Innovative Non-Thermal Processing Technologies for Shelf Life Extension and Retention of Bioactive Compounds in Liquid Foods: Current Status and Future Prospects
Abstract
1. Introduction
2. Literature Search and Selection of Studies for Review Article Preparation
3. Basics of Innovative Non-Thermal Processing Technologies
3.1. High-Pressure Processing
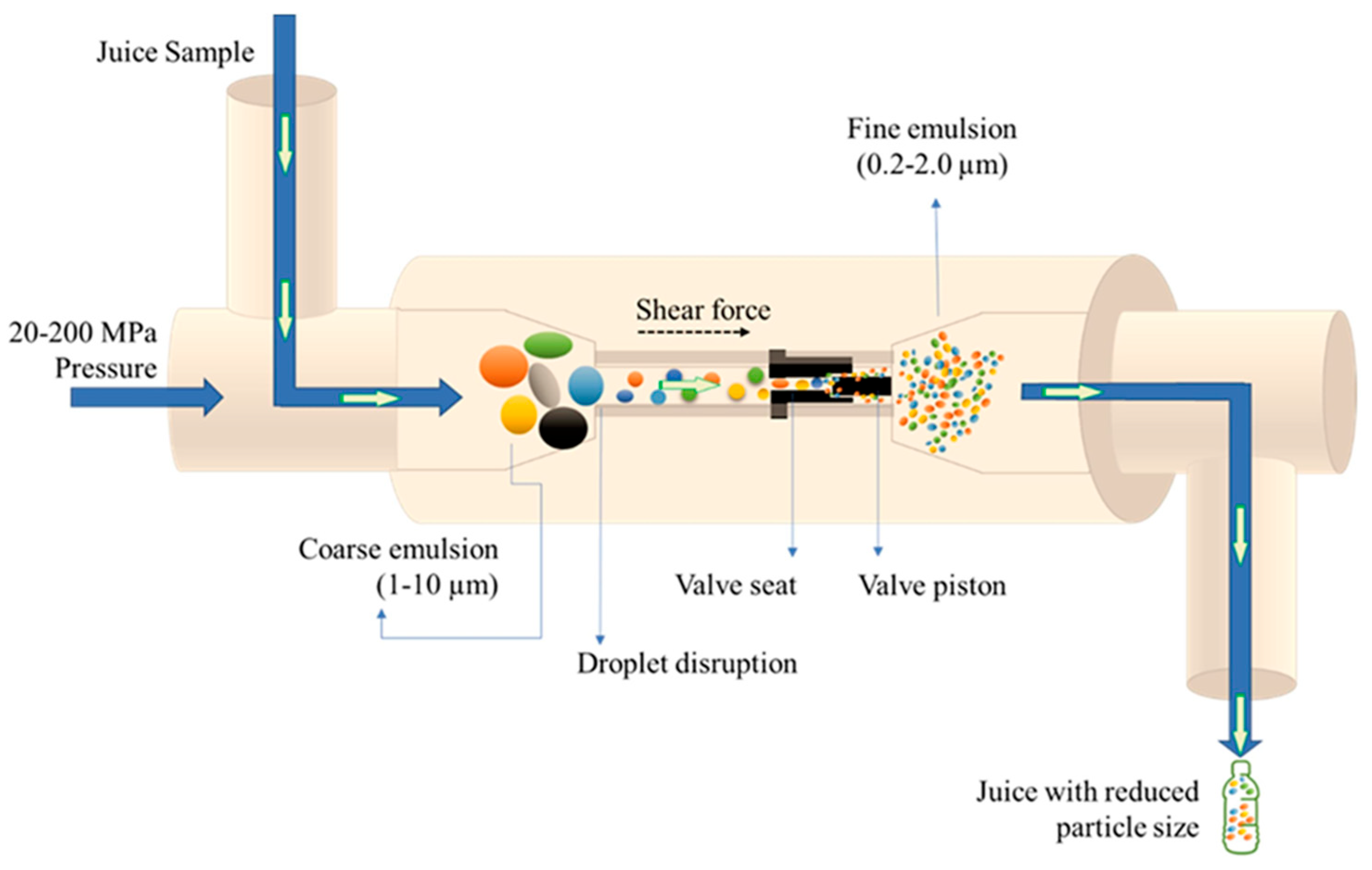
3.2. High-Pressure Homogenization
3.3. Pulsed Electric Field
3.4. Pulse Magnetic Field
3.5. High-Pressure Carbon Dioxide
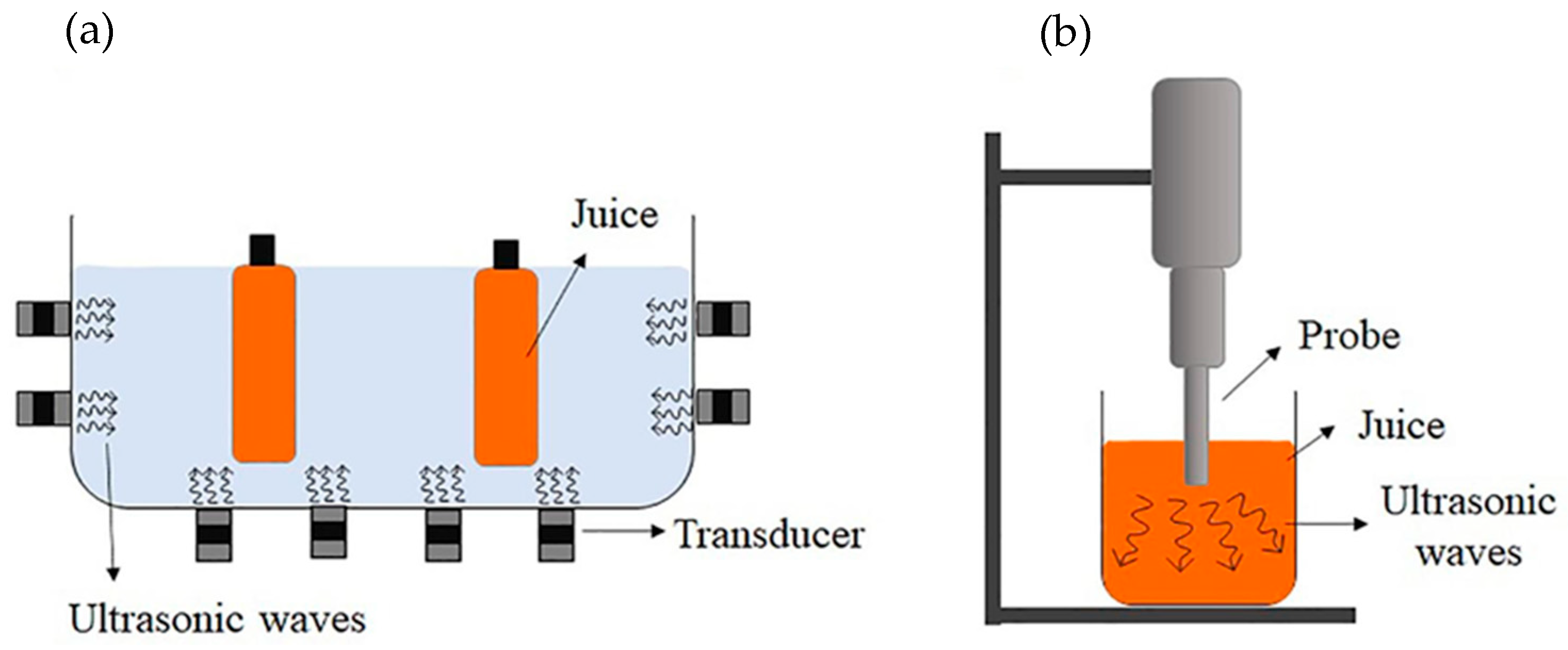
3.6. Ultrasound Treatment
3.7. Radiation Processing
3.8. Ozone Processing
3.9. Cold Plasma
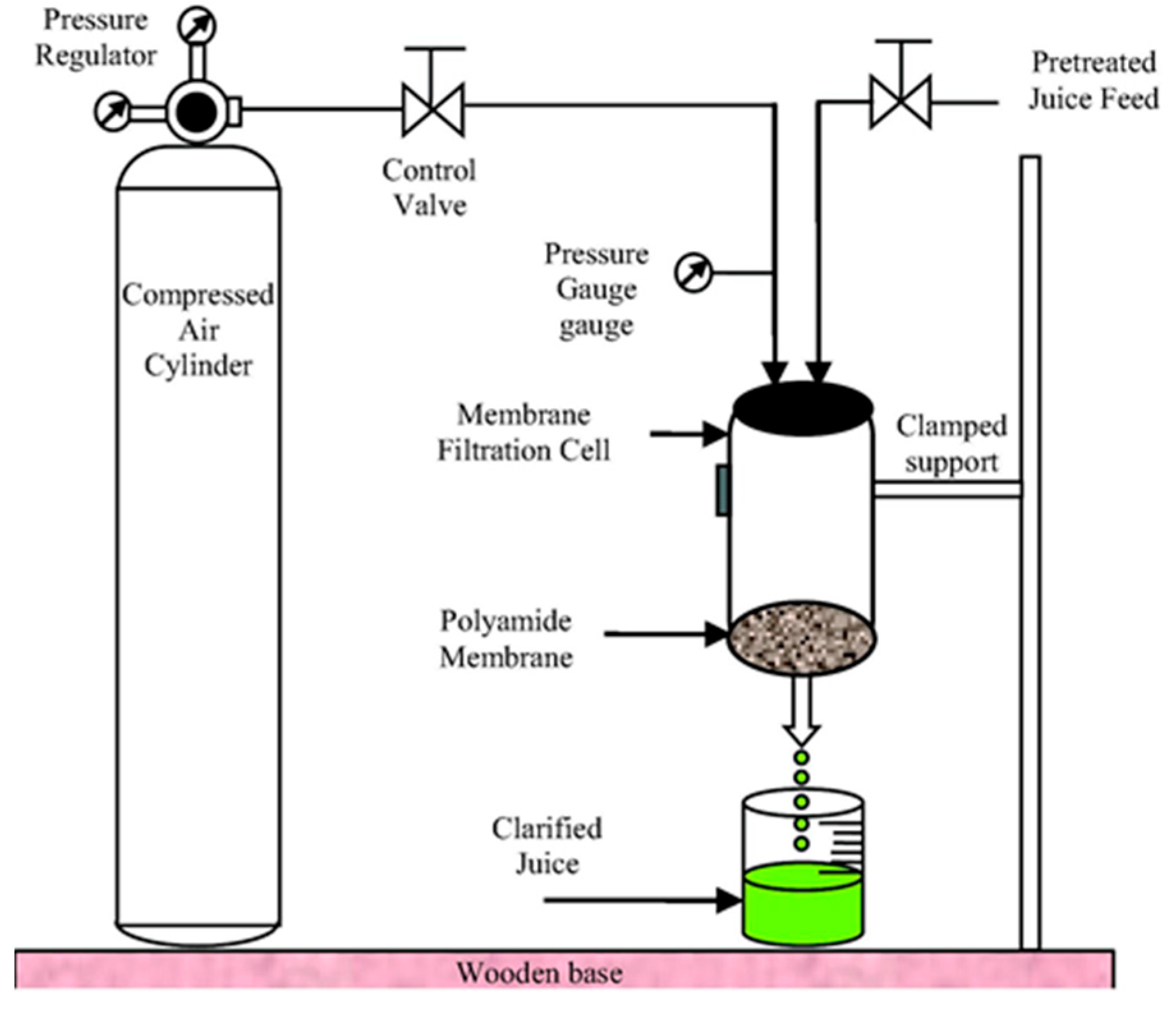
3.10. Membrane Processing
3.11. Fundamentals of Using Combined Technologies
4. Application of Innovative Non-Thermal Processing Technologies
4.1. High-Pressure Processing
4.2. High-Pressure Homogenization
4.3. Pulsed Electric Field
4.4. Pulsed Magnetic Field
4.5. High-Pressure Carbon Dioxide
4.6. Ultrasound Treatment
4.7. Radiation Processing
4.8. Ozone Processing
4.9. Cold Plasma
4.10. Membrane Processing
4.11. Other Non-Thermal Processing Technologies
4.12. Combination of Various Techniques
5. Technical Challenges and Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koutchma, T. Advances in ultraviolet light technology for non-thermal processing of liquid foods. Food Bioprocess Technol. 2009, 2, 138–155. [Google Scholar] [CrossRef]
- Khan, M.R.; Syed, A.; Zia, S.; Ahmed, W.; Aadil, R.M.; Manzoor, M.F.; Inam-Ur-Raheem, M.; Abid, M.; Shabbir, M.A.; Qureshi, S. Stabilization and attributive amelioration of sugarcane juice by naturally derived preservatives using aonla and moringa extract. Food Sci. Nutr. 2021, 9, 3048–3058. [Google Scholar] [CrossRef] [PubMed]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Xiao, L.; Li, X.; Hu, M. Effect of fermentation parameters and their optimization on the phytochemical properties of lactic-acid-fermented mulberry juice. J. Food Meas. Charact. 2017, 11, 1462–1473. [Google Scholar] [CrossRef]
- Xia, X.; Dai, Y.; Wu, H.; Liu, X.; Wang, Y.; Yin, L.; Wang, Z.; Li, X.; Zhou, J. Kombucha fermentation enhances the health-promoting properties of soymilk beverage. J. Funct. Foods 2019, 62, 103549. [Google Scholar] [CrossRef]
- Gao, X.; Feng, T.; Liu, E.; Shan, P.; Zhang, Z.; Liao, L.; Ma, H. Ougan juice debittering using ultrasound-aided enzymatic hydrolysis: Impacts on aroma and taste. Food Chem. 2021, 345, 128767. [Google Scholar] [CrossRef] [PubMed]
- Marafon, K.; Prestes, A.A.; Carvalho, A.C.F.; de Souza, C.K.; Prudencio, E.S. Bioactive compounds’ importance in plant-based beverages: A review. Curr. Opin. Food Sci. 2025, 63, 101304. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Herbal beverages: Bioactive compounds and their role in disease risk reduction-A review. J. Tradit. Complement. Med. 2018, 8, 451–458. [Google Scholar] [CrossRef]
- Sperber, W.H. Introduction to the microbiological spoilage of foods and beverages. In Compendium of the Microbiological Spoilage of Foods and Beverages; Springer: New York, NY, USA, 2009; pp. 1–40. [Google Scholar]
- Ceylan, E.; Amezquita, A.; Anderson, N.; Betts, R.; Blayo, L.; Garces-Vega, F.; Gkogka, E.; Harris, L.J.; McClure, P.; Winkler, A. Guidance on validation of lethal control measures for foodborne pathogens in foods. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2825–2881. [Google Scholar] [CrossRef]
- Rahman, M.S. Food preservation: An overview. In Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2020; pp. 7–18. [Google Scholar]
- Huang, G.; Sun, W.; Dai, C.; Sun, L.; Tang, Y.; He, R.; Ma, H. Sterilization of Bacillus tequilensis isolated from aerogenic vinegar by intense pulsed light. LWT 2020, 118, 108811. [Google Scholar] [CrossRef]
- Lewis, M.J.; Jun, S. Thermal processing. In Food Processing Handbook; Wiley-VCH: Weinheim, Germany, 2006; Volume 2, p. 33. [Google Scholar]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Wahia, H.; Fakayode, O.A.; Mustapha, A.T.; Zhou, C.; Dabbour, M. Application and potential of multifrequency ultrasound in juice industry: Comprehensive analysis of inactivation and germination of Alicyclobacillus acidoterrestris spores. Crit. Rev. Food Sci. Nutr. 2024, 64, 4561–4586. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Sackey, A.S.; Wu, M.; Xiao, L. Impact of ultrasonication and pulsed light treatments on phenolics concentration and antioxidant activities of lactic-acid-fermented mulberry juice. LWT 2018, 92, 61–66. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Petruzzi, L.; Perricone, M.; Speranza, B.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R. Nonthermal technologies for fruit and vegetable juices and beverages: Overview and advances. Compr. Rev. Food Sci. Food Saf. 2018, 17, 2–62. [Google Scholar] [CrossRef]
- Zhou, C.; Okonkwo, C.E.; Inyinbor, A.A.; Yagoub, A.E.A.; Olaniran, A.F. Ultrasound, infrared and its assisted technology, a promising tool in physical food processing: A review of recent developments. Crit. Rev. Food Sci. Nutr. 2023, 63, 1587–1611. [Google Scholar] [CrossRef]
- Kwaw, E.; Tchabo, W.; Ma, Y.; Apaliya, M.T.; Sackey, A.S.; Mintah, B.K.; Farooq, M.; Ma, S. Effect of storage on quality attributes of lactic-acid-fermented mulberry juice subjected to combined pulsed light and ultrasonic pasteurization treatment. J. Food Meas. Charact. 2018, 12, 1763–1771. [Google Scholar] [CrossRef]
- Subaitha, Z.A.; Santhoshkumar, P.; Shubham, N.; Moses, J. Environmental Impact of Novel Non-Thermal Technologies. In Non-Thermal Technologies for the Food Industry; CRC Press: Boca Raton, FL, USA, 2024; pp. 335–344. [Google Scholar]
- Balasubramaniam, V.; Martinez-Monteagudo, S.I.; Gupta, R. Principles and application of high pressure–based technologies in the food industry. Annu. Rev. Food Sci. Technol. 2015, 6, 435–462. [Google Scholar] [CrossRef]
- Sehrawat, R.; Kaur, B.P.; Nema, P.K.; Tewari, S.; Kumar, L. Microbial inactivation by high pressure processing: Principle, mechanism and factors responsible. Food Sci. Biotechnol. 2021, 30, 19–35. [Google Scholar] [CrossRef]
- Pou, K.J.; Raghavan, V. Recent advances in the application of high pressure processing-based hurdle approach for enhancement of food safety and quality. J. Biosyst. Eng. 2020, 45, 175–187. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Z.; Wu, D.; Fei, X.; Ei-Seedi, H.R.; Wang, C. High-pressure homogenization influences the functional properties of protein from oyster (Crassostrea gigas). LWT 2021, 151, 112107. [Google Scholar] [CrossRef]
- Qayum, A.; Rashid, A.; Liang, Q.; Wu, Y.; Cheng, Y.; Kang, L.; Liu, Y.; Zhou, C.; Hussain, M.; Ren, X. Ultrasonic and homogenization: An overview of the preparation of an edible protein–polysaccharide complex emulsion. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4242–4281. [Google Scholar] [CrossRef]
- Wei, B.; Cai, C.; Xu, B.; Jin, Z.; Tian, Y. Disruption and molecule degradation of waxy maize starch granules during high pressure homogenization process. Food Chem. 2018, 240, 165–173. [Google Scholar] [CrossRef]
- Sherman, I.M.; Mounika, A.; Srikanth, D.; Shanmugam, A.; Ashokkumar, M. Leveraging new opportunities and advances in high-pressure homogenization to design non-dairy foods. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13282. [Google Scholar] [CrossRef]
- Chandel, A.; Xavier, J.R.; Chauhan, O.P. Applications of High Pressure Homogenization in Food Industry for Ensuring Quality and Safety. J. Food Process Eng. 2025, 48, e70105. [Google Scholar] [CrossRef]
- Mukhtar, K.; Nabi, B.G.; Arshad, R.N.; Roobab, U.; Yaseen, B.; Ranjha, M.M.A.N.; Aadil, R.M.; Ibrahim, S.A. Potential impact of ultrasound, pulsed electric field, high-pressure processing and microfludization against thermal treatments preservation regarding sugarcane juice (Saccharum officinarum). Ultrason. Sonochem. 2022, 90, 106194. [Google Scholar] [CrossRef]
- Beebe, S.J.; Sain, N.M.; Ren, W. Induction of cell death mechanisms and apoptosis by nanosecond pulsed electric fields (nsPEFs). Cells 2013, 2, 136–162. [Google Scholar] [CrossRef] [PubMed]
- Naliyadhara, N.; Kumar, A.; Girisa, S.; Daimary, U.D.; Hegde, M.; Kunnumakkara, A.B. Pulsed electric field (PEF): Avant-garde extraction escalation technology in food industry. Trends Food Sci. Technol. 2022, 122, 238–255. [Google Scholar] [CrossRef]
