Physicochemical and Sensory Properties of Davidson Plum (Davidsonia jerseyana) Sorbet, a Potential for New Functional Food Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Product Development
2.2. Sorbet Samples
2.3. Macronutrient Analysis
2.4. Colour Analysis
2.5. Melting Rate
2.6. Texture Analysis
2.7. Phytochemical Composition
2.8. Methanolic Extraction
2.9. Total Phenolic Content
2.10. Total Flavonoid Content
2.11. Anthocyanin Content
2.12. Total Proanthocyanidins Content
2.13. 2,2-diphenyl-1-picrylhydrazyl (DPPH) Assay
2.14. Ferric Reducing Antioxidant (FRAP) Assay
2.15. Cupric Ion Reducing Antioxidant Capacity (CUPRAC) Assay
2.16. Taste Evaluation and Product Acceptability
2.17. Data Analysis
3. Results
3.1. Macronutrient Analysis of Sorbets
3.2. Colourimetry
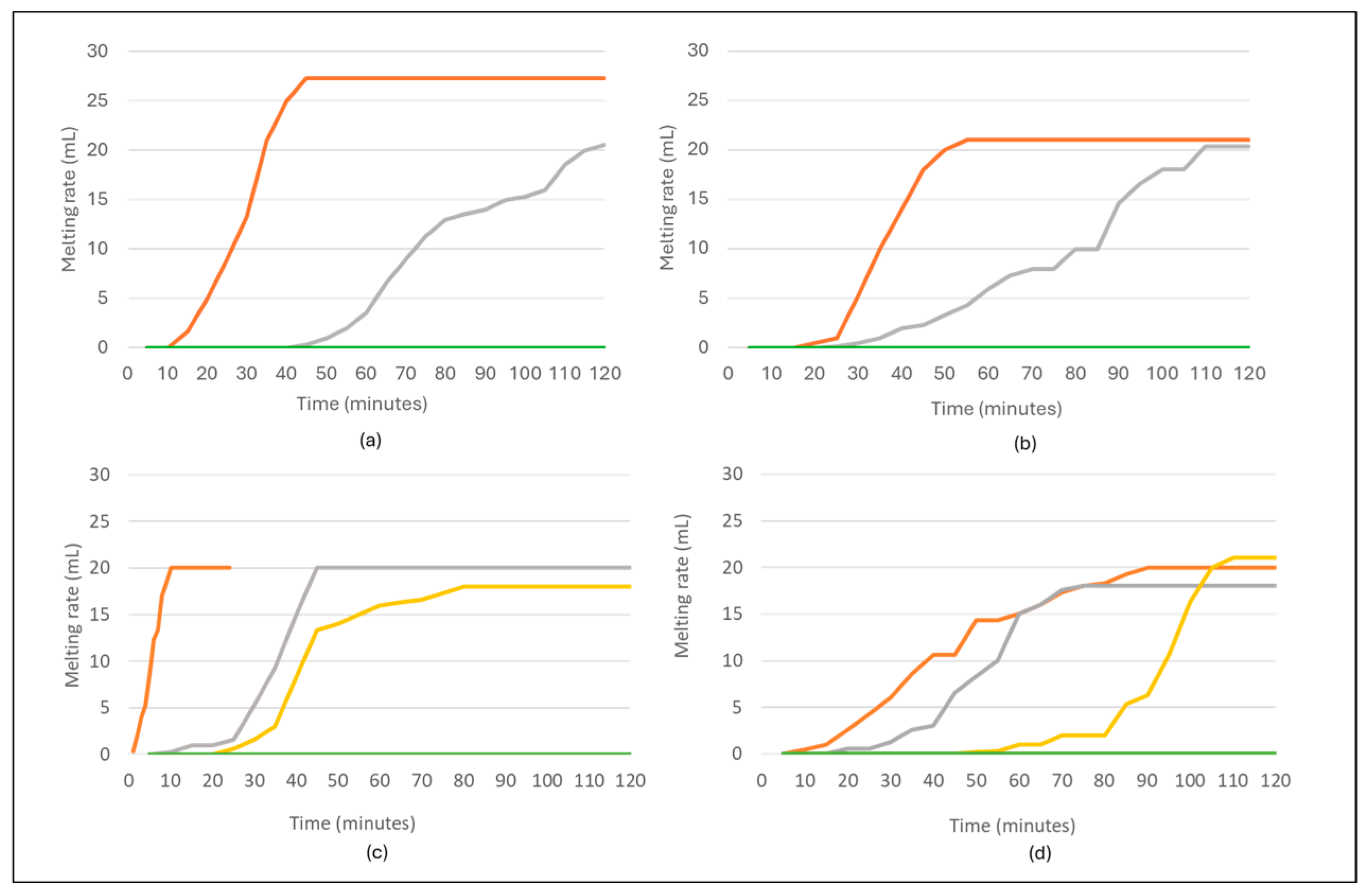
3.3. Melting Point
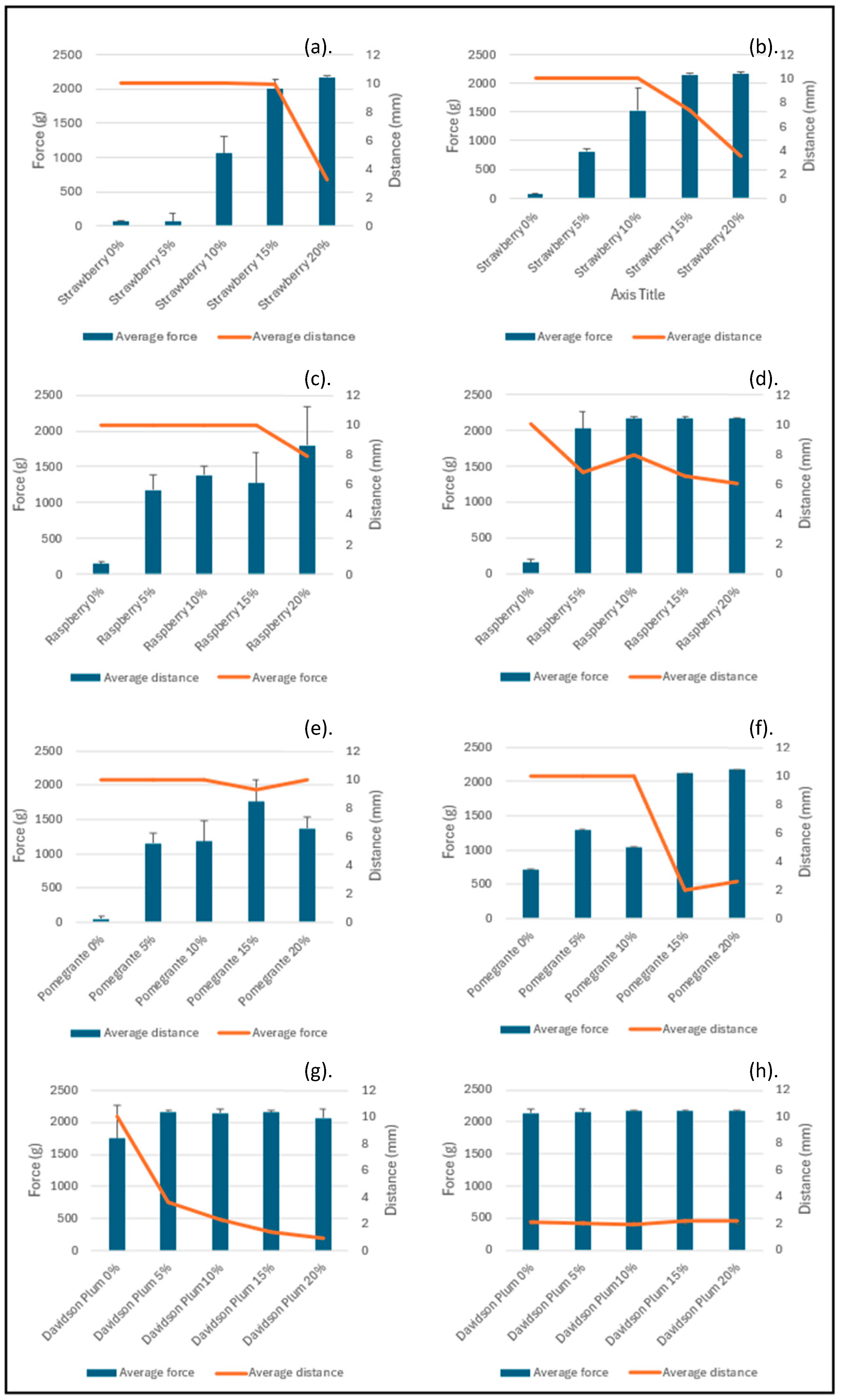
3.4. Texture Analysis
3.5. Phytochemical Content
3.5.1. Total Polyphenol and Flavonoid Analysis
3.5.2. Antioxidant Analysis
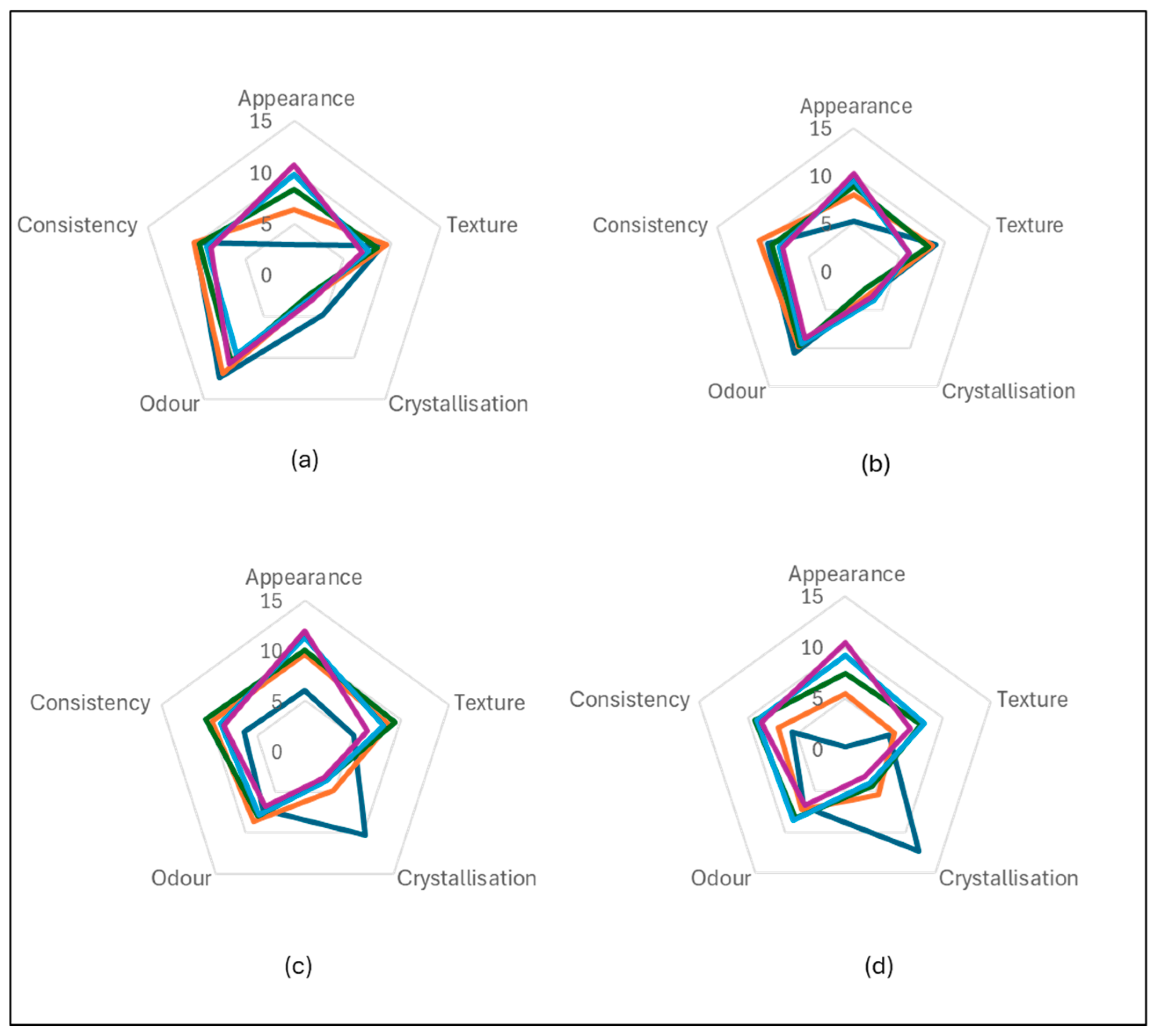
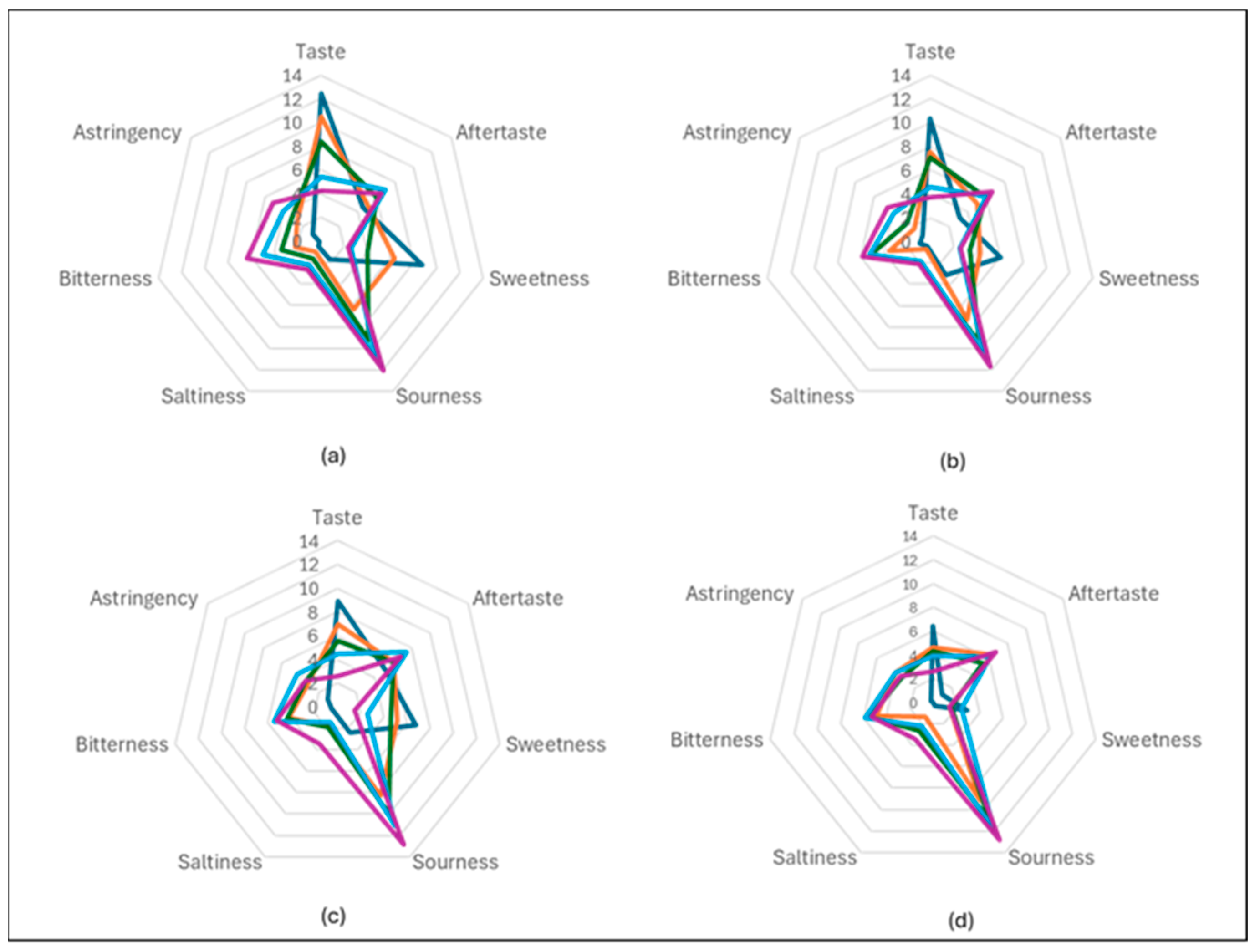
3.6. Sensory Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| TPTZ | 2,4,6-Tri(2-pyridyl)-s-triazine |
| FRAP | Ferric Reducing Antioxidant Power |
| CUPRAC | Cupric Reducing Antioxidant Capacity |
| TPC | Total polyphenol content |
| TFC | Total Flavonoid Content |
References
- Sarkar, D.; Walker-Swaney, J.; Shetty, K. Food Diversity and Indigenous Food Systems to Combat Diet-Linked Chronic Diseases. Curr. Dev. Nutr. 2020, 4, 3–11. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Identification and characterization of anthocyanins and non-anthocyanin phenolics from Australian native fruits and their antioxidant, antidiabetic, and anti-Alzheimer potential. Food Res. Int. 2022, 162, 111951. [Google Scholar] [CrossRef] [PubMed]
- Porykali, B.; Davies, A.; Brooks, C.; Melville, H.; Allman-Farinelli, M.; Coombes, J. Effects of Nutritional Interventions on Cardiovascular Disease Health Outcomes in Aboriginal and Torres Strait Islander Australians: A Scoping Review. Nutrients 2021, 13, 4084. [Google Scholar] [CrossRef] [PubMed]
- Richmond, R.; Bowyer, M.; Vuong, Q. Australian native fruits: Potential uses as functional food ingredients. J. Funct. Foods 2019, 62, 103547. [Google Scholar] [CrossRef]
