Key Odorant Identification Confirms 3-Oxododecanal as the Most Important Contributor to the Characteristic Aroma of Fresh Rhizomes and Leaves of Houttuynia cordata
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Reference Odorants
2.3. Stable Isotopically Substituted Odorants
2.4. Miscellaneous Chemicals
2.5. Gas Chromatography
2.6. Enantioselective Odorant Analysis
2.7. GC–MS Quantitations
2.8. GC–FID Quantitations
2.9. Determination of Odor Threshold Concentrations (OTCs)
2.10. Preparation of Aroma Reconstitution Models
2.11. Quantitative Olfactory Profile Analyses
2.12. Omission Tests
3. Results and Discussion
3.1. Odorant Concentrations and OAVs
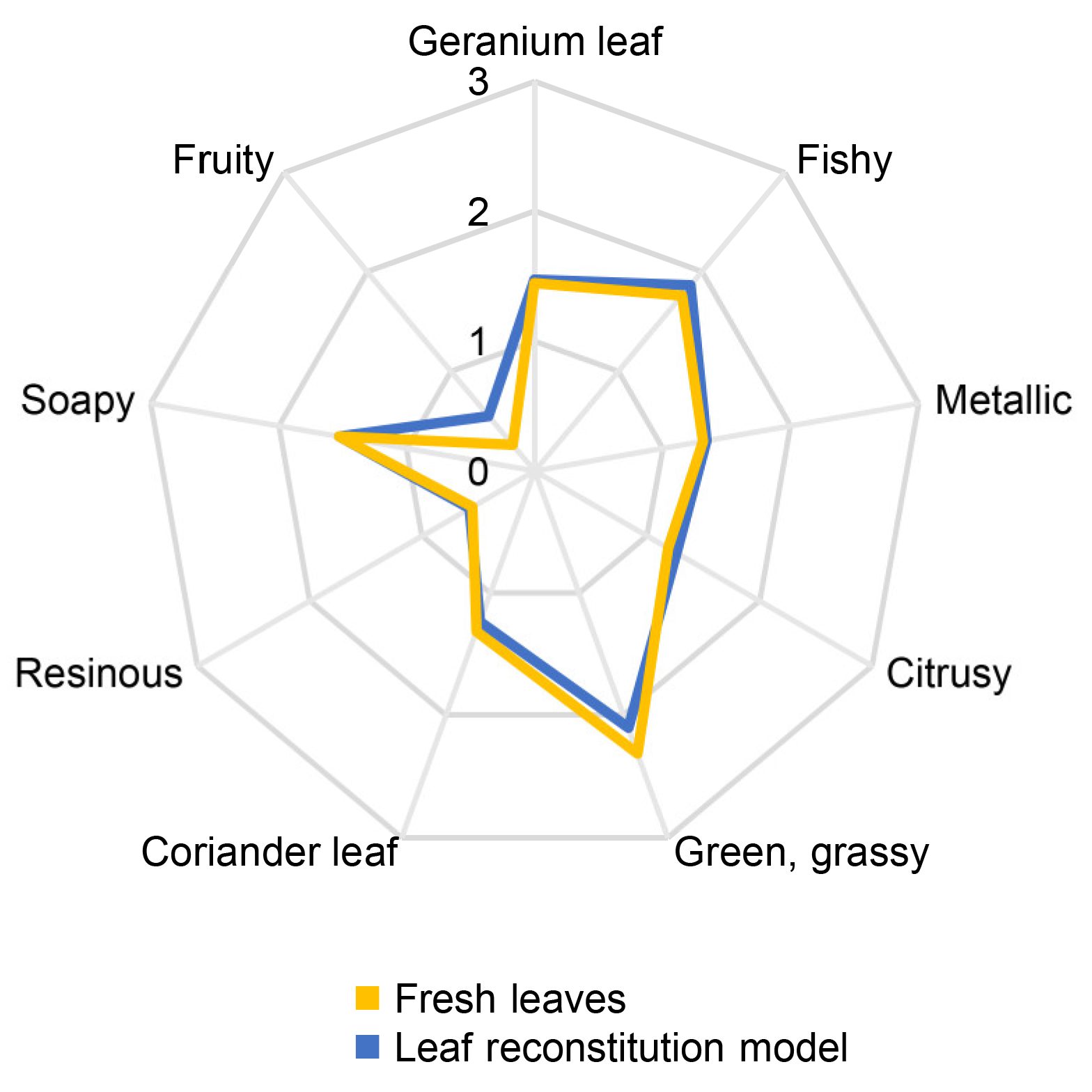
3.2. Aroma Reconstitution
3.3. Key Odorants
3.4. Effect of Tissue Disruption on 3-Oxododecanal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Nomenclature
| 3-AFC | Three-alternative forced choice |
| aSAFE | Automated solvent-assisted flavor evaporation |
| cAEDA | Comparative aroma extract dilution analysis |
| (E)-β-damascenone | (2E)-1-(2,6,6-trimethylcyclohexa-1,3-dien-1-yl)but-2-en-1-one |
| trans-4,5-epoxy-(2E)-dec-2-enal | (2E)-3-[(2R,3R)/(2S,3S)-3-pentyloxiran-2-yl]prop-2-enal |
| eugenol | 2-methoxy-4-(prop-2-en-1-yl)phenol |
| FD | Flavor dilution |
| FID | Flame ionization detector |
| GC | Gas chromatography |
| GC–O | Gas chromatography–olfactometry |
| geraniol | (2E)-3,7-dimethylocta-2,6-dien-1-ol |
| geranyl acetate | (2E)-3,7-dimethylocta-2,6-dien-1-yl acetate |
| HR | High resolution |
| i.d. | Inner diameter |
| trans-isoeugenol | 2-methoxy-4-[(1E)-prop-1-en-1-yl]phenol |
| limonene | 1-methyl-4-(prop-1-en-2-yl)cyclohex-1-ene |
| MS | Mass spectrometry |
| myrcene | 7-methyl-3-methylideneocta-1,6-diene |
| OAV | Odor activity value |
| OTC | Odor threshold concentration |
| α-pinene | 2,6,6-trimethylbicyclo[3.1.1]hept-2-ene |
| RI | Retention index |
| TOF | Time-of-flight |
| vanillin | 4-hydroxy-3-methoxybenzaldehyde |
References
- Wei, P.; Luo, Q.; Hou, Y.; Zhao, F.; Li, F.; Meng, Q. Houttuynia Cordata Thunb.: A comprehensive review of traditional applications, phytochemistry, pharmacology and safety. Phytomedicine 2024, 123, 155195. [Google Scholar] [CrossRef]