- Qian, J.; Zhou, C.; Ma, H.; Li, S.; Yagoub, A.E.A.; Abdualrahman, M.A. Proteomics analyses and morphological structure of Bacillus subtilis inactivated by pulsed magnetic field. Food Biophys. 2016, 11, 436–445. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, M.; Dai, C.; Huo, S.; Ma, H. Transcriptomic analysis of Listeria monocytogenes under pulsed magnetic field treatment. Food Res. Int. 2020, 133, 109195. [Google Scholar] [CrossRef]
- Guo, L.; Azam, S.R.; Guo, Y.; Liu, D.; Ma, H. Germicidal efficacy of the pulsed magnetic field against pathogens and spoilage microorganisms in food processing: An overview. Food Control 2022, 136, 108496. [Google Scholar] [CrossRef]
- Yu, T.; Niu, L.; Iwahashi, H. High-pressure carbon dioxide used for pasteurization in food industry. Food Eng. Rev. 2020, 12, 364–380. [Google Scholar] [CrossRef]
- Hart, A.; Anumudu, C.; Onyeaka, H.; Miri, T. Application of supercritical fluid carbon dioxide in improving food shelf-life and safety by inactivating spores: A review. J. Food Sci. Technol. 2022, 59, 417–428. [Google Scholar] [CrossRef]
- Wahia, H.; Zhang, L.; Zhou, C.; Mustapha, A.T.; Fakayode, O.A.; Amanor-Atiemoh, R.; Ma, H.; Dabbour, M. Pulsed multifrequency thermosonication induced sonoporation in Alicyclobacillus acidoterrestris spores and vegetative cells. Food Res. Int. 2022, 156, 111087. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, A.T.; Wahia, H.; Ji, Q.; Fakayode, O.A.; Zhang, L.; Zhou, C. Multiple-frequency ultrasound for the inactivation of microorganisms on food: A review. J. Food Process Eng. 2024, 47, e14587. [Google Scholar] [CrossRef]
- Li, M.; Zhou, C.; Wang, B.; Zeng, S.; Mu, R.; Li, G.; Li, B.; Lv, W. Research progress and application of ultrasonic-and microwave-assisted food processing technology. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3707–3731. [Google Scholar] [CrossRef]
- Lopes, S.J.; Sant’Ana, A.S.; Freire, L. Non-thermal emerging processing technologies: Mitigation of microorganisms and mycotoxins, sensory and nutritional properties maintenance in clean label fruit juices. Food Res. Int. 2023, 168, 112727. [Google Scholar] [CrossRef]
- Gayán, E.; Serrano, M.; Monfort, S.; Álvarez, I.; Condón, S. Combining ultraviolet light and mild temperatures for the inactivation of Escherichia coli in orange juice. J. Food Eng. 2012, 113, 598–605. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Alternatives to conventional thermal treatments in fruit-juice processing. Part 1: Techniques and applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 501–523. [Google Scholar] [CrossRef]
- Ren, M.; Yu, X.; Mujumdar, A.S.; Yagoub, A.E.-G.A.; Chen, L.; Zhou, C. Visualizing the knowledge domain of pulsed light technology in the food field: A scientometrics review. Innov. Food Sci. Emerg. Technol. 2021, 74, 102823. [Google Scholar] [CrossRef]
- Lung, H.-M.; Cheng, Y.-C.; Chang, Y.-H.; Huang, H.-W.; Yang, B.B.; Wang, C.-Y. Microbial decontamination of food by electron beam irradiation. Trends Food Sci. Technol. 2015, 44, 66–78. [Google Scholar] [CrossRef]
- Asokapandian, S.; Periasamy, S.; Swamy, G.J. Ozone for fruit juice preservation. In Fruit Juices; Elsevier: Amsterdam, The Netherlands, 2018; pp. 511–527. [Google Scholar]
- Panigrahi, C.; Kaur, G.; Sahoo, M. Recent advancements in applications of ozone technology in juice and beverage processing: A review. J. Food Process Eng. 2025, 48, e70074. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Feasibility of cold plasma for the control of biofilms in food industry. Trends Food Sci. Technol. 2020, 99, 142–151. [Google Scholar] [CrossRef]
- Cui, H.; Li, W.; Li, C.; Lin, L. Synergistic effect between Helichrysum italicum essential oil and cold nitrogen plasma against Staphylococcus aureus biofilms on different food-contact surfaces. Int. J. Food Sci. Technol. 2016, 51, 2493–2501. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Saxena, V.; Dutta, S. Fruit juice processing using membrane technology: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 136–153. [Google Scholar] [CrossRef]
- Singh, V.; Das, C. Clarification of Citrus fruit (Mosambi) juice by hybrid (Pretreatment and Membrane) process. Mater. Today Proc. 2021, 47, 1384–1388. [Google Scholar] [CrossRef]
- Jan, A.; Sood, M.; Sofi, S.; Norzom, T. Non-thermal processing in food applications: A review. Int. J. Food Sci. Nutr. 2017, 2, 171–180. [Google Scholar]
- Xia, Q.; Liu, C.; Cao, Y.; Zhao, Y.; Lu, S.; Wu, D.; Guan, R. Improving quality of sea buckthorn juice by high-pressure processing. LWT 2023, 185, 115149. [Google Scholar] [CrossRef]
- Lim, S.H.; Chin, N.L.; Sulaiman, A.; Tay, C.H.; Wong, T.H. Microbiological, physicochemical and nutritional properties of fresh cow milk treated with industrial high-pressure processing (HPP) during storage. Foods 2023, 12, 592. [Google Scholar] [CrossRef]
- Lou, X.; Jin, Y.; Tian, H.; Yu, H.; Chen, C.; Hanna, M.; Lin, Y.; Yuan, L.; Wang, J.; Xu, H. High-pressure and thermal processing of cloudy hawthorn berry (Crataegus pinnatifida) juice: Impact on microbial shelf-life, enzyme activity and quality-related attributes. Food Chem. 2022, 372, 131313. [Google Scholar] [CrossRef]
- Yi, T.; Fang, W.; Xie, X.; Yuan, B.; Lu, M.; Xu, C. High pressure processing (HPP) improved safety and quality of emerging aronia berry juice: A pilot scale shelf-life study. J. Food Sci. Technol. 2022, 59, 755–767. [Google Scholar] [CrossRef]
- Rios-Corripio, G.; Welti-Chanes, J.; Rodriguez-Martinez, V.; Guerrero-Beltrán, J.Á. Influence of high hydrostatic pressure processing on physicochemical characteristics of a fermented pomegranate (Punica granatum L.) beverage. Innov. Food Sci. Emerg. Technol. 2020, 59, 102249. [Google Scholar] [CrossRef]
- Pokhrel, P.R.; Boulet, C.; Yildiz, S.; Sablani, S.; Tang, J.; Barbosa-Canovas, G.V. Effect of high hydrostatic pressure on microbial inactivation and quality changes in carrot-orange juice blends at varying pH. LWT 2022, 159, 113219. [Google Scholar] [CrossRef]
- Raghubeer, E.V.; Phan, B.N.; Onuoha, E.; Diggins, S.; Aguilar, V.; Swanson, S.; Lee, A. The use of High-Pressure Processing (HPP) to improve the safety and quality of raw coconut (Cocos nucifera L) water. Int. J. Food Microbiol. 2020, 331, 108697. [Google Scholar] [CrossRef]
- Ozkan, G.; Kamiloglu, S.; Demir, N.; Capanoglu, E. Impact of High Pressure Processing on the In Vitro Bioaccessibility of Polyphenols in Sour Cherry (Prunus cerasus L.) Juice. ACS Omega 2025, 10, 20038–20046. [Google Scholar] [CrossRef]
- Engmann, F.; Ma, Y.-K.; Sanful, R. The impact of high hydrostatic pressure treatment on anthocyanins, colour, microorganisms, and enzyme activity of mulberry (Morus nigra) juice. Int. Food Res. J. 2020, 27, 88–95. [Google Scholar]
- Li, F.; Yang, S.; Liu, L.; Fu, H.; Ming, J. Variations of bioactive compounds, physicochemical and sensory properties of Rosa roxburghii Tratt juice after high pressure processing. LWT 2023, 184, 114932. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, M.; Rao, L.; Zhao, L.; Wang, Y.; Liao, X. Effect of ultra-high pressure homogenization on microorganism and quality of composite pear juice. Food Sci. Nutr. 2022, 10, 3072–3084. [Google Scholar] [CrossRef] [PubMed]
- Wellala, C.K.D.; Bi, J.; Liu, X.; Liu, J.; Lyu, J.; Zhou, M. Effect of high pressure homogenization on mixed juice stability, rheology, physicochemical properties and microorganism reduction. J. Food Sci. Technol. 2020, 57, 1944–1953. [Google Scholar] [CrossRef]
- Patrignani, F.; Mannozzi, C.; Tappi, S.; Tylewicz, U.; Pasini, F.; Castellone, V.; Riciputi, Y.; Rocculi, P.; Romani, S.; Caboni, M.F. (Ultra) high pressure homogenization potential on the shelf-life and functionality of kiwifruit juice. Front. Microbiol. 2019, 10, 246. [Google Scholar] [CrossRef]
- Antony, A.; Soni, A.; Samuelsson, L.M.; Weeks, M.; Woo, M.W.; Quek, S.-Y.; Farid, M.; Gupta, T. Impact of Ultra-High-Pressure Homogenisation on the Inactivation of Bacillus pumilus and Bacillus subtilis Spores in Sheep and Cow Milk. Foods 2024, 13, 3452. [Google Scholar] [CrossRef]
- Jalali, K.; Pastor-Villaescusa, B.; Flores-Rojas, K.; Pleguezuelos, V.; Pérez-Cano, F.J.; Franch-Masferrer, À.; Trujillo-Mesa, A.J.; Hernández-Herrero, M.M.; Roig-Sagués, A.X. Evaluation of Ultra-High Pressure Homogenization Treatments to Ensure the Microbiological Safety and Immunoglobulin Preservation in Donor Human Milk. Foods 2025, 14, 1310. [Google Scholar] [CrossRef]