- Dissanayake, I.H.; Zak, V.; Kaur, K.; Jaye, K.; Ayati, Z.; Chang, D.; Li, C.G.; Bhuyan, D.J. Australian native fruits and vegetables: Chemical composition, nutritional profile, bioactivity and potential valorization by industries. Crit. Rev. Food Sci. Nutr. 2022, 63, 8511–8544. [Google Scholar] [CrossRef] [PubMed]
- Yeshi, K.; Turpin, G.; Jamtsho, T.; Wangchuk, P. Indigenous Uses, Phytochemical Analysis, and Anti-Inflammatory Properties of Australian Tropical Medicinal Plants. Molecules 2022, 27, 3849. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Lopes, C.V.A.; Mihrshahi, S.; Ronto, R.; Hunter, J. Aboriginal Food Practices and Australian Native Plant-Based Foods: A Step toward Sustainable Food Systems. Sustainability 2023, 15, 11569. [Google Scholar] [CrossRef]
- Sultanbawa, Y.; Sivakumar, D. Enhanced nutritional and phytochemical profiles of selected underutilized fruits, vegetables, and legumes. Curr. Opin. Food Sci. 2022, 46, 100853. [Google Scholar] [CrossRef]
- Donno, D.; Turrini, F. Plant Foods and Underutilized Fruits as Source of Functional Food Ingredients: Chemical Composition, Quality Traits, and Biological Properties. Foods 2020, 9, 1474. [Google Scholar] [CrossRef]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant Polyphenols and Their Potential Benefits on Cardiovascular Health: A Review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef] [PubMed]
- Sommano, S.; Caffin, N.; Kerven, G. Screening for Antioxidant Activity, Phenolic Content, and Flavonoids from Australian Native Food Plants. Int. J. Food Prop. 2013, 16, 1394–1406. [Google Scholar] [CrossRef]
- John, O.D.; Mouatt, P.; Prasadam, I.; Xiao, Y.; Panchal, S.K.; Brown, L. The edible native Australian fruit, Davidson’s plum (Davidsonia pruriens), reduces symptoms in rats with diet-induced metabolic syndrome. J. Funct. Foods 2019, 56, 204–215. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Mazhar, M.S.; Quddus, S.; Agar, O.T.; Suleria, H.A.R. Australian Native Plum: A Review of the Phytochemical and Health Effects. Food Rev. Int. 2023, 40, 504–532. [Google Scholar] [CrossRef]
- Sommano, S.; Caffin, N.; McDonald, J.; Cocksedge, R. Food safety and standard of Australian native plants. Qual. Assur. Saf. Crops Foods 2011, 3, 176–184. [Google Scholar] [CrossRef]
- Grigalius, S.; McPhee, D.P. In conclusion, something to chew on: Native plant foods of the Gold Coast. In The Gold Coast Transformed; CSIRO Publishing: Clayton, Australia, 2015; pp. 183–193. [Google Scholar]