- Huang, X.; Yu, P.; Luo, Y.; Guo, Z.; Wang, Z.; Ma, C.; Dong, L.; Luo, P.; Wang, G.; Hu, X.; et al. The nutritional value, application status and challenges of Houttuynia cordata Thunb (H. cordata). Phytochem. Rev. 2025. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, J.G. Bioactive components and functional properties of Hottuynia cordata and its applications. Pharm. Biol. 2009, 47, 1154–1161. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, J.; Kreissl, J.; Oellig, C.; Vetter, W.; Steinhaus, M.; Frank, S. Characterization of the major odor-active compounds in fresh rhizomes and leaves of Houttuynia cordata by comparative aroma extract dilution analysis. Foods 2025, 14, 2303. [Google Scholar] [CrossRef]
- Guth, H.; Grosch, W. Quantitation of potent odorants of virgin olive oil by stable-isotope dilution assays. J. Am. Oil Chem. Soc. 1993, 70, 513–518. [Google Scholar] [CrossRef]
- Frank, S.; Schieberle, P. Changes in the major odorants of grape juice during manufacturing of Dornfelder red wine. J. Agric. Food Chem. 2022, 70, 13979–13986. [Google Scholar] [CrossRef]
- Sellami, I.; Mall, V.; Schieberle, P. Changes in the key odorants and aroma profiles of Hamlin and Valencia orange juices not from concentrate (NFC) during chilled storage. J. Agric. Food Chem. 2018, 66, 7428–7440. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.E.; Steinhaus, M. Characterization of the major odor-active compounds in jackfruit pulp. J. Agric. Food Chem. 2019, 67, 5838–5846. [Google Scholar] [CrossRef]
- Neiens, S.D.; Steinhaus, M. Odor-active compounds in the special flavor hops Huell Melon and Polaris. J. Agric. Food Chem. 2018, 66, 1452–1460. [Google Scholar] [CrossRef]
- Kubícková, J.; Grosch, W. Quantification of potent odorants in camembert cheese and calculation of their odour activity values. Int. Dairy J. 1998, 8, 17–23. [Google Scholar] [CrossRef]
- Frank, S.; Reglitz, K.; Mall, V.; Morgenstern, U.; Steinhaus, M. Molecular background of the undesired odor of polypropylene materials and insights into the sources of key odorants. Indoor Air 2021, 31, 1038–1049. [Google Scholar] [CrossRef]
- Guth, H.; Grosch, W. Deterioration of soya-bean oil: Quantification of primary flavour compounds using a stable isotope dilution assay. Lebensm.-Wiss. Technol. 1990, 23, 513–522. [Google Scholar]
- Hausch, B.J.; Lorjaroenphon, Y.; Cadwallader, K.R. Flavor chemistry of lemon-lime carbonated beverages. J. Agric. Food Chem. 2015, 63, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Laskawy, G.; Schieberle, P.; Grosch, W. Quantitative determination of β-damascenone in foods using a stable isotope dilution assay. J. Agric. Food Chem. 1991, 39, 757–759. [Google Scholar] [CrossRef]