- Adhikari, J.; Araghi, L.R.; Singh, R.; Adhikari, K.; Patil, B.S. Continuous-Flow High-Pressure Homogenization of Blueberry Juice Enhances Anthocyanin and Ascorbic Acid Stability during Cold Storage. J. Agric. Food Chem. 2024, 72, 11629–11639. [Google Scholar] [CrossRef]
- Turan, E.; Aslantaş, R.; Bilgin, J.; Aksu, M.I. High-Pressure Homogenization of Pomegranate Juice: Impact on Physicochemical, Antioxidant, Antimicrobial, and In Vitro Bioaccessibility Properties. Food Sci. Nutr. 2024, 12, 10315–10329. [Google Scholar] [CrossRef]
- Liu, J.; Bi, J.; Liu, X.; Liu, D.; Verkerk, R.; Dekker, M.; Lyu, J.; Wu, X. Modelling and optimization of high-pressure homogenization of not-from-concentrate juice: Achieving better juice quality using sustainable production. Food Chem. 2022, 370, 131058. [Google Scholar] [CrossRef]
- Fabroni, S.; Platania, G.M.; Amenta, M.; Ballistreri, G.; Galvano, F.; Nges, I.A.; Timpanaro, N. Pulsed electric field as a mild treatment for extended shelf-life and preservation of bioactive compounds in blood orange juice. Appl. Sci. 2024, 14, 7275. [Google Scholar] [CrossRef]
- Jin, T.Z.; Aboelhaggag, R.M. Combined pulsed electric field with antimicrobial caps for extending shelf life of orange juice. Beverages 2022, 8, 72. [Google Scholar] [CrossRef]
- Öztürk, H.İ.; Buzrul, S.; Bilge, G.; Yurdakul, M. Pulsed electric field for shalgam juice: Effects on fermentation, shelf-life, and sensory quality. J. Sci. Food Agric. 2024, 104, 1784–1792. [Google Scholar] [CrossRef]
- Rios-Corripio, G.; Morales-de la Peña, M.; Welti-Chanes, J.; Guerrero-Beltrán, J.Á. Pulsed electric field processing of a pomegranate (Punica granatum L.) fermented beverage. Innov. Food Sci. Emerg. Technol. 2022, 79, 103045. [Google Scholar] [CrossRef]
- Ziaiifar, A.M.; Dezyani, A.; Mokhtari, Z.; Aghajanzadeh, S.; Arjeh, E. Response surface optimization of pulsed electric field processed kiwi–carrot juice: Enzyme inactivation and evaluation of physicochemical and nutritional properties. J. Food Meas. Charact. 2024, 18, 489–499. [Google Scholar] [CrossRef]
- Li, L.; Yang, R.; Zhao, W. The effect of pulsed electric fields (PEF) combined with temperature and natural preservatives on the quality and microbiological shelf-life of cantaloupe juice. Foods 2021, 10, 2606. [Google Scholar] [CrossRef]
- Younis, M.; Mohamed Ahmed, I.A.; Ahmed, K.A.; Yehia, H.M.; Abdelkarim, D.O.; Alhamdan, A.; Elfeky, A. The effects of thermal and pulsed electric field processing on the physicochemical and microbial properties of a high-fiber, nutritious beverage from a milk-based date powder. AgriEngineering 2023, 5, 2020–2031. [Google Scholar] [CrossRef]
- Debbarma, M.; Srivastava, B. Impact of Pulsed Electric Field Pretreatment on Extraction Efficiency and Quality Attributes of Sohiong Fruit Juice. J. Food Process Eng. 2025, 48, e70038. [Google Scholar] [CrossRef]
- Cai, R.; Jing, Z.; Li, Y.; Zhong, X.; Sheng, Q.; Yue, T.; Wang, Z.; Yuan, Y. Inactivation activity and mechanism of high-voltage pulsed electric fields combined with antibacterial agents against Alicyclobacillus spp. in apple juice. Int. J. Food Microbiol. 2025, 431, 111079. [Google Scholar] [CrossRef]
- Qian, J.; Yan, G.; Huo, S.; Dai, C.; Ma, H.; Kan, J. Effects of pulsed magnetic field on microbial and enzymic inactivation and quality attributes of orange juice. J. Food Process. Preserv. 2021, 45, e15533. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; He, R.; Cui, H. Action mechanism of pulsed magnetic field against E. coli O157: H7 and its application in vegetable juice. Food Control 2019, 95, 150–156. [Google Scholar] [CrossRef]
- Qian, J.; Chen, S.; Huo, S.; Dai, C.; Zhou, C.; Ma, H. Impact of pulsed magnetic field treatment on enzymatic inactivation and quality of cloudy apple juice. J. Food Sci. Technol. 2021, 58, 2982–2991. [Google Scholar] [CrossRef]
- Pandiarajan, T.; Dharani, S.; Patra, A.; Ganapathy, S.; Balakrishnan, M.; Prasath, V.A. Optimizing Pulsed Magnetic Field Parameters for Microbial Safety and Quality in Orange Juice. J. Food Process Eng. 2025, 48, e70123. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; Cui, H. Synergistic efficacy of pulsed magnetic fields and Litseacubeba essential oil treatment against Escherichia coli O157: H7 in vegetable juices. Food Control 2019, 106, 106686. [Google Scholar] [CrossRef]
- Pimenta, F.C.; Moraes, T.C.K.; Dacanal, G.C.; Oliveira, A.L.d.; Petrus, R.R. The potential use of supercritical carbon dioxide in sugarcane juice processing. npj Sci. Food 2024, 8, 6. [Google Scholar] [CrossRef]
- Tang, Y.; Jiang, Y.; Jing, P.; Jiao, S. Dense phase carbon dioxide treatment of mango in syrup: Microbial and enzyme inactivation, and associated quality change. Innov. Food Sci. Emerg. Technol. 2021, 70, 102688. [Google Scholar] [CrossRef]
- Bertolini, F.M.; Morbiato, G.; Facco, P.; Marszałek, K.; Pérez-Esteve, É.; Benedito, J.; Zambon, A.; Spilimbergo, S. Optimization of the supercritical CO2 pasteurization process for the preservation of high nutritional value of pomegranate juice. J. Supercrit. Fluids 2020, 164, 104914. [Google Scholar] [CrossRef]
- Guangsen, T.; Jiahu, G.; Xiang, L.; Yuanju, G.; Tian, M.; Fei, F.; Xiaolong, H. Enzymatic activity, browning, physiochemical and phenolic evaluation of fruit juices subjected to high pressure-CO2 processing at different temperatures. Food Sci. Technol. Res. 2022, 28, 467–478. [Google Scholar] [CrossRef]
- Silva, E.K.; Bargas, M.A.; Arruda, H.S.; Vardanega, R.; Pastore, G.M.; Meireles, M.A.A. Supercritical CO2 processing of a functional beverage containing apple juice and aqueous extract of Pfaffia glomerata roots: Fructooligosaccharides chemical stability after non-thermal and thermal treatments. Molecules 2020, 25, 3911. [Google Scholar] [CrossRef]
- Trych, U.; Buniowska, M.; Skąpska, S.; Kapusta, I.; Marszałek, K. Bioaccessibility of antioxidants in blackcurrant juice after treatment using supercritical carbon dioxide. Molecules 2022, 27, 1036. [Google Scholar] [CrossRef]
- Juliato, R.A.; Brito, I.P.C.; Silva, E.K. Ultrasound-driven chemical and biochemical changes in jabuticaba juice: Phenolic compounds, volatile profile and inactivation of polyphenol oxidase, peroxidase and pectin methylesterase. Food Chem. 2025, 481, 144037. [Google Scholar] [CrossRef]
- Alvarado-López, D.A.; Parralejo-Sanz, S.; Lobo, M.G.; Cano, M.P. Ultrasound Pasteurization of Brazil Nut Beverage Enriched with Opuntia stricta var. dillenii Extract: Effects on Quality and Bioactives. LWT 2025, 228, 118055. [Google Scholar] [CrossRef]
- Feng, M.; Chitrakar, B.; Chen, J.; Islam, M.N.; Wei, B.; Wang, B.; Zhou, C.; Ma, H.; Xu, B. Effect of multi-mode thermosonication on the microbial inhibition and quality retention of strawberry clear juice during storage at varied temperatures. Foods 2022, 11, 2593. [Google Scholar] [CrossRef]
- Wei, L.; Shi, C.; Hu, C.Y.; Alharbi, N.K.; Shami, A.; Al-Asmari, F.; Al-Joufi, F.A.; Meng, Y. Inactivation effect of phloretin with ultrasound on Staphylococcus aureus and Escherichia coli extended the shelf-life of cloudy apple juice. Food Biosci. 2025, 68, 106498. [Google Scholar] [CrossRef]
- Li, L.; Su, H.; Pang, L.; Pan, Y.; Li, X.; Xu, Q.; Song, J.; Qiao, L. Thermosonication enhanced the bioactive, antioxidant, and flavor attributes of freshly squeezed tomato juice. Ultrason. Sonochem. 2025, 115, 107299. [Google Scholar] [CrossRef]
- Hasheminya, S.-M.; Dehghannya, J. Non-thermal processing of black carrot juice using ultrasound: Intensification of bioactive compounds and microbiological quality. Int. J. Food Sci. Technol. 2022, 57, 5848–5858. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Xu, B.; Khan, S.; Shukat, R.; Ahmad, N.; Imran, M.; Rehman, A.; Karrar, E.; Aadil, R.M.; Korma, S.A. Impact of high-intensity thermosonication treatment on spinach juice: Bioactive compounds, rheological, microbial, and enzymatic activities. Ultrason. Sonochem. 2021, 78, 105740. [Google Scholar] [CrossRef]
- Rodríguez-Rico, D.; Sáenz-Esqueda, M.d.l.Á.; Meza-Velázquez, J.A.; Martínez-García, J.J.; Quezada-Rivera, J.J.; Umaña, M.M.; Minjares-Fuentes, R. High-intensity ultrasound processing enhances the bioactive compounds, antioxidant capacity and microbiological quality of melon (Cucumis melo) juice. Foods 2022, 11, 2648. [Google Scholar] [CrossRef] [PubMed]
