Integrated Approach Reveals Fermented Moringa oleifera Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Screening and Compounding of Bacterial Strains
2.3. Preparation of Moringa Fermentation Extracts
2.4. Targeted Quantification of Amino Acids
2.5. Animals and Experimental Design
2.6. Sleep Behavioral Assessment
2.7. Measurement of Neurotransmitters
2.8. Brain Tissue Metabolomics Analysis
2.9. Web-Based Pharmacology
2.10. Molecular Docking
2.11. 16S rRNA Gene Sequencing Analysis
2.12. Data Analysis
3. Results
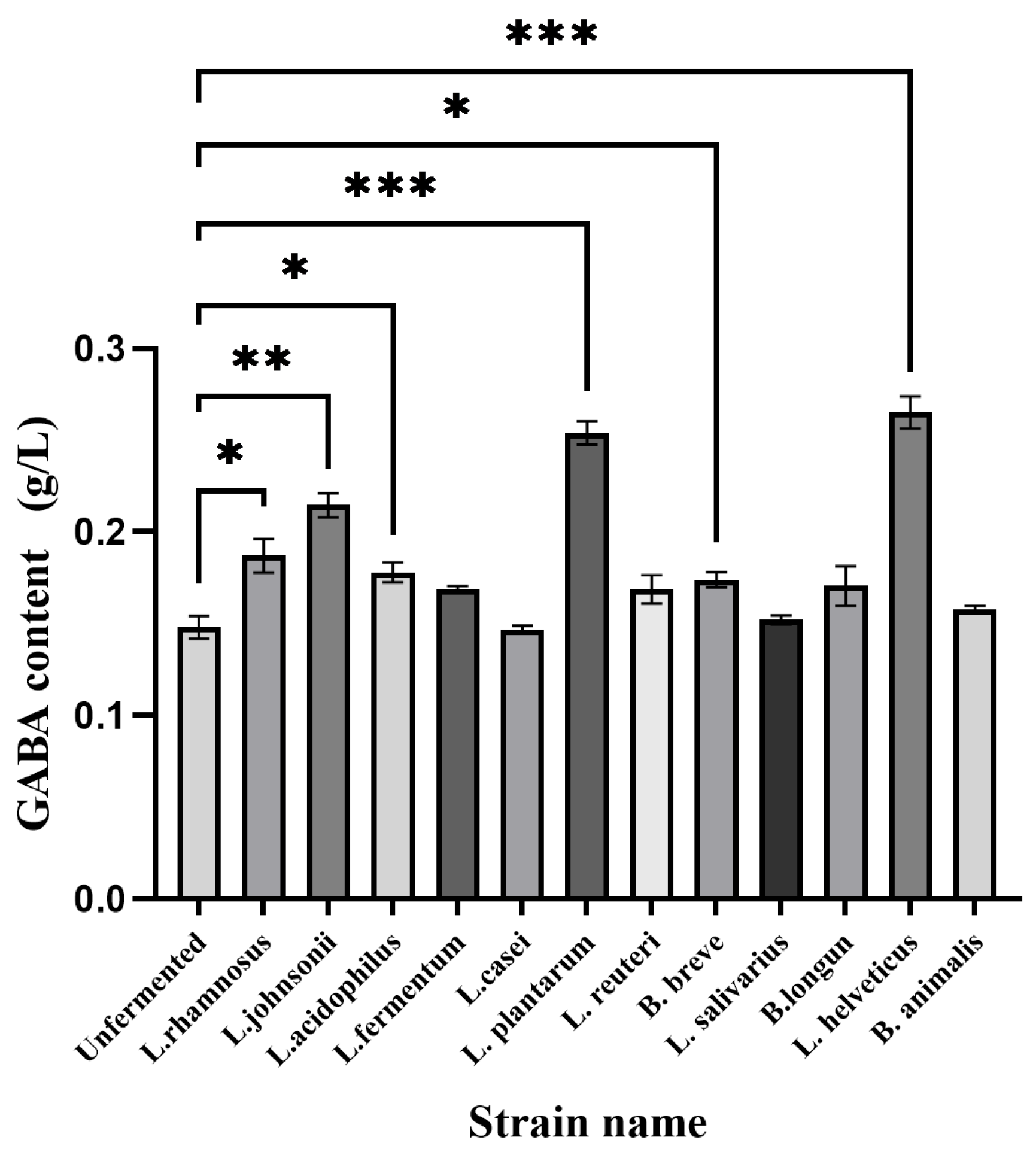
3.1. Screening and Compounding of Bacterial Strains
3.2. Quantitative Results of Amino Acids
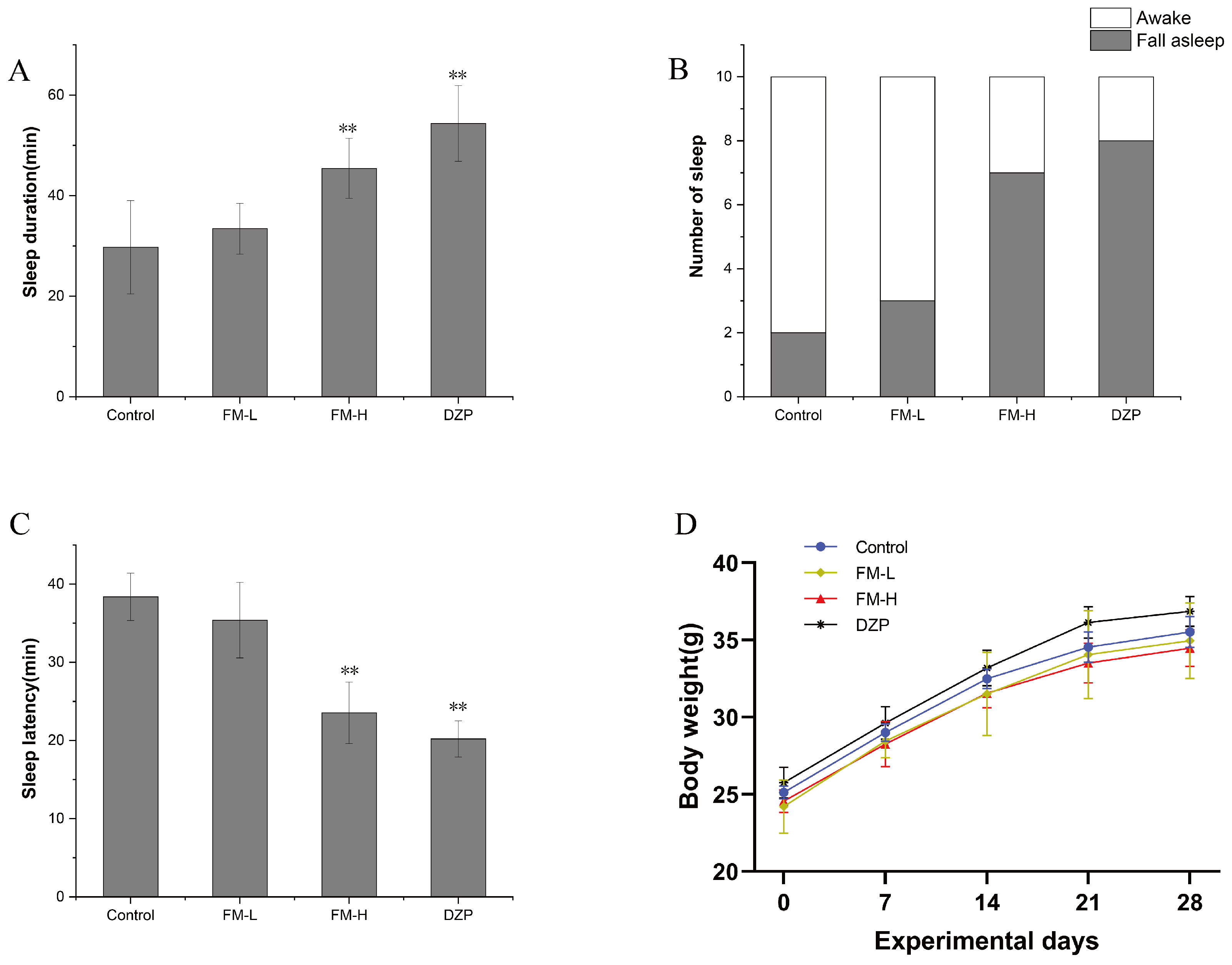
3.3. Improvement of Sleep Quality by FM in Mice
3.4. Effect of FM on Hypothalamic Neurotransmitters in Mice
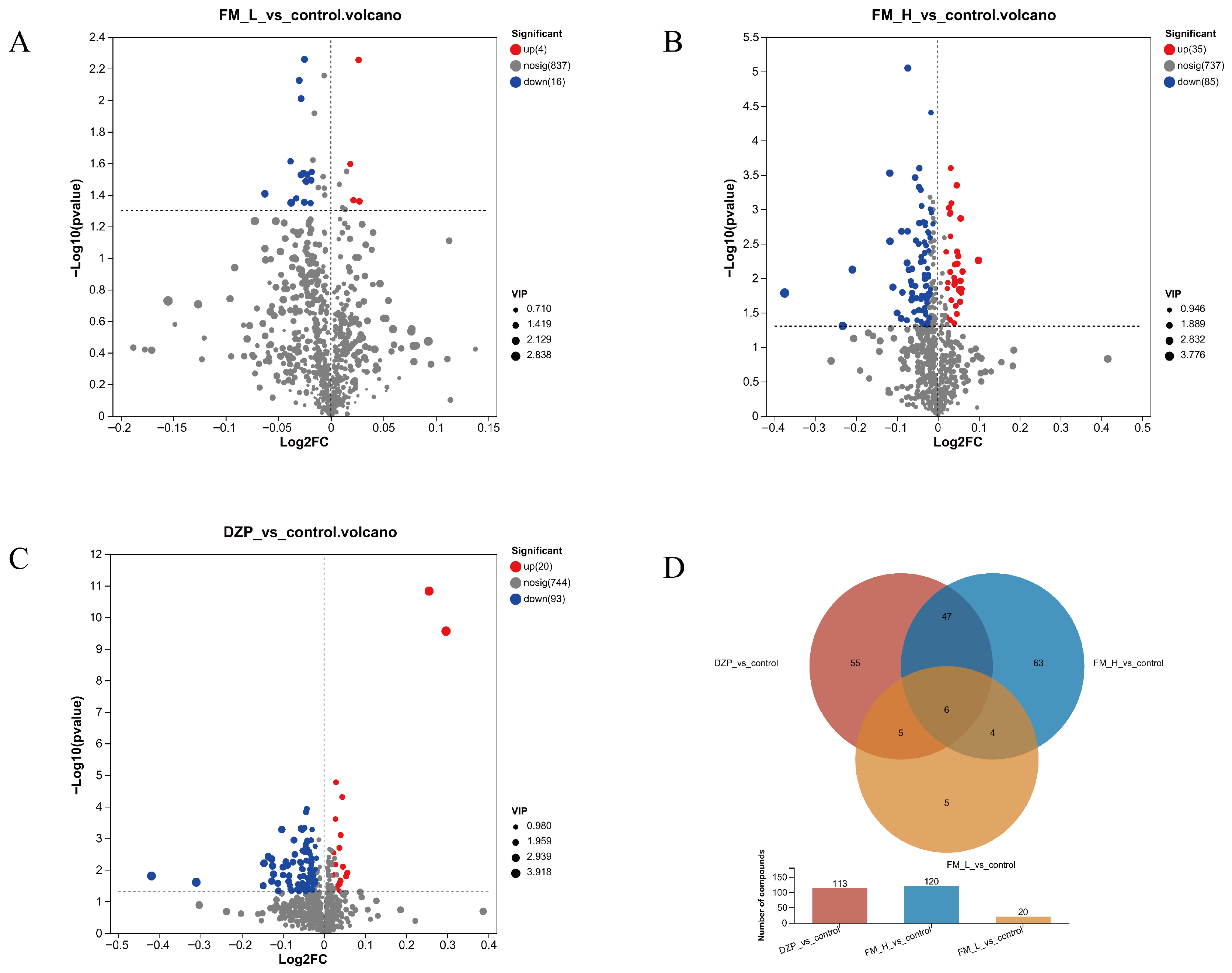
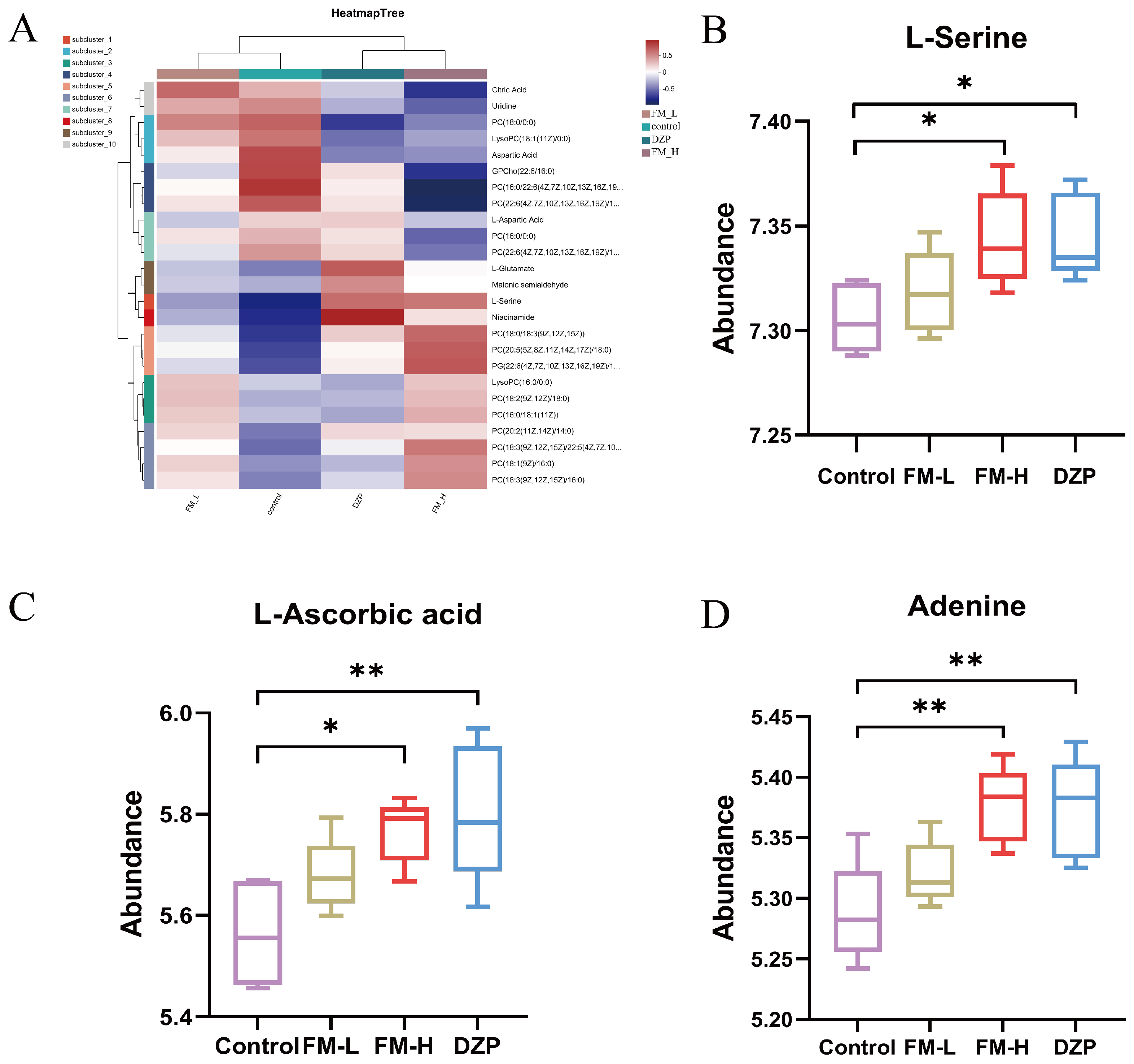
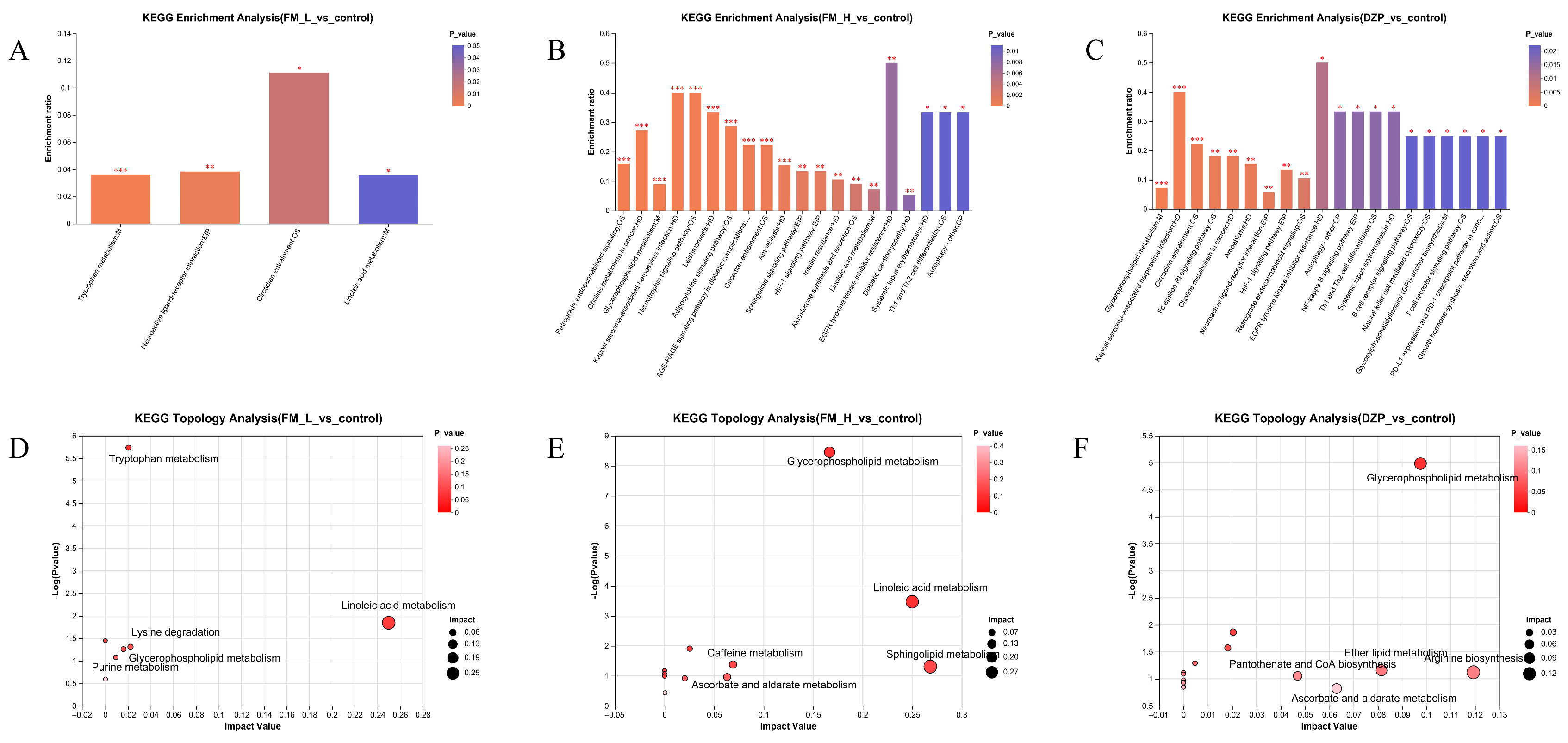
3.5. Detection and Analysis of LC-MC
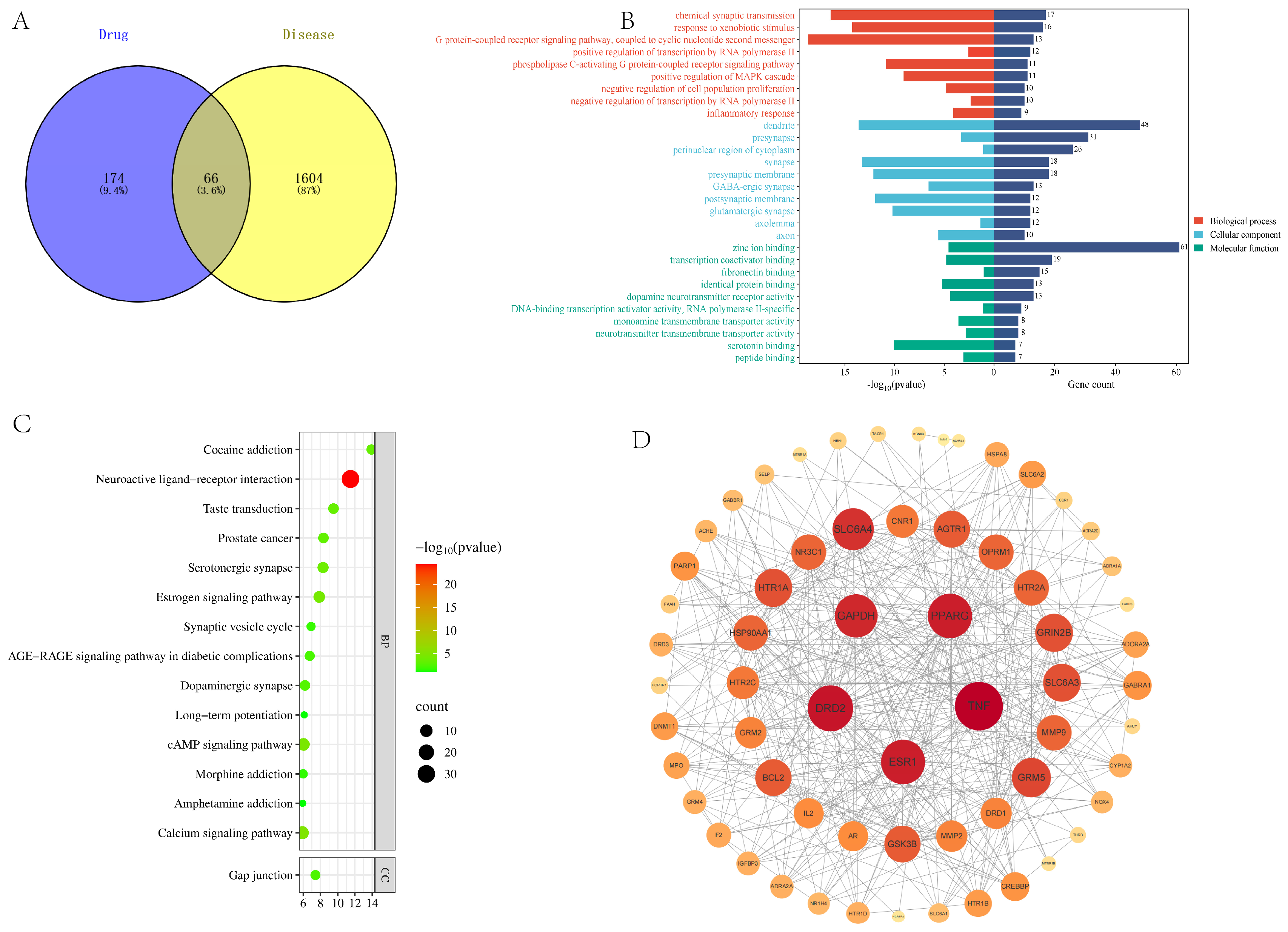
3.6. Network Pharmacology Predicts Sleep-Aiding Targets
3.7. Molecular Docking Validation
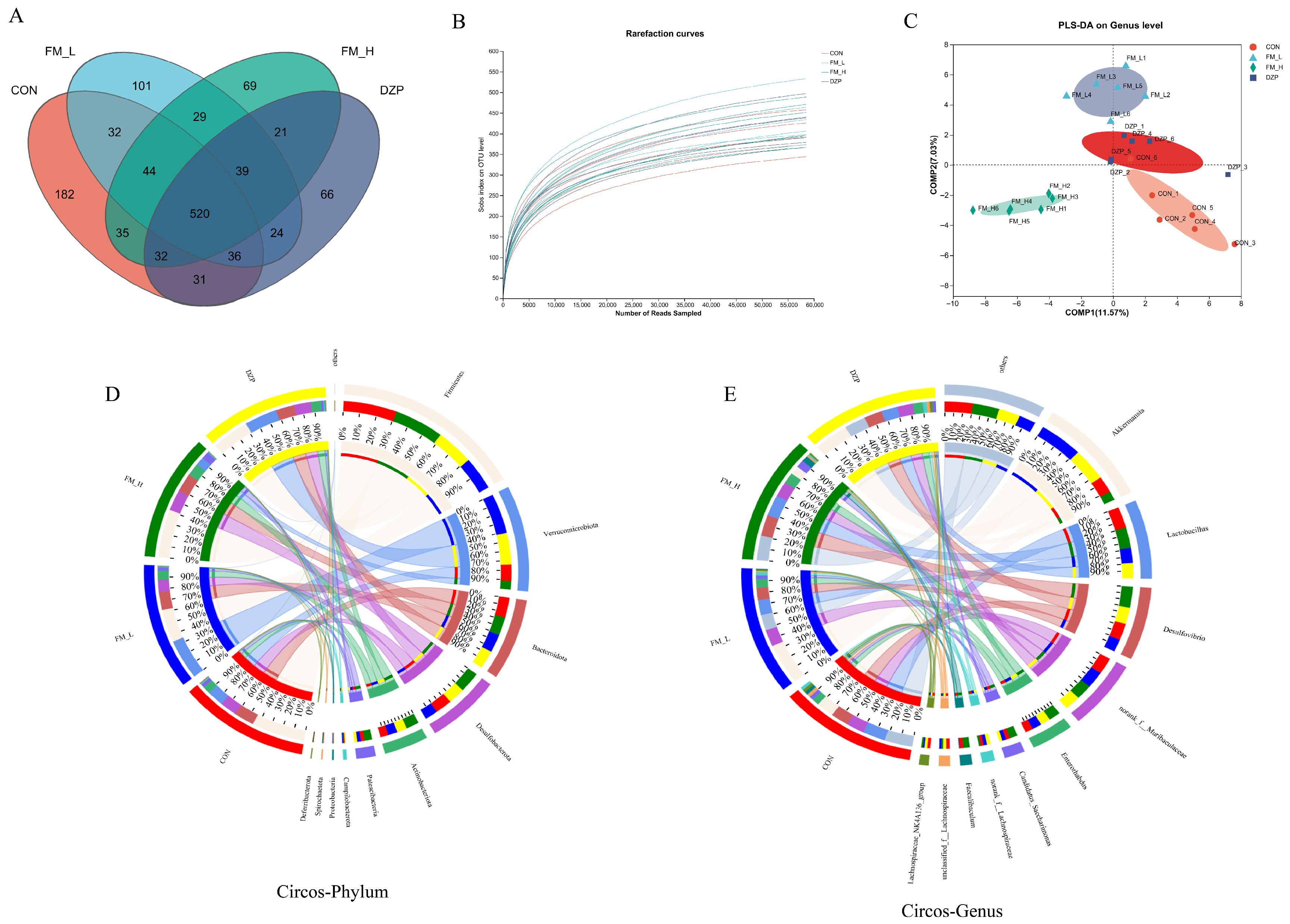
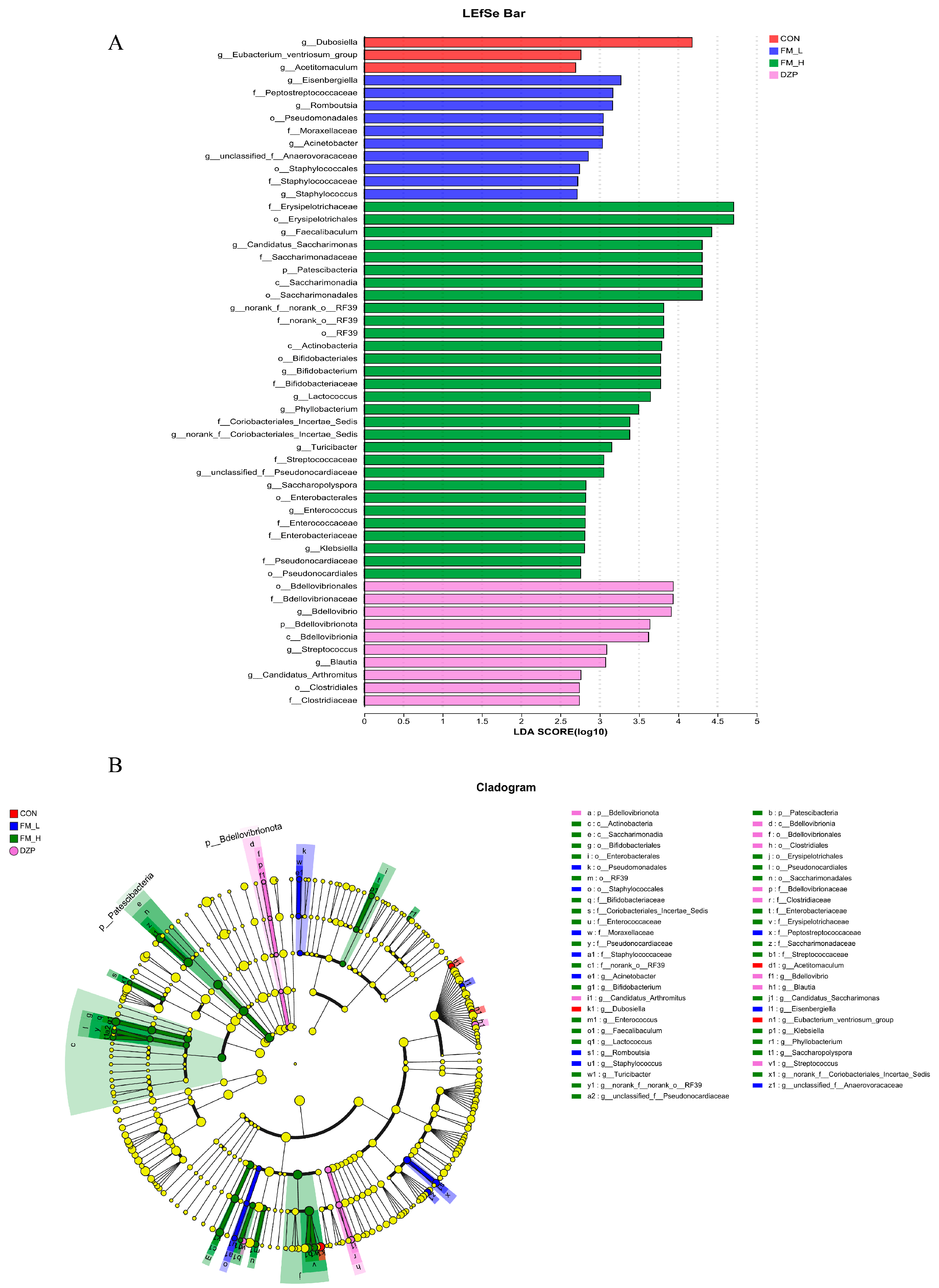
3.8. 16. S rRNA Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalish-Schur, G.B.; Somayaji, M.; Meltzer, L.J.; Cielo, C.M. Sleep problems in youth with WAGR syndrome. Sleep Med. 2025, 129, 101–104. [Google Scholar] [CrossRef]
- Palagini, L.; Manni, R.; Liguori, C.; De Gennaro, L.; Gemignani, A.; Fanfulla, F.; Ferri, R.; Nobili, L.; Ferini-Strambi, L. Evaluation and management of insomnia in the clinical practice in Italy: A 2023 update from the Insomnia Expert Consensus Group. J. Neurol. 2024, 271, 1668–1679. [Google Scholar] [CrossRef]