- Sakulnarmrat, K.; Srzednicki, G.; Konczak, I. Composition and inhibitory activities towards digestive enzymes of polyphenolic-rich fractions of Davidson’s plum and quandong. LWT—Food Sci. Technol. 2014, 57, 366–375. [Google Scholar] [CrossRef]
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public. Health 2022, 19, 1217. [Google Scholar] [CrossRef]
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N.; et al. Functional importance of bioactive compounds of foods with Potential Health Benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Majdan, M.; Bobrowska-Korczak, B. Active Compounds in Fruits and Inflammation in the Body. Nutrients 2022, 14, 2496. [Google Scholar] [CrossRef]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012, 143, 397–405. [Google Scholar] [CrossRef]
- Golovinskaia, O.; Wang, C.-K. Review of Functional and Pharmacological Activities of Berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef]
- Hurst, R.D.; Lyall, K.A.; Wells, R.W.; Sawyer, G.M.; Lomiwes, D.; Ngametua, N.; Hurst, S.M. Daily Consumption of an Anthocyanin-Rich Extract Made From New Zealand Blackcurrants for 5 Weeks Supports Exercise Recovery Through the Management of Oxidative Stress and Inflammation: A Randomized Placebo Controlled Pilot Study. Front. Nutr. 2020, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sports Med. 2019, 49, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Bloedon, T.K.; Braithwaite, R.E.; Carson, I.A.; Klimis-Zacas, D.; Lehnhard, R.A. Impact of anthocyanin-rich whole fruit consumption on exercise-induced oxidative stress and inflammation: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 630–645. [Google Scholar] [CrossRef]
- Topolska, K.; Filipiak-Florkiewicz, A.; Florkiewicz, A.; Cieślik, E. Organoleptic quality of fruit sorbets containing yacon (Smallanthus sonchifolius Poepp. and Endl.). J. Microbiol. Biotechnol. Food Sci. 2015, 4, 161. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.Q.; Weber, C.; Lee, C.Y.; Brown, J.; Liu, R.H. Antioxidant and Antiproliferative Activities of Raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [Google Scholar] [CrossRef]
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry As a Functional Food: An Evidence-Based Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 790–806. [Google Scholar] [CrossRef]
