kinship effects in quasi-social parasitoids I: Co-foundress number and relatedness affect suppression of dangerous hosts. Biol. J. Linn. Soc. 2020, 130, 627–641. [Google Scholar] [CrossRef]
- Aitkin, M.; Anderson, D.; Francis, B.; Hinde, J. Statistical Modelling in GLIM; Clarendon Press: Oxford, UK, 1989. [Google Scholar]
- Crawley, M.J. GLIM for Ecologists; Blackwell Scientific Publications: Oxford, UK, 1993. [Google Scholar]
- Sodhi, S.K. Impact of wheat variety on the post embryonic growth pattern of Corcyra cephalonica (Stainton) (Galleriinae: Pyralidae: Lepidoptera). Environ. Ecol. 1992, 10, 518–520. [Google Scholar]
- Locatelli, D.P.; Limonta, L.; Stampini, M. Development of Cadra cautella (Walker), Corcyra cephalonica (Stainton), and Plodia interpunctella (Hübner) (Lepidoptera Pyralidae) on Triticum monococcum L., T. dicoccum Schrank ex Schubler, and T. spelta L. In Proceedings of the 9th International Working Conference on Stored Product Protection, Sao Paulo, Brazil, 15–18 October 2006; pp. 469–475. [Google Scholar]
- Haines, C.P. Grain storage in the tropics. In Stored-Grain Ecosystems; Marcel Dekker Inc.: New York, NY, USA, 1995; pp. 55–100. [Google Scholar]
- Silhacek, D.; Murphy, C. Moisture content in a wheat germ diet and its effect on the growth of Plodia interpunctella (Hubner). J. Stored Prod. Res. 2008, 44, 36–40. [Google Scholar] [CrossRef]
- Limonta, L.; Locatelli, D.P. Are Dried and Powdered Moringa oleifera Lam. Leaves Susceptible to Moths That Feed on Stored Products? Insects 2021, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- Almaši, R. The effect of nutrition on fertility and number of generations of Indianmeal moth Plodia interpunctella Hbn. (Lepidoptera, Pyralidae). Master’s thesis, University of Novi Sad-Faculty of Agriculture, Novi Sad, Serbia, 1984; 92p. [Google Scholar]
- Vukajlovic, F.N.; Pesic, S. Contribution to the studies of the indian meal moth Plodia interpunctella Hbn. (Lepidoptera: Pyralidae) fecundity depending on diet type. Kragujevac J. Sci. 2012, 34, 107–115. [Google Scholar]
- Borzoui, E.; Bandani, A.R.; Goldansaz, S.H.; Talaei-Hassanlouei, R. Dietary Protein and Carbohydrate Levels Affect Performance and Digestive Physiology of Plodia interpunctella (Lepidoptera: Pyralidae). J. Econ. Entomol. 2018, 111, 942–949. [Google Scholar] [CrossRef]
- Kurtuluş, A.; Pehlivan, S.; Achiri, T.D.; Atakan, E. Influence of different diets on some biological parameters of the Mediterranean flour moth, Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2020, 85, 101554. [Google Scholar] [CrossRef]
- Locatelli, D.P.; Limonta, L.; Stampini, M. Effect of particle size of soft wheat flour on the development of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2008, 44, 269–272. [Google Scholar] [CrossRef][Green Version]
- Fraenkel, G.; Blewett, M. The basic food requirements of several insects. J. Exp. Biol. 1943, 20, 28–34. [Google Scholar] [CrossRef]
- Nawrot, J.; Warchalewski, J.R.; Stasinska, B.; Nowakowska, K. The effect of grain albumins, globulins and gliadins on larval development and longevity and fecundity of some stored product pests. Entomol. Exp. Appl. 1985, 37, 187–192. [Google Scholar] [CrossRef]
- Bauerfeind, S.S.; Fischer, K. Effects of food stress and density in different life stages on reproduction in a butterfly. Oikos 2005, 111, 514–524. [Google Scholar] [CrossRef]
- Maciá, A. Effects of larval crowding on development time, survival and weight at metamorphosis in Aedes aegypti (Diptera: Culicidae). Rev. Soc. Entomol. Arg. 2009, 68, 107–114. [Google Scholar]
