Preparation and Evaluation of Anti-Fatigue Effects of Sea Buckthorn–Wolfberry Compound Coffee
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of SWCC
2.2.1. Single Factor Tests
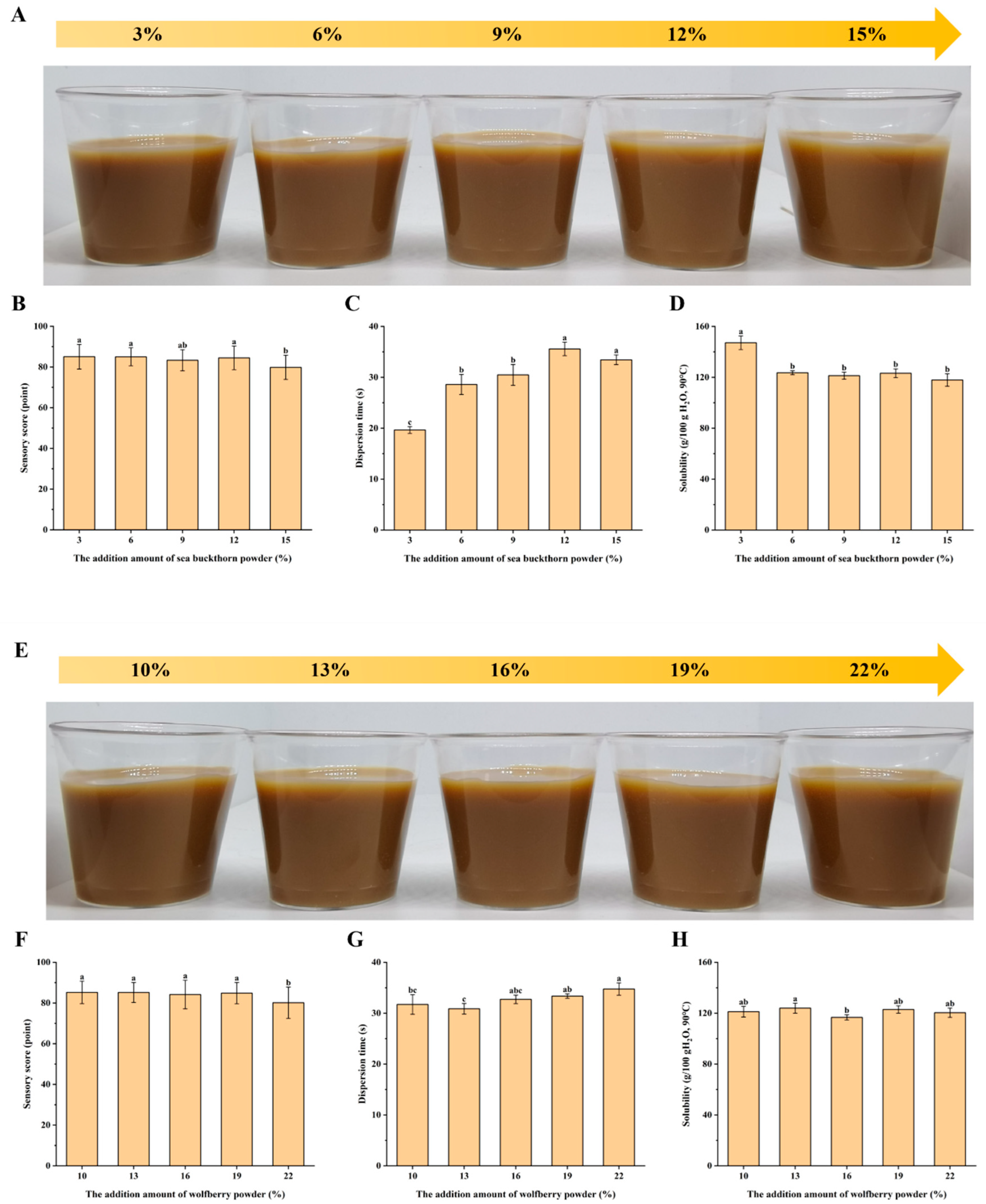
Determination of the Addition Amount of Sea Buckthorn Powder
Determination of the Addition Amount of Wolfberry Powder
2.2.2. Orthogonal Tests
2.2.3. Comprehensive Evaluation of SWCC
The Sensory Test
The Dispersion Test
The Water Solubility Test
2.3. Evaluation of Nutritional Quality and Antioxidant Activity of SWCC
2.3.1. Determination of Physical Properties
Determination of Fluidity
Determination of Clarity
Determination of Soluble Solids Content (SSC)
Determination of Chromaticity
2.3.2. Evaluation of Nutritional Quality of Compound Coffee
Preparation of the Extract
Determination of Total Flavonoid Content (TFC)
Determination of Total Phenol Content (TPC)
2.3.3. Determination of Antioxidant Capacity of SWCC
Preparation of Extract
Determination of DPPH Radical Scavenging Rate
Determination of ABTS Radical Scavenging Rate
Determination of Ferric-Reducing Antioxidant Power (FRAP)
2.4. Evaluation of Anti-Fatigue Function of SWCC
2.4.1. Experimental Animals and Treatments
2.4.2. Animal Grouping and Administration
2.4.3. Determination of Body Weight and Organ Index of Mice
2.4.4. The Weight-Bearing Swimming Test
2.4.5. Determination of Fatigue-Related Biochemical Indicators
2.5. Statistical Analysis
3. Results and Discussions
3.1. Preparation of SWCC
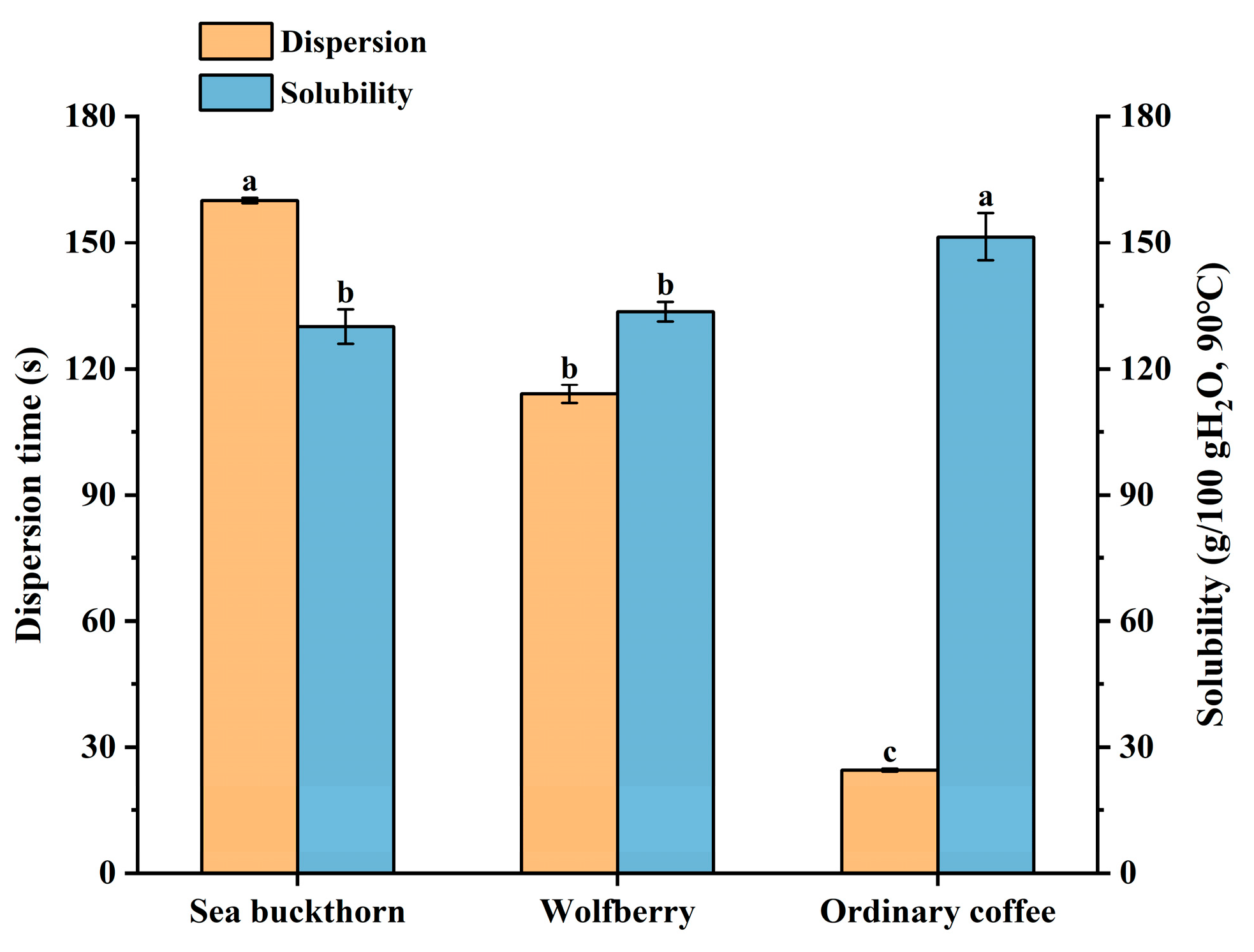
3.1.1. Properties of Raw Material Powder
3.1.2. Single Factor Test Results
3.1.3. Orthogonal Tests
3.2. Evaluation of Nutritional Quality and Antioxidant Activity of SWCC
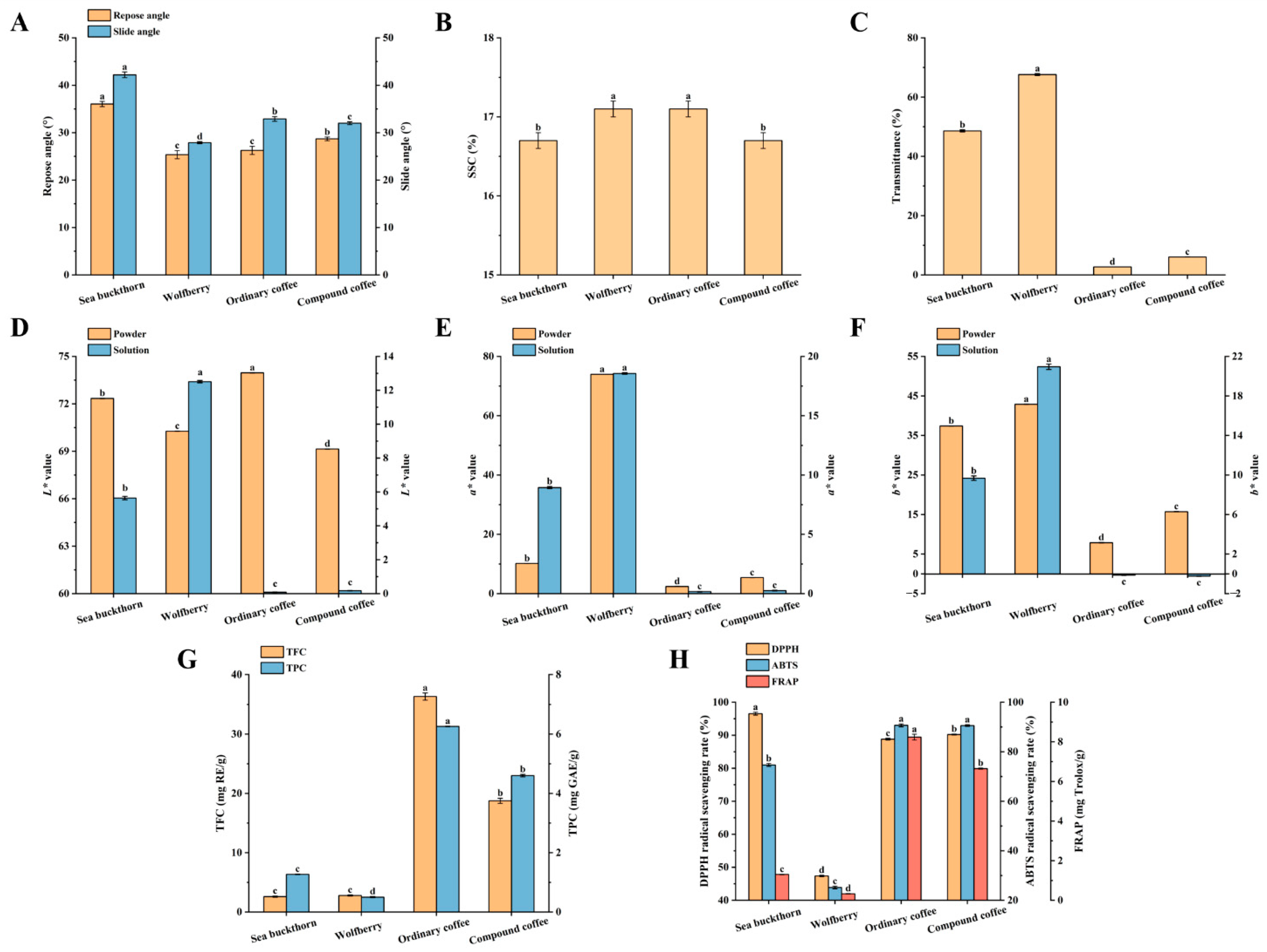
3.2.1. Determination of Physical Properties
Determination of Fluidity
Determination of Clarity
Determination of SSC
Determination of Chromaticity
3.2.2. Evaluation of Nutritional Quality of SWCC
3.2.3. Determination of Antioxidant Capacity of SWCC
3.3. Evaluation of Anti-Fatigue Function of SWCC
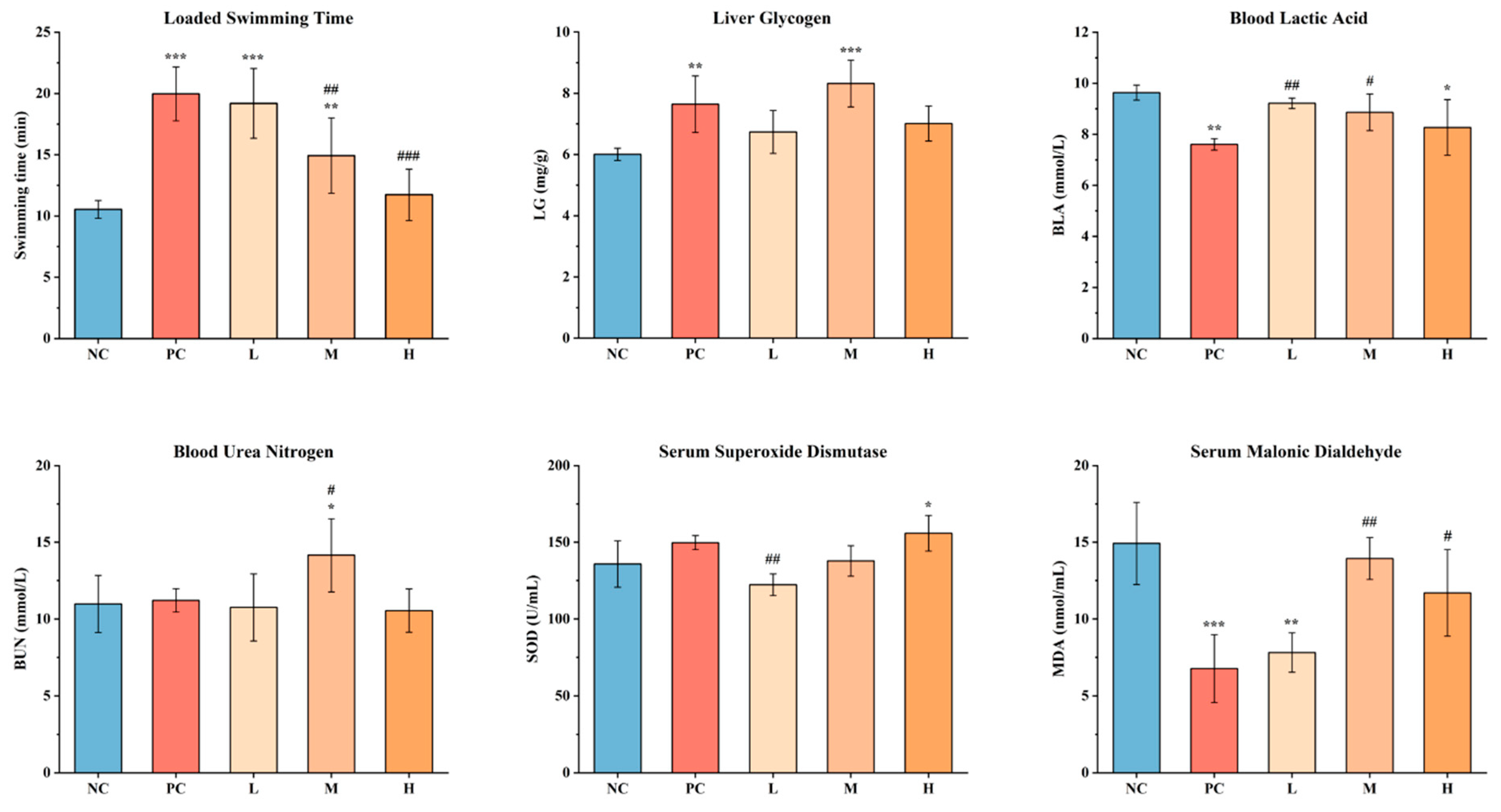
3.3.1. Effects of SWCC on Physiological Indexes of Mice
3.3.2. Effects of SWCC on Exercise Capacity of Mice
3.3.3. Effects of SWCC on Energy Metabolism in Mice
3.3.4. Effects of SWCC on the Metabolite Accumulation in Mice
3.3.5. Effects of SWCC on Oxidative Stress in Mice
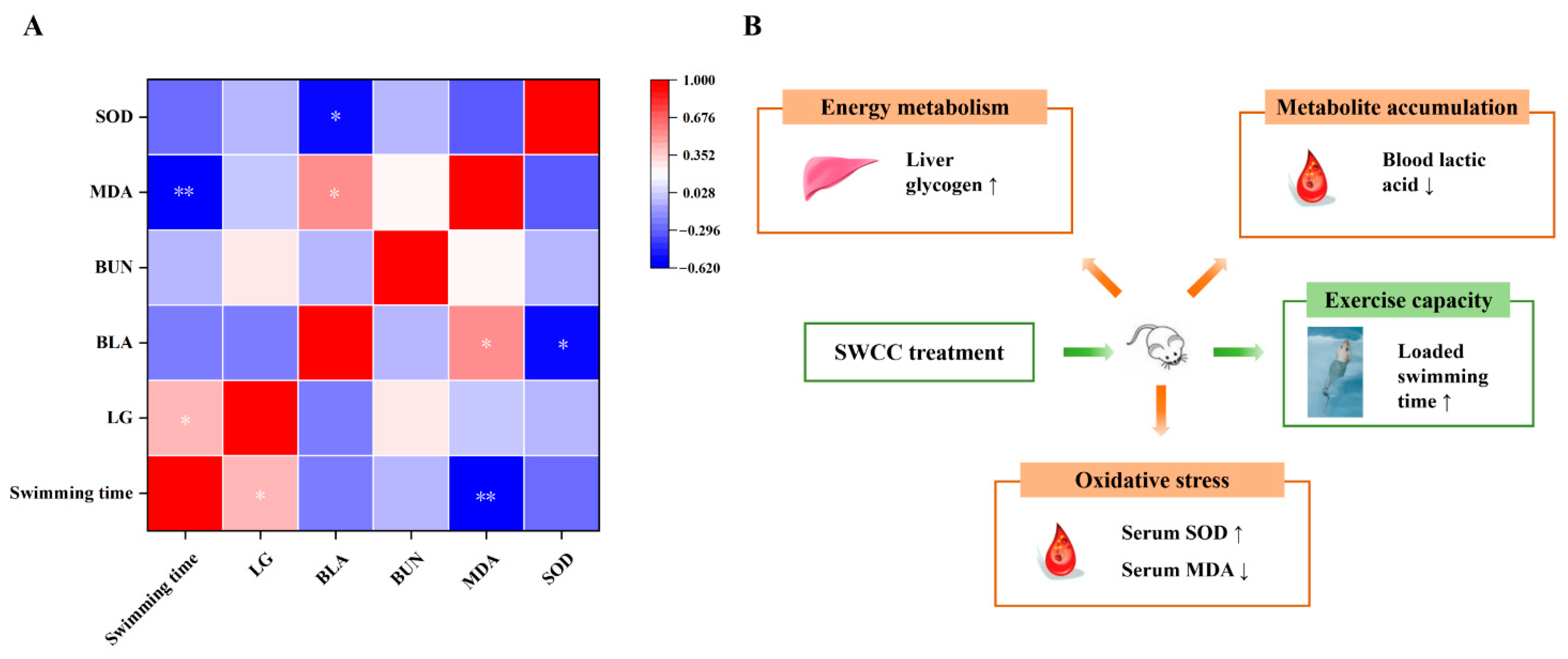