- Urbaniak, A.; Basta, P.; Ast, K.; Wołoszyn, A.; Kuriańska—Wołoszyn, J.; Latour, E.; Skarpańska—Stejnborn, A. The impact of supplementation with pomegranate fruit (Punica granatum L.) juice on selected antioxidant parameters and markers of iron metabolism in rowers. J. Int. Soc. Sports Nutr. 2018, 15, 35. [Google Scholar] [CrossRef]
- Naumovski, N.; Blades, B.L.; Roach, P.D. Food Inhibits the Oral Bioavailability of the Major Green Tea Antioxidant Epigallocatechin Gallate in Humans. Antioxidants 2015, 4, 373–393. [Google Scholar] [CrossRef]
- Williams, J.; D’Cunha, N.M.; Kellett, J.; Georgousopoulou, E.N.; McKune, A.J.; Mellor, D.D.; Naumovski, N. Physicochemical, antioxidant and sensory properties of Mango Sorbet containing L-theanine as a potential functional food product. J. Food Sci. Technol. 2022, 59, 4833–4843. [Google Scholar] [CrossRef]
- Ng, F.S.K.; Chiang, J.H.; Ng, G.C.F.; Lee, C.S.H.; Henry, C.J. Effects of proteins and fats on the physicochemical, nutritional and sensory properties of plant-based frozen desserts. Int. J. Food Sci. Technol. 2023, 58, 3912–3923. [Google Scholar] [CrossRef]
- Malgor, M.; Sabbione, A.C.; Scilingo, A. Amaranth Lemon Sorbet, Elaboration of a Potential Functional Food. Plant Foods Hum. Nutr. 2020, 75, 404–412. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Petry, R.D.; Ortega, G.G.; Silva, W.B. Flavonoid content assay: Influence of the reagent concentration and reaction time on the spectrophotometric behavior of the aluminium chloride-flavonoid complex. Pharmazie 2001, 56, 465–470. [Google Scholar]
- Apak, R.; Güçlü, K.; Özyürek, M.; Çelik, S.E. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 2008, 160, 413–419. [Google Scholar] [CrossRef]
- Smyth, H.; Sanderson, J.; Sultanbawa, Y. Lexicon for the sensory description of a ustralian native plant foods and ingredients. J. Sens. Stud. 2012, 27, 471–481. [Google Scholar] [CrossRef]
- Suwonsichon, S. The Importance of Sensory Lexicons for Research and Development of Food Products. Foods 2019, 8, 27. [Google Scholar] [CrossRef]
- Society of Sensory Professionals. Quantitative Descriptive Analysis. Available online: https://www.sensorysociety.org/knowledge/sspwiki/Pages/Quantitative%20Descriptive%20Analysis.aspx (accessed on 5 October 2023).
