Effects of Consuming Ultraviolet Light-Exposed Mushrooms on Self-Reported Indices of Brain Health and Performance-Based Cognition in Middle-Aged and Older Adults
Abstract
1. Introduction
2. Materials and Methods
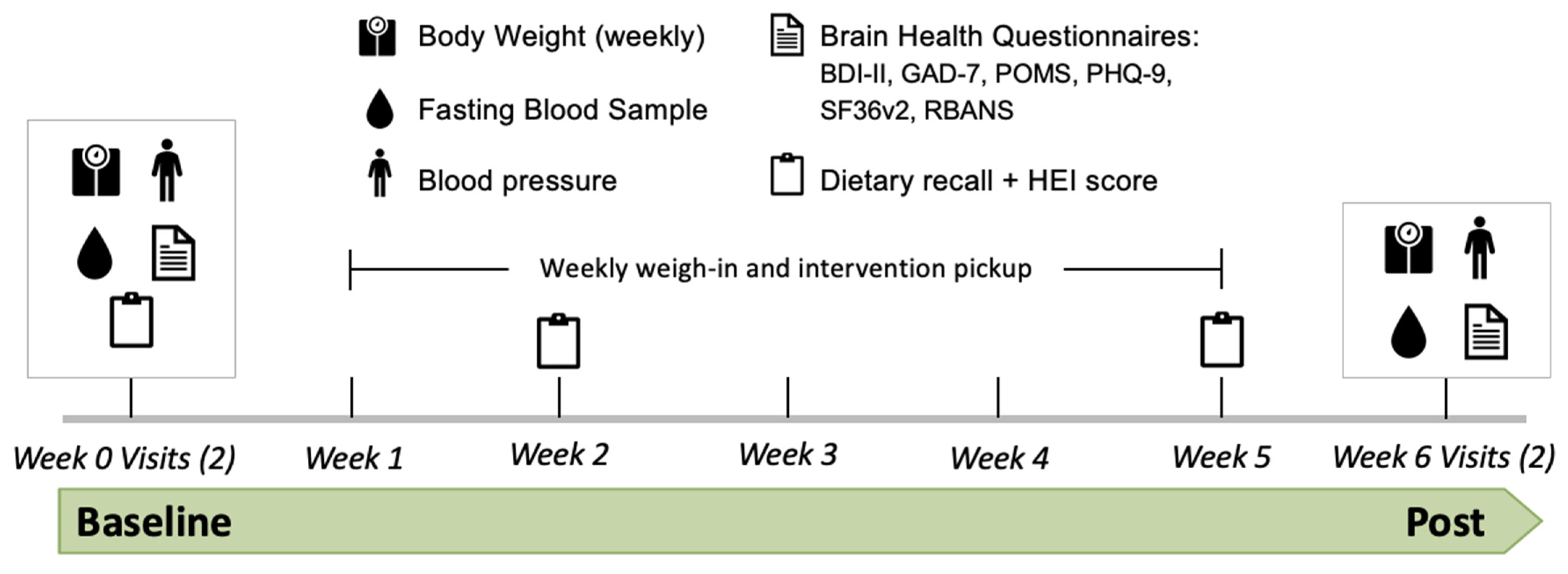
2.1. Experimental Design
2.2. Participant Inclusion Criteria
2.3. Study Intervention and Dietary Assessment
2.4. Clinical Assessments
2.4.1. Assessment of Symptoms of Generalized Anxiety Disorder
2.4.2. Assessment of Symptoms of Depression
2.4.3. Assessment of Mood
2.4.4. Assessment of Perceived Quality of Life
2.4.5. Neuropsychological Assessment
2.5. Statistical Analysis
3. Results
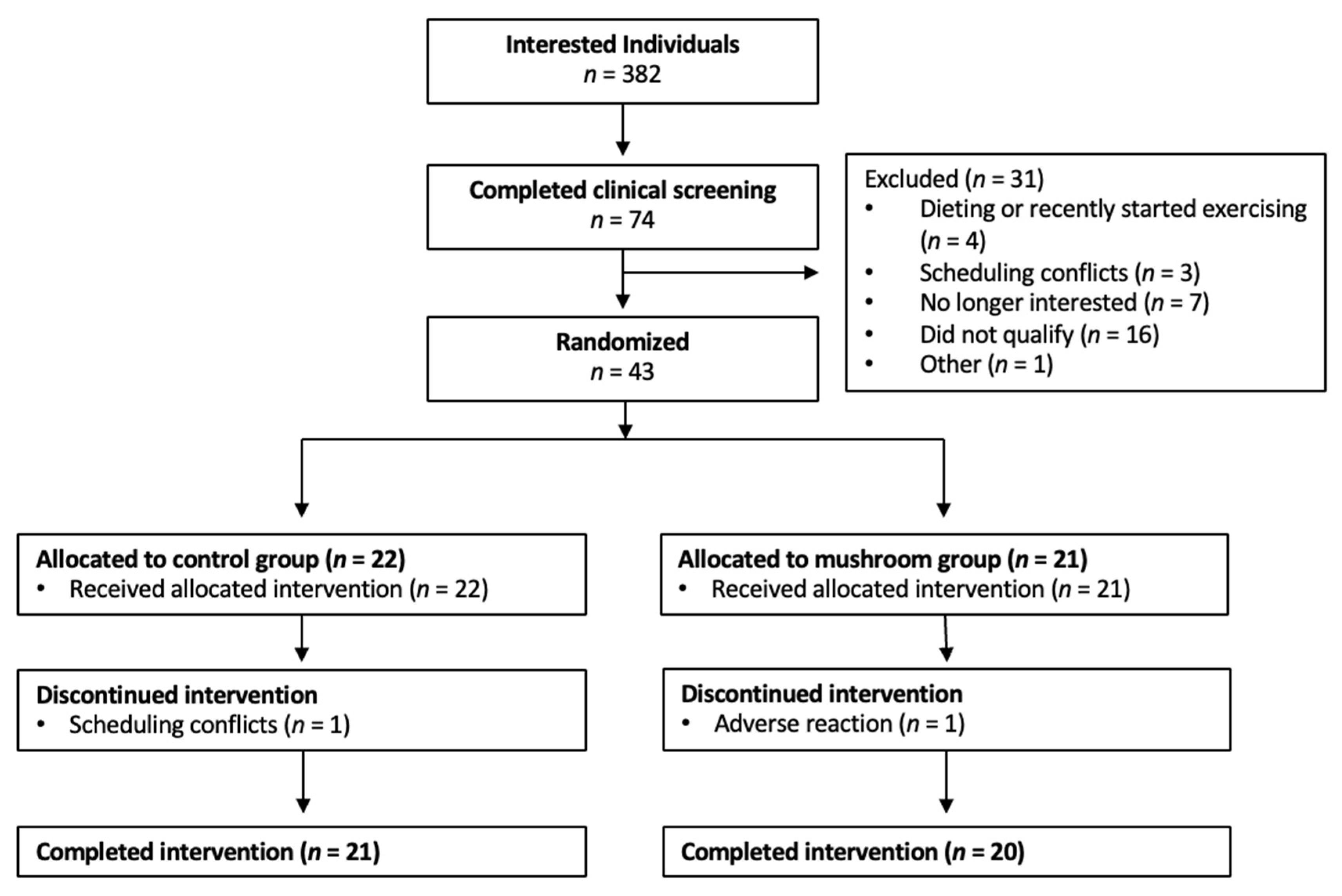
3.1. Participants
3.2. Dietary Assessment and Adherence to the Dietary Intervention
3.3. Anxiety and Depression
3.4. Mood
3.5. Perceived Quality of Life
3.6. Neuropsychological Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ejtahed, H.-S.; Mardi, P.; Hejrani, B.; Mahdavi, F.S.; Ghoreshi, B.; Gohari, K.; Heidari-Beni, M.; Qorbani, M. Association between junk food consumption and mental health problems in adults: A systematic review and meta-analysis. BMC Psychiatry 2024, 24, 438. [Google Scholar] [CrossRef]
- Ferreiro, A.L.; Choi, J.H.; Ryou, J.; Newcomer, E.P.; Thompson, R.; Bollinger, R.M.; Hall-Moore, C.; Ndao, I.M.; Sax, L.; Benzinger, T.L.S.; et al. Gut microbiome composition may be an indicator of preclinical Alzheimer’s disease. Sci. Transl. Med. 2023, 15, eabo2984. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, R.; Selvamani, T.Y.; Zahra, A.; Malla, J.; Dhanoa, R.K.; Venugopal, S.; Shoukrie, S.I.; Hamouda, R.K.; Hamid, P. Association Between Dietary Habits and Depression: A Systematic Review. Cureus 2022, 14, e32359. Available online: https://www.cureus.com/articles/91449-association-between-dietary-habits-and-depression-a-systematic-review (accessed on 6 March 2024). [CrossRef] [PubMed]