- Fischer, A.; Schieberle, P. Characterisation of the key aroma compounds in the peel oil of Pontianak oranges (Citrus nobilis Lour. var. microcarpa Hassk.) by aroma reconstitution experiments. Eur. Food Res. Technol. 2009, 229, 319–328. [Google Scholar] [CrossRef]
- Lin, J.; Fay, L.B.; Welti, D.H.; Blank, I. Synthesis of trans-4,5-epoxy-(E)-2-decenal and its deuterated analog used for the development of a sensitive and selective quantification method based on isotope dilution assay with negative chemical ionization. Lipids 1999, 34, 1117–1126. [Google Scholar] [CrossRef]
- Kiefl, J.; Pollner, G.; Schieberle, P. Sensomics analysis of key hazelnut odorants (Corylus avellana L. ‘Tonda Gentile’) using comprehensive two-dimensional gas chromatography in combination with time-of-flight mass spectrometry (GCxGC–TOF–MS). J. Agric. Food Chem. 2013, 61, 5226–5235. [Google Scholar] [CrossRef]
- Schaller, T.; Schieberle, P. Quantitation of key aroma compounds in fresh, raw ginger (Zingiber officinale Roscoe) from China and roasted ginger by stable isotope dilution assays and aroma profiling by recombination experiments. J. Agric. Food Chem. 2020, 68, 15284–15291. [Google Scholar] [CrossRef]
- Semmelroch, P.; Laskawy, G.; Blank, I.; Grosch, W. Determination of potent odourants in roasted coffee by stable isotope dilution assays. Flavour Fragr. J. 1995, 10, 1–7. [Google Scholar] [CrossRef]
- Schmitt, R. On the Role of Ingredients as Sources of Key Aroma Compounds in Crumb Chocolate. Ph.D. Thesis, Technical University of Munich, Munich, Germany, 2005. [Google Scholar]
- Schlumpberger, P.; Stübner, C.A.; Steinhaus, M. Development and evaluation of an automated solvent-assisted flavour evaporation (aSAFE). Eur. Food Res. Technol. 2022, 248, 2591–2602. [Google Scholar] [CrossRef]
- Bemelmanns, J.M.H. Review of isolation and concentration techniques. In Progress in Flavour Research; Land, D.G., Nursten, H.E., Eds.; Applied Science Publishers: London, UK, 1979; pp. 79–88. [Google Scholar]
- E679-19; Standard Practice for Determination of Odor and Taste Thresholds By a ForcedChoice Ascending Concentration Series Method of Limits. ASTM: West Conshohocken, PA, USA, 2019.