- Sarber, M.K.; Patil, D.R. Comorbid Insomnia and Sleep Apnea: Challenges and Treatments. Otolaryngol. Clin. N. Am. 2024, 57, 385–393. [Google Scholar] [CrossRef]
- Qian, J.; Zheng, L.; Huang, M.; Zhao, M. Potential Mechanisms of Casein Hexapeptide YPVEPF on Stress-Induced Anxiety and Insomnia Mice and Its Molecular Effects and Key Active Structure. J. Agric. Food Chem. 2024, 72, 6189–6202. [Google Scholar] [CrossRef]
- Brandt, J.; Bressi, J.; Lê, M.-L.; Neal, D.; Cadogan, C.; Witt-Doerring, J.; Witt-Doerring, M.; Wright, S. Prescribing and deprescribing guidance for benzodiazepine and benzodiazepine receptor agonist use in adults with depression, anxiety, and insomnia: An international scoping review. E Clin. Med. 2024, 70, 102507. [Google Scholar] [CrossRef]
- Tamilselvi, A.N.; Arumugam, T. Health Benefits, Therapeutic and Pharmacological Properties of Moringa- A Review. J. Pharm. Res. Int. 2019, 25, 1–11. [Google Scholar] [CrossRef]
- Wei, S.; Chen, R.; Liu, X.; Ma, H.; Peng, Y.; Wu, X.; An, Y.; Wang, X.; Luo, P. Aromatherapy was used to explore the sedative and hypnotic effects of Moringa seed essential oil on insomnia rats. Food Sci. Nutr. 2024, 12, 10463–10476. [Google Scholar] [CrossRef]
- de Siqueira Patriota, L.L.; de Lima, B.R.F.; de Oliveira Marinho, A.; da Costa, J.A.; Coelho, L.C.B.B.; Paiva, P.M.G.; da Rosa, M.M.; Napoleão, T.H. The anxiolytic-like activity of water-soluble Moringa oleifera Lam. lectin is mediated via serotoninergic, noradrenergic, and dopaminergic neurotransmission. Brain Disord. 2023, 9, 100066. [Google Scholar] [CrossRef]