- Challana, V.; Kaimal, A.M.; Shirkole, S.; Sahoo, A.K. Comparative analysis and investigation of ultrasonication on juice yield and bioactive compounds of kinnow fruit using RSM and ANN models. Sci. Rep. 2025, 15, 9859. [Google Scholar] [CrossRef]
- Xu, B.; Feng, M.; Chitrakar, B.; Cheng, J.; Wei, B.; Wang, B.; Zhou, C.; Ma, H. Multi-frequency power thermosonication treatments of clear strawberry juice: Impact on color, bioactive compounds, flavor volatiles, microbial and polyphenol oxidase inactivation. Innov. Food Sci. Emerg. Technol. 2023, 84, 103295. [Google Scholar] [CrossRef]
- Silva, C.N.d.; Carmo, J.R.d.; Nunes, B.V.; Demoliner, F.; Souza, V.R.d.; Bastos, S.C. Synergistic Effect of Thermosonication on the Stability of Bioactive Compounds and Antioxidant Activity of Blackberry Juice. Foods 2025, 14, 901. [Google Scholar] [CrossRef]
- Amaro, K.C.; Popović, V.; Tadini, C.C.; Koutchma, T. Ultraviolet processing of coconut water at multiple wavelengths in continuous flow: Shelf-life and quality effects. J. Food Eng. 2025, 392, 112469. [Google Scholar] [CrossRef]
- Orjuela, M.A.; Moreno, F.L.; Cordoba, N.; Osorio, C.; Ruiz-Pardo, R.Y. Effect of ultraviolet irradiation on the shelf life and chemical composition of cold brew coffee. Food Bioprod. Process. 2025, 149, 58–69. [Google Scholar] [CrossRef]
- Ceballos, M.W.; Jafari, S.; Fikry, M.; Shiekh, K.A.; Kijpatanasilp, I.; Assatarakul, K. Changes in quality attributes of coconut water treated with UV-radiation and nisin during cold storage: Kinetics modelling and shelf-life prediction. Food Control 2025, 167, 110801. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Sackey, A.S.; Wu, M.; Xiao, L. Effect of pulsed light treatment on the phytochemical, volatile, and sensorial attributes of lactic-acid-fermented mulberry juice. Int. J. Food Prop. 2018, 21, 213–228. [Google Scholar] [CrossRef]
- Kwaw, E.; Osae, R.; Apaliya, M.T.; Alolga, R.N.; Sackey, A.S.; Yongkun, M.; Tchabo, W.; Obikyembi, V. Effect of optimized pulsed light treatment conditions on microbiological safety, phytochemical and sensory properties of lactic-acid-fermented mulberry juice. J. Food Meas. Charact. 2024, 18, 1878–1888. [Google Scholar] [CrossRef]
- Wang, B.; Wei, W.; Zhang, Y.; Xu, H.; Ma, H. Decontamination and quality assessment of freshly squeezed grape juice under spiral continuous flow-through pulsed light (SCFPL) treatment. J. Food Process. Preserv. 2022, 46, e16186. [Google Scholar] [CrossRef]
- Balaji, A.S.; Allahdad, Z.; Lacroix, M. Effect of γ-irradiation in combination with natural antimicrobial formulation on microbial inactivation, protein digestibility and quality of mothers’ milk. Int. Dairy J. 2022, 131, 105386. [Google Scholar] [CrossRef]
- Yan, J.; Li, Y.; He, H.; Liu, G.; Tang, X.; Wang, Y.; Peng, X. Effects of electron beam irradiation on the sensory qualities and bioactive compounds of broccoli sprout juice. Food Res. Int. 2025, 199, 115365. [Google Scholar] [CrossRef]
- Wen, C.; Peng, Y.; Zhang, L.; Chen, Y.; Yu, J.; Bai, J.; Yang, K.; Ding, W. Effect of electron beam irradiation on raw goat milk: Microbiological, physicochemical and protein structural analysis. J. Sci. Food Agric. 2024, 104, 7713–7721. [Google Scholar] [CrossRef]
- Wahia, H.; Zhou, C.; Sarpong, F.; Mustapha, A.T.; Liu, S.; Yu, X.; Li, C. Simultaneous optimization of Alicyclobacillus acidoterrestris reduction, pectin methylesterase inactivation, and bioactive compounds enhancement affected by thermosonication in orange juice. J. Food Process. Preserv. 2019, 43, e14180. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Sackey, A.S.; Apaliya, M.T.; Xiao, L.; Wu, M.; Sarpong, F. Ultrasonication effects on the phytochemical, volatile and sensorial characteristics of lactic acid fermented mulberry juice. Food Biosci. 2018, 24, 17–25. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, D.; Xi, P.; Cai, T.; Cao, X.; Liu, H.; Li, J. Effects of temperature-controlled ultrasound treatment on sensory properties, physical characteristics and antioxidant activity of cloudy apple juice. LWT 2021, 142, 111030. [Google Scholar] [CrossRef]
- Mu, Q.; Su, H.; Zhou, Q.; Xiao, S.; Zhu, L.; Xu, X.; Pan, S.; Hu, H. Effect of ultrasound on functional properties, flavor characteristics, and storage stability of soybean milk. Food Chem. 2022, 381, 132158. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Hong, C.; Telebielaigen, S.; Nsor-Atindana, J.; Duan, Y.; Zhong, F. Optimization of spiral continuous flow-through pulse light sterilization for Escherichia coli in red grape juice by response surface methodology. Food Control 2019, 105, 8–12. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Antimicrobial mechanism of pulsed light for the control of Escherichia coli O157: H7 and its application in carrot juice. Food Control 2019, 106, 106751. [Google Scholar] [CrossRef]
- Wang, B.; Mahoney, N.E.; Khir, R.; Wu, B.; Zhou, C.; Pan, Z.; Ma, H. Degradation kinetics of aflatoxin B1 and B2 in solid medium by using pulsed light irradiation. J. Sci. Food Agric. 2018, 98, 5220–5224. [Google Scholar] [CrossRef]
- Zheng, Q.; Tian, W.; Yue, L.; Wang, S.; Zhang, Y.; Chen, Z.; Qi, W.; Zhang, C.; Yan, W.; Kong, Q. Effect of gamma irradiation on sensory and aroma compounds of soaked bayberry jiu. Radiat. Phys. Chem. 2024, 224, 111990. [Google Scholar] [CrossRef]
- Akbari, S.; Pajohi-Alamoti, M.; Karami, M. Ozonation of the pasteurized skim milk to extend the shelf life: Evaluation of the chemical and microbial properties. Sci. Res. 2023, 142, 15–30. [Google Scholar]
- Iqbal, A.; Nadeem, M.; Ainee, A.; Ameer, K.; Ather Nadeem, M.; Sultan, M.; Malik, F.; Siddeeg, A. The impact of ozonation on the physicochemical properties, antioxidant potential and shelf life of Kinnow (Citrus Reticulata Blanco) juice. Int. J. Food Prop. 2022, 25, 2551–2560. [Google Scholar] [CrossRef]
- Iqbal, A.; Nadeem, M.; Ainee, A.; Qureshi, T.M.; Khalid, W.; Malik, F.; Rehman, S.-U.; Rehman, A.; Zubair Khalid, M.; Ahmad, N. Quality evaluation of ozone-processed Kinnow (Citrus reticulata Blanco) juice at ambient temperature. Int. J. Food Prop. 2023, 26, 2420–2432. [Google Scholar] [CrossRef]
- Lee, B.J.; Ting, A.S.Y.; Thoo, Y.Y. Impact of ozone treatment on the physico-chemical properties, bioactive compounds, pectin methylesterase activity and microbiological properties of watermelon juice. J. Food Sci. Technol. 2022, 59, 979–989. [Google Scholar] [CrossRef]
- Morsy, M.K. Impact of Thermal Treatment and Ozone on Quality Parameters and Shelf-Life of Mango Nectar. Ann. Agric. Sci. Moshtohor 2021, 59, 933–948. [Google Scholar] [CrossRef]
- EL-Geddawy, Y.I.; Badr, S.A.; EL-Geddawy, D.I. Effect Of Ozone and Combined Ozone-Freezing Treatments on The Physico-Chemical Properties and Enzymatic Activity of Sugarcane Juice. Food Technol. Res. J. 2024, 6, 83–98. [Google Scholar] [CrossRef]
- Kumar, S.; Pipliya, S.; Srivastav, P.P.; Srivastava, B. Shelf life and storage stability of cold plasma treated kiwifruit juice: Kinetic models. Int. J. Food Prop. 2024, 27, 1–23. [Google Scholar] [CrossRef]
- Pipliya, S.; Kumar, S.; Srivastav, P.P. Impact of cold plasma and thermal treatment on the storage stability and shelf-life of pineapple juice: A comprehensive postharvest quality assessment. Food Phys. 2024, 1, 100025. [Google Scholar] [CrossRef]
- Pipliya, S.; Kumar, S.; Srivastav, P.P. Effect of thermal and non-thermal plasma treatment on particle size distribution, protein secondary structure, fuzzy logic sensory evaluation, rheological, and selected quality attributes of pineapple juice: A comparative analysis. Food Bioproc. Technol. 2024, 17, 3615–3636. [Google Scholar] [CrossRef]
- Garimella, J.N.; Jaddu, S.; Pradhan, R.C. Effect of non–thermal plasma on physiochemical properties, antioxidant activities, morphological and crystalline structures of red dragon fruit (Hylocereus polyrhizus) juice during storage. J. Food Meas. Charact. 2025, 19, 4368–4384. [Google Scholar] [CrossRef]
- Wang, X.; Hou, M.; Liu, T.; Ren, J.; Li, H.; Yang, H.; Hu, Z.; Gao, Z. Continuous cold plasma reactor for the processing of NFC apple juice: Effect on quality control and preservation stability. Innov. Food Sci. Emerg. Technol. 2025, 100, 103905. [Google Scholar] [CrossRef]