- Konishi, K. Associations between healthy Japanese dietary patterns and depression in Japanese women. Public Health Nutr. 2021, 24, 1753–1765. [Google Scholar] [CrossRef]
- Nanri, A.; Mizoue, T.; Poudel-Tandukar, K.; Noda, M.; Kato, M.; Kurotani, K.; Goto, A.; Oba, S.; Inoue, M.; Tsugane, S.; et al. Dietary patterns and suicide in Japanese adults: The Japan Public Health Center-based Prospective Study. Br. J. Psychiatry 2013, 203, 422–427. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, M.-S.; Lee, H.-J. The association between dietary pattern and depression in middle-aged Korean adults. Nutr. Res. Pract. 2019, 13, 316. [Google Scholar] [CrossRef]
- Feeney, M.J.; Miller, A.M.; Roupas, P. Mushrooms—Biologically Distinct and Nutritionally Unique: Exploring a “Third Food Kingdom”. Nutr. Today 2014, 49, 301–307. [Google Scholar] [CrossRef]
- Torres, L.L.; Quaglio, N.B.; De Souza, G.T.; Garcia, R.T.; Dati, L.M.M.; Moreira, W.L.; de Melo Loureiro, A.P.; de Souza-Talarico, J.N.; Smid, J.; Porto, C.S.; et al. Peripheral Oxidative Stress Biomarkers in Mild Cognitive Impairment and Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 26, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Kato, Y. Ergothioneine in the brain. FEBS Lett. 2022, 596, 1290–1298. [Google Scholar] [CrossRef]
- Halliwell, B.; Cheah, I.K.; Drum, C.L. Ergothioneine, an adaptive antioxidant for the protection of injured tissues? A hypothesis. Biochem. Biophys. Res. Commun. 2016, 470, 245–250. [Google Scholar] [CrossRef]
- Cheah, I.K.; Feng, L.; Tang, R.M.Y.; Lim, K.H.C.; Halliwell, B. Ergothioneine levels in an elderly population decrease with age and incidence of cognitive decline; a risk factor for neurodegeneration? Biochem. Biophys. Res. Commun. 2016, 478, 162–167. [Google Scholar] [CrossRef]
- Katsube, M.; Ishimoto, T.; Fukushima, Y.; Kagami, A.; Shuto, T.; Kato, Y. Ergothioneine promotes longevity and healthy aging in male mice. GeroScience 2024, 46, 3889–3909. [Google Scholar] [CrossRef]