3.3.6. Comprehensive Analysis of Physiological and Biochemical Indexes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| BLA | Blood lactic acid |
| BUN | Blood urea nitrogen |
| BW | Body weight |
| DPPH | Free radical scavenger activity on 2,2-diphenyl-1-picrylhydrazy |
| FRAP | Ferric-reducing antioxidant power |
| LG | Liver glycogen |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| SSC | Soluble solids content |
| SWCC | Sea buckthorn–wolfberry compound coffee |
| TFC | Total flavonoid content |
| TPC | Total phenol content |
References
- Xi, H.; Zhu, R.; Li, C.; Wu, H.; Zhou, W.; Lu, Y.; Wang, S.; Liu, W.; Xiong, X.; Guo, L. Effect of self-acupressure on fatigue-predominant subhealth in young and middle-aged adults: A randomized controlled trial. Eur. J. Integr. Med. 2023, 62, 102274. [Google Scholar] [CrossRef]
- Jeon, H.; Lee, K.; Kim, Y.-T.; Kim, J.-Y.; Shim, J.-J.; Lee, J.-H. Effect of HY7602 fermented deer antler on physical fatigue and antioxidant activity in mice. Int. J. Mol. Sci. 2024, 25, 3318. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, C.; Pan, K.; Xiong, H. Acupuncture and moxibustion for chronic fatigue syndrome in traditional Chinese medicine: A systematic review and meta-analysis. BMC Complement. Altern. Med. 2017, 17, 163. [Google Scholar] [CrossRef]
- Zhou, J.; Chang, S.; Zhang, Q.; Lu, L.; Li, L.; Li, Z. Five natural active ingredients achieve anti-fatigue function by synergistic antioxidation and regulating the structure of intestinal flora. J. Food Nutr. Res. 2022, 10, 655–663. [Google Scholar] [CrossRef]
- Yu, W.; Song, C.; Lei, Z.; Li, Y.; He, X.; Yu, J.; Yang, X. Anti-fatigue effect of traditional Chinese medicines: A review. Saudi Pharm. J. 2023, 31, 597–604. [Google Scholar] [CrossRef]
- Zheng, X.; Long, W.; Liu, G.; Zhang, X.; Yang, X. Effect of seabuckthorn (Hippophae rhamnoides ssp. sinensis) leaf extract on the swimming endurance and exhaustive exercise-induced oxidative stress of rats. J. Sci. Food Agric. 2012, 92, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Liu, X.; Liu, Y.; Wang, M.; Shan, Y.; Yin, Y.; Meng, X.; Sun, F.; Li, H.; Li, Z. Molecular and biochemical investigations of the anti-fatigue effects of tea polyphenols and fruit extracts of Lycium ruthenicum Murr. on mice with exercise-induced fatigue. Front. Mol. Biosci. 2023, 10, 1223411. [Google Scholar] [CrossRef] [PubMed]
- Patay, É.B.; Bencsik, T.; Papp, N. Phytochemical overview and medicinal importance of coffea species from the past until now. Asian Pac. J. Trop. Med. 2016, 9, 1127–1135. [Google Scholar] [CrossRef]
