Effect of Food Matrix and Administration Timing on the Survival of Lactobacillus rhamnosus GG During In Vitro Gastrointestinal Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Probiotic Culture, Pasta, and Soy Milk
2.2. Chemicals for Simulated Static In Vitro Digestion
2.3. In Vitro Static Digestion
2.4. Microbiological Analysis
2.5. pH Measurement
2.6. Starch Hydrolysis of Pasta
2.7. Protein Digestibility of Soy Milk
2.8. Statistical Analysis
3. Results
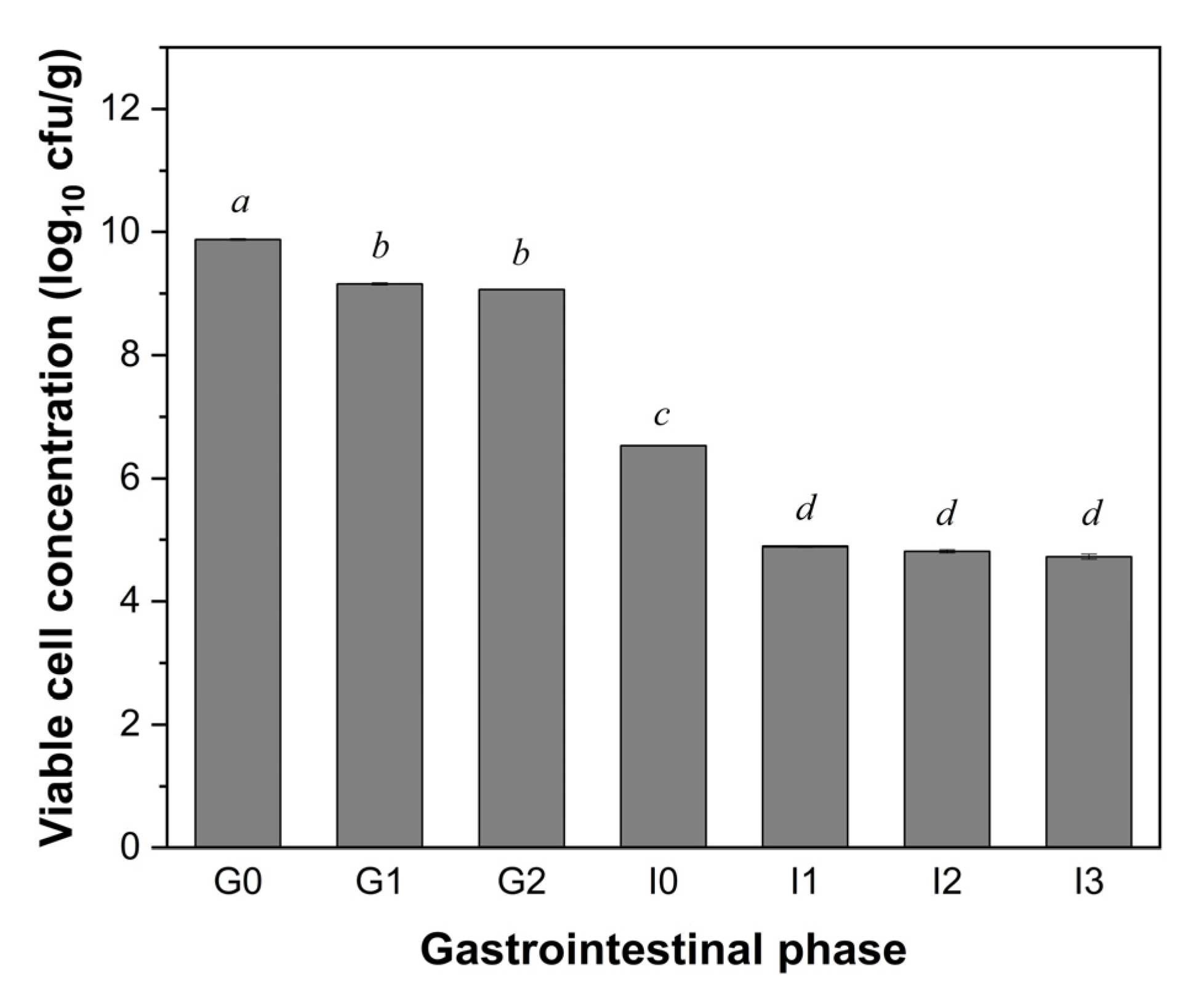
3.1. Viability of LGG During Simulated In Vitro Static Digestion
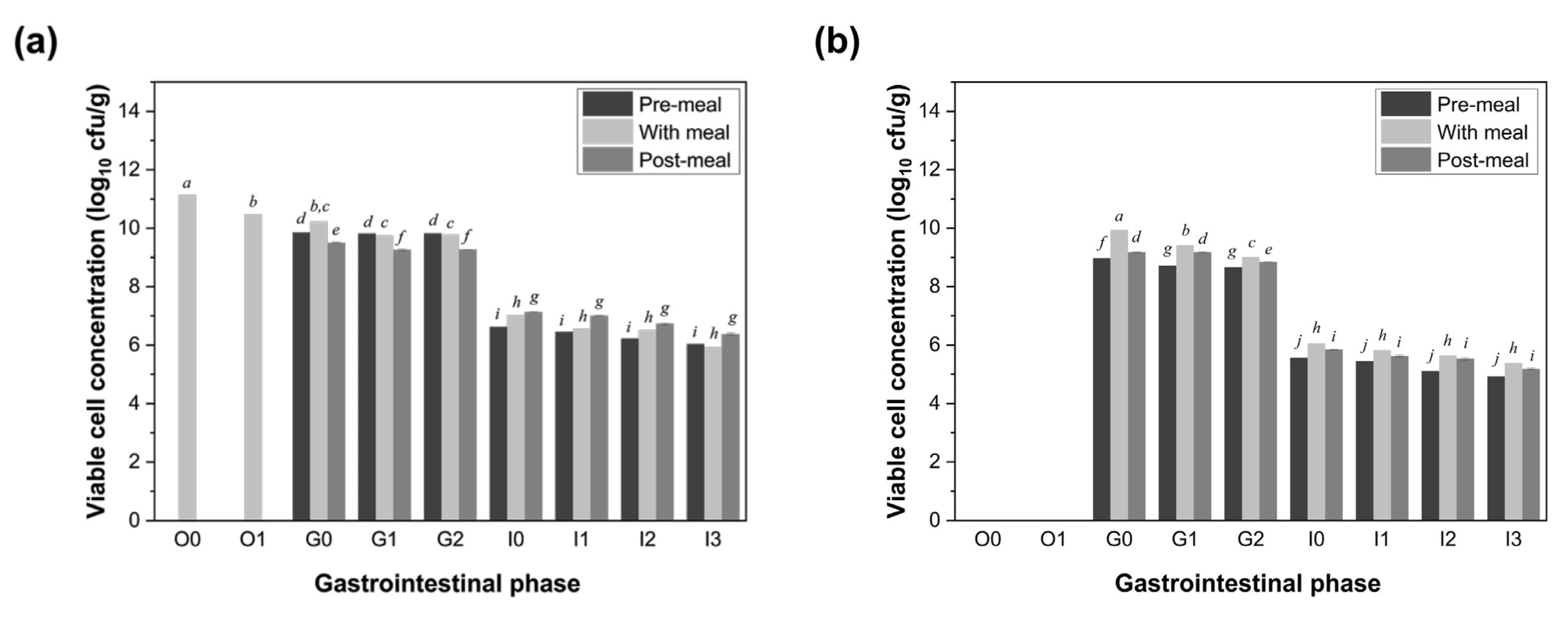
3.2. Viability of LGG Co-Ingested with Food During Simulated Digestion
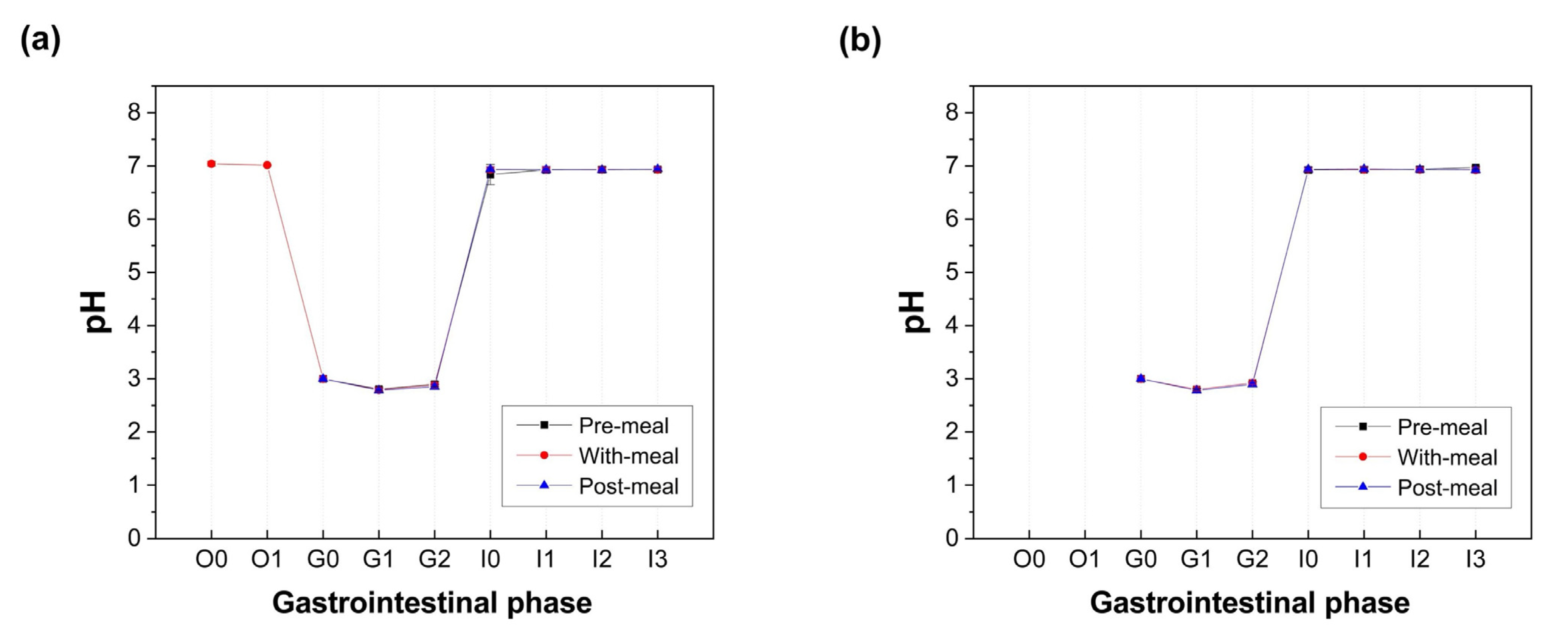
3.3. pH, Starch Hydrolysis, and Protein Digestibility
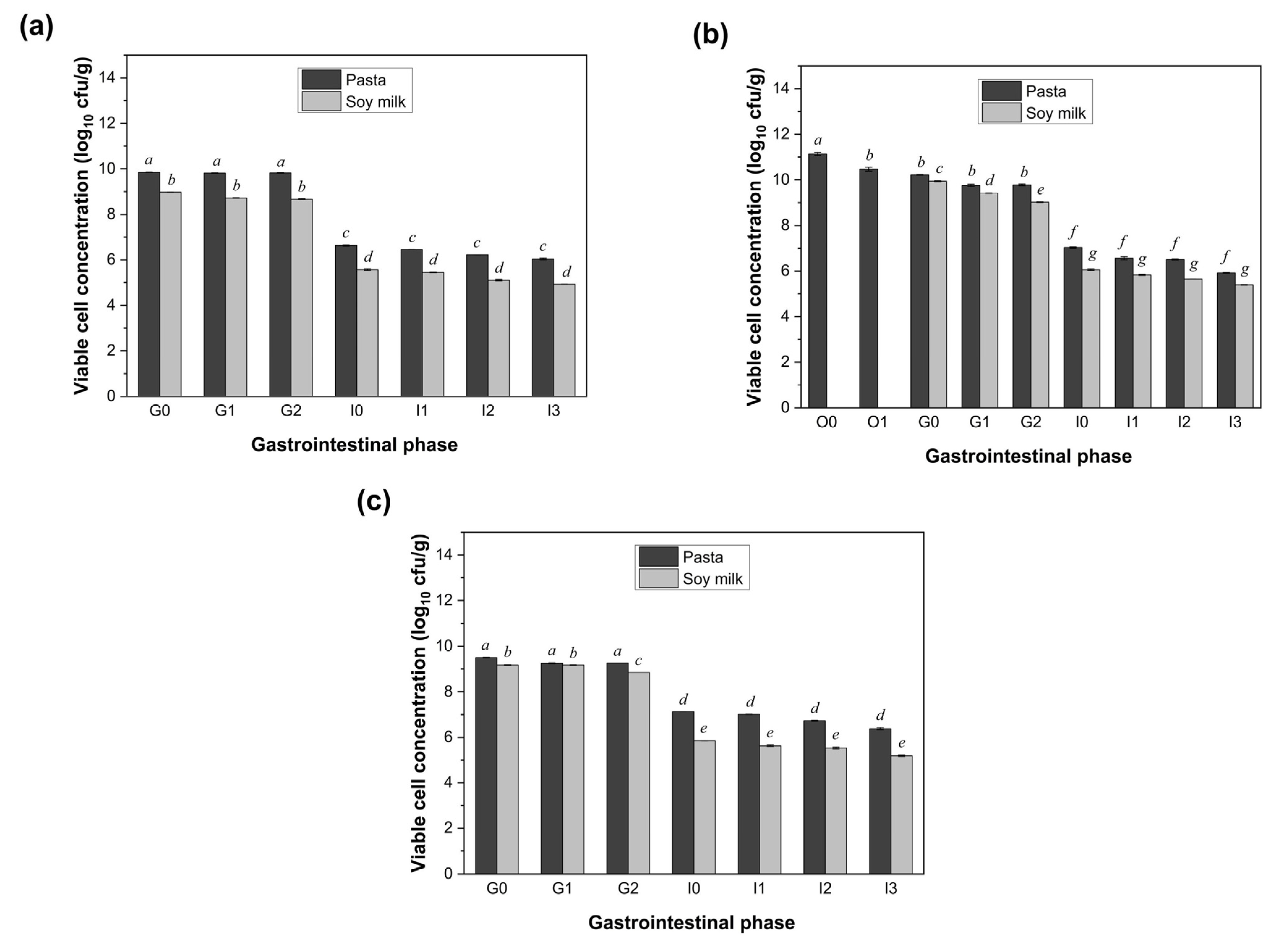
3.4. Impact of Different Food Matrices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GI | gastrointestinal |
| CFU | colony-forming unit |
| LGG | Lactobacillus rhamnosus GG |
| SSF | simulated salivary fluid |
| SGF | simulated gastric fluid |
| SIF | simulated intestinal fluid |
| v/v | volume by volume |
| w/v | weight by volume |
| MRS | de Man Rogosa Sharpe |
| PABAH | p-hydroxybenzoic acid hydrazide |
| BCA | bicinchoninic acid |
| BSA | bovine serum albumin |
| SD | standard deviation |
| ANOVA | analysis of variance |
Appendix A
| Component | Quantity per 100 g |
|---|---|
| Energy | |
| – kJ | 1530 kJ |
| – kcal | 366 kcal |
| Protein | 12.5 g |
| Fat, total | 2.0 g |
| – Trans | <1 g |
| – Saturated | <1 g |
| – Polyunsaturated | 1.2 g |
| – Monounsaturated | <1 g |
| Carbohydrates | 72.0 g |
| – Sugars | 2.5 g |
| Dietary Fiber | 3.0 g |
| Sodium | 30 mg |
| Component | Quantity per 100 mL |
|---|---|
| Energy | |
| – kJ | 134 kJ |
| – kcal | 32 kcal |
| Protein | 1.9 g |
| Fat, total | 2.3 g |
| – Trans | <0.6 g |
| – Saturated | 0.3 g |
| – Polyunsaturated | 0.8 g |
| – Monounsaturated | 1.2 g |