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Choudhury, B.H.; Baruah, A.M.; Sarmah, T.C.; Baishya, S. Nutritional and antinutritional composition of twenty five indigenous leafy vegetables of Jorhat district of Assam state, India. Asian J. Chem. 2017, 29, 65–68. [Google Scholar] [CrossRef]
- Fu, M.; Wu, X.; Chen, C. Quantitative analysis of nutritive composition from Houttuynia cordata Thunb. J. Huaihua Univ. 2006, 25, 70–71. [Google Scholar]
- Sjödin, K.; Persson, M.; Borg-Karlson, A.-K.; Norin, T. Enantiomeric compositions of monoterpene hydrocarbons in different tissues of four individuals of Pinus sylvestris. Phytochemistry 1996, 41, 439–445. [Google Scholar] [CrossRef]
- Li, J.X.; Schieberle, P.; Steinhaus, M. Characterization of the major odor-active compounds in Thai durian (Durio zibethinus L. ‘Monthong’) by aroma extract dilution analysis and headspace gas chromatography–olfactometry. J. Agric. Food Chem. 2012, 60, 11253–11262. [Google Scholar] [CrossRef]
- Lytra, G.; Tempere, S.; Revel, D.G.; Barbe, J.C. Distribution and organoleptic impact of ethyl 2-methylbutanoate enantiomers in wine. J. Agric. Food Chem. 2014, 62, 5005–5010. [Google Scholar] [CrossRef]
- Frank, S.; Wollmann, N.; Schieberle, P.; Hofmann, T. Reconstitution of the flavor signature of Dornfelder red wine on the basis of the natural concentrations of its key aroma and taste compounds. J. Agric. Food Chem. 2011, 59, 8866–8874. [Google Scholar] [CrossRef] [PubMed]
- Matheis, K.; Granvogl, M.; Schieberle, P. Quantitation and enantiomeric ratios of aroma compounds formed by an Ehrlich degradation of L-isoleucine in fermented foods. J. Agric. Food Chem. 2016, 64, 646–652. [Google Scholar] [CrossRef]
- Jagella, T.; Grosch, W. Flavour and off-flavour compounds of black and white pepper (Piper nigrum L.) III. Desirable and undesirable odorants of white pepper. Eur. Food Res. Technol. 1999, 209, 27–31. [Google Scholar] [CrossRef]
- Kreissl, J.; Mall, V.; Steinhaus, P.; Steinhaus, M. Leibniz-LSB@TUM Odorant Database, version 1.2; Leibniz Institute for Food Systems Biology at the Technical University of Munich: Freising, Germany, 2022. Available online: https://www.leibniz-lsb.de/en/databases/leibniz-lsbtum-odorant-database (accessed on 8 August 2025).
- Steinhaus, M. Confirmation of 1-phenylethane-1-thiol as the character impact aroma compound in curry leaves and its behavior during tissue disruption, drying, and frying. J. Agric. Food Chem. 2017, 65, 2141–2146. [Google Scholar] [CrossRef]
- Pan, X.; Li, H.; Chen, D.; Zheng, J.; Yin, L.; Zou, J.; Zhang, Y.; Deng, K.; Xiao, M.; Meng, L.; et al. Comparison of essential oils of Houttuynia cordata Thunb. from different processing methods and harvest seasons based on GC–MS and chemometric analysis. Int. J. Anal. Chem. 2021, 1, 8324169. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Luo, S.; Ma, J.; Wu, D.; Hong, L.; Yu, Z. GC-MS analyses of the volatiles of Houttuynia cordata Thunb. Pak. J. Pharm. Sci. 2016, 29, 1591–1600. [Google Scholar]