- Mandal, P.K.; Goel, A.; Bush, A.I.; Punjabi, K.; Joon, S.; Mishra, R.; Tripathi, M.; Garg, A.; Kumar, N.K.; Sharma, P.; et al. Hippocampal glutathione depletion with enhanced iron level in patients with mild cognitive impairment and Alzheimer’s disease compared with healthy elderly participants. Brain Commun. 2022, 4, fcac215. [Google Scholar] [CrossRef]
- Park, S.K.; Oh, C.-M.; Ryoo, J.-H.; Jung, J.Y. The protective effect of mushroom consumption on depressive symptoms in Korean population. Sci. Rep. 2022, 12, 21914. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Cheah, I.K.-M.; Ng, M.M.-X.; Li, J.; Chan, S.M.; Lim, S.L.; Mahendran, R.; Kua, E.-H.; Halliwell, B. The Association between Mushroom Consumption and Mild Cognitive Impairment: A Community-Based Cross-Sectional Study in Singapore. J. Alzheimer’s Dis. 2019, 68, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Inatomi, S.; Ouchi, K.; Azumi, Y.; Tuchida, T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: A double-blind placebo-controlled clinical trial. Phytother. Res. 2009, 23, 367–372. [Google Scholar] [CrossRef]
- Saitsu, Y.; Nishide, A.; Kikushima, K.; Shimizu, K.; Ohnuki, K. Improvement of cognitive functions by oral intake of Hericium erinaceus. Biomed. Res. 2019, 40, 125–131. [Google Scholar] [CrossRef]
- Cha, S.; Bell, L.; Shukitt-Hale, B.; Williams, C.M. A review of the effects of mushrooms on mood and neurocognitive health across the lifespan. Neurosci. Biobehav. Rev. 2024, 158, 105548. [Google Scholar] [CrossRef] [PubMed]
- Uffelman, C.N.; Harold, R.; Hodson, E.S.; Chan, N.I.; Foti, D.; Campbell, W.W. Effects of Consuming White Button and Oyster Mushrooms within a Healthy Mediterranean-Style Dietary Pattern on Changes in Subjective Indexes of Brain Health or Cognitive Function in Healthy Middle-Aged and Older Adults. Foods 2024, 13, 2319. [Google Scholar] [CrossRef]