- Lupi, D.; Savoldelli, S.; Leonardi, M.G.; Jucker, C. Feeding in the adult of Hermetia illucens (Diptera Stratiomyidae): Reality or fiction? JEAR 2019, 51, 8046. [Google Scholar] [CrossRef]
- Marks, R.J. Mating behaviour and fecundity of the red boll-worm Diparopsis castanea Hmps. (Lepidoptera, Noctuidae). Bull. Ent. Res. 1976, 66, 145–158. [Google Scholar] [CrossRef]
- Miller, T.A.; Cooper, W.J.; Highfill, J.W. Relationships between pupal size and egg production in reared female Antheraea polyphemus. Ann. Entomol. Soc. Am. 1982, 75, 107–108. [Google Scholar] [CrossRef]
- Miller, T.A.; Cooper, W.J.; Highfill, J.W. Egg production in female Callosamia promethea (Lepidoptera: Saturniidae) as a function of pupal size and adult longevity. Ann. Entomol. Soc. Am. 1983, 76, 668–670. [Google Scholar] [CrossRef]
- Leather, S.R. The effect of adult feeding on the fecundity, weight loss and survival of the pine beauty moth, Panolis flammea (D & S). Oecologia 1984, 65, 70–74. [Google Scholar] [CrossRef]
- Carroll, A.L.; Quiring, D.T. Interactions between size and temperature influence fecundity and longevity of a tortricid moth, Zeiraphera canadensis. Oecologia 1993, 93, 233–241. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Q. Male moths undertake both pre- and in-copulation mate choice based on female age and weight. Behav. Ecol. Sociobiol. 2009, 63, 801–808. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Q. Trade-off between adult body size and juvenile survival: An experimental test of parental effects in the Mediterranean flour moth. Aust. J. Entomol. 2013, 52, 403–406. [Google Scholar] [CrossRef]
- Xu, J. Reproductive Behaviour of Ephestia Kuehniella Zeller (Lepidoptera: Pyralidae). Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 2010. [Google Scholar]
- Horak, M. A Review of Cadra Walker in Australia: Five New Native Species and the Two Introduced Pest Species (Lepidoptera: Pyralidae: Phycitinae). Aust. J. Entomol. 1994, 33, 245–262. [Google Scholar] [CrossRef]
- Stillwell, R.C.; Blanckenhorn, W.U.; Teder, T.; Davidowitz, G.; Fox, C.W. Sex differences in phenotypic plasticity affect variation in sexual size dimorphism in insects: From physiology to evolution. Annu. Rev. Entomol. 2010, 55, 227–245. [Google Scholar] [CrossRef]
- Ramanaji, N.; Dabhi, M.V.; Thangavel, S. Bio-ecology of Rice Moth Corcyra cephalonica (Stainton) on Groundnut Seeds. J. Entomol. Zool. Stud. 2020, 8, 2406–2410. [Google Scholar]
- Bankapur, R.; Mule, R.S.; Gurav, S.S.; Kadam, J.J.; Munj, S.S.; Dethe, S.R. Comparative biology of rice moth, Corcyra cephalonica (Stainton) (Lepidoptera: Pyralidae) on rice varieties. Pharma Innov. 2022, 11, 2263–2268. [Google Scholar]
- Russell, V.M.; Schulten, G.G.M.; Roorda, F.A. Laboratory observations on the development of the rice moth Corcyra cephalonica (Stainton) (Lepidoptera: Galleriinae) on millet and sorghum at 28 and different relative humidities. J. Appl. Entomol. 1980, 89, 488–498. [Google Scholar] [CrossRef]
- Bhandari, G.; Regmi, R. Effect of different diets on body length, adult life span and biomass of Corcyra cephalonica (stainton) under laboratory condition in Chitwan, Nepal. IJR 2014, 1, 1432–1436. [Google Scholar]
- Jaenike, J. Environmental modification of oviposition behavior in Drosophila. Am. Nat. 1982, 119, 784–802. [Google Scholar] [CrossRef]
- Kalajdzic, P.; Kenig, B.; Andjelkovic, M. Drosophila subobscura flies adapted to low lead concentration carry no fitness cost. Environ. Pollut. 2015, 204, 90–98. [Google Scholar] [CrossRef]