- Bian, X.; Wang, L.; Ma, Y.; Yu, Y.; Guo, C.; Gao, W. A Flavonoid Concentrate from Moringa Oleifera Lam. Leaves Extends Exhaustive Swimming Time by Improving Energy Metabolism and Antioxidant Capacity in Mice. J. Med. Food 2024, 27, 887–894. [Google Scholar] [CrossRef]
- Shi, H.; Yang, E.; Yang, H.; Huang, X.; Zheng, M.; Chen, X.; Zhang, J. Dynamic changes in the chemical composition and metabolite profiles of drumstick (Moringa oleifera Lam.) leaf flour during fermentation. LWT 2022, 155, 112973. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, Z.-Y.; Chen, J.; Chen, K.; Mao, X.; Liu, Q.; Sun, Y. Acute Sleep-Wake Cycle Shift Results in Community Alteration of Human Gut Microbiome. mSphere 2020, 5, e00914–e00919. [Google Scholar] [CrossRef]
- Su, H.; Zhang, C.; Zou, X.; Lu, F.; Zeng, Y.; Guan, H.; Ren, Y.; Yuan, F.; Xu, L.; Zhang, M.; et al. Jiao-tai-wan inhibits inflammation of the gut-brain-axis and attenuates cognitive impairment in insomnic rats. J. Ethnopharmacol. 2020, 250, 112478. [Google Scholar] [CrossRef]
- Molina, G.E.S.; Ras, G.; Barone, G.; Fernández-Varela, R.; da Silva, D.F.; Jacobsen, C.; Duedahl-Olesen, L.; Hansen, E.B.; Bang-Berthelsen, C.H. Multiphasic and mixture lactic acid bacteria screening approach for the removal of antinutrients and off-flavors present in a pea, oat and potato blend. Food Res. Int. 2024, 197, 115200. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.; Zhou, R.; Ma, M. Comprehensive identification of molecular profiles related to sensory and nutritional changes in Mongolian cheese during storage by untargeted metabolomics coupled with quantification of free amino acids. Food Chem. 2022, 386, 132740. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.; Zhang, Y.; Ding, M.; Sun, B.; Su, G.; Zhao, Y. The effects of linalool acupoint application therapy on sleep regulation. RSC Adv. 2021, 11, 5896–5902. [Google Scholar] [CrossRef]