| Carbohydrates | 0.4 g |
| – Sugars | 0.4 g |
| – Lactose | 0.0 g |
| – Galactose | 0.0 g |
| Dietary Fiber | <0.5 g |
| Sodium | 70 mg |
References
- Quercia, S.; Candela, M.; Giuliani, C.; Turroni, S.; Luiselli, D.; Rampelli, S.; Brigidi, P.; Franceschi, C.; Bacalini, M.G.; Garagnani, P. From lifetime to evolution: Timescales of human gut microbiota adaptation. Front. Microbiol. 2014, 5, 587. [Google Scholar] [CrossRef]
- Ikeda, T.; Nishida, A.; Yamano, M.; Kimura, I. Short-chain fatty acid receptors and gut microbiota as therapeutic targets in metabolic, immune, and neurological diseases. Pharmacol. Ther. 2022, 239, 108273. [Google Scholar] [CrossRef]
- Tompkins, T.; Mainville, I.; Arcand, Y. The impact of meals on a probiotic during transit through a model of the human upper gastrointestinal tract. Benef. Microbes 2011, 2, 295–304. [Google Scholar] [CrossRef]
- Panwar, H.; Rokana, N.; Aparna, S.V.; Kaur, J.; Singh, A.; Singh, J.; Singh, K.S.; Chaudhary, V.; Puniya, A.K. Gastrointestinal stress as innate defence against microbial attack. J. Appl. Microbiol. 2021, 130, 1035–1061. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, P.; Dhital, S.; Yu, A.; Chen, X.D. Impact of Freezing and Freeze Drying on Lactobacillus rhamnosus GG Survival: Mechanisms of Cell Damage and the Role of Pre-Freezing Conditions and Cryoprotectants. Foods 2025, 14, 1817. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, P.; Chen, X.; Yu, A.; Dhital, S. Fortification of Cereal-Based Food with Lactobacillus rhamnosus GG and Bacillus coagulans GBI-30 and Their Survival During Processing. Foods 2025, 14, 2250. [Google Scholar] [CrossRef] [PubMed]
- Division, N. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Han, S.; Lu, Y.; Xie, J.; Fei, Y.; Zheng, G.; Wang, Z.; Liu, J.; Lv, L.; Ling, Z.; Berglund, B.; et al. Probiotic Gastrointestinal Transit and Colonization After Oral Administration: A Long Journey. Front. Cell. Infect. Microbiol. 2021, 11, 609722. [Google Scholar] [CrossRef]