- Abbas, H.M.; Altamim, E.A.; Salama, M.; Fouad, M.T.; Zahran, H.A. Cold plasma technology: A sustainable approach to milk preservation by reducing pathogens and enhancing oxidative stability. Sustainability 2024, 16, 8754. [Google Scholar] [CrossRef]
- Ozen, E.; Mishra, A.; Singh, R.K. Atmospheric cold plasma treatment effects on quality of cloudy apple juice during storage. J. Food Sci. 2025, 90, e70158. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Fang, Y.; Pei, Z.; Zhang, W. Coconut milk treated by atmospheric cold plasma: Effect on quality and stability. Food Chem. 2024, 430, 137045. [Google Scholar] [CrossRef]
- Pankaj, S.; Misra, N.; Alzahrani, K.J.; Alamri, A.S.; Galanakis, C.M. Cold Plasma Treatment of Orange Juice Using Multipin-Plane Electrical Discharge. J. Food Process Eng. 2025, 48, e70072. [Google Scholar] [CrossRef]
- de Castro, D.R.G.; Mar, J.M.; da Silva, L.S.; da Silva, K.A.; Sanches, E.A.; de Araújo Bezerra, J.; Rodrigues, S.; Fernandes, F.A.; Campelo, P.H. Dielectric barrier atmospheric cold plasma applied on camu-camu juice processing: Effect of the excitation frequency. Food Res. Int. 2020, 131, 109044. [Google Scholar] [CrossRef]
- Lovato, F.; Kowaleski, J.; da Silva, S.Z.; Kottwitz, L.B.M.; Martin, C.A.; Tiuman, T.S.; Zara, R.F. Watermelon juice microfiltration: Optimization to maximize antioxidant compounds and influence mineral content. J. Food Sci. Technol. 2025, 1–10. [Google Scholar] [CrossRef]
- Abdullah, S.; Karmakar, S.; Mishra, S.; Pradhan, R.C. Ultrafiltration of cashew apple juice using hollow fibers for shelf life extension: Process optimization, flux modelling and storage study. J. Food Meas. Charact. 2023, 17, 2182–2192. [Google Scholar] [CrossRef]
- Panigrahi, C.; Mondal, M.; Karmakar, S.; Mishra, H.N.; De, S. Shelf life extension of sugarcane juice by cross flow hollow fibre ultrafiltration. J. Food Eng. 2020, 274, 109880. [Google Scholar] [CrossRef]
- Tangüler, H.; Erten, H. Effect of Microfiltration, Storage Time and Temperature on Properties of Shalgam Juices. Akad. Gıda 2023, 21, 211–219. [Google Scholar] [CrossRef]
- Kallel, F.; Chaibi, Z.; Neifar, M.; Chaabouni, S.E. Effect of enzymatic treatments and microfiltration on the physicochemical properties and antioxidant activities of two Tunisian prickly pear juices. Process Biochem. 2023, 132, 140–151. [Google Scholar] [CrossRef]
- Daneluz, J.; da Silva, G.F.; Duarte, J.; Turossi, T.C.; dos Santos, V.; Baldasso, C.; Daneluz, A.C. Membrane separation process of microfiltration applied to the filtration of kombuchas. Food Chem. Adv. 2023, 3, 100451. [Google Scholar] [CrossRef]
- Abdullah, S.; Karmakar, S.; Pradhan, R.C.; Mishra, S. Pressure-driven crossflow microfiltration coupled with centrifugation for tannin reduction and clarification of cashew apple juice: Modeling of permeate flux decline and optimization of process parameters. J. Food Process. Preserv. 2022, 46, e16497. [Google Scholar] [CrossRef]
- Zhang, J.; Chitrakar, B.; Wang, Y.; Adhikari, B.; Xu, B.; Gao, X.; Zhou, C.; Xu, T.; Wang, B. Application of high-voltage electrospray system for non-thermal microbial inactivation of raw bovine milk. J. Food Eng. 2023, 342, 111372. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.; Wang, B.; Yang, D.; Liao, J.; Zhang, M. Effect of high-voltage electrospray on the inactivation, induced damage and growth of microorganisms and flavour components of honey raspberry wine. Int. J. Food Microbiol. 2023, 388, 110060. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, B.; Xu, J. Selective enzyme inactivation in a simulated system and in cabbage juice using electrospray technology. Innov. Food Sci. Emerg. Technol. 2022, 75, 102875. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.; Wang, B.; Liao, J.; Zhang, M. Non-thermal microbial inactivation of honey raspberry wine through the application of high-voltage electrospray technology. Food Bioproc. Technol. 2022, 15, 177–189. [Google Scholar] [CrossRef]
- Xue, L.; Xiao, Z.; Luo, Y.; Chen, Y.; Zhang, H.; Yang, N.; Xu, X.; Jin, Y. Effects of induced electric field on quality enhancement and shelf-life extension of bayberry juice during storage. Innov. Food Sci. Emerg. Technol. 2025, 100, 103917. [Google Scholar] [CrossRef]
- Rajeswari; Vidyalakshmi, R.; Radhakrishnan, M.; Tito Anand, M. Exploring the Impact of Moderate Electric Field Treatment on Grape Juice: Physicochemical Characteristics and Microbial Reduction. J. Food Process Eng. 2025, 48, e70067. [Google Scholar] [CrossRef]
- Alsaedi, A.W.M.; Al-Hilphy, A.R.; Al-Mousawi, A.J.; Gavahian, M. Non-thermal pasteurization of milk by elongated electrode moderate electrical field: Chemical and sensory analysis during cold storage and shelf-life determination. Innov. Food Sci. Emerg. Technol. 2024, 94, 103647. [Google Scholar] [CrossRef]
- Zhu, W.-L.; Chang, C.-K.; Tsai, S.-Y.; Gavahian, M.; Santoso, S.P.; Hsieh, C.-W. High voltage electric field as a green technology preserves the appearance of apple juice during cold storage. Sustain. Chem. Pharm. 2024, 41, 101676. [Google Scholar] [CrossRef]
- Chaudhary, K.; Khalid, S.; AlMasoud, N.; Alomar, T.S.; Ansar, S.; Ghazal, A.F.; Aït-Kaddour, A.; Aadil, R.M. Impact of ultrasonication, ozonation, and their combination on the preservation of novel clean-label functional drink of strawberry-cantaloupe incorporated with Spirulina platensis and orange peel extracts. Ultrason. Sonochem. 2025, 120, 107455. [Google Scholar] [CrossRef]
- Markovinović, A.B.; Stulić, V.; Putnik, P.; Janči, T.; Pavlić, B.; Milošević, S.; Herceg, Z.; Khaneghah, A.M.; Kovačević, D.B. Optimizing pulsed electric field and high-power ultrasound treatments to preserve anthocyanin stability and physicochemical quality in stored strawberry juice. Qual. Assur. Saf. Crops Foods 2025, 17, 129–142. [Google Scholar] [CrossRef]
- Panigrahi, C.; Mishra, H.N.; De, S. Combined ultrafiltration and ozone processing of sugarcane juice: Quantitative assessment of polyphenols, investigation of storage effects by multivariate techniques and shelf life prediction. Food Chem. Adv. 2023, 2, 100214. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Li, Z.; Xu, S.; Hettinga, K.; Zhou, P. Retaining bioactive proteins and extending shelf life of skim milk by microfiltration combined with Ultraviolet-C treatment. LWT 2021, 141, 110945. [Google Scholar] [CrossRef]
- Bebek Markovinović, A.; Stulić, V.; Putnik, P.; Birkić, A.; Jambrović, M.; Šaško, D.; Ljubičić, J.; Pavlić, B.; Herceg, Z.; Bursać Kovačević, D. Pulsed electric field (PEF) and high-power ultrasound (HPU) in the hurdle concept for the preservation of antioxidant bioactive compounds of strawberry juice—A chemometric evaluation—Part I. Foods 2023, 12, 3172. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, Y.; Zhao, L.; Liao, X. Processing of chestnut rose juice using three-stage ultra-filtration combined with high pressure processing. LWT 2021, 143, 111127. [Google Scholar] [CrossRef]
- Faisal Manzoor, M.; Ahmed, Z.; Ahmad, N.; Karrar, E.; Rehman, A.; Muhammad Aadil, R.; Al-Farga, A.; Waheed Iqbal, M.; Rahaman, A.; Zeng, X.A. Probing the combined impact of pulsed electric field and ultra-sonication on the quality of spinach juice. J. Food Process. Preserv. 2021, 45, e15475. [Google Scholar] [CrossRef]
- Atchaya, P.; Anandakumar, S.; Kirankumar, M.; Santhosh, K. Membrane assisted Pulsed Electric Field (M-PEF) a novel technique for preservation of functional and biological properties of sugarcane juice. J. Phys. Conf. Ser. 2024, 2801, 012025. [Google Scholar] [CrossRef]
- Li, Y.; Padilla-Zakour, O.I. Evaluation of pulsed electric field and high-pressure processing on the overall quality of refrigerated Concord grape juice. LWT 2024, 198, 116002. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Li, Z.; Xu, S.; Zhang, J.; Hettinga, K.; Zhou, P. Effects of microfiltration combined with ultrasonication on shelf life and bioactive protein of skim milk. Ultrason. Sonochem. 2021, 77, 105668. [Google Scholar] [CrossRef]
- Liu, G.; Carøe, C.; Qin, Z.; Munk, D.M.; Crafack, M.; Petersen, M.A.; Ahrné, L. Comparative study on quality of whole milk processed by high hydrostatic pressure or thermal pasteurization treatment. LWT 2020, 127, 109370. [Google Scholar] [CrossRef]
- Panigrahi, C.; Mishra, H.N.; De, S. Ozone treatment of ultrafiltered sugarcane juice: Process optimization using multi-objective genetic algorithm and correlation analysis by multivariate technique. LWT 2022, 154, 112861. [Google Scholar] [CrossRef]