- Presset, A.; Bodard, S.; Lefèvre, A.; Millet, A.; Oujagir, E.; Dupuy, C.; Iazourène, T.; Bouakaz, A.; Emond, P.; Escoffre, J.-M.; et al. Metabolomic profile of cerebral tissue after acoustically-mediated blood-brain barrier opening in a healthy rat model: A focus on the contralateral side. Front. Mol. Neurosci. 2024, 17, 1383963. [Google Scholar] [CrossRef]
- Ahmed, M.; Hamed, M.M.; Sayed, M.; El-Rashedy, A.A.; El-Dean, A.M.K.; Hassan, M.H.A.; Mady, M.F.; Tolba, M.S. Synthesis, antimicrobial activity, molecular docking and molecular dynamics studies of novel bioactive compounds derived from propylthiouracil. J. Mol. Struct. 2025, 1333, 141779. [Google Scholar] [CrossRef]
- Kong, X.; Zhou, X.; Li, R.; Kang, Q.; Hao, L.; Zhu, J.; Lu, J. Sleep-improving effect and the potential mechanism of Morus alba L. on mice. Fitoterapia 2024, 179, 106205. [Google Scholar] [CrossRef]
- Olia, J.B.H.; Raman, A.; Hsu, C.Y.; Alkhayyat, A.; Nourazarian, A. A comprehensive review of neurotransmitter modulation via artificial intelligence: A new frontier in personalized neurobiochemistry. Comput. Biol. Med. 2025, 189, 109984. [Google Scholar] [CrossRef]
- Blusztajn, K.J.; Slack, E.B.; Mellott, J.T. Neuroprotective Actions of Dietary Choline. Nutrients 2017, 9, 815. [Google Scholar] [CrossRef]
- Echten-Deckert, V.G.; Herget, T. Sphingolipid metabolism in neural cells. BBA-Biomembr. 2006, 1758, 1978–1994. [Google Scholar] [CrossRef]