- Shori, A.B. Microencapsulation Improved Probiotics Survival During Gastric Transit. HAYATI J. Biosci. 2017, 24, 1–5. [Google Scholar] [CrossRef]
- Faye, T.; Tamburello, A.; Vegarud, G.E.; Skeie, S. Survival of lactic acid bacteria from fermented milks in an in vitro digestion model exploiting sequential incubation in human gastric and duodenum juice. J. Dairy Sci. 2012, 95, 558–566. [Google Scholar] [CrossRef]
- Matouskova, P.; Hoova, J.; Rysavka, P.; Marova, I. Stress effect of food matrices on viability of probiotic cells during model digestion. Microorganisms 2021, 9, 1625. [Google Scholar] [CrossRef]
- Soares, M.B.; Martinez, R.C.; Pereira, E.P.; Balthazar, C.F.; Cruz, A.G.; Ranadheera, C.S.; Sant’Ana, A.S. The resistance of Bacillus, Bifidobacterium, and Lactobacillus strains with claimed probiotic properties in different food matrices exposed to simulated gastrointestinal tract conditions. Food Res. Int. 2019, 125, 108542. [Google Scholar] [CrossRef]
- Blaiotta, G.; De Sena, M.; De Girolamo, F.; Aponte, M.; Romano, R. Probiotic bacilli incorporation in foods: Is really so easy? Food Microbiol. 2023, 115, 104342. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Hussain, M.; Ismail, Z.; Siddeeg, A.; Ammar, A.-F.; Aljobair, M.O. Influence of encapsulation on the survival of probiotics in food matrix under simulated stress conditions. Saudi J. Biol. Sci. 2022, 29, 103394. [Google Scholar] [CrossRef]
- Champagne, C.; Raymond, Y.; Guertin, N.; Martoni, C.; Jones, M.; Mainville, I.; Arcand, Y. Impact of a yogurt matrix and cell microencapsulation on the survival of Lactobacillus reuteri in three in vitro gastric digestion procedures. Benef. Microbes 2015, 6, 753–763. [Google Scholar] [CrossRef]
- da Cruz Rodrigues, V.C.; da Silva, L.G.S.; Simabuco, F.M.; Venema, K.; Antunes, A.E.C. Survival, metabolic status and cellular morphology of probiotics in dairy products and dietary supplement after simulated digestion. J. Funct. Foods 2019, 55, 126–134. [Google Scholar] [CrossRef]