- Asakawa, Y.; Tomiyama, K.; Sakurai, K.; Kawakami, Y.; Yaguchi, Y. Volatile compounds from the different organs of Houttuynia cordata and Litsea cubeba (L. citriodora). J. Oleo Sci. 2017, 66, 889–895. [Google Scholar] [CrossRef]
- Lin, C.H.; Chao, L.K.; Lin, L.Y.; Wu, C.S.; Chu, L.P.; Huang, C.H.; Chen, H.C. Analysis of volatile compounds from different parts of Houttuynia cordata Thunb. Molecules 2022, 27, 8893. [Google Scholar] [CrossRef]
- Liang, M.; Qi, M.; Zhang, C.; Zhou, S.; Fu, R.; Huang, J. Gas chromatography-mass spectrometry analysis of volatile compounds from Houttuynia cordata Thunb after extraction by solid-phase microextraction, flash evaporation and steam distillation. Anal. Chim. Acta 2005, 531, 97–104. [Google Scholar] [CrossRef]
- Xu, Y.W.; Liu, L.; Zhao, D.; Zou, Y.T.; Zeng, J.W.; Wu, W. Aliphatic aldehyde rich volatile constituents of Houttuynia cordata from southwest China. J. Med. Plant Res. 2011, 5, 5844–5847. [Google Scholar]
- Lu, H.; Wu, X.; Liang, Y.; Zhang, J. Variation in chemical composition and antibacterial activities of essential oils from two species of Houttuynia THUNB. Chem. Pharm. Bull. 2006, 54, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Steinhaus, M. Gas chromatography–olfactometry: Principles, practical aspects and applications in food analysis. In Advanced Gas Chromatography in Food Analysis; Tranchida, P.Q., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2019; pp. 337–399. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Y.; Cao, S. Effect of trace water on enzyme catalysis in organic solvents and its control method. Prog. Nat. Sci. 2002, 12, 130–134. [Google Scholar]
- Ni, X.L.; Peng, L.; Liu, W.Z. Structures, components and functions of secretory tissues in Houttuynia cordata. J. Integr. Plant Biol. 2007, 49, 1734–1745. [Google Scholar] [CrossRef]
- Steinhaus, M.; Sinuco, D.; Polster, J.; Osario, C.; Schieberle, P. Characterization of the aroma-active compounds in pink guava (Psidium guajava L.) by application of the aroma extract dilution analysis. J. Agric. Food Chem. 2008, 56, 4120–4127. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A. 1963, 11, 463–471. [Google Scholar] [CrossRef]


| Odorant | Replicate 1 | Extract Portion 2 | Concentration (μg/kg) 3 | Concentration (%) 4 | |||
|---|---|---|---|---|---|---|---|
| Rhizomes | Leaves | Rhizomes | Leaves | ||||
| α-pinene | 1 | 1 | 143,097 | 12,628 | 100 | 100 | |
| 2 | 0 | 0 | 0 | 0 | |||
| 3 | 0 | 0 | 0 | 0 | |||
| 2 | 1 | 152,590 | 11,728 | 100 | 100 | ||
| 2 | 0 | 0 | 0 | 0 | |||
| 3 | 0 | 0 | 0 | 0 | |||
| 3 | 1 | 161,436 | 15,378 | 100 | 100 | ||
| 2 | 0 | 0 | 0 | 0 | |||
| 3 | 0 | 0 | 0 | 0 | |||
| myrcene | 1 | 1 | 103,576 | 139,970 | 100 | 100 | |
| 2 | 0 | 0 | 0 | 0 | |||
| 3 | 0 | 0 | 0 | 0 | |||
| 2 | 1 | 94,347 | 137,496 | 100 | 100 | ||