- Sato, Y.; Inoue, M.; Yoshizawa, T.; Yamagata, K. Moderate hypoxia induces β-cell dysfunction with HIF-1-independent gene expression changes. PLoS ONE 2014, 9, e114868. [Google Scholar] [CrossRef]
- Huang, Z.; Qu, W. Dopamine D2 Receptor and Sleep-Wake Regulation. In Proceedings of the 10th National Academic Conference of Chinese Academy of Neuroscience, Beijing, China, 8 December 2013–10 December 2025; 2013; pp. 136–137. [Google Scholar]
- Peng, W.; Wu, Z.; Song, K.; Zhang, S.; Li, Y.; Xu, M. Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons. Science 2020, 369, eabb0556. [Google Scholar] [CrossRef]
- Wen-Ting, C.; Xiakun, C.; Jin, W. Binding mechanism and dynamic conformational change of C subunit of PKA with different pathways. Proc. Natl. Acad. Sci. USA 2017, 114, E7959–E7968. [Google Scholar]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Li, J.; Yang, G.; Zhang, Q.; Liu, Z.; Jiang, X.; Xin, Y. Function of Akkermansia muciniphila in type 2 diabetes and related diseases. Front. Microbiol. 2023, 141, 172400. [Google Scholar] [CrossRef] [PubMed]
- Kagemasa, S.; Kuroda, K.; Nakai, R.; Li, Y.Y.; Kubota, K. Diversity of Candidatus Patescibacteria in Activated Sludge Revealed by a Size-Fractionation Approach. Microbes Environ. 2022, 37, ME22027. [Google Scholar] [CrossRef]
- Kaakoush, O.N. Insights into the role of Erysipelotrichaceae in the human host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef]
- Ren, Y.-M.; Xia, T.-X.; Xie, Y.-L.; Liu, Z.-H.; Kang, Z.-R.; Leng, X.-X.; Lu, S.-Y.; Zhang, L.; Chen, J.-X.; Xue, J.; et al. BCAA-producing Clostridium symbiosum promotes colorectal tumorigenesis through the modulation of host cholesterol metabolism. Cell Host Microbe 2024, 32, 1519–1535.e7. [Google Scholar] [CrossRef]