- Society of Sensory Professionals. The 9-Point Hedonic Scale. Available online: https://www.sensorysociety.org/knowledge/sspwiki/Pages/The%209-point%20Hedonic%20Scale.aspx (accessed on 30 August 2023).
- Wojdyło, A.; Oszmiański, J.; Teleszko, M.; Sokół-Łętowska, A. Composition and quantification of major polyphenolic compounds, antioxidant activity and colour properties of quince and mixed quince jams. Int. J. Food Sci. Nutr. 2013, 64, 749–756. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess. Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Thai, S.; Sinrod, A.J.G.; Chiou, B.-S.; McHugh, T. Functionality of freeze-dried berry powder on frozen dairy desserts. J. Food Process. Preserv. 2019, 43, e14076. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Sinrod, A.J.G.; Chiou, B.-S.; McHugh, T. Functionality of strawberry powder on frozen dairy desserts. J. Texture Stud. 2019, 50, 556–563. [Google Scholar] [CrossRef]
- Salehi, F. Quality, physicochemical, and textural properties of dairy products containing fruits and vegetables: A review. Food Sci. Nutr. 2021, 9, 4666–4686. [Google Scholar] [CrossRef] [PubMed]
- Tawfek, M.A.E.-M. Properties of Low Fat Bio-frozen Yoghurt Fortified with Extract and Powder of Pomegranate Peel (Punica ganatum L.). Egypt. J. Food Sci. 2021, 49, 267–286. [Google Scholar]
- Analianasari; Apriyani, M. Characteristics of Frozen Yoghurt Enriched with Red Dragon Fruit Skin Extracts (Hylocereus polyrhizus). J. Phys. Conf. Ser. 2018, 953, 012036. [Google Scholar] [CrossRef]
- Williams, J.; McKune, A.J.; Naumovski, N. Sorbets as Functional Food Products, Unexplored Food Matrices, Their Challenges, and Advancements. Appl. Sci. 2023, 13, 11945. [Google Scholar] [CrossRef]
- El-Hawary, M.; Ragab, W.; OM, A.E.-B. Effect of Adding Beetroot Powder on Chemical, Nutritional, Rheological, and Organoleptic Properties of Frozen Yogurt. Asian J. Food Res. Nutr. 2023, 4, 511–521. [Google Scholar]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Barkaoui, S.; Madureira, J.; Boudhrioua, N.; Cabo Verde, S. Berries: Effects on health, preservation methods, and uses in functional foods: A review. Eur. Food Res. Technol. 2023, 249, 1689–1715. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef]
- Palka, A.; Skotnicka, M. The Health-Promoting and Sensory Properties of Tropical Fruit Sorbets with Inulin. Molecules 2022, 27, 4239. [Google Scholar] [CrossRef] [PubMed]
- Nemli, E.; Ozkan, G.; Gultekin Subasi, B.; Çavdar, H.; Lorenzo, J.M.; Zhao, C.; Capanoglu, E. Interactions between proteins and phenolics: Effects of food processing on the content and digestibility of phenolic compounds. J. Sci. Food Agric. 2024, 104, 2535–2550. [Google Scholar] [CrossRef]