- Sampedro, F.; McAloon, A.; Yee, W.; Fan, X.; Geveke, D. Cost analysis and environmental impact of pulsed electric fields and high pressure processing in comparison with thermal pasteurization. Food Bioprocess Technol. 2014, 7, 1928–1937. [Google Scholar] [CrossRef]
- Soni, A.; Samuelsson, L.M.; Loveday, S.M.; Gupta, T.B. Applications of novel processing technologies to enhance the safety and bioactivity of milk. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4652–4677. [Google Scholar] [CrossRef]
- Picart-Palmade, L.; Cunault, C.; Chevalier-Lucia, D.; Belleville, M.-P.; Marchesseau, S. Potentialities and limits of some non-thermal technologies to improve sustainability of food processing. Front. Nutr. 2019, 5, 130. [Google Scholar] [CrossRef]
| Liquid Food | Processing Conditions | Key Findings | Ref. |
|---|---|---|---|
| High-pressure processing | |||
| Sea buckthorn juice | 500 MPa for 5 min | Total plate count, mold, and yeast count were ≤10 CFU/mL for 0 to 9 days. Total phenols, total carotenoids, and vitamin C were higher in high-pressure processing. | [51] |
| Cow milk | 600 MPa for 10 min | Successfully retained all vitamins, minerals, and extend the shelf life beyond 60 days of storage. | [52] |
| Cloudy hawthorn berry juice | 300 and 600 MPa for 2 and 6 min | High-pressure processing enhanced the shelf life for at least 150 days. | [53] |
| Aronia berry juice | 600 MPa for 5 min | Improved microbial shelf life by at least 5 times at refrigerated storage and 10 times at room temperature. Successfully retained hydroxycinnamic acid, anthocyanin, and flavonols content for 24 weeks. | [54] |
| Fermented pomegranate beverage | 500, and 550 for 10 min, and 600 MPa for 5 min | High hydrostatic pressure successfully contained microbial growth for 42 days of storage. Total flavonoids, total phenolic compounds, total anthocyanins, and antioxidant activity were slightly increased after processing. | [55] |
| Carrot-orange juice blends | 200, 300, and 400 MPa for 1 to 5 min | High-pressure processing attained higher than 6 log reduction in L. innocua, and blends were more stable during 28 days of storage. Total phenolic, total carotenoid, and ascorbic acid content did not change significantly. | [56] |
| Raw coconut water | 593 MPa for 3 min | No detection of inoculated pathogens was reported and microbial count remained about 2log with no detectable sign of deterioration through 120 days of storage. | [57] |
| Sour cherry juice | 300, 400, and 500 MPa for 5, 10, and 20 min | Reported higher total flavonoid bioaccessibility and improved bioaccessibility for most phenolic fractions at 500 MPa. Revealed lower total antioxidant capacity except at 500 MPa for 20 min conditions. | [58] |
| Mulberry juice | 200, 400, and 600 MPa for 10, 20, and 30 min | Treatment at 200 MPa/10 min significantly inactivated PPO and POD enzymes and retained anthocyanin content. | [59] |
| Rosa roxburghii Tratt juice | 100, 200, 300, 400 and 500 MPa for 5,10, 15, 20 and 25 min | High-pressure processing (>100 MPa) increased the level of ascorbic acid, total phenolics, total flavonoids, gallic acid, catechin, and ferulic acid. | [60] |
| High-pressure homogenization | |||
| Pear juice | 50, 100, 150, and 200 MPa at 4, 20, 30, 40, 60, and 80 °C | Ultra-high-pressure homogenization treatment reduced the bacteria, yeast, and mold count, and total phenolic content and antioxidant activity was increased. | [61] |
| Mixed juice (carrot, apple, peach) | 25, 100, 140, 180 MPa, Pass 1 and 2, inlet temperature 25 °C, and 40 °C | High-pressure homogenization reduced total plate count, yeast, and mold by 4 log10 and 3 log10, respectively. | [62] |
| kiwifruit juice | 200 MPa for 2 and 3 cycles | Treatment at 200 MPa for 3 cycles greatly increased the shelf life for more than 40 days at refrigeration storage and increased the total phenolic content availability. | [63] |
| Sheep and cow milk | 200, and 250 MPa, inlet temperature 85 °C | Treatment at 250 MPa achieved more than 5 log CFU/mL reduction for B. subtilis and B. pumilus spores. | [64] |
| Human milk | 150 to 300 MPa | Ultra-high-pressure homogenization at 200 MPA attained a lethality > 5 log and did not significantly reduce the immunoglobulin. | [65] |
| Blueberry juice | 200, 250, and 300 MPa, 4 °C and 22 °C, flow rates (0.75, 1.125, and 1.5 L/min) | Treatment at 300 MPa, 4 °C, and 1.5 L/min yielded 54% more anthocyanins. In addition, 22 °C favored ascorbic acid retention. Lower PPO activity was also recorded. | [66] |
| Pomegranate Juice | 50, 100, and 150 MPa | High-pressure homogenization treatment at 150 MPa successfully preserved the total phenolic content and antioxidant capacity (DPPH-ARA, FRAP, ABTS-ARA). | [67] |
| Peach and carrot juices | 25, 50, 100, 150, 200, 250, and 300 MPa | Combined juice treated at 200 MPa retained more concentration of polyphenols and carotenoids. | [68] |
| Pulsed electric field | |||
| Blood orange juice | Energy density 180 kJ/kg and treatment time of ≤ 3000 µs | Pulsed electric field significantly inactivated the microbes, extended the shelf life (15–20 days), and preserved the bioactive compounds. | [69] |
| Orange juice | Flow rate 60 mL/min, field strengths 19 kV/cm, 1250 pulses per second, pulse width 2 µs, and total treatment time of 181 µs | Pulsed electric field combined with antimicrobial caps exhibited lowest mold and yest populations. No significant differences in vitamin C and total phenolic compounds after treatments. Stability was maintained for 5 weeks at 10 °C but vitamin C was lost. | [70] |
| Shalgam juice | Field strength of 1 kV cm−1 | Pulsed electric field-treated sample exhibited lower lactic acid bacteria and total aerobic mesophilic bacteria after 70 days of storage. Insignificant effect was recorded for antioxidant activity and total phenolic content. | [71] |
| Pomegranate fermented beverage | Field strength of 11.7 and 18.8 kV/cm, and 15 and 20 µs of pulse-width | A reduced microbial load and slight reduction in bioactive compounds were observed during storage. | [72] |
| Kiwi-carrot juice | Field strength 35.86 kV/cm for 2400 µs | PME was successfully inactivated, and degradation of ascorbic acid and phenolic compounds was observed. | [73] |
| Cantaloupe juice | Electric field intensity 15, 20, 25, and 30 kV/cm for 400 µs and treatment times 200, 400, 600 and 800 µs with 20 kV/cm electric field strength | Pulsed electric field combined with natural preservatives improved the inactivation of S. cerevisiae, prolonged shelf life, and better preserved vitamin C levels. | [74] |
| Milk-based beverage | Levels of pulses 20, 50, and 80 | Beverage treatment was performed at 80 pulses with no preservative, and stored for 6 days at 5 °C. | [75] |
| Sohiong juice | Field strength 10 kV/cm for 60 s | Significant increase of 11%, 20%, 12% and 89% was recorded for total anthocyanin content, total phenolic content, DPPH inhibition, and ascorbic acid, respectively. | [76] |
| Apple juice | 9.6 kV/cm field strength, 20 min treatment time, 1000 Hz frequency, and 50% duty ratio | Pulsed electric fields combined with antibacterial agents successfully reduced bacteria and maintained organic acids in apple juice. | [77] |
| Pulsed magnetic field | |||
| Orange juice | 5 to 7 T and 5 to 30 pulses | Pulsed magnetic field reduced the activity of yeast, mesophilic bacteria, and mold. Partially inactivated POD and PME activities and slightly reduced the antioxidant capacity, phenolic compounds, and ascorbic acid. | [78] |
| Tomato, lettuce, carrot, and cucumber juices | 0 to 8 T and 10 to 60 pulses | Pulsed magnetic field indicated superior antibacterial effect against E. coli O157:H7 in all the vegetable juices. | [79] |
| Cloudy apple juice | 5 to 7 T and 5 to 30 pulses | PPO, POD, and PME activities were successfully inhibited, and significant decrease in ascorbic acid was observed. No change in DPPH activity and phenols was recorded. | [80] |
| Orange Juice | 2, 4, and 6 T for 5, 10, and 15 min | Successfully reduced the bacterial load, yeast counts, and mold counts at 4 T for 15 min. | [81] |
| Cucumber, carrot, spinach, and bitter gourd juices | 3 times under 8 T and 60 pulses | Pulsed magnetic fields combined with Litseacubeba essential oil completely destroyed the E. coli O157:H7. | [82] |