- Musazadeh, V.; Keramati, M.; Ghalichi, F.; Kavyani, Z.; Ghoreishi, Z.; Alras, K.A.; Albadawi, N.; Salem, A.; Albadawi, M.I.; Salem, R.; et al. Vitamin D protects against depression: Evidence from an umbrella meta-analysis on interventional and observational meta-analyses. Pharmacol. Res. 2023, 187, 106605. [Google Scholar] [CrossRef] [PubMed]
- Anglin, R.E.S.; Samaan, Z.; Walter, S.D.; McDonald, S.D. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 100–107. [Google Scholar] [CrossRef]
- Annweiler, C.; Annweiler, T.; Montero-Odasso, M.; Bartha, R.; Beauchet, O. Vitamin D and brain volumetric changes: Systematic review and meta-analysis. Maturitas 2014, 78, 30–39. [Google Scholar] [CrossRef]
- Navale, S.S.; Mulugeta, A.; Zhou, A.; Llewellyn, D.J.; Hyppönen, E. Vitamin D and brain health: An observational and Mendelian randomization study. Am. J. Clin. Nutr. 2022, 116, 531–540. [Google Scholar] [CrossRef]
- Outila, T.A.; Mattila, P.H.; Piironen, V.I.; Lamberg-Allardt, C.J. Bioavailability of vitamin D from wild edible mushrooms (Cantharellus tubaeformis) as measured with a human bioassay. Am. J. Clin. Nutr. 1999, 69, 95–98. [Google Scholar] [CrossRef]
- Keegan, R.-J.H.; Lu, Z.; Bogusz, J.M.; Williams, J.E.; Holick, M.F. Photobiology of vitamin D in mushrooms and its bioavailability in humans. Derm. Endocrinol. 2013, 5, 165–176. [Google Scholar] [CrossRef]
- Zajac, I.T.; Barnes, M.; Cavuoto, P.; Wittert, G.; Noakes, M. The Effects of Vitamin D-Enriched Mushrooms and Vitamin D3 on Cognitive Performance and Mood in Healthy Elderly Adults: A Randomised, Double-Blinded, Placebo-Controlled Trial. Nutrients 2020, 12, 3847. [Google Scholar] [CrossRef]
- Generalised Anxiety Disorder Assessment (GAD-7). Available online: https://www.corc.uk.net/outcome-experience-measures/generalised-anxiety-disorder-assessment-gad-7/ (accessed on 23 March 2025).