- Shen, F.; Niu, F.; Li, J.; Su, Y.; Liu, Y.; Yang, Y. Interactions between tea polyphenol and two kinds of typical egg white proteins—Ovalbumin and lysozyme: Effect on the gastrointestinal digestion of both proteins in vitro. Food Res. Int. 2014, 59, 100–107. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Czech, A.; Malik, A.; Sosnowska, B.; Domaradzki, P. Bioactive Substances, Heavy Metals, and Antioxidant Activity in Whole Fruit, Peel, and Pulp of Citrus Fruits. Int. J. Food Sci. 2021, 2021, 6662259. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Netzel, M.; Netzel, G.; Tian, Q.; Schwartz, S.; Konczak, I. Native Australian fruits—a novel source of antioxidants for food. Innov. Food Sci. Emerg. Technol. 2007, 8, 339–346. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.P.; Pereira, L.; Domingues, F. Interactions between the major bioactive polyphenols of berries: Effects on antioxidant properties. Eur. Food Res. Technol. 2018, 244, 175–185. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W. Effects of Production and Processing Factors on Major Fruit and Vegetable Antioxidants. J. Food Sci. 2005, 70, R11–R19. [Google Scholar] [CrossRef]
- Kårlund, A.; Moor, U.; Sandell, M.; Karjalainen, R.O. The Impact of Harvesting, Storage and Processing Factors on Health-Promoting Phytochemicals in Berries and Fruits. Processes 2014, 2, 596–624. [Google Scholar] [CrossRef]
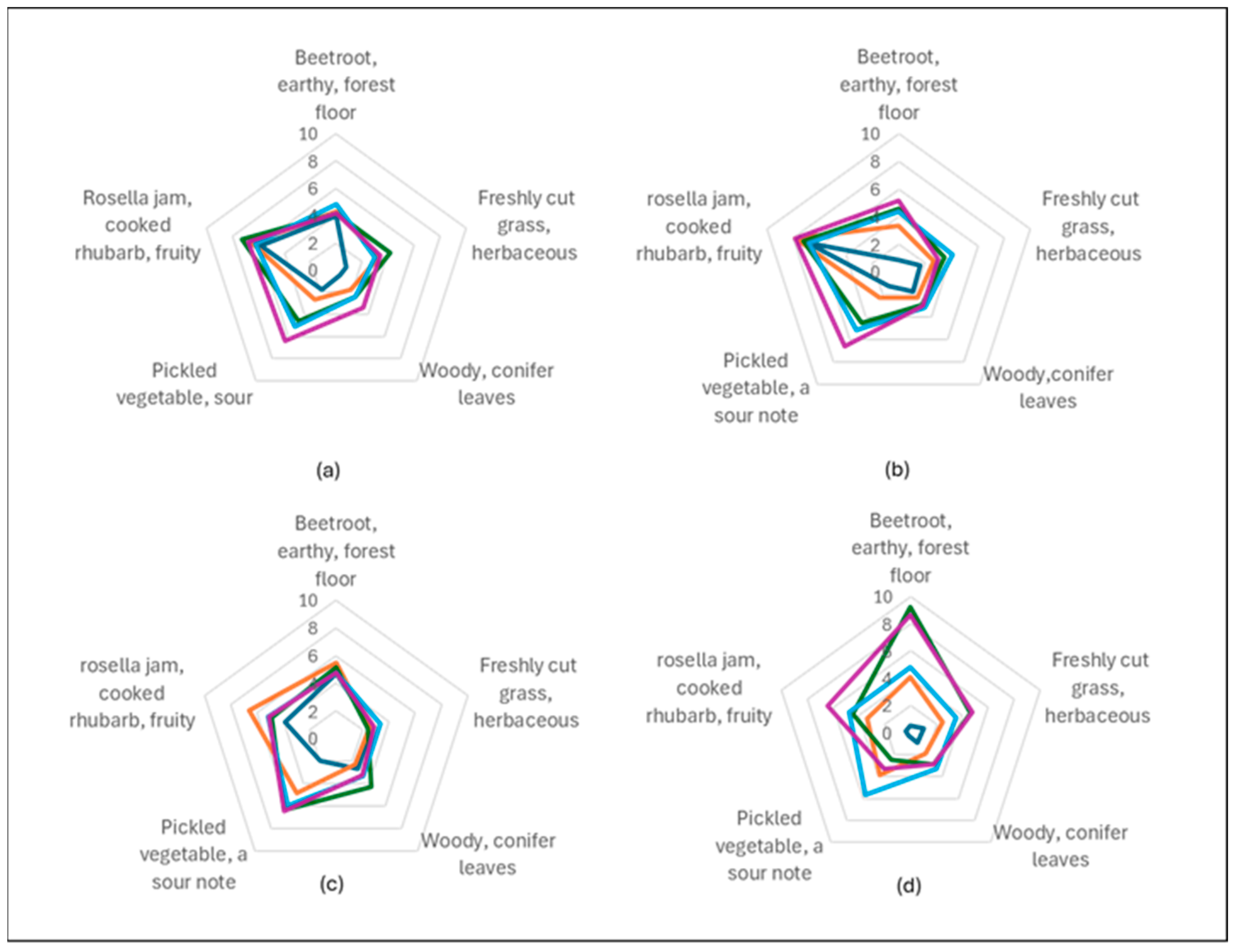
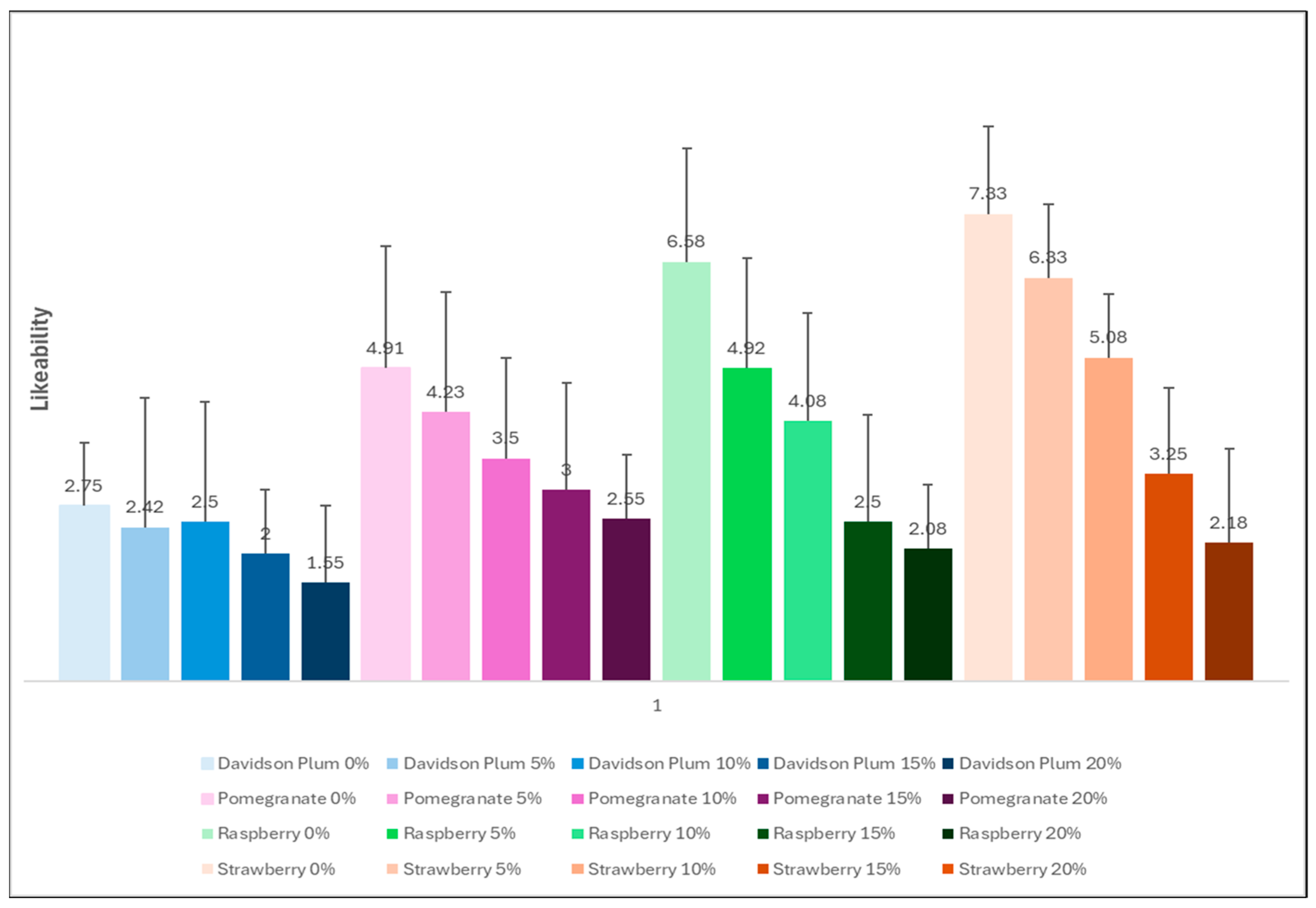
| Sorbet | Energy (kJ) | Protein (g) | Total Fat (g) | Carbohydrates (g) | Sugar (g) | Fibre (g) |
|---|---|---|---|---|---|---|
| Strawberry 0% | 234.2 | 0.59 | 0.22 | 12.5 | 12.4 | 1.8 |
| Strawberry 5% | 301.3 | 0.83 | 0.32 | 14.2 | 12.5 | 5.7 |
| Strawberry 10% | 369.4 | 1.04 | 0.50 | 15.6 | 12.5 | 9.2 |
| Strawberry 15% | 438.25 | 1.28 | 0.65 | 17.4 | 12.6 | 12.8 |
| Strawberry 20% | 502.2 | 1.46 | 0.805 | 18.9 | 12.7 | 16.2 |
| Raspberry 0% | 291.3 | 0.87 | 0.22 | 14.5 | 9.8 | 3.91 |
| Raspberry 5% | 366.7 | 1.09 | 0.32 | 16.0 | 9.8 | 7.73 |
| Raspberry 10% | 429.2 | 1.30 | 0.48 | 17.4 | 9.9 | 11.2 |
| Raspberry 15% | 489.7 | 1.50 | 0.64 | 18.9 | 10.0 | 14.5 |
| Raspberry 20% | 548.8 | 1.67 | 0.79 | 20.3 | 10.2 | 17.7 |
| Pomegranate 0% | 347.9 | 0.30 | 0.00 | 20.2 | 19.4 | 0.36 |
| Pomegranate 5% | 408.8 | 0.54 | 0.18 | 21.5 | 19.4 | 4.2 |
| Pomegranate 10% | 469.0 | 0.77 | 0.52 | 22.7 | 19.1 | 7.78 |
| Pomegranate 15% | 534.6 | 1.00 | 0.5 | 24.3 | 19.1 | 11.3 |
| Pomegranate 20% | 593.3 | 1.19 | 0.67 | 25.6 | 19.1 | 14.6 |
| Davidson Plum 0% | 523.1 | 0.28 | 0.00 | 30.4 | 30.4 | 0.0 |
| Davidson Plum 5% | 696.0 | 1.04 | 0.56 | 29.5 | 29.5 | 12 |
| Davidson Plum 10% | 857.6 | 1.68 | 1.02 | 37.5 | 28.3 | 21.8 |
| Davidson Plum 15% | 986.7 | 2.20 | 1.40 | 39.7 | 27.0 | 30 |
| Davidson Plum 20% | 1063.0 | 2.50 | 1.67 | 40.4 | 25.3 | 35 |
| Sorbet Flavor | L* | a* | b* | Chroma* (c) |
|---|---|---|---|---|
| Strawberry 0% | 27.12 ± 5.20 a | 17.01 ± 3.68 a | 5.27 ± 1.50 a | 17.81 ± 3.54 a |
| Strawberry 5% | 17.86 ± 2.47 b | 13.86 ± 3.57 a | 3.45 ± 1.12 b | 14.24 ± 3.49 b |