- Klu, Y.A.K.; Chen, J. Effect of peanut butter matrices on the fate of probiotics during simulated gastrointestinal passage. LWT-Food Sci. Technol. 2015, 62, 983–988. [Google Scholar] [CrossRef]
- Treven, P.; Paveljšek, D.; Matijašić, B.B.; Lorbeg, P.M. The Effect of Food Matrix Taken with Probiotics on the Survival of Commercial Probiotics in Simulation of Gastrointestinal Digestion. Foods 2024, 13, 3135. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Influence of foods and nutrition on the gut microbiome and implications for intestinal health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- García-Moncayo, A.I.; Ochoa-Reyes, E.; Sáenz-Hidalgo, H.K.; González-Pérez, P.; Muñoz-Castellanos, L.N.; Sepúlveda-Ahumada, D.R.; Buenrostro-Figueroa, J.J.; Alvarado-González, M. Wheat Flour Pasta Combining Bacillus coagulans and Arthrospira platensis as a Novel Probiotic Food with Antioxidants. Foods 2024, 13, 3381. [Google Scholar] [CrossRef] [PubMed]
- Sissons, M. Development of Novel Pasta Products with Evidence Based Impacts on Health—A Review. Foods 2022, 11, 123. [Google Scholar] [CrossRef]
- Olías, R.; Delgado-Andrade, C.; Padial, M.; Marín-Manzano, M.C.; Clemente, A. An Updated Review of Soy-Derived Beverages: Nutrition, Processing, and Bioactivity. Foods 2023, 12, 2665. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Fu, N.; Woo, M.W.; Chen, X.D. Exploring the interactions between Lactobacillus rhamnosus GG and whey protein isolate for preservation of the viability of bacteria through spray drying. Food Funct. 2021, 12, 2995–3008. [Google Scholar] [CrossRef]
- Jia, B.; Devkota, L.; Sissons, M.; Dhital, S. Degradation of starch in pasta induced by extrusion below gelatinization temperature. Food Chem. 2023, 426, 136524. [Google Scholar] [CrossRef]