- Gupta, A.; Sanwal, N.; Bareen, M.A.; Barua, S.; Sharma, N.; Joshua Olatunji, O.; Prakash Nirmal, N.; Sahu, J.K. Trends in functional beverages: Functional ingredients, processing technologies, stability, health benefits, and consumer perspective. Food Res. Int. 2023, 170, 113046. [Google Scholar] [CrossRef]
- Liang, B. Fermented plant-based cereal beverages as a good alternative to fermented milk beverages: From nutritional composition, microorganisms, and sensory properties. ICBioMed 2022, 14–21. [Google Scholar]
- GB 2760-2024; National Food Safety Standard—Use Standard of Food Additives. China National Standards Publishing House: Beijing, China, 2024.
- Mohd Aris, H.; Mohd Kasim, Z.; Zubairi, S.I.; Babji, A.S. Antioxidant capacity and sensory quality of soy-based powder drink mix enriched with functional hydrolysates of swiftlet (Aerodramus fuciphagus). Arab. J. Chem. 2023, 16, 104553. [Google Scholar] [CrossRef]
- Covacevich, L.; Aguilera, J.M.; Moreno, M.C.; Brossard, N.; Osorio, F. Rheological and tribological properties of seaweed powders as thickeners for liquid foods. Food Hydrocolloid 2024, 154, 110116. [Google Scholar] [CrossRef]
- Henao-González, D.; David, D.; Torres-Oquendo, J.; Sobral, P.J.D.A.; Vega-Castro, O. Design and optimization of a strawberry-based dispersion to produce a spray drying functional powdered product, fortified with folic acid and zinc. Food Bioprod. Process. 2024, 147, 105–114. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Chen, S.-C.; Wang, Q.-L.; Liu, C.-Q.; Xiao, J.-H.; Huang, D.-W. Effects of traditional grinding and superfine grinding technologies on the properties and volatile components of Protaetia brevitarsis larvae powder. LWT-Food Sci. Technol. 2023, 173, 114307. [Google Scholar] [CrossRef]
- Huang, X.; Liang, K.; Liu, Q.; Qiu, J.; Wang, J.; Zhu, H. Superfine grinding affects physicochemical, thermal and structural properties of moringa oleifera leaf powders. Ind. Crops Prod. 2020, 151, 112472. [Google Scholar] [CrossRef]
- Saifullah, M.; Yusof, Y.A.; Chin, N.L.; Aziz, M.G. Physicochemical and flow properties of fruit powder and their effect on the dissolution of fast dissolving fruit powder tablets. Powder Technol. 2016, 301, 396–404. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Wang, H.; Wang, S.; Lin, Z.; Zhao, L.; Xu, H. Ethanol and blanching pretreatments change the moisture transfer and physicochemical properties of apple slices via microstructure and cell-wall polysaccharides nanostructure modification. Food Chem. 2022, 381, 132274. [Google Scholar] [CrossRef]