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II. 2011. Available online: https://doi.apa.org/doi/10.1037/t00742-000 (accessed on 24 June 2024).
- Wang, Y.-P.; Gorenstein, C. Psychometric properties of the Beck Depression Inventory-II: A comprehensive review. Rev. Bras. Psiquiatr. 2013, 35, 416–431. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Curran, S.L.; Andrykowski, M.A.; Studts, J.L. Short Form of the Profile of Mood States (POMS-SF): Psychometric Information. Psychol. Assess. 1995, 7, 80–83. [Google Scholar] [CrossRef]
- Randolph, C. Repeatable Battery for the Assessment of Neuropsychological Status Update; Pearson: London, UK, 2012; Available online: https://www.pearsonassessments.com/store/usassessments/en/Store/Professional-Assessments/Cognition-%26-Neuro/Repeatable-Battery-for-the-Assessment-of-Neuropsychological-Status-Update/p/100000726.html (accessed on 24 June 2024).
- Shura, R.D.; Brearly, T.W.; Rowland, J.A.; Martindale, S.L.; Miskey, H.M.; Duff, K. RBANS Validity Indices: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2018, 28, 269–284. [Google Scholar] [CrossRef]
- Gualtierotti, R.; Bressi, C.; Garavaglia, B.; Brambilla, P. Exploring the Impact of Sex and Gender in Brain Function: Implications and Considerations. Adv. Ther. 2024, 41, 4377–4383. [Google Scholar] [CrossRef]
- Docherty, S.; Doughty, F.L.; Smith, E.F. The Acute and Chronic Effects of Lion’s Mane Mushroom Supplementation on Cognitive Function, Stress and Mood in Young Adults: A Double-Blind, Parallel Groups, Pilot Study. Nutrients 2023, 15, 4842. [Google Scholar] [CrossRef]
- Vigna, L.; Morelli, F.; Agnelli, G.M.; Napolitano, F.; Ratto, D.; Occhinegro, A.; Di Iorio, C.; Savino, E.; Girometta, C.; Brandalise, F.; et al. Hericium erinaceus Improves Mood and Sleep Disorders in Patients Affected by Overweight or Obesity: Could Circulating Pro-BDNF and BDNF Be Potential Biomarkers? Evid. Based Complement. Altern. Med. 2019, 2019, 7861297. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Shimizu, K.; Kondo, R.; Hayashi, C.; Sato, D.; Kitagawa, K.; Ohnuki, K. Reduction of depression and anxiety by 4 weeks Hericium erinaceus intake. Biomed. Res. 2010, 31, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Anno, N.; Tsuda, A.; Inokuchi, T.; Uchimura, N.; Inanaga, K. The effects of Hericium erinaceus (Amyloban® 3399) on sleep quality and subjective well-being among female undergraduate students: A pilot study. Pers. Med. Universe 2015, 4, 76–78. [Google Scholar] [CrossRef]