| 2 | 0 | 0 | 0 | 0 | |||
| 3 | 0 | 0 | 0 | 0 | |||
| 3 | 1 | 135,502 | 141,241 | 100 | 100 | ||
| 2 | 0 | 0 | 0 | 0 | |||
| 3 | 0 | 0 | 0 | 0 | |||
| limonene | 1 | 1 | 40,709 | 230 | 100 | 100 | |
| 2 | 0 | 0 | 0 | 0 | |||
| 3 | 0 | 0 | 0 | 0 | |||
| 2 | 1 | 40,055 | 169 | 100 | 100 | ||
| 2 | 0 | 0 | 0 | 0 | |||
| 3 | 0 | 0 | 0 | 0 | |||
| 3 | 1 | 45,106 | 178 | 100 | 100 | ||
| 2 | 0 | 0 | 0 | 0 | |||
| 3 | 0 | 0 | 0 | 0 | |||
| 3-oxododecanal | 1 | 1 | 2,377,222 | 1,266,578 | 100 | 100 | |
| 2 | 0 | 0 | 0 | 0 | |||
| 3 | 0 | 0 | 0 | 0 | |||
| 2 | 1 | 2,406,408 | 1,304,849 | 100 | 100 | ||
| 2 | 0 | 0 | 0 | 0 | |||
| 3 | 0 | 0 | 0 | 0 | |||
| 3 | 1 | 2,662,308 | 1,453,164 | 100 | 100 | ||
| 2 | 0 | 0 | 0 | 0 | |||
| 3 | 0 | 0 | 0 | 0 | |||
| Odorant | Odor 1 | RI 2 | Enantiomeric Distribution 3 (%) in | |
|---|---|---|---|---|
| BGB-176 | Rhizomes | Leaves | ||
| (1S,5S)-α-pinene | resinous | 987 | 94 | 46 |
| (1R,5R)-α-pinene | resinous | 994 | 6 | 54 |
| ethyl (2R)-2-methylbutanoate | fruity | 873 | 0 | 0 |
| ethyl (2S)-2-methylbutanoate | fruity | 882 | 100 | 100 |
| (4S)-limonene | geranium leaf, citrusy | 1065 | 74 | 98 |
| (4R)-limonene | citrusy | 1076 | 26 | 2 |
| No. 1 | Odorant 2 | Concentration 3 (µg/kg) | OTC 4 (μg/kg) | OAV 5 | ||
|---|---|---|---|---|---|---|
| Rhizomes | Leaves | Rhizomes | Leaves | |||
| 7 | myrcene | 111,000 | 140,000 | 1.2 6 | 93,000 | 120,000 |
| 36 | 3-oxododecanal | 2,480,000 | 1,340,000 | 92 7 | 27,000 | 15,000 |
| 8a | (4S)-limonene 8 | 31,000 | 188 | 8.0 7 | 3900 | 24 |
| 12 | (5Z)-octa-1,5-dien-3-one | 1.16 | 1.05 | 0.00034 6 | 3400 | 3100 |
| 17 | decanal | 5070 | 13,600 | 9.3 6 | 550 | 1500 |
| 2b | (1R,5R)-α-pinene 8 | 9140 | 7150 | 9.0 9 | 1000 | 790 |
| 6 | (3Z)-hex-3-enal | 15.1 | 102 | 0.12 6 | 130 | 850 |
| 2a | (1S,5S)-α-pinene 8 | 143,000 | 6090 | 170 6 | 840 | 36 |
| 8b | (4R)-limonene 8 | 10,900 | 3.84 | 13 6 | 840 | <1 |
| 35 | (2E)-dodec-2-enal | 128 | 91.9 | 0.22 7 | 580 | 420 |
| 11 | oct-1-en-3-one | 4.7 | 5.38 | 0.016 6 | 290 | 340 |
| 3b | ethyl (2S)-2-methylbutanoate 8 | 0.149 | 1.64 | 0.0080 6 | 19 | 200 |
| 31 | geranyl acetate | 2050 | 4960 | 31 7 | 66 | 160 |
| 32 | (E)-β-damascenone | 0.109 | 0.826 | 0.0060 6 | 18 | 140 |
| 22 | undecan-2-one | 2040 | 686 | 24 10 | 85 | 29 |
| 28 | 3-methylnonane-2,4-dione | 0.885 | 2.41 | 0.046 6 | 19 | 52 |
| 33 | geraniol | 40.5 | 4.23 | 1.1 6 | 37 | 3.8 |
| 42 | eugenol | 59.1 | 16.1 | 1.8 6 | 33 | 9 |
| 45 | trans-isoeugenol | 15.9 | 13.2 | 0.716 | 22 | 19 |
| 16 | 3-(methylsulfanyl)propanal | 7.18 | 0.165 | 0.43 6 | 17 | <1 |
| 10 | octanal | 39.9 | 24.1 | 3.4 6 | 12 | 7.1 |
| 27 | (2E,4E)-nona-2,4-dienal | <0.025 | 0.286 | 0.046 6 | <1 | 6.2 |
| 40 | 4-methylphenol | 6.42 | 16.1 | 3.9 6 | 1.6 | 4.1 |
| 47 | vanillin | 149 | 13.9 | 53 6 | 2.8 | <1 |
| 38 | 4-methoxybenzaldehyde | 1.44 | 4.04 | 2.6 6 | <1 | 1.6 |
| 23 | butanoic acid | 1660 | 352 | 2400 6 | <1 | <1 |