- Jiang, G.; Ramachandraiah, K.; Wu, Z.; Li, S.; Eun, J.-B. Impact of ball-milling time on the physical properties, bioactive compounds, and structural characteristics of onion peel powder. Food Biosci. 2020, 36, 100630. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Zhang, Q.; Deng, L.; Miao, S.; Zhong, G. Konjac-mulberry leaf compound powder alleviates ova-induced allergic rhinitis in BALB/c mice. Food Sci. Hum. Wellness 2023, 12, 1674–1682. [Google Scholar] [CrossRef]
- Ciganović, P.; Jakupović, L.; Momchev, P.; Nižić Nodilo, L.; Hafner, A.; Zovko Končić, M. Extraction optimization, antioxidant, cosmeceutical and wound healing potential of Echinacea purpurea Glycerolic extracts. Molecules 2023, 28, 1177. [Google Scholar] [CrossRef] [PubMed]
- Turcov, D.; Barna, A.S.; Trifan, A.; Blaga, A.C.; Tanasă, A.M.; Suteu, D. Antioxidants from Galium verum as ingredients for the design of new dermatocosmetic products. Plants 2022, 11, 2454. [Google Scholar] [CrossRef]
- Navoda, N.P.G.D.; Samaranayake, M.D.W.; Liyanage, S.L.; Herath, H.M.T.; Jayasinghe, J.M.J.K. Determination of functional properties of Sri Lankan Ambarella (Spondias dulcis Forst. syn. Spondias cytherea Sonn.) fruit and development of vacuum dried Ambarella fruit powder and incorporated soup mix. Asian Food Sci. J. 2021, 20, 113–122. [Google Scholar]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Zhao, W.; Zhang, W.; Liu, L.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Fractionation, characterization and anti-fatigue activity of polysaccharides from Brassica rapa L. Process Biochem. Process Biochem. 2021, 106, 163–175. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Zeng, L.; Yang, Z.-S.; Huang, F.-F.; Ding, G.-F.; Wang, B. Anti-fatigue effect by peptide fraction from protein hydrolysate of croceine croaker (Pseudosciaena crocea) swim bladder through inhibiting the oxidative reactions including DNA damage. Mar. Drugs 2016, 14, 221. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Chen, L.; Qin, X.; Du, G.; Li, Z. Astragali Radix–Codonopsis Radix–Jujubae Fructus water extracts ameliorate exercise-induced fatigue in mice via modulating gut microbiota and its metabolites. J. Sci. Food Agric. 2022, 102, 5141–5152. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Tang, M.; Sun, H.; Ye, Y.; Cao, X.; Wang, J. Potential of water dropwort (Oenanthe javanica DC.) powder as an ingredient in beverage: Functional, thermal, dissolution and dispersion properties after superfine grinding. Powder Technol. 2019, 353, 516–525. [Google Scholar] [CrossRef]