- Grozier, C.D.; Alves, V.A.; Killen, L.G.; Simpson, J.D.; O’neal, E.K.; Waldman, H.S. Four Weeks of Hericium erinaceus Supplementation Does Not Impact Markers of Metabolic Flexibility or Cognition. Int. J. Exerc. Sci. 2022, 15, 1366. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Wang, C.; Qin, L. Spore powder of Ganoderma lucidum for the treatment of Alzheimer disease. Medicine 2018, 97, e0636. [Google Scholar] [CrossRef]
- Li, I.-C.; Chang, H.-H.; Lin, C.-H.; Chen, W.-P.; Lu, T.-H.; Lee, L.-Y.; Chen, Y.-W.; Chen, Y.-P.; Chen, C.-C.; Lin, D.P.-C. Prevention of Early Alzheimer’s Disease by Erinacine A-Enriched Hericium erinaceus Mycelia Pilot Double-Blind Placebo-Controlled Study. Front. Aging Neurosci. 2020, 12, 155. [Google Scholar] [CrossRef]
| Demographic Characteristics | Control (n = 21) | Mushroom (n = 20) | Total (n = 41) |
|---|---|---|---|
| Age (y) | 42.2 ± 11.2 | 44.0 ± 11.1 | 43.1 ± 11.1 |
| Female, n (%) | 12 (57) | 10 (50) | 22 (54) |
| White, n (%) | 11 (52) | 15 (75) | 26 (63) |
| Hispanic or Latino, n (%) | 5 (24) | 2 (10) | 7 (17) |
| Asian, n (%) | 4 (19) | 3 (15) | 7 (17) |
| Black, n (%) | 1 (5) | 1 (5) | 2 (5) |
| Other (not specified/not reported), n (%) | 5 (24) | 1 (5) | 6 (15) |
| Weight (kg) | 82.3 ± 10.0 | 87.3 ± 15.6 | 84.7 ± 13.1 |
| BMI (kg/m2) | 29.9 ± 7.9 | 29.7 ± 2.7 | 29.8 ± 5.9 |
| Fasted clinical characteristics | |||
| 25(OH)D3 (ng/mL) | 23.3 ± 7.5 | 21.7 ± 5.6 | 22.5 ± 6.6 |
| 25(OH)D2 (ng/mL) | 0.2 ± 0.9 | 0.0 ± 0.0 | 0.1 ± 0.6 |
| * Total 25OHD (ng/mL) | 23.5 ± 7.8 | 21.7 ± 5.6 | 22.6 ± 6.8 |
| BUN (mg/dL) | 15.1 ± 5.0 | 12.3 ± 3.0 | 15.1 ± 4.3 |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| BUN/Creatinine ratio | 15.1 ± 5.0 | 15.1 ± 3.4 | 15.1 ± 4.3 |
| eGFR (mL/min/1.73 m2) | 98.1 ± 13.0 | 101.6 ± 13.3 | 99.7 ± 13.1 |
| ALT (U/L) | 19.1 ± 13.7 | 18.6 ± 9.4 | 18.9 ± 11.7 |
| AST (U/L) | 19.6 ± 7.3 | 20.1 ± 5.2 | 19.8 ± 6.3 |
| Depression Characteristics | |||
| Beck’s Depression Inventory (0–63) | 5.14 ± 7.04 | 5.25 ± 4.79 | 5.20 ± 6.0 |
| Levels of depression, n (%) | |||
| Normal (1–10) | 18 (86) | 17 (85) | 35 (85) |
| Mild Mood Disturbance (11–16) | 2 (10) | 3 (15) | 5 (12) |
| Borderline Clinical Depression (17–20) | 0 (0) | 0 (0) | 0 (0) |
| Moderate Depression (21–30) | 1 (5) | 0 (0) | 1 (2) |
| Severe Depression (31–40) | 0 (0) | 0 (0) | 0 (0) |
| Extreme Depression (>40) | 0 (0) | 0 (0) | 0 (0) |
| Timepoint | Mushroom | Control | Mushroom + Control | p-Value |
|---|---|---|---|---|