| Strawberry 10% | 17.95 ± 3.32 b | 11.42 ± 2.67 b | 2.27 ± 1.09 b | 11.64 ± 2.63 c |
| Strawberry 15% | 17.27 ± 3.80 b | 9.33 ± 3.0 b | 1.47 ± 0.92 c | 9.46 ± 2.96 c |
| Strawberry 20% | 22.38 ± 5.15 b | 17.64 ± 5.44 a | 4.47 ± 1.70 a | 18.18 ± 5.30 a |
| Raspberry 0% | 15.90 ± 4.11 a | 15.00 ± 5.71 a | 4.13 ± 2.58 a | 15.55 ± 5.56 a |
| Raspberry 5% | 18.71 ± 3.11 b | 14.71 ± 3.45 a | 3.33 ± 0.92 a | 15.08 ± 3.37 a |
| Raspberry 10% | 14.00 ± 3.07 a | 10.01 ± 3.45 b | 1.65 ± 1.20 b | 10.14 ± 3.42 b |
| Raspberry 15% | 14.28 ± 3.45 a | 7.2 ± 3.48 c | 2.51 ± 1.12 b | 7.64 ± 3.31 c |
| Raspberry 20% | 16.54 ± 2.38 a | 10.30 ± 4.03 b | 1.89 ± 1.28 c | 10.47 ± 3.98 b |
| Pomegranate 0% | 21.50 ± 3.77 a | 10.44 ± 1.48 a | 3.83 ± 0.67 a | 11.12 ± 1.41 a |
| Pomegranate 5% | 16.48 ± 4.31 b | 9.80 ± 2.71 b | 1.73 ± 0.79 b | 9.95 ± 2.68 a |
| Pomegranate 10% | 17.49 ± 3.70 b | 10.57 ± 2.99 b | 1.82 ± 0.78 b | 10.73 ± 2.94 a |
| Pomegranate 15% | 18.53 ± 2.38 b | 11.74 ± 2.30 b | 1.42 ± 0.58 b | 11.83 ± 2.27 a |
| Pomegranate 20% | 15.26 ± 4.96 b | 6.17 ± 1.99 c | −0.04 ± 1.12 c | 6.17 ± 1.99 b |
| Davidson Plum 0% | 44.69 ± 4.65 a | −0.17 ± 0.12 a | 0.59 ± 0.29 a | 0.61 ± 0.28 a |
| Davidson Plum 5% | 25.12 ± 2.32 b | 16.97 ± 2.66 b | 2.70 ± 0.56 b | 17.22 ± 2.62 b |
| Davidson Plum 10% | 23.13 ± 3.84 b | 15.06 ± 4.25 b | 2.40 ± 1.01 b | 15.25 ± 4.20 b |
| Davidson Plum 15% | 19.77 ± 2.85 c | 16.68 ± 2.82 b | 3.14 ± 0.74 b | 16.97 ± 2.77 b |
| Davidson Plum 20% | 19.93 ± 4.24 c | 14.18 ± 2.85 b | 2.25 ± 0.92 b | 14.33 ± 2.83 c |
| Sorbet Flavour | Bioactive Compounds | ||||||
|---|---|---|---|---|---|---|---|
| TPC | TFC | FRAP | DPPH | CUPRAC | Proanthocyanins | Anthocyanins | |
| (μgGAE/mL) | (μgCE/mL) | (µMTE) | (µMTE) | (µMTE) | (µgCE/mL) | (µg/mL) | |
| Strawberry 0% | 87.19 ± 0.00 a | 2.19 ± 0.05 a | 0.97 ± 0.01 a | 33.79 ± 0.02 a | 1.41 ± 0.00 a | 61.68 ± 0.00 a | 5.91 ± 0.00 a |
| Strawberry 5% | 200.16 ± 0.16 b | 2.12 ± 0.10 a | 1.3 ± 0.01 b | 1135.11 ± 0.04 b | 1.97 ± 0.00 a | 39.51 ± 0.01 b | 5.77 ± 0.00 a |
| Strawberry 10% | 270.16 ± 0.16 c | 2.34 ± 0.07 a | 2.04 ± 0.04 c | 1894.50 ± 0.05 b | 3.24 ± 0.02 b | 45.30 ± 0.00 b | 6.32 ± 0.00 b |
| Strawberry 15% | 338.92 ± 0.09 c | 2.63 ± 0.01 a | 2.46 ± 0.03 d | 3214.49 ± 0.06 c | 3.46 ± 0.00 b | 87.91 ± 0.00 c | 2.37 ± 0.00 c |
| Strawberry 20% | 447.29 ± 0.09 d | 3.01 ± 0.04 b | 2.85 ± 0.05 e | 3415.27 ± 0.04 c | 4.06 ± 0.00 c | 99.28 ± 0.01 c | 4.80 ± 0.00 d |
| Raspberry 0% | 150.74 ± 0.08 a | 2.16 ± 0.07 a | 1.19 ± 0.02 a | 99.40 ± 0.06 a | 1.40 ± 0.00 a | 97.35 ± 0.00 a | 0.634 ± 0.00 a |
| Raspberry 5% | 195.10 ± 0.06 b | 2.73 ± 0.03 a | 1.80 ± 0.02 b | 805.11 ± 0.05 b | 2.29 ± 0.00 b | 33.74 ± 0.00 b | 0.067 ± 0.00 b |
| Raspberry 10% | 321.73 ± 0.06 c | 2.71 ± 0.06 a | 2.27 ± 0.03 c | 2769.19 ± 0.03 c | 3.25 ± 0.00 c | 59.76 ± 0.00 c | 0.7 ± 0.00 a |