- Wu, P.; Bhattarai, R.R.; Dhital, S.; Deng, R.; Chen, X.D.; Gidley, M.J. In vitro digestion of pectin- and mango-enriched diets using a dynamic rat stomach-duodenum model. J. Food Eng. 2017, 202, 65–78. [Google Scholar] [CrossRef]
- da Silva, M.N.; Tagliapietra, B.L.; do Amaral Flores, V.; dos Santos Richards, N.S.P. In vitro test to evaluate survival in the gastrointestinal tract of commercial probiotics. Curr. Res. Food Sci. 2021, 4, 320–325. [Google Scholar] [CrossRef]
- Aziz, G.; Zaidi, A.; Tariq, M. Compositional quality and possible gastrointestinal performance of marketed probiotic supplements. Probiotics Antimicrob. Proteins 2022, 14, 288–312. [Google Scholar] [CrossRef]
- Millette, M.; Nguyen, A.; Amine, K.M.; Lacroix, M. Gastrointestinal survival of bacteria in commercial probiotic products. Int. J. Probiotics Prebiotics 2013, 8, 149–156. [Google Scholar]
- Ayyash, M.M.; Abdalla, A.K.; AlKalbani, N.S.; Baig, M.A.; Turner, M.S.; Liu, S.-Q.; Shah, N.P. Invited review: Characterization of new probiotics from dairy and nondairy products—Insights into acid tolerance, bile metabolism and tolerance, and adhesion capability. J. Dairy Sci. 2021, 104, 8363–8379. [Google Scholar] [CrossRef]
- D’Amico, V.; Cavaliere, M.; Ivone, M.; Lacassia, C.; Celano, G.; Vacca, M.; la Forgia, F.M.; Fontana, S.; De Angelis, M.; Denora, N.; et al. Microencapsulation of Probiotics for Enhanced Stability and Health Benefits in Dairy Functional Foods: A Focus on Pasta Filata Cheese. Pharmaceutics 2025, 17, 185. [Google Scholar] [CrossRef]
- Ouwehand, A. A review of dose-responses of probiotics in human studies. Benef. Microbes 2017, 8, 143–151. [Google Scholar] [CrossRef]
- Li, M.j.; Cao, R.g.; Tong, L.t.; Fan, B.; Sun, R.q.; Liu, L.y.; Wang, F.z.; Wang, L.l. Effect of freezing treatment of soybean on soymilk nutritional components, protein digestibility, and functional components. Food Sci. Nutr. 2021, 9, 5997–6005. [Google Scholar] [CrossRef] [PubMed]