| Baseline | 50 ± 15.3 | 55 ± 15.3 | 53 ± 15.4 | 0.288 |
| Intervention | 50 ± 10.9 | 52 ± 14.1 | 51 ± 12.5 | 0.730 |
| Baseline + Intervention | 50 ± 10.3 | 53 ± 12.1 | 52 ± 11.2 | 0.475 |
| Control (n = 21) | Mushroom (n = 20) | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Baseline | Week 6 | Change | Baseline | Week 6 | Change | Time | Time × Group |
| Generalized Anxiety Disorder-7 (0–21) | 3.10 ± 0.92 | 2.33 ± 0.57 | −0.65 ± 0.72 | 2.70 ± 0.67 | 2.30 ± 0.79 | −0.40 ± 0.50 | 0.200 | 0.698 |
| Beck’s Depression Inventory (0–63) | 5.14 ± 1.54 | 4.90 ± 1.43 | −0.24 ± 0.47 | 5.25 ± 1.07 | 3.40 ± 0.82 | −1.85 ± 0.75 | 0.019 * | 0.092 |
| Patient Health Questionnaire-9 (0–27) | 3.48 ± 0.70 | 3.10 ± 0.77 | −0.38 ± 0.51 | 3.45 ± 0.70 | 3.15 ± 0.81 | −0.30 ± 0.51 | 0.277 | 0.796 |
| Control (n = 21) | Mushroom (n = 20) | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|
| Mood | Baseline | Week 6 | Change | Baseline | Week 6 | Change | Time | Time × Group |
| Depression (0–32) | 1.10 ± 0.44 | 1.76 ± 0.54 | 0.67 ± 0.52 | 1.35 ± 0.42 | 1.70 ± 0.82 | 0.35 ± 0.89 | 0.389 | 0.853 |
| Vigor (0–24) | 10.3 ± 1.43 | 11.8 ± 1.44 | 1.43 ± 1.07 | 10.1 ± 1.39 | 10.0 ± 1.25 | −0.63 ± 0.98 | 0.484 | 0.100 |
| Confusion (0–20) | 1.71 ± 0.40 | 1.95 ± 0.41 | 0.24 ± 0.31 | 2.20 ± 0.47 | 2.15 ± 0.51 | −0.05 ± 0.46 | 0.685 | 0.560 |
| Tension (0–24) | 1.75 ± 0.48 | 2.10 ± 0.55 | 0.45 ± 0.57 | 1.45 ± 0.41 | 2.10 ± 0.79 | 0.65 ± 0.37 | 0.251 | 0.821 |
| Fatigue (0–20) | 2.90 ± 0.74 | 3.57 ± 0.82 | 0.70 ± 0.38 | 3.45 ± 0.76 | 3.35 ± 0.69 | −0.10 ± 0.45 | 0.293 | 0.482 |
| Anger (0–48) | 0.60 ± 0.27 | 0.95 ± 0.43 | 0.35 ± 0.52 | 0.75 ± 0.37 | 1.80 ± 0.70 | 1.05 ± 0.71 | 0.145 | 0.373 |
| Control (n = 21) | Mushroom (n = 20) | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|
| SF-36v2 Scale (0–100) | Baseline | Week 6 | Change | Baseline | Week 6 | Change | Time | Time × Group |
| Physical Functioning | 91.0 ± 2.38 | 89.8 ± 2.70 | −1.25 ± 2.40 | 91.0 ± 3.66 | 91.1 ± 2.95 | 0.53 ± 1.57 | 0.939 | 0.679 |
| Physical RL | 91.3 ± 4.54 | 88.1 ± 6.13 | −3.75 ± 5.22 | 92.5 ± 5.47 | 92.5 ± 3.19 | 0.00 ± 6.28 | 0.757 | 0.757 |
| Emotional RL | 88.9 ± 5.79 | 80.9 ± 7.12 | −7.94 ± 6.04 | 83.3 ± 6.17 | 75.0 ± 8.33 | −8.33 ± 7.20 | 0.115 | 0.884 |
| Energy/Fatigue | 60.7 ± 4.49 | 60.7 ± 4.66 | 0.00 ± 2.60 | 63.5 ± 4.81 | 63.3 ± 5.35 | −0.25 ± 2.53 | 0.864 | 0.751 |
| Emotional Well-being | 78.1 ± 3.62 | 78.1 ± 2.86 | 0.00 ± 2.19 | 78.8 ± 2.84 | 80.8 ± 3.33 | 2.00 ± 3.04 | 0.502 | 0.687 |