- Yang, B.; Sanches-Padilla, J.; Kondapalli, J.; Morison, S.L.; Delpire, E.; Awatramani, R.; Surmeier, D.J. Locus coeruleus anchors a trisynaptic circuit controlling fear-induced suppression of feeding. Neuron 2021, 109, 823–838.e6. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, Y.; Ou, S.; Jiang, T.; Wang, L.; Ma, N. Correction to: The effect of total sleep deprivation on working memory: Evidence from diffusion model. Sleep 2024, 47, zsae006. [Google Scholar] [CrossRef]
- Bannai, M.; Kawai, N. New Therapeutic Strategy for Amino Acid Medicine: Glycine Improves the Quality of Sleep. J. Pharmacol. Sci. 2019, 118, 145–148. [Google Scholar] [CrossRef]
- Nazarian, B.; Moghadam, E.F.; Asbaghi, O.; Khosroshahi, M.Z.; Choghakhori, R.; Abbasnezhad, A. Effect of l-arginine supplementation on C-reactive protein and other inflammatory biomarkers: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 47, 102226. [Google Scholar] [CrossRef]
- Titos, I.; Juginović, A.; Vaccaro, A.; Nambara, K.; Gorelik, P.; Mazor, O.; Rogulja, D. A gut-secreted peptide suppresses arousability from sleep. Cell 2023, 186, 1382–1397.e21. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Xu, L.; Zhu, X.-Y.; Tang, Z.-H.; Dong, Y.-B.; Yu, Z.-P.; Shang, Q.; Wang, Z.-C.; Shen, H.-W. D-serine reconstitutes synaptic and intrinsic inhibitory control of pyramidal neurons in a neurodevelopmental mouse model for schizophrenia. Nat. Commun. 2023, 14, 8255. [Google Scholar] [CrossRef]
- He, X.; Wang, Q.; Cheng, X.; Wang, W.; Li, Y.; Nan, Y.; Wu, J.; Xiu, B.; Jiang, T.; Johann, S.; et al. Lysine vitcylation is a novel vitamin C-derived protein modification that enhances STAT1-mediated immune response. Biorxiv Prepr. Serv. Biol. 2023, 188, 1858–1877. [Google Scholar]