- Bochnak-Niedzwiecka, J.; Świeca, M. Quality of new functional powdered beverages enriched with lyophilized fruits—Potentially bioaccessible antioxidant properties, nutritional value, and consumer analysis. Appl. Sci. 2020, 10, 3668. [Google Scholar] [CrossRef]
- LaClair, C.E.; Etzel, M.R. Ingredients and pH are key to clear beverages that contain whey protein. J. Food Sci. 2010, 75, C21–C27. [Google Scholar] [CrossRef]
- Cao, M.; Zeng, S.; Wang, J.; Guo, W. Assessment of SSC and soluble sugar content of three pear cultivars during storage using dielectric method. Postharvest Biol. Technol. 2024, 212, 112906. [Google Scholar] [CrossRef]
- Kim, S.; Kim, N.; Lee, S.; Lee, S. Determination of the sugar content in high-sugar beverages. Prev. Nutr. Food Sci. 2022, 27, 309–314. [Google Scholar] [CrossRef]
- Chen, L.; Wu, W.; Zhang, N.; Bak, K.H.; Zhang, Y.; Fu, Y. Sugar reduction in beverages: Current trends and new perspectives from sensory and health viewpoints. Food Res. Int. 2022, 162, 112076. [Google Scholar] [CrossRef]
- Raal, A.; Rusalepp, L.; Chiru, T.; Ciobanu, N.; Talvistu, K.; Shusta, M.; Koshovyi, O.; Püssa, T. Polyphenolic compounds and antioxidant activity of sea buckthorn (Hippophae Rhamnoides L.). Phyton-Int. J. Exp. Bot. 2023, 92, 2965–2979. [Google Scholar] [CrossRef]
- Huang, H.; Ni, Z.-J.; Wu, Z.-F.; Ma, Y.-L.; Hu, F.; Thakur, K.; Zhang, J.-G.; Khan, M.R.; Wei, Z.-J. Comparison of polyphenols in Goji (Lycium barbarum L.) leaves at different leaf positions under different extraction methods. Ind. Crops Prod. 2024, 209, 117982. [Google Scholar] [CrossRef]
- Geertsema, J.; Kratochvil, M.; González-Domínguez, R.; Lefèvre-Arbogast, S.; Low, D.Y.; Du Preez, A.; Lee, H.; Urpi-Sarda, M.; Sánchez-Pla, A.; Aigner, L.; et al. Coffee polyphenols ameliorate early-life stress-induced cognitive deficits in male mice. Neurobiol. Stress 2024, 31, 100641. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-T.; Wu, M.-F.; Lee, M.-C.; Wu, J.-H.; Huang, C.-C.; Huang, W.-C. Antifatigue activity and exercise performance of phenolic-rich extracts from Calendula officinalis, Ribes nigrum, and Vaccinium myrtillus. Nutrients 2019, 11, 1715. [Google Scholar] [CrossRef] [PubMed]