| Social Functioning | 91.7 ± 3.03 | 92.3 ± 3.17 | 0.60 ± 2.20 | 90.6 ± 2.99 | 91.9 ± 3.30 | 1.25 ± 3.50 | 0.584 | 0.960 |
| Pain | 79.9 ± 4.54 | 82.4 ± 4.23 | 2.50 ± 5.21 | 86.1 ± 3.87 | 87.8 ± 4.59 | 1.62 ± 2.36 | 0.550 | 0.957 |
| General Health | 72.8 ± 3.69 | 71.0 ± 3.56 | −1.90 ± 2.79 | 72.0 ± 3.78 | 75.8 ± 2.91 | 3.75 ± 2.43 | 0.564 | 0.157 |
| Control (n = 21) | Mushroom (n = 20) | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Baseline | Week 6 | Change | Baseline | Week 6 | Change | Time | Time × Group |
| Immediate Memory | 103.8 ± 3.21 | 109.5 ± 4.17 | 6.76 ± 3.12 | 99.6 ± 2.81 | 108.0 ± 2.40 | 8.45 ± 2.66 | <0.001 *** | 0.616 |
| Visuospatial/Constructional | 84.4 ± 2.59 | 83.5 ± 3.18 | −0.95 ± 2.63 | 88.5 ± 3.18 | 88.8 ± 3.34 | 0.30 ± 2.63 | 0.852 | 0.728 |
| Language | 98.6 ± 3.23 | 101.1 ± 3.68 | 2.52 ± 3.02 | 95.5 ± 3.34 | 101.9 ± 2.50 | 6.40 ± 2.73 | 0.035 * | 0.339 |
| Attention | 103.8 ± 3.03 | 106.1 ± 3.63 | 2.29 ± 2.41 | 106.6 ± 3.68 | 110.6 ± 3.89 | 4.00 ± 2.61 | 0.074 | 0.705 |
| Delayed Memory | 98.6 ± 2.06 | 98.6 ± 2.99 | 0.00 ± 2.53 | 98.1 ± 1.50 | 100.2 ± 2.38 | 2.10 ± 1.90 | 0.610 | 0.437 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glover, E.S.; Napolitano, S.C.; Comboni, L.M.; Fleet, J.C.; Olson, M.R.; Foti, D.; Campbell, W.W. Effects of Consuming Ultraviolet Light-Exposed Mushrooms on Self-Reported Indices of Brain Health and Performance-Based Cognition in Middle-Aged and Older Adults. Foods 2025, 14, 3148. https://doi.org/10.3390/foods14183148
Glover ES, Napolitano SC, Comboni LM, Fleet JC, Olson MR, Foti D, Campbell WW. Effects of Consuming Ultraviolet Light-Exposed Mushrooms on Self-Reported Indices of Brain Health and Performance-Based Cognition in Middle-Aged and Older Adults. Foods. 2025; 14(18):3148. https://doi.org/10.3390/foods14183148
Chicago/Turabian StyleGlover, Emily S., Skye C. Napolitano, Luz M. Comboni, James C. Fleet, Matthew R. Olson, Dan Foti, and Wayne W. Campbell. 2025. "Effects of Consuming Ultraviolet Light-Exposed Mushrooms on Self-Reported Indices of Brain Health and Performance-Based Cognition in Middle-Aged and Older Adults" Foods 14, no. 18: 3148. https://doi.org/10.3390/foods14183148
APA StyleGlover, E. S., Napolitano, S. C., Comboni, L. M., Fleet, J. C., Olson, M. R., Foti, D., & Campbell, W. W. (2025). Effects of Consuming Ultraviolet Light-Exposed Mushrooms on Self-Reported Indices of Brain Health and Performance-Based Cognition in Middle-Aged and Older Adults. Foods, 14(18), 3148. https://doi.org/10.3390/foods14183148