- Fang, T.; Dong, H.; Xu, X.H.; Yuan, X.S.; Chen, Z.K.; Chen, J.F.; Qu, W.M.; Huang, Z.L. Adenosine A2A receptor mediates hypnotic effects of ethanol in mice. Sci. Rep. 2017, 7, 12678. [Google Scholar] [CrossRef]

| Amino Acid Types | UFM (ug/g) | FM (ug/g) |
|---|---|---|
| Gly | 84.13 ± 2.76 | 157.51 ± 3.73 |
| Ala | 4320.29 ± 32.35 | 3817.81 ± 104.13 |
| Ser | 2184.95 ± 50.05 | 2734.49 ± 67.93 |
| Pro | 1904.77 ± 56.07 | 1755.85 ± 48.79 |
| Val | 2306.58 ± 61.21 | 1977.63 ± 27.11 |
| Thr | 2381.01 ± 62.15 | 2055.19 ± 40.44 |
| Ile | 1566.61 ± 29.95 | 1419.81 ± 39.63 |
| Leu | 2035.86 ± 45.37 | 1954.83 ± 24.83 |
| Asn | 9664.22 ± 81.62 | 10,652.51 ± 267.69 |
| Orn | 2637.48 ± 41.99 | 22.51 ± 1.30 |
| Asp | 1845.35 ± 46.61 | 1333.30 ± 23.00 |
| Gln | 1563.28 ± 19.90 | 4220.03 ± 55.37 |
| Lys | 314.63 ± 4.65 | 602.95 ± 16.51 |
| Glu | 3433.15 ± 65.13 | 2337.14 ± 85.89 |
| Met | 18.48 ± 0.85 | 78.38 ± 0.84 |
| His | 1094.90 ± 14.81 | 1159.44 ± 19.09 |
| Phe | 3524.23 ± 64.26 | 3616.08 ± 88.18 |
| Arg | 24.35 ± 1.48 | 3315.39 ± 27.02 |
| Tyr | 806.17 ± 12.36 | 899.82 ± 27.88 |
| Trp | 1402.16 ± 37.66 | 1447.53 ± 26.25 |
| Groups | 5-HT (n/g) | Glu (µg/g) | GABA (µg/g) |
|---|---|---|---|
| Control | 183.557 ± 42.512 | 19.865 ± 7.734 | 4.702 ± 0.556 |
| FM-L | 216.362 ± 53.114 | 17.956 ± 6.498 | 4.979 ± 0.726 |
| FM-H | 272.322 ± 76.095 * | 15.814 ± 2.389 ** | 6.261 ± 0.766 ** |
| DZP | 293.082 ± 35.910 ** | 13.771 ± 2.274 ** | 6.568 ± 1.078 ** |
| Name | Formula | HD:MD | Source |
|---|---|---|---|
| 3,6,7-Trihydroxy-4′-methoxyflavone 7-rhamnoside | C22H22O10 | up | hrysanthemum morifolium |