- Binita, R.; Khetarpaul, N. Probiotic fermentation: Effect on antinutrients and digestibility of starch and protein of indigenously developed food mixture. Nutr. Health 1997, 11, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.C.; Khetarpaul, N. Probiotic fermentation of indigenous food mixture: Effect on antinutrients and digestibility of starch and protein. J. Food Compos. Anal. 2001, 14, 601–609. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Wang, P.; Zhao, Y.; Zhu, Y.; Xiao, X. The Effect of Protein-Starch Interaction on the Structure and Properties of Starch, and Its Application in Flour Products. Foods 2025, 14, 778. [Google Scholar] [CrossRef]
- Bhattarai, R.R.; Dhital, S.; Wu, P.; Chen, X.D.; Gidley, M.J. Digestion of isolated legume cells in a stomach-duodenum model: Three mechanisms limit starch and protein hydrolysis. Food Funct. 2017, 8, 2573–2582. [Google Scholar] [CrossRef]
- Ranadheera, R.; Baines, S.K.; Adams, M.C. Importance of food in probiotic efficacy. Food Res. Int. 2010, 43, 1–7. [Google Scholar] [CrossRef]
- Mennah-Govela, Y.A.; Singh, R.P.; Bornhorst, G.M. Buffering capacity of protein-based model food systems in the context of gastric digestion. Food Funct. 2019, 10, 6074–6087. [Google Scholar] [CrossRef]
- Yang, S.; Hu, Z.; Wu, P.; Kirk, T.; Chen, X.D. In vitro release and bioaccessibility of oral solid preparations in a dynamic gastrointestinal system simulating fasted and fed states: A case study of metformin hydrochloride tablets. Int. J. Pharm. 2024, 652, 123869. [Google Scholar] [CrossRef]
- Meybodi, N.; Mortazavian, A. Probiotic supplements and food products: A comparative approach. Biochem. Pharmacol. 2017, 6, 227. [Google Scholar]
- de Vos, W.M.; Vaughan, E.E. Genetics of lactose utilization in lactic acid bacteria. FEMS Microbiol. Rev. 1994, 15, 217–237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, S.M.; Onilude, A.A. Enzymatic Reduction of Anti-nutritional Factors in Fermenting Soybeans by Lactobacillus plantarum Isolates from Fermenting Cereals. Niger. Food J. 2013, 31, 84–90. [Google Scholar] [CrossRef]