- Bernays, E.A.; Chapman, R.E. Host-Plant Selection by Phytophagous Insects; Springer: Boston, MA, USA, 1994. [Google Scholar]
- Sambaraju, K.R.; Phillips, T.W. Effects of Physical and Chemical Factors on Oviposition by Plodia interpunctella (Lepidoptera: Pyralidae). Ann. Entomol. Soc. Am. 2008, 101, 955–963. [Google Scholar]
- Wang, C.; Wang, D.; Zeng, F.; Chen, L.; Zhao, X.; Zhu, X.; Li, Y. Identification on Key Volatiles Contributed to Oviposition Preference of Plodia interpunctella (Hübner, 1813) (Lepidoptera: Pyralidae) from High and Normal Oleic Varieties of Peanut. Insects 2024, 15, 866. [Google Scholar] [CrossRef] [PubMed]
| Diets | Protein(%) | Lipid(%) | Fiber (%) | Ash(%) | Carbohydrate(%) * |
|---|---|---|---|---|---|
| Standard diet (SD) | 12.48 | 3.98 | 19.41 | 2.25 | 61.88 |
| Cricket powder (CP) | 56.07 | 27.45 | 5.56 | 4.56 | 11.92 |
| Biscuit Name | Powder Mixture (%) |
|---|---|
| W100 | 100% wheat flour |
| W95N5 | 95% wheat flour + 5% hazelnut flour |
| W85C10N5 | 85% wheat flour + 10% cricket powder + 5% hazelnut flour |
| W90C10 | 90% wheat flour + 10% cricket powder |
| P. interpunctella | C. cephalonica | |
|---|---|---|
| W85C10N5 | 110 ± 7 ab | 92 ± 11 |
| W90C10 | 137 ± 8 b | 83 ± 11 |
| W95N5 | 97 ± 70 a | // |
| DIET | P. interpunctella | C. cephalonica | ||
|---|---|---|---|---|
| M | F | M | F | |
| SD | 14.17 ± 0.05 Bb | 16.56 ± 0.06 Ab | 18.29 ± 0.07 Ba | 22.33 ± 0.09 Aa |
| (n = 156) | (n = 129) | (n = 162) | (n = 202) | |
| CP | 14.79 ± 0.04 Ba | 17.09 ± 0.09 Aa | 16.15 ± 0.11 Bb | 19.80 ± 0.16 Ab |
| (n = 148) | (n = 227) | (n = 64) | (n = 59) | |
| BISCUITS | P. interpunctella | C. cephalonica | ||
|---|---|---|---|---|
| M | F | M | F | |
| W85C10N5 | 13.49 ± 0.2 Ba | 14.85 ± 0.16 Aa | 15.28 | 19.36 ± 0.85 a |
| (n = 3) | (n = 5) | (n = 1) | (n = 2) | |
| W90C10 | 11.61 ± 0.44 Bb | 14.60 ± 0.31 Aa | // | 17.78 ± 0.86 a |
| (n = 2) | (n = 4) | (n = 3) | ||
| W95N5 | 11.02 ± 0.11 Bb | 12.41 ± 0.11 Ab | // | // |
| (n = 2) | (n = 2) | |||
| Species | W100 | W85C10N5 | W90C10 | W95N5 | Tot. |
|---|---|---|---|---|---|
| P. interpunctella | 100% (5/5) | 100% (5/5) | 100% (5/5) | 100% (5/5) | 100% (20/20) |
| C. cephalonica | 80% (4/5) | 100% (5/5) | 100% (5/5) | 100% (5/5) | 95% (19/20) |
| E. kuehniella | 60% (3/5) | 40% (2/5) | 20% (1/5) | 20% (1/5) | 35% (7/20) |
| Tot. | 80% (12/15) | 80% (12/15) | 73.33% (11/15) | 73.33% (11/15) | 80% (48/60) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malabusini, S.; Savoldelli, S.; Bresciani, A.; Marti, A.; Lupi, D.; Jucker, C. Development of Stored-Product Moths on Cricket Powder and Insect-Enriched Biscuits. Foods 2025, 14, 3154. https://doi.org/10.3390/foods14183154
Malabusini S, Savoldelli S, Bresciani A, Marti A, Lupi D, Jucker C. Development of Stored-Product Moths on Cricket Powder and Insect-Enriched Biscuits. Foods. 2025; 14(18):3154. https://doi.org/10.3390/foods14183154
Chicago/Turabian StyleMalabusini, Serena, Sara Savoldelli, Andrea Bresciani, Alessandra Marti, Daniela Lupi, and Costanza Jucker. 2025. "Development of Stored-Product Moths on Cricket Powder and Insect-Enriched Biscuits" Foods 14, no. 18: 3154. https://doi.org/10.3390/foods14183154
APA StyleMalabusini, S., Savoldelli, S., Bresciani, A., Marti, A., Lupi, D., & Jucker, C. (2025). Development of Stored-Product Moths on Cricket Powder and Insect-Enriched Biscuits. Foods, 14(18), 3154. https://doi.org/10.3390/foods14183154