| (S)C(S)S-S-Methylcysteine sulfoxide | C4H9NO3S | up | Allium sativum, Allium cepa |
| Cinncassiol A | C20H30O7 | down | Cinnamomum cassia |
| Palmitoyl Ethanolamide | C18H37NO2 | down | Glycine max, Helianthus annuus |
| Nepetaside | C16H26O8 | down | Nepeta cataria |
| 8(R)-Hydroperoxylinoleic acid | C18H32O4 | down | Glycine max, Helianthus annuus |
| Dodecyl Hydrogen Sulfate | C12H26O4S | down | Gleditsia sinensis, Cocos nucifera |
| Melleolide | C23H28O6 | down | Armillaria ostoyae, Armillaria gallica |
| Melatonin | C13H16N2O2 | down | Vitis vinifera, Olea europaea |
| Uric Acid | C5H4N4O3 | down | Adenine, Guanine |
| Complexes | Binding Energy (kcal/mol) | Hydrophobic Interactions | Hydrogen Bonding | Electrostatic |
|---|---|---|---|---|
| GABA/GBRA1 | −4.3 | \ | Asn414, Asp420, Arg424 | Glu277 |
| 5-HT/HTR1A | −6.8 | Tyr96 | Ala93, Gln97, Asp116 | \ |
| 5-HT/HTR2A | −6.5 | Val156, Phe340 | Phe234, Val235, Ser239 | \ |
| Glu/GRM5 | −4.8 | \ | Lys186, Phe188, Glu458, Asn459 | \ |
| 5-HT/HTR1B | −6.3 | Ile130, Cys133, Ala216, Phe330, Phe331 | Thr134 | \ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Wu, K.; Guo, Y.; Mu, H.; Sheng, J.; Tian, Y.; Liu, J.; Zhao, C.
Integrated Approach Reveals Fermented Moringa oleifera Leaves
Huang S, Wu K, Guo Y, Mu H, Sheng J, Tian Y, Liu J, Zhao C.
Integrated Approach Reveals Fermented Moringa oleifera Leaves
Huang, Si, Kuan Wu, Yuwei Guo, Hongyu Mu, Jun Sheng, Yang Tian, Jia Liu, and Cunchao Zhao.
2025. "Integrated Approach Reveals Fermented Moringa oleifera Leaves
Huang, S., Wu, K., Guo, Y., Mu, H., Sheng, J., Tian, Y., Liu, J., & Zhao, C.
(2025). Integrated Approach Reveals Fermented Moringa oleifera Leaves