- Burri, S.C.M.; Ekholm, A.; Håkansson, Å.; Tornberg, E.; Rumpunen, K. Antioxidant capacity and major phenol compounds of horticultural plant materials not usually used. J. Funct. Foods 2017, 38, 119–127. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Li, J.; Pei, Y.; Liang, Y. Antioxidant properties of tartary buckwheat extracts as affected by different thermal processing methods. LWT-Food Sci. Technol. 2010, 43, 181–185. [Google Scholar] [CrossRef]
- Imran, A.; Sadiq Butt, M.; Saeed, F.; Sajid Arshad, M.; Sultan, T.; Sohaib, M. Effect of different time-solvent interactions on polyphenol content of milky tea: Antioxidant potential of milky tea. J. Food Process. Preserv. 2017, 41, e13039. [Google Scholar] [CrossRef]
- Hue, H.T.; Thai, N.M.; Dao, P.T.A. Survey on different polyphenol extraction methods from avocado seed (Persea americana Mill) and assay on their antioxidant and acute toxicity activities. Vietnam J. Chem. 2021, 59, 870–876. [Google Scholar] [CrossRef]
- Miao, X.; Xiao, B.; Shui, S.; Yang, J.; Huang, R.; Dong, J. Metabolomics analysis of serum reveals the effect of Danggui Buxue Tang on fatigued mice induced by exhausting physical exercise. J. Pharm. Biomed. 2018, 151, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Xu, F.; Liu, G.; Pang, B.; Liao, N.; Li, H.; Shi, J. Gut microbiota as a potential target for developing anti-fatigue foods. Crit. Rev. Food Sci. Nutr. 2023, 63, 3065–3080. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wei, Y.; Xu, Y.; Zhang, R.; Li, M.; Qin, H.; Gu, R.; Cai, M. The anti-fatigue activity of corn peptides and their effect on gut bacteria. J. Sci. Food Agric. 2022, 102, 3456–3466. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jo, K.; Byun, B.S.; Han, S.H.; Yu, K.-W.; Suh, H.J.; Hong, K.-B. Chemical and biological properties of puffed Dendrobium officinale extracts: Evaluation of antioxidant and anti-fatigue activities. J. Funct. Foods 2020, 73, 104144. [Google Scholar] [CrossRef]
- Fu, G.; Zhang, M.; Huang, Y.; Han, R.; Qi, K.; Yin, L.; Zhao, D.; Huang, Y.; Ma, T.; Wang, L. Effects of different addition levels of CHM-JM113 on growth performance, antioxidant capacity, organ index, and intestinal health of AA broilers. Front. Vet. Sci. 2024, 11, 1388173. [Google Scholar] [CrossRef]
- Hong, H.-D.; Kim, J.-C.; Lim, T.-G.; Song, Y.-R.; Cho, C.-W.; Jang, M. Mixing ratio optimization for functional complex extracts of Rhodiola crenulata, Panax quinquefolius, and Astragalus membranaceus using mixture design and verification of immune functional efficacy in animal models. J. Funct. Foods 2018, 40, 447–454. [Google Scholar] [CrossRef]