| Raspberry 15% | 329.87 ± 0.04 c | 2.86 ± 0.04 a | 2.51 ± 0.01 d | 3709.48 ± 0.04 d | 3.61 ± 0.00 d | 82.89 ± 0.00 d | 0.133 ± 0.00 c |
| Raspberry 20% | 357.50 ± 0.04 c | 2.89 ± 0.03 a | 2.63 ± 0.02 d | 4051.41 ± 0.03 e | 3.64 ± 0.00 d | 91.57 ± 0.01 d | 0.55 ± 0.00 a |
| Pomegranate 0% | 185.88 ± 0.01 a | 0.60 ± 0.04 a | 0.95 ± 0.01 a | 1119.30 ± 0.06 a | 1.28 ± 0.00 a | 86.60 ± 0.00 a | 0.350 ± 0.00 a |
| Pomegranate 5% | 217.72 ± 0.01 a | 0.83 ± 0.08 a | 1.27 ± 0.02 b | 1983.96 ± 0.05 a | 1.85 ± 0.00 a | 152.99 ± 0.00 b | 0.650 ± 0.00 b |
| Pomegranate 10% | 478.99 ± 0.01 b | 0.81 ± 0.06 a | 1.91 ± 0.03 c | 3946.05 ± 0.03 b | 3.25 ± 0.00 b | 196.29 ± 0.00 c | 1.15 ± 0.00 c |
| Pomegranate 15% | 563.09 ± 0.03 c | 0.89 ± 0.03 a | 2.15 ± 0.01 c | 4492.73 ± 0.04 b | 3.39 ± 0.00 b | 233.81 ± 0.00 d | 0.095 ± 0.00 d |
| Pomegranate 20% | 730.74 ± 0.04 d | 1.21 ± 0.04 b | 2.81 ± 0.08 d | 5588.08 ± 0.02 c | 5.29 ± 0.05 c | 288.66 ± 0.00 e | 1.6 ± 0.00 c |
| Davidson Plum 0% | 47.36 ± 0.02 a | 0.60 ± 0.02 a | 0.09 ± 0.00 a | 137.17 ± 0.03 a | 0.12 ± 0.00 a | 98.15 ± 0.00 a | 0.061 ± 0.00 a |
| Davidson Plum 5% | 108.59 ± 0.01 b | 0.75 ± 0.04 a | 0.70 ± 0.00 b | 314.10 ± 0.04 b | 1.06 ± 0.00 b | 115.47 ± 0.00 a | 2.116 ± 0.00 b |
| Davidson Plum 10% | 326.58 ± 0.02 c | 0.89 ± 0.04 a | 1.54 ± 0.00 c | 1970.04 ± 0.04 c | 2.12 ± 0.00 c | 229.49 ± 0.00 b | 4.115 ± 0.00 c |
| Davidson Plum 15% | 442.85 ± 0.02 d | 1.08 ± 0.03 b | 2.44 ± 0.02 d | 2135.04 ± 0.07 c | 3.63 ± 0.01 d | 268.46 ± 0.00 c | 4.97 ± 0.00 d |
| Davidson Plum 20% | 456.68 ± 0.02 d | 1.21 ± 0.02 b | 2.99 ± 0.03 e | 3292.01 ± 0.06 d | 4.39 ± 0.00 e | 281.44 ± 0.00 c | 2.87 ± 0.00 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harriden, B.; Stathopoulos, C.; Chockchaisawasdee, S.; McKune, A.J.; Naumovski, N. Physicochemical and Sensory Properties of Davidson Plum (Davidsonia jerseyana) Sorbet, a Potential for New Functional Food Product. Foods 2025, 14, 2902. https://doi.org/10.3390/foods14162902
Harriden B, Stathopoulos C, Chockchaisawasdee S, McKune AJ, Naumovski N. Physicochemical and Sensory Properties of Davidson Plum (Davidsonia jerseyana) Sorbet, a Potential for New Functional Food Product. Foods. 2025; 14(16):2902. https://doi.org/10.3390/foods14162902
Chicago/Turabian StyleHarriden, Brittany, Costas Stathopoulos, Suwimol Chockchaisawasdee, Andrew J. McKune, and Nenad Naumovski. 2025. "Physicochemical and Sensory Properties of Davidson Plum (Davidsonia jerseyana) Sorbet, a Potential for New Functional Food Product" Foods 14, no. 16: 2902. https://doi.org/10.3390/foods14162902
APA StyleHarriden, B., Stathopoulos, C., Chockchaisawasdee, S., McKune, A. J., & Naumovski, N. (2025). Physicochemical and Sensory Properties of Davidson Plum (Davidsonia jerseyana) Sorbet, a Potential for New Functional Food Product. Foods, 14(16), 2902. https://doi.org/10.3390/foods14162902