| 37 | trans-4,5-epoxy-(2E)-dec-2-enal | 0.069 | 0.141 | 0.22 6 | <1 | <1 |
| Test | Odorants in the Incomplete Model 1 | Correct Answers/ Assessors 2 | p-Value (%) | Level of Significance |
|---|---|---|---|---|
| OR-A1 | 7, 36, 8a, 12, 2b, 2a, 8b, 35, 17, 11, 6 | 6/16 | 45 | not significant (p > 5%) |
| OR-A2 | 7, 36, 8a, 12, 2b | 8/16 | 13 | not significant (p > 5%) |
| OR-B1 | 36, 8a, 12, 2b | 7/19 | 46 | not significant (p > 5%) |
| OR-B2 | 7, 8a, 12, 2b | 16/19 | 0.00073 | very highly significant (p < 0.1%) |
| OR-B3 | 7, 36, 12, 2b | 7/19 | 46 | not significant (p > 5%) |
| OR-B4 | 7, 36, 8a, 2b | 9/19 | 15 | not significant (p > 5%) |
| OR-B5 | 7, 36, 8a, 12 | 10/19 | 6.5 | not significant (p > 5%) |
| Test | Odorants in the Incomplete Model 1 | Correct Answers/ Assessors 2 | p-Value (%) | Level of Significance |
|---|---|---|---|---|
| OL-A1 | 7, 36, 12, 17, 6, 2b, 35, 11, 3b, 31, 32 | 5/16 | 66 | not significant (p > 5%) |
| OL-A2 | 7, 36, 12, 17, 6 | 8/15 | 8.8 | not significant (p > 5%) |
| OL-B1 | 36, 12, 17, 6 | 14/20 | 0.088 | very highly significant (p < 0.1%) |
| OL-B2 | 7, 12, 17, 6 | 18/20 | 0.000023 | very highly significant (p < 0.1%) |
| OL-B3 | 7, 36, 17, 6 | 6/20 | 70 | not significant (p > 5%) |
| OL-B4 | 7, 36, 12, 6 | 7/20 | 52 | not significant (p > 5%) |
| OL-B5 | 7, 36, 12, 17 | 9/20 | 19 | not significant (p > 5%) |
| Odorant | Concentration (μg/kg) | |
|---|---|---|
| Rhizome Strips 1 | Whole Rhizomes 2 | |
| α-pinene | 152,000 ± 9200 | 162,000 ± 19,000 |
| myrcene | 111,000 ± 22,000 | 129,000 ± 33,000 |
| limonene | 42,000 ± 2700 | 45,000 ± 5200 |
| 3-oxododecanal | 2,480,000 ± 160,000 | 2,760,000 ± 250,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Oellig, C.; Vetter, W.; Steinhaus, M.; Frank, S. Key Odorant Identification Confirms 3-Oxododecanal as the Most Important Contributor to the Characteristic Aroma of Fresh Rhizomes and Leaves of Houttuynia cordata. Foods 2025, 14, 3147. https://doi.org/10.3390/foods14183147
Xu Z, Oellig C, Vetter W, Steinhaus M, Frank S. Key Odorant Identification Confirms 3-Oxododecanal as the Most Important Contributor to the Characteristic Aroma of Fresh Rhizomes and Leaves of Houttuynia cordata. Foods. 2025; 14(18):3147. https://doi.org/10.3390/foods14183147
Chicago/Turabian StyleXu, Zhenli, Claudia Oellig, Walter Vetter, Martin Steinhaus, and Stephanie Frank. 2025. "Key Odorant Identification Confirms 3-Oxododecanal as the Most Important Contributor to the Characteristic Aroma of Fresh Rhizomes and Leaves of Houttuynia cordata" Foods 14, no. 18: 3147. https://doi.org/10.3390/foods14183147
APA StyleXu, Z., Oellig, C., Vetter, W., Steinhaus, M., & Frank, S. (2025). Key Odorant Identification Confirms 3-Oxododecanal as the Most Important Contributor to the Characteristic Aroma of Fresh Rhizomes and Leaves of Houttuynia cordata. Foods, 14(18), 3147. https://doi.org/10.3390/foods14183147