- Luo, C.; Xu, X.; Wei, X.; Feng, W.; Huang, H.; Liu, H.; Xu, R.; Lin, J.; Han, L.; Zhang, D. Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol. Res. 2019, 148, 104409. [Google Scholar] [CrossRef]
- Yang, D.; Lian, J.; Wang, L.; Liu, X.; Wang, Y.; Zhao, X.; Zhang, X.; Hu, W. The anti-fatigue and anti-anoxia effects of tremella extract. Saudi J. Biol. Sci. 2019, 26, 2052–2056. [Google Scholar] [CrossRef]
- Xiao, M.; Lin, L.; Chen, H.; Ge, X.; Huang, Y.; Zheng, Z.; Li, S.; Pan, Y.; Liu, B.; Zeng, F. Anti-fatigue property of the oyster polypeptide fraction and its effect on gut microbiota in mice. Food Funct. 2020, 11, 8659–8669. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Lyu, C.; Li, Q.; Kou, F.; Jiang, M.; Wei, H. Metabolomics study on the intervention effect of Radix Salviae Miltiorrhizae extract in exercise-induced exhaustion rat using gas chromatography coupled to mass spectrometry. J. Chromatogr. B 2021, 1178, 122805. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shi, C.; Xia, P.; Ning, K.; Xiang, H.; Xie, Q. Fermented deer blood ameliorates intense exercise-induced fatigue via modulating small intestine microbiota and metabolites in mice. Nutrients 2021, 13, 1543. [Google Scholar] [CrossRef]
- An, Z.; Wang, Y.; Li, X.; Jin, H.; Gong, Y. Antifatigue effect of sea buckthorn seed oil on swimming fatigue in mice. J. Food Sci. 2023, 88, 1482–1494. [Google Scholar] [CrossRef]
- Chai, X.; Pan, M.; Wang, J.; Feng, M.; Wang, Y.; Zhang, Q.; Sun, Y. Cordycepin exhibits anti-fatigue effect via activating TIGAR/SIRT1/PGC-1α signaling pathway. Biochem. Biophys. Res. Commun. 2022, 637, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Deng, X.; Li, M.; Qiu, M.; Zhang, Y.; Li, G.; Jiang, Y.; Tan, P.; Fan, S.; Zheng, Y.; et al. Thermal treatment enhances the resisting exercise fatigue effect of Phyllanthus emblica L.: Novel evidence from tannin conversion in vitro, metabolomics, and gut microbiota community analysis. Chin. Med. 2023, 18, 127. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, D.; Hu, J.; Tan, B.K.; Zhang, Y.; Lin, S. Natural bioactive peptides to beat exercise-induced fatigue: A review. Food Biosci. 2021, 43, 101298. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, L.; Hu, K.; Yang, Y.; Ma, J.; Zhai, Y.; Jiang, Y.; Zhang, D. Anti-fatigue effects of Lycium barbarum polysaccharide and effervescent tablets by regulating oxidative stress and energy metabolism in rats. Int. J. Mol. Sci. 2022, 23, 10920. [Google Scholar] [CrossRef] [PubMed]