| Liquid Food | Processing Conditions | Key Findings | Ref. |
|---|---|---|---|
| High-pressure carbon dioxide | |||
| Sugarcane juice | Pressure 74–351 bar, temperature 33–67 °C, and holding time 30–70 min | Successful reduction was observed for yeast, molds, mesophiles, lactic acid bacteria, PPO, and POD. Supercritical carbon dioxide combined with mild temperature preserved the cane juice. | [83] |
| Mango in syrup | 20 MPa, 60 °C for 30 min | Dense phase carbon dioxide completely inactivated pathogens, PPO, and POD. The shelf life was documented to be less than 86 days, and higher content of total phenols and vitamin C was recorded. | [84] |
| Pomegranate juice | 12.7 MPa, 45 °C, 40 min | Optimal conditions produced a microbiological stable juice up to 28 days of storage at 4 °C. Total phenol content was maintained, while antioxidant activity was decreased during storage. | [85] |
| Peach, apple, and pear juices | 20 MPa, 20, 30, 40, 50, 60 and 70 °C, 20 min | Lower enzymatic activity and slight decline in phenolic compounds were documented. | [86] |
| Apple juice enriched with Pfaffia glomerata root extract | Pressure 8 and 21 MPa, temperature 40 and 60 °C, CO2 volume ratio 20 and 50% | Supercritical carbon dioxide treatment successfully maintained the fructooligosaccharide content. | [87] |
| Blackcurrant juice | 10, 30, and 60 MPa for 10 min at 45 °C | Supercritical carbon dioxide successfully enhanced the stability of total anthocyanins, vitamin C, and antioxidant capacity. | [88] |
| Ultrasound treatment | |||
| Jabuticaba juice | 6.3, 15.9, 25.5, and 36 W/cm2 | HIUS effectively inactivated PPO, POD, and PME, retained anthocyanin, exhibit phenolic stability, increased gallic acid, and maintained ellagic acid levels. | [89] |
| Brazil nut beverage enriched with Opuntia stricta var. dillenii extract | Amplitude 20–80%, 2–12 min | Ultrasounds effectively inactivated microbes and preserved the total phenolic, total flavonoid, and total betaxanthin content. | [90] |
| Strawberry clear juice | DEUP 20/40 kHz frequency at 60 °C for 5 min (Sequential operation mode), FSDUP 20 + 40 kHz at 60 °C for 5 min (simultaneous operation mode) | DEUP and FSDUP ultrasound treatment increased the storage period up to 14 and 21 days, respectively. DPPH activity, ascorbic acid, anthocyanins, flavonoids, and phenols were reduced during storage. | [91] |
| Cloudy apple juice | 300, 500, and 700 W for 10 min | Ultrasound combined with phloretin resulted in reduction in Staphylococcus aureus and Escherichia coli and maintained the quality after 14 days of storage. | [92] |
| Tomato juice | 50, 60, and 70 °C for 5, 10, and 15 min | Thermosonication greatly inactivated the microbes and enhanced the content of total phenols, flavonoids, ascorbic acid, and lycopene. Antioxidant capacity was maintained. | [93] |
| Black carrot juice | 0, 4, 8 and 12 min at 24 kHz | Considerably decreased the microbial population and increased the antioxidant properties, ascorbic acid, and phenolic content. | [94] |
| Spinach juice | 200 W, 400 W, and 600 W, 30 kHz, 20 min, 60 °C | Thermosonication significantly reduced the microbial load, and inactivated PPO and POD. Improved the total flavonols, total phenolic, total flavonoid, anthocyanin, chlorophyll, carotenoid, and antioxidant activities (FRAP and DPPH assay). | [95] |
| Melon juice | Intensities 27 and 52 W/cm2, time 10 and 30 min, duty cycle 30 and 75% | Microbial load was greatly reduced, total carotenoids and antioxidant capacities were enhanced, total phenolic content was reduced. | [96] |
| Kinnow fruit juice | Frequency 40 kHz, power 120 W, time 30–90 min, temperature 30–70 °C | Optimal ultrasound treatment enhanced the total phenolic content, whereas vitamin C and antioxidant activity decreased. | [97] |
| Clear strawberry juice | 60 °C for 5 min, 55 °C for 15 min (DCU), 60 °C for 15 min, 55 °C for 20 min (SDU) | Thermosonication (SDU-60 °C) reduced the microbial load, inactivated the PPO enzyme, and reduced the loss of total phenolic content. | [98] |
| Blackberry juice | Temperature 64 and 86 °C, amplitude 60% and 90%, and time 114 s and 517 s | Thermosonication (60%, 86 °C) delivered excellent retention of antioxidant activity, phenolic compounds, and anthocyanins. | [99] |
| Radiation processing | |||
| Coconut water | Excimer lamp 222 nm, UV LED sources at 257, 267, and 286 nm | UV-treated sample achieved 12 days of shelf life. Ascorbic acid and phenolics were decreased with increase in fluence. | [100] |
| Cold brew coffee | UV wavelength of 254 nm, flux 12 L min−1, 18 °C | UV-C extended the shelf life up to 14 days (4 °C) and presented higher content of chlorogenic acid and total polyphenols. | [101] |
| Coconut water | Dose of 11.52 J/mL, and flow rate of 20.8 mL/s | UV-C radiation combined with nisin produced microbiologically stable coconut water up to 24 days of refrigerated storage. A consistent reduction in total flavonoids, total phenolic compounds, and antioxidant activity was documented. | [102] |
| Mulberry juice | 14 J/cm2 for 2, 4, and 8 s | Pulsed light decreased the microbial load and slightly reduced the anthocyanin content at exposure time of 8 s. | [103] |
| Mulberry juice | Optimized conditions: Exposure length of 6.5 s, light source 10 cm, and sample width 1 mm. | Pulsed light at optimal condition increased the total phenolics and total flavonoids, whereas no difference was observed in total anthocyanin content. | [104] |
| Grape juice | Fluences of 13, 40, and 66 J/cm2 and flow rate 60 mL/min | Pulsed light successfully reduced E. coli and slightly affected total phenolic, ascorbic acid, and anthocyanins content. | [105] |
| Mothers’ milk | 5 kGy combined with 4 antimicrobial formulations | γ-irradiation combined with 3 and 4 formulations eliminated all tested pathogens, and immunoglobulins was not altered significantly. | [106] |
| Broccoli sprout juice | 2, 4, 6, 8, 10, and 12 kGy | Sulforaphane content was reduced with the increase in electron beam irradiation dose. | [107] |
| Goat milk | 2, 3, 5, and 7 kGy | Electron beam irradiation (2 kGy) significantly reduced the total microbial count. | [108] |
| Liquid Food | Processing Conditions | Key Findings | Ref. |
|---|---|---|---|
| Ozone processing | |||
| Pasteurized skim milk | 1.5, 5, and 10 ppm | Total viable count was reduced and shelf life was extended up to 15 days of storage. | [117] |
| Kinnow juice | 150 mg/h for 5, 10, and 15 min | Microbiologically stable juice was produced and stored for 3 months. Total flavonoids, phenolics, and antioxidant potential was increased. | [118] |
| Kinnow juice | 150 mg/h for 5, 10, 15 min | Microbial population was reduced and total flavonoids, phenols, antioxidant activity, and DPPH activity were moderately enhanced. | [119] |
| Watermelon juice | Flow rate of 1 L/min for 5, 10, 15, 20, and 25 min | Microorganism were reduced, PME activity was not altered, and lycopene, ascorbic acid, and total phenolic contents were degraded. | [120] |
| Mango nectar | 40 ppm, 70 °C for 30 min and 20 ppm, 76 °C for 30 min | Microbial load was reduced and highest vitamin C, total carotenoids, and total phenolic values were recorded. | [121] |
| Sugarcane juice | Flow rate 10 g/h, time 5, 10, 15 min, and temperature 18–20 °C | PPO activity was reduced with longer exposure time, and total phenol and flavonoid contents were reduced. | [122] |
| Cold plasma | |||
| Kiwifruit juice | Optimized: 30 kV/5 mm/6.7 min Extreme: 30 kV/2 mm/10 min | Shelf life of optimized cold plasma-treated sample was 60, 80, and 100 days at 25 °C, 15 °C, and 5 °C, respectively. More than 50% ascorbic acid loss was documented. | [123] |
| Pineapple juice | Optimized: 38 kV/631 s Extreme: 45 kV/900 s | Shelf life of optimized cold plasma-treated sample was 25, 50, and 90 days at 25 °C, 15 °C, and 5 °C, respectively. Bioactive substances reduced during storage. | [124] |
| Pineapple juice | Optimized: 38 kV/631 s Extreme: 45 kV/900 s | Optimized non-thermal plasma extend the shelf life and retained ascorbic acid, total phenol, antioxidant capacity, and flavonoids. | [125] |
| Dragon fruit juice | Voltage level 10, 20, 30 kV and time 10, 20, 30 min | Atmospheric cold plasma produced microbiologically stable juice for 28 days at 4 °C and increased total phenols. Antioxidant levels and flavonoids were decreased. | [126] |