- Wang, J.; Wu, P.; Wang, J.; Wang, J.; Gu, B.; Ge, F.; Chen, X.D. In vitro gastric digestion and emptying of cooked white and brown rice using a dynamic human stomach system. Food Struct. 2022, 31, 100245. [Google Scholar] [CrossRef]
- Zhang, P.; Iqbal, S.; Deng, R.; Duan, X.; Han, H.; Chen, X.D.; Wu, P. Impact of elderly gastrointestinal alterations on gastric emptying and enzymatic hydrolysis of skim milk: An in vitro study using a dynamic stomach system. Food Chem. 2023, 402, 134365. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, P.; Chen, X.D. Chapter Five—Assessing food digestion in the elderly using in vitro gastrointestinal models. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2025; Volume 114, pp. 273–300. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wu, P.; Chen, X.D.; Yu, A.; Dhital, S. Effect of Food Matrix and Administration Timing on the Survival of Lactobacillus rhamnosus GG During In Vitro Gastrointestinal Digestion. Foods 2025, 14, 3076. https://doi.org/10.3390/foods14173076
Wang J, Wu P, Chen XD, Yu A, Dhital S. Effect of Food Matrix and Administration Timing on the Survival of Lactobacillus rhamnosus GG During In Vitro Gastrointestinal Digestion. Foods. 2025; 14(17):3076. https://doi.org/10.3390/foods14173076
Chicago/Turabian StyleWang, Junyan, Peng Wu, Xiao Dong Chen, Aibing Yu, and Sushil Dhital. 2025. "Effect of Food Matrix and Administration Timing on the Survival of Lactobacillus rhamnosus GG During In Vitro Gastrointestinal Digestion" Foods 14, no. 17: 3076. https://doi.org/10.3390/foods14173076
APA StyleWang, J., Wu, P., Chen, X. D., Yu, A., & Dhital, S. (2025). Effect of Food Matrix and Administration Timing on the Survival of Lactobacillus rhamnosus GG During In Vitro Gastrointestinal Digestion. Foods, 14(17), 3076. https://doi.org/10.3390/foods14173076