| Level | Factor | |||
|---|---|---|---|---|
| (A) Sea Buckthorn Powder/% | (B) Wolfberry Powder/% | (C) Coffee Powder/% | (D) Additive Mixture/% | |
| 1 | 6 | 13 | 59 | 1 |
| 2 | 9 | 16 | 62 | 2 |
| 3 | 12 | 19 | 65 | 4 |
| Group | Initial Weight/g | 1st Weight/g | 2nd Weight/g | 3rd Weight/g | 4th Weight/g | Weight Change/g |
|---|---|---|---|---|---|---|
| NC | 31.50 ± 1.86 | 34.15 ± 2.48 | 36.07 ± 2.95 | 36.48 ± 3.30 | 37.10 ± 3.03 | 5.60 ± 2.27 ab |
| PC | 31.82 ± 1.43 | 33.93 ± 1.64 | 35.14 ± 1.74 | 35.53 ± 2.05 | 36.64 ± 2.21 | 4.82 ± 2.25 b |
| L | 31.93 ± 1.79 | 34.26 ± 2.43 | 35.85 ± 2.76 | 36.89 ± 2.74 | 37.72 ± 2.92 | 5.79 ± 1.91 ab |
| M | 31.83 ± 1.70 | 34.47 ± 1.58 | 36.29 ± 2.39 | 37.58 ± 2.28 | 38.73 ± 2.59 | 6.89 ± 2.84 a |
| H | 31.24 ± 2.11 | 33.61 ± 2.12 | 35.06 ± 2.59 | 36.10 ± 2.90 | 37.26 ± 2.69 | 6.02 ± 1.82 ab |
| Group | Liver Index/% | Kidney Index/% | Spleen Index/% | Heart Index/% | Lung Index/% |
|---|---|---|---|---|---|
| NC | 4.3 ± 0.38 | 1.56 ± 0.14 | 0.22 ± 0.05 b | 0.78 ± 0.12 | 0.70 ± 0.06 |
| PC | 3.85 ± 0.57 | 1.52 ± 0.08 | 0.20 ± 0.04 b | 0.85 ± 0.10 | 0.78 ± 0.12 |
| L | 3.88 ± 0.52 | 1.54 ± 0.17 | 0.27 ± 0.04 ab | 0.89 ± 0.12 | 0.83 ± 0.08 |
| M | 3.66 ± 0.46 | 1.44 ± 0.08 | 0.25 ± 0.09 b | 0.86 ± 0.06 | 0.81 ± 0.08 |
| H | 4.30 ± 0.73 | 1.57 ± 0.16 | 0.34 ± 0.07 a | 0.80 ± 0.16 | 0.77 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhao, L.; Wang, Q.; Yang, X.; Wang, J. Preparation and Evaluation of Anti-Fatigue Effects of Sea Buckthorn–Wolfberry Compound Coffee. Foods 2025, 14, 2818. https://doi.org/10.3390/foods14162818
Chen Y, Zhao L, Wang Q, Yang X, Wang J. Preparation and Evaluation of Anti-Fatigue Effects of Sea Buckthorn–Wolfberry Compound Coffee. Foods. 2025; 14(16):2818. https://doi.org/10.3390/foods14162818
Chicago/Turabian StyleChen, Yuxian, Lili Zhao, Qinghui Wang, Xuhai Yang, and Jun Wang. 2025. "Preparation and Evaluation of Anti-Fatigue Effects of Sea Buckthorn–Wolfberry Compound Coffee" Foods 14, no. 16: 2818. https://doi.org/10.3390/foods14162818
APA StyleChen, Y., Zhao, L., Wang, Q., Yang, X., & Wang, J. (2025). Preparation and Evaluation of Anti-Fatigue Effects of Sea Buckthorn–Wolfberry Compound Coffee. Foods, 14(16), 2818. https://doi.org/10.3390/foods14162818