| NFC apple juice | Voltage 65 kV, Air 25%, and gas flow rate 470 L/h. | Successfully reduced E. coli for 30 days of storage. Ascorbic acid, phenolics, and antioxidant properties were better preserved. Also, chlorogenic acid, l-epicatechin, and cianidanol were preserved. | [127] |
| Raw buffalo milk | Voltage 70 kV for 15 min | Cold plasma reduced the microbial load, enhanced the microbial stability, and prolonged the shelf life. | [128] |
| Cloudy apple juice | Gas feed-simulated air (20% oxygen, 80% nitrogen, Gas feed-combined gas (10% oxygen, 90% oxygen), and duration 30 to 150 s | Atmospheric cold plasma reduced A. acidoterrestris spores, mold and yeast counts increased during storage, and antioxidant activity remained stable, whereas total phenolic content increased post-storage. | [129] |
| Coconut milk | Voltage 50 kV, 60 kV, 70 kV, and time 30 s, 60 s, and 90 s | Atmospheric cold plasma successfully reduced the colony count. | [130] |
| Orange juice | Voltage 20, 25, and 30 kV for 10 min | Enzymes and microbes were successfully inactivated, and ascorbic acid content was degraded. | [131] |
| Camu-camu juice | Frequency 200, 420, 583, 698 and 960 Hz for 15 min | PPO, POD, and anthocyanins considerably degraded, and concentration of ascorbic acid was increased at higher excitation frequencies. | [132] |
| Membrane processing | |||
| Watermelon juice | Pore size 0.05 mm, area 4 m2, pressure 1 bar, and temperature 37 °C | Successfully reduced the microbial load and comparable shelf life to pasteurized juice (14 days). Effectively concentrated flavonoids, lycopene, and phenolic compounds. | [133] |
| Cashew apple juice | Molecular weight cut-offs 5, 10, 30, 50 kDa, and pressures 35, 69, 103, and 138 kPa | Ultrafiltered juice was markedly stored for 12 weeks. Tannic acid increased, whereas ascorbic acid and total polyphenol content reduced during storage. | [134] |
| Sugarcane juice | Molecular weight cut-offs 30 kDA, pressure 104 kPa, and cross flow rate 30 L/h | Clarified juice was stable for 9 weeks (4 °C) with 80% recovery of polyphenols. | [135] |
| Shalgam Juice | Filter diameter 0.45 µm | Microfiltration reduced the microbial population and increased the shelf life when stored at 4 °C. | [136] |
| Prickly pear juices (red/green fruit) | Enzyme concentration 4.75 U/mL and 4.65 U/mL; Time 37 min and 35 min; Temperature 55 °C for both fruits | Microfiltration combined with enzymatic treatment yielded microbiologically safe juices for 90 days of storage (4 °C). | [137] |
| Kombuchas drink | Porosity 10−7 to 10−5 m, pressure 1 bar, rejection rate 99.99%, filtration surface 0.027 m2, and hydraulic permeability 127.20 L h−1 m−2 bar−1 | Microfiltration reduced the fermentation microorganism (53.93%). | [138] |
| Cashew apple juice | Optimal conditions: pore size 0.2 µm and 138 kPa | Microfiltration combined with centrifugation preserved the 87% ascorbic acid content. | [139] |
| Combined Technologies | Liquid Food | Processing Conditions | Key Findings | Ref. |
|---|---|---|---|---|
| Ultrasonication, Ozonation | Strawberry-cantaloupe functional drink | Power 300 W, frequency 25 kHz, 25 °C, and 10 min; Concentration 300 mg/L, flow rate 1 L/min, 25 °C, and 10 min | Combined treatment extended the shelf life for two months and retained the bioactive compounds (total phenolics, total flavonoids, total carotenes, ascorbic acid, DPPH, ABTS, and FRAP). | [148] |
| Pulsed electric field, High-power ultrasound | Strawberry juice | 30 kV/cm, 100 Hz, 1.5–4.5 min; Amplitude 25%, pulse 50%, 2.5–7.5 min | Combined treatment markedly improved anthocyanin stability during 7 days of storage. | [149] |
| Ultrafiltration, Ozone processing | Sugarcane juice | 30 kDa polysulphone hollow fiber, transmembrane pressure 104 kPa, cross flow rate 30 L/h; Concentration 3 ppm, flow rate 4.6 L/min, time 8.2 min | Combination of ultrafiltration and ozone successfully achieved microbial and enzyme inactivation. Caffeic acid was decreased and vitexin and its derivatives did not reduce significantly. | [150] |
| Microfiltration, UV-C | Skim milk | Pressure 75 kPa, cross-flow velocity 7 m/s, membrane area 0.312 m2, pore size 1.4 µm; UV-C dosage 13.1–39.3 mJ/cm2 | Combination of microfiltration and UV-C extended the refrigerated shelf life for 40 days. Bioactive serum proteins were not damaged. | [151] |
| Pulsed electric field, High-power ultrasound | Strawberry juice | 30 kV cm−1, 100 Hz for 1.5, 3, and 4.5 min; Amplitude 25%, Pulse 50%, for 2.5, 5.0, and 7.5 min. | Combined technologies documented that hydroxycinnamic acids and total phenols were the most stable bioactive compounds. Condensed tannin and flavonol stability depend upon duration of both treatments. | [152] |
| Ultra-filtration, High-pressure processing | Chestnut rose juice | 100, 5, and 5 kDa; 500 MPa for 6 min | Combination of both technologies retained the total flavonoids, but significant reduction in kaempferol, quercetin, catechin, and myricetin was documented. | [153] |
| Pulsed electric field, Ultrasonication | Spinach juice | Pulse frequency 1 kHz, electric field strength 9 kV/cm, temperature 30 °C, flow rate 60 mL/min, and time 335 µs; Frequency 40 kHz, temperature 30 °C, time 21 min, and radiating power 200 W | Inactivation of PPO and POD was enhanced, and highest value of vitamin C, carotenoids, flavonols, anthocyanins, flavonoids, antioxidant capacity, DPPH, phenolic, and total chlorophyll was achieved with combined technologies. | [154] |
| Ultra-filter membrane, Pulsed electric field | Sugarcane juice | Pressure 1 bar, and pore size 10 kDa; Field strength 20, 30, and 40 kV/cm, pulse width 100, 150, and 200 µs | Reduction in PPO and POD activity and retention of ascorbic acid content were documented. | [155] |
| High-pressure processing, Pulsed electric field | Concord grape juice | 600 MPa, 5 °C, and 3 min; 0.85 kV/cm and 300 pulses | Combined treatment reduced the microbial load, enhanced the shelf life, and increased the antioxidant activity and antioxidant contents. | [156] |
| Microfiltration, Ultrasonication | Skim milk | Pore size 1.4 µm, length 500 mm, area 0.312 m2, pressure 75 kPa, and cross flow velocity 7.0 m/s; Output power 720 W, pulse time 5 s, intermittent time 5 s, and total ultrasonic time 3, 9, or 15 min | Combination of the two techniques removed the bacteria, extended the shelf life for at least 40 days, and retained the bioactive proteins (IgG, IgA, and IgM). | [157] |
| Microfiltration, High-pressure processing | whole milk | Pore size 1.4 µm; 600 MPa for 5 min | Combined treatment reduced the total bacterial and E. coli count to 4 log units. | [158] |
| Ultrafiltration, Ozone treatment | Sugarcane juice | Hollow fiber membranes 30 kDa (TMP of 104 kPa and CFR of 30 L/h); Ozone concentration 3.12 ppm, flow rate 4.58 L/min, and time 8.2 min. | Hurdle technology reduced the spoilage rate and phenolic content degradation. | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arslan, M.; Zareef, M.; Afzal, M.; Tahir, H.E.; Li, Z.; Aalim, H.; Abaker, H.M.A.; Zou, X. Innovative Non-Thermal Processing Technologies for Shelf Life Extension and Retention of Bioactive Compounds in Liquid Foods: Current Status and Future Prospects. Foods 2025, 14, 2953. https://doi.org/10.3390/foods14172953
Arslan M, Zareef M, Afzal M, Tahir HE, Li Z, Aalim H, Abaker HMA, Zou X. Innovative Non-Thermal Processing Technologies for Shelf Life Extension and Retention of Bioactive Compounds in Liquid Foods: Current Status and Future Prospects. Foods. 2025; 14(17):2953. https://doi.org/10.3390/foods14172953
Chicago/Turabian StyleArslan, Muhammad, Muhammad Zareef, Mubrrah Afzal, Haroon Elrasheid Tahir, Zhihua Li, Halah Aalim, Hamza M. A. Abaker, and Xiaobo Zou. 2025. "Innovative Non-Thermal Processing Technologies for Shelf Life Extension and Retention of Bioactive Compounds in Liquid Foods: Current Status and Future Prospects" Foods 14, no. 17: 2953. https://doi.org/10.3390/foods14172953
APA StyleArslan, M., Zareef, M., Afzal, M., Tahir, H. E., Li, Z., Aalim, H., Abaker, H. M. A., & Zou, X. (2025). Innovative Non-Thermal Processing Technologies for Shelf Life Extension and Retention of Bioactive Compounds in Liquid Foods: Current Status and Future Prospects. Foods, 14(17), 2953. https://doi.org/10.3390/foods14172953





